Abstract
Immune signalling pathways need to be tightly regulated as overactivation of these pathways can result in chronic inflammatory diseases and cancer. NF-κB signalling and associated innate immune pathways are crucial in the first line of defense against infection in all animals. In a genome-wide RNAi screen for modulators of Drosophila immune deficiency (IMD)/NF-κB signalling, we identified components of the Ras/MAPK pathway as essential for suppression of IMD pathway activity, even in the absence of an immune challenge. Downregulation of Ras/MAPK activity mimics the induction of innate immune responses by microbial patterns. Conversely, ectopic Ras/MAPK pathway activation results in the suppression of Drosophila IMD/NF-κB signalling. Mechanistically, we show that the Ras/MAPK pathway acts by inducing transcription of the IMD pathway inhibitor Pirk/Rudra/PIMS. Finally, in vivo experiments demonstrate a requirement for Ras/MAPK signalling in restricting innate immune responses in haemocytes, fat body and adult intestinal stem cells. Our observations provide an example of a pathway that promotes cell proliferation and has simultaneously been utilized to limit the immune response.
Keywords: Drosophila , innate immunity, intestinal stem cells, MAPK, RNAi
Introduction
Drosophila is a well-established model for the study of innate immune responses and has led to the identification of evolutionarily conserved signalling pathways involved in both cellular and humoural aspects of host defense. Screens for Drosophila mutants in which the immune induction of antimicrobial peptides is abolished revealed two signalling pathways that regulate NF-κB-dependent immune gene transcription: the Toll and immune deficiency (IMD) pathways (Lemaitre and Hoffmann, 2007).
The IMD pathway is activated by recognition of DAP-type (meso-diaminopimelic acid) peptidoglycans by the PGRP-LC and PGRP-LE receptors (Choe et al, 2002; Gottar et al, 2002; Takehana et al, 2004). Receptor activation brings about the association of the adaptor protein IMD, a homologue of human RIPK, with DmelFADD and the apical caspase Dredd (Leulier et al, 2002; Naitza et al, 2002). Drosophila equivalents of the mammalian IKK complex, ird5/IKKβ and Kenny/IKKγ, phosphorylate and transactivate the NF-κB homologue Relish (Rel) (Rutschmann et al, 2000; Silverman et al, 2000; Lu et al, 2001; Erturk-Hasdemir et al, 2009). Rel is cleaved, possibly by Dredd, whereupon the activated Rel domain translocates to the nucleus to activate target gene expression (Dushay et al, 1996; Hedengren et al, 1999). The transforming growth factor β-activated kinase 1 kinase and TAB are thought to activate the Drosophila IKK complex in an IMD- and possibly DmelFADD-dependent manner (Kleino et al, 2005; Zhuang et al, 2006).
Genomic analysis of signal transduction, the identification of novel pathway components and the dissection of cross-regulatory mechanisms have advanced in recent years to offer more integrated models of how signalling pathways elicit coordinated responses (Fraser and Germain, 2009). Large-scale RNAi screens have been employed successfully in the identification of novel components and interconnections between signalling pathways in cultured cell lines as well as in model organisms (Boutros and Ahringer, 2008). Recently, several large-scale RNAi screens were conducted to find novel regulators of IMD signalling (Foley and O’Farrell, 2004; Gesellchen et al, 2005; Kleino et al, 2005). In an RNAi screen for regulators of IMD signalling, in cultured Drosophila cells of haematopoetic origin, several components of the Ras/MAPK signalling pathway were found to act as negative regulators (Gesellchen et al, 2005).
Most of our knowledge of Ras/MAPK signalling has been derived from work on receptor tyrosine kinases (RTKs), although Ras signalling can also be activated through other classes of membrane receptors. In Drosophila, RTKs initiate signalling by activation of the small GTPase Ras oncogene at 85D (Ras85D) (Simon et al, 1991). Activated Ras85D then triggers the sequential phosphorylation of the three MAPKs within the cascade; Pole hole (phl, MAP3K/Raf1 homologue), which phosphorylates Downstream of raf1 (Dsor1, MAP2K1/MEK homologue) which then activates Rolled (Rl, MAPK/ERK1/2 homologue) (Nishida et al, 1988; Tsuda et al, 1993; Brunner et al, 1994b). Activated Rl modulates the expression of target genes through Ets transcription factors including the Pointed (Pnt) protein (O’Neill et al, 1994; Brunner et al, 1994a).
Here, we characterize the involvement of the Ras/MAPK pathway in the Drosophila innate immune response and show that active Ras/MAPK signalling is required for intrinsic suppression of IMD signalling in cultured cells and in all immune tissues examined in vivo, including haemocytes, fat body and the adult midgut. Concomitant with a role in immune suppression in the gut, we find that Ras/MAPK is also required for proliferation of intestinal stem cells (ISCs) and enteroblasts (EBs). We propose a model where Ras/MAPK signalling acts cell autonomously to repress PGRP-LC activity by modulating the expression of Pirk/Rudra/PIMS. Our results provide evidence for a novel interaction of Ras/MAPK and NF-κB signalling that limits the strength of innate immune responses.
Results
Ras/MAPK pathway components are negative regulators of IMD signalling
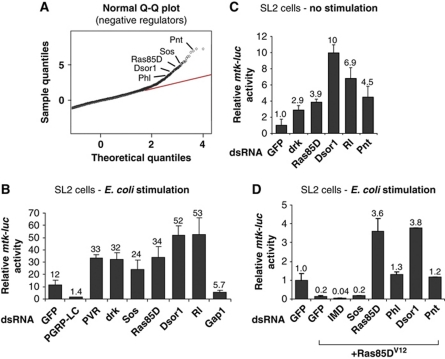
We have previously described an RNAi screening approach to discover novel regulators of IMD signalling in SL2 cells (Gesellchen et al, 2005). Briefly, IMD pathway activity was monitored in SL2 cells, stimulated with heat inactivated Escherichia coli, using a luciferase reporter consisting of a minimal promoter of Metchnikowin, fused to firefly luciferase (mtk-luc). The mtk-luc reporter contains Rel-binding sites that specifically respond to IMD/Rel but not to Toll signalling (Senger et al, 2004). The genome-wide RNAi screen identified 29 putative negative regulators of IMD signalling; among them eight were factors of the Ras/MAPK pathway (Figure 1A; Supplementary Table I). RNAi of the Ras/MAPK cascade components downstream of receptor kinase (drk), Son of sevenless (Sos), Ras85D, phl, Dsor1, rl and pnt resulted in increased mtk-luc reporter activity and z-scores ranging from 7.2 for pnt to 3.5 for phl. The z-score indicates the number of standard deviations an observed phenotype is above or below the mean observations in the screen. Consistent with the finding that knockdown of positive Ras/MAPK regulators enhanced IMD signalling, RNAi against GTPase-activating protein 1 (Gap1), a negative regulator of Ras/MAPK signalling, led to significantly reduced IMD signalling activity (Gesellchen et al, 2005). The overrepresentation of Ras/MAPK cascade members (8 out of 29) among the candidate repressors in the screen strongly suggested a role for the Ras/MAPK cascade in the negative regulation of IMD signalling.
Figure 1.
Ras/MAPK signalling is required and sufficient for suppression of IMD signalling. (A) Identification of negative regulators of IMD signalling. Q-Q plot of normally distributed quantiles against screening result quantiles. A perfect fit to a normal distribution is represented by red line. Shown is the tail of negatively interacting dsRNAs. (B) Confirmation of Ras/MAPK pathway knockdown phenotypes in the IMD pathway luciferase reporter assay (mtk-luc) with independent dsRNAs in Drosophila SL2 cells stimulated with E. coli. (C) Depletion of Ras/MAPK components is sufficient to induce mtk-luc reporter activity in the absence of an immune stimulus. (D) Ectopic activation of Ras/MAPK pathway by expression of dominant active Ras (Ras85DV12) suppresses the induction of IMD signalling by E. coli. Knockdown of Ras85D or factors acting downstream of Ras85D (Phl, Dsor1, Pnt) are able to rescue mtk-luc suppression. Data are shown as the mean (values above bars) and standard deviation of at least six replicates, and is representative of at least three independent experiments.
To confirm that the observed phenotypes were not caused by unintended knockdowns due to unspecific double-stranded RNA (dsRNA)-sequence matches in secondary genes (off-target effects) independent dsRNAs, and additional dsRNAs against Ras/MAPK signalling components, were used to validate the results from the screen. As shown in Figure 1B, RNAi of platelet-derived growth factor (PDGF)/vascular endothelial growth factor (VEGF) receptor (PVR) and the signalling components Drk, Sos, Ras85D, Dsor1 and rl increased mtk-luc reporter activity after stimulation with E. coli. In contrast, depletion of Gap1 significantly reduced mtk-luc activity. These results confirm and extend previous RNAi screen results implicating this pathway as a possible regulator of IMD signalling (Foley and O’Farrell, 2004; Gesellchen et al, 2005; Kleino et al, 2005; Bond and Foley, 2009).
Ras/MAPK signalling mediates intrinsic control of IMD signalling
Depletion of negative regulators of immune signalling can result in spurious immune responses in the absence of infection. We therefore asked whether disruption of Ras/MAPK pathway activity is able to induce IMD signalling in SL2 cells in the absence of immune stimulation. We monitored mtk-luc activity in cells depleted for members of the Ras/MAPK pathway in the absence of E. coli treatment. As shown in Figure 1C, depletion of Ras/MAPK signalling components resulted in an induction of the mtk-luc reporter induction without stimulation. This indicates that the IMD pathway in SL2 cells requires constitutive repression in the absence of immune stimulation to remain quiescent and that Ras/MAPK signalling is involved in this repression.
To further confirm a role of the Ras/MAPK pathway in the regulation of IMD signalling, we ectopically expressed dominant active Ras85DV12 in SL2 cells and monitored IMD activity. Activation of Ras/MAPK signalling by expression of Ras85DV12 strongly suppressed IMD pathway activity, upon stimulation, compared with the control (Figure 1D). Consistently, knockdown of pathway components at the level of Ras85D or downstream was able to rescue this phenotype. RNAi of sos was unable to reverse reporter repression while depletion of Ras85D, phl, Dsor1 and pnt, in samples overexpressing Ras85DV12, resulted in normal or increased reporter induction (Figure 1D).
Ras/MAPK signalling can be activated by several RTKs including epidermal growth factor receptor (EGFR), Torso, Sev (Sevenless) and PVR in Drosophila (Perrimon, 1994; Duchek et al, 2001; Cho et al, 2002). Our data suggested that the RTK PVR acts as the Ras/MAPK cascade initiating receptor in Drosophila SL2 cells. Pvf1–3 (PDGF- and VEGF-related factors 1–3) are proposed ligands for PVR and Pvf2 is described as a specific ligand for PVR in haemocytes (Munier et al, 2002; Bruckner et al, 2004). To examine whether PVR ligands are able to suppress IMD activity, Pvf1–3 were ectopically expressed in the mtk-luc reporter assay. To similarly occupy protein expression and ligand secretion, overexpression of Wingless (Wg) (Bartscherer et al, 2006) was used as a negative control. As shown in Supplementary Figure S1, overexpression of Pvf1, Pvf2 and Pvf3 all reduced mtk-luc activity while simultaneous RNAi of PVR in these cells was able to rescue this repression. RNAi of PVR in the control samples (control vector and Wg) resulted in ectopic mtk-luc activity, indicating that Pvf growth factors, signalling through PVR, are able to mediate Ras/MAPK repression of the IMD pathway in Drosophila haemocyte-like cells.
Modulation of Ras/MAPK signalling affects expression of immune responsive genes
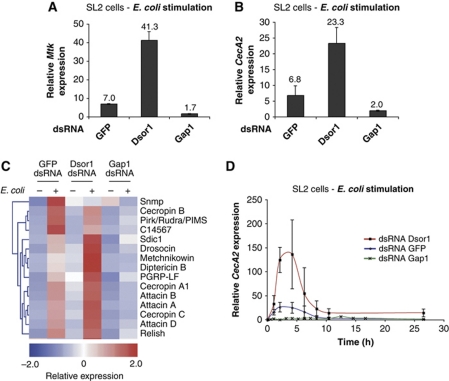
To confirm that the RNAi phenotypes of Ras/MAPK components were not limited to the mtk-luc reporter assay, we examined the induction of endogenous IMD pathway target genes, Metchnikowin (Mtk) and Cecropin A2 (CecA2), by quantitative RT–PCR (qRT–PCR). SL2 cells were treated with dsRNA targeting Dsor1 or Gap1 before induction with E. coli. Knockdown of Dsor1 enhanced the expression of Mtk and CecA2 (Figure 2A and B). Conversely, RNAi of Gap1 decreased Mtk and CecA2 expression below GFP control levels. These results demonstrate that Ras/MAPK mediated repression of IMD signalling affects the expression of endogenous targets of the pathway.
Figure 2.
Ras/MAPK pathway activity modulates the expression of antimicrobial peptides upon infection. The relative expression levels of antimicrobial peptides Mtk (A) and CecA2 (B) were monitored by qRT–PCR, upon treatment with E. coli, in SL2 cells depleted for Dsor1 and Gap1. RNAi of Dsor1 results in increased expression, while Gap1 RNAi results in decreased expression levels compared with the control (GFP dsRNA). (C) Cluster analysis of the transcriptional response of SL2 cells to E. coli treatment and Ras/MAPK modulation. Antimicrobial peptide and other IMD target genes are activated upon stimulation of SL2 cells with E. coli. Activation of Ras/MAPK signalling by depletion of Gap1 potently suppresses the induction of IMD target genes by E. coli. RNAi of Dsor1 results in mild induction of target genes in the absence of stimulation and increased target gene expression upon stimulation compared with GFP control. In the cluster, red represents expression levels above the mean expression of a gene across all samples, white represents mean expression and blue represents expression lower than the mean (see colour scale at the bottom of the clustering). (D) Ras/MAPK activity alters the magnitude of IMD signalling but does not affect the kinetics of antimicrobial peptide expression. CecA2 expression was monitored by qRT–PCR upon infection with E. coli in SL2 cells depleted for Dsor1 and Gap1. Expression data for each dsRNA sample are normalized to the 0-h time point. Error bars represent the s.d. of three replicates. Data shown are representative of at least three independent experiments.
To gain a more comprehensive view of the changes in IMD target gene expression upon modulation of Ras/MAPK signalling, and to elucidate whether Ras/MAPK signalling additionally interferes with other signalling routes, such as Toll immune signalling, we performed microarray expression profiling analysis. We compared transcript profiles from SL2 cells depleted for GFP, Dsor1 or Gap1 with samples treated with dsRNAs against GFP as a control. In addition, we compared the expression profiles of these cells in the presence or absence of E. coli stimulation. Transcriptome analysis revealed 39 genes that were significantly differentially expressed across all samples and could be grouped into three major clusters upon hierarchical clustering (Supplementary Figure S2, see Materials and methods). Hierarchical cluster analysis of differentially expressed genes in response to immune stimulation and to modulation of Ras/MAPK activity grouped all antimicrobial peptides together in one cluster. Figure 2C shows the cluster of genes enriched for known IMD pathway target genes including the Attacins, Cecropins and Diptericin. The IMD signalling component Rel and known negative regulators PGRP-LF and Pirk/Rudra/PIMS also clustered with the antimicrobial peptides. RNAi of Dsor1 combined with E. coli treatment resulted in increased expression levels of this cluster of genes (Figure 2C). Conversely, upon Gap1 RNAi IMD target gene induction was strongly repressed in the E. coli stimulated samples. Gap1 depletion also induced upregulation of known direct target genes of Ras/MAPK signalling such as the negative Ras/MAPK regulator sprouty (Supplementary Figure S2). In summary, expression of several IMD target genes is linked to the levels of Ras/MAPK signalling in Drosophila SL2 cells.
Ras/MAPK signalling regulates the strength of the immune response
The expression of antimicrobial peptides is tightly regulated both in terms of magnitude of mRNA levels and kinetics of expression (Aggarwal and Silverman, 2008). Given that Ras/MAPK modulation affects IMD target gene induction, we next asked whether the altered levels of IMD target expression were due to a longer sustained immune response, that is loss of negative feedback on the pathway resulting in higher and/or prolonged expression of antimicrobial peptides.
We therefore examined the kinetics of expression of Mtk and CecA2 by qRT–PCR in SL2 cells treated with dsRNA against Dsor1 or Gap1. Upon E. coli stimulation, Mtk and CecA2 display similar expression kinetics with a peak of expression at ∼5 h after E. coli stimulation (Figure 2D and data not shown). Samples depleted for Dsor1 displayed normal expression kinetics but a significant increase in the induction of CecA2 (Figure 2D). Depletion of Gap1 strongly suppressed the expression of CecA2 as compared with the control. Similar results were observed with Mtk expression (data not shown), indicating that Ras/MAPK signalling is involved in restricting the magnitude of induction of antimicrobial peptides but not the kinetics of the immune response.
Ras/MAPK signalling represses IMD signalling in larval immune tissues in a cell autonomous manner
In order to assess the role of Ras/MAPK signalling in IMD pathway suppression in vivo, we first examined the larval immune response. We ectopically expressed components of the pathway specifically in the immune responsive organs of Drosophila third instar larvae using the Cg-Gal4 driver, which is expressed in the lymph gland, and circulating haemocytes and the fat body (Asha et al, 2003).
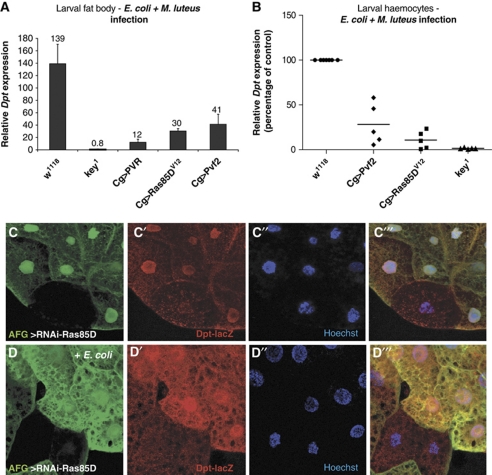
Third instar larvae were infected with a mixture of E. coli and Micrococcus luteus to induce the IMD and Toll pathways, respectively. Six hours after infection, haemocytes and fat body were isolated and the induction of the antimicrobal peptides, Diptericin (Dpt), an IMD pathway target, and Drosomycin (Drs), a Toll pathway target, was monitored by qRT–PCR. As shown in Figure 3A, overexpression of Ras85DV12, PVR or Pvf2 resulted in robust repression of Dpt expression in the fat body, while Drs expression remained comparable to the control (Supplementary Figure S3A). This phenotype is also seen in haemocytes isolated from infected larvae (Figure 3B; Supplementary Figure S3B). As the expression of Drs was not affected by Ras85D, PVR or Pvf2 expression, we can conclude that the general immune function of the fat body or haemocytes is not compromised. These results show that Ras/MAPK activity specifically inhibits IMD signalling in vivo in the larval haemocytes and fat body.
Figure 3.
Modulating MAPK signalling alters IMD pathway activity in vivo in the larval fat body and haemocytes. Ectopic activation of the MAPK pathway by overexpression of UAS-Ras85DV12, PVR and Pvf2 with Cg-Gal4 (Cg>). Dpt expression was monitored by qRT–PCR in isolated fat body (A) or haemocytes (B) 6 h after infection with a mixture of E. coli and M. luteus. (A) Overexpression of Ras/MAPK components results in significant decreases in Dpt induction. kenny mutant flies (key1) are included as a positive control. Mean values are shown above the bars (N=12 for all genotypes, except key1, N=6). Data are representative of at least four independent experiments. (B) Dpt expression is also reduced in haemocytes overexpressing Ras85DV12 and Pvf2. Data are shown as Dpt expression levels relative to the w1118 control. (C, D) Ras signalling modulates the IMD pathway in a cell autonomous manner. Depletion of Ras85D in flipout Gal4 clones in the larval fat body in the absence of infection (C) or upon infection with E. coli (D) results in upregulation of Dpt-lacZ specifically in the Ras85D-depleted clones but not neighbouring wild-type cells. AFG, actin flipout Gal4.
To confirm our observations in the larval fat body, we used a Diptericin-lacZ (Dpt-lacZ) reporter to monitor IMD pathway activity. We specifically depleted Ras85D in actin flipout clones in the larval fat body using a UAS-RNAi construct. As shown in Figure 3C and Supplementary Figure S4, in the absence of infection, RNAi of Ras85D results in a slight induction of the Dpt-lacZ reporter specifically in the Ras85D-depleted cells but not the neighbouring wild-type cells. Upon E. coli infection, Dpt-lacZ expression is increased in the Ras85D-depleted clones relative to the surrounding cells (Figure 3D; Supplementary Figure S4). This supports our observations in vitro that Ras signalling is required for intrinsic repression of basal IMD activity. Our results also suggest that the Ras pathway acts cell autonomously to repress IMD signalling.
Ras/MAPK signalling modulates IMD signalling in adult flies
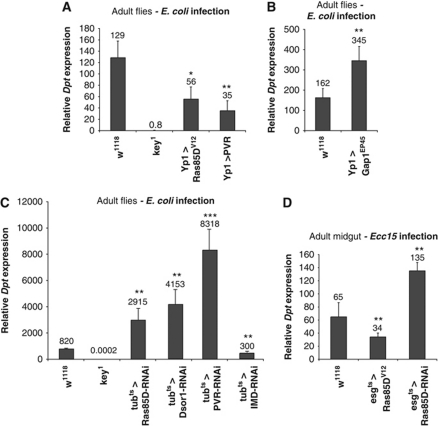
To rule out that Ras/MAPK mediated repression of IMD signalling occurs solely in haemocytes or the fat body in developing larvae, we also monitored antimicrobial peptide expression in adult flies. To this end, we used the Yolk protein 1 (Yp1-Gal4) driver (Georgel et al, 2001). This driver is expressed 3 days after eclosion in the female fat body thereby avoiding any effects on the development of the fat body. Four-day-old adult females were infected with either E. coli or M. luteus, and expression of Dpt and Drs was monitored 6 and 24 h after infection, respectively. Overexpression of Ras85DV12 resulted in significantly reduced Dpt expression while the expression of Drs was unaffected (Figure 4A; Supplementary Figure S5A). A stronger reduction in Dpt transcript levels was seen with overexpression of PVR. Flies containing Yp1-Gal4 or any of the UAS constructs on their own did not display changes in AMP expression demonstrating the specificity of our results (Supplementary Figure S6).
Figure 4.
Modulation of Ras/MAPK signalling alters IMD pathway activity in vivo in the adult fat body and gut. (A) Ectopic activation of the Ras/MAPK pathway in the adult fat body by overexpression of UAS-Ras85DV12 and PVR using Yp1-Gal4 (Yp1>) results in a strong decrease in Dpt induction upon infection compared with the wild-type control (N=12 for all genotypes, except key1, N=6). (B) Reduction of Ras/MAPK pathway activity through overexpression of Gap1EP45 results in increased induction of Dpt expression upon infection with E. coli (w1118 and Gap1EP45, N=15). (C) Depletion of Ras pathway components in adult flies using RNAi constructs and tubGal4ts (tubts>) also results in increased Dpt expression upon infection. kenny mutant flies (key1) and UAS-IMD-RNAi are positive controls (all genotypes N=4). Dpt expression was monitored by qRT–PCR in flies 6 h post-infection with E. coli. (D) In addition, modulation of Ras/MAPK activity using esgGal4ts (esgts>) in the adult midgut affects Dpt induction. RNAi of Ras85D results in increased Dpt expression while overexpression of Ras85DV12 results in Dpt repression. Relative expression levels of Dpt were monitored by qRT–PCR in flies 18 h after infection with E. carotova carotova (Ecc15) (all genotypes N=4). Mean values are shown above the bars and data are representative of at least two independent experiments. *P<0.05, **P<0.01, ***P<0.001.
In order to examine the effect of downregulation of Ras/MAPK signalling on IMD pathway activity in vivo, we overexpressed the negative regulator Gap1 using Yp1-Gal4 and the EP insertion line Gap1EP45 (Rorth, 1996). Ras activity is regulated by GAPs, which stimulate the conversion of activated GTP-bound Ras to GDP-bound Ras, and thus function as a negative regulator of the pathway (Gaul et al, 1992). As shown in Figure 4B, overexpression of Gap1 resulted in a stronger induction of Dpt expression compared with the wild-type control, while Drs expression was not affected (Supplementary Figure S5B).
To confirm that Ras pathway downregulation results in increased AMP expression, we expressed UAS-RNAi constructs targeting Ras pathway components, using the tubGal4; tubGal80ts system (tubulin-Gal4 combined with a temperature-sensitive Gal4 inhibitor, Gal80 (tubulin-Gal80ts)). This inducible system allows us to bypass lethality arising from loss of Ras signalling during development. Flies were raised at 18°C and then shifted to 29°C (3 days after eclosion) for 10 days to deplete Ras pathway components before infection with either E. coli or M. luteus. Expression of Dpt and Drs were monitored by qRT–PCR as above.
Upon RNAi of PVR, Dsor1 and Ras85D, we observe increased expression of Dpt (Figure 4C). In addition, we observe increased expression of CecA2 and Mtk in flies depleted for Ras signalling components (Supplementary Figure S7). These observations are in agreement with previous results showing that RNAi of PVR upregulates Attacin expression in adult flies (Bond and Foley, 2009). Drs expression was not affected in flies depleted for Ras pathway components (Supplementary Figure S5C). These results demonstrate that the Ras pathway specifically modulates IMD signalling activity in vivo but does not affect the ability of the fat body to produce antimicrobial peptides induced by Toll signalling.
Ras/MAPK signalling is required for immune suppression and stem cell proliferation in the Drosophila gut
The intestine constitutes one of the barrier epithelia against outside infection. The Drosophila midgut is of particular interest as a delicate balance between immune suppression against indigenous gut flora while a robust immune response against invading microbes is required. Recently, expression profiling and other studies of the immune response, specifically in the adult Drosophila midgut, has revealed the major role of the IMD pathway in antimicrobial peptide production (Liehl et al, 2006; Ryu et al, 2006; Nehme et al, 2007; Buchon et al, 2009b). In addition, signalling pathways previously associated with the immune response have also been shown to be important for gut homeostasis. JNK and JAK/STAT signalling are required for gut epithelium renewal upon infection through ISC proliferation (Biteau et al, 2008; Cronin et al, 2009; Jiang et al, 2009; Buchon et al, 2009a).
To examine whether the modulation of Ras/MAPK signalling in the gut also affects the IMD mediated immune response, flies were orally infected with Erwinia carotova carotova (Ecc15), a Gram-negative bacterium. As expression of the Ras85-RNAi construct was lethal, we used the temperature-sensitive esgGal4ts system (esgGal4 combined with tubGal80ts) (Jiang et al, 2009). As shown in Figure 4D, esgGal4ts flies expressing a Ras85D-RNAi construct showed increased Dpt expression upon infection. Conversely, overexpression of Ras85DV12 resulted in repression of Dpt expression consistent with our results in adult flies.
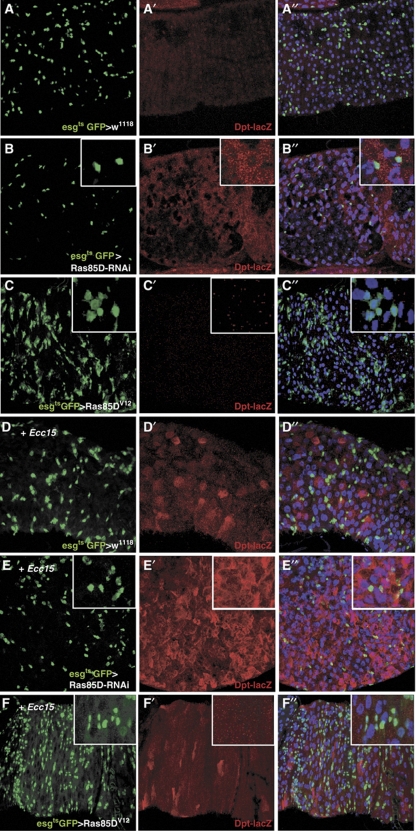
The adult Drosophila midgut is made up of mature enterocytes (ECs), EBs and ISCs (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). In order to examine at a cellular level which intestinal cell types are involved in Ras-mediated repression of IMD signalling, we monitored Dpt-lacZ expression by immunofluorescence microscopy. EsgGal4ts was employed to specifically deplete or overexpress Ras85D in ISCs and EBs (Figure 5). MyoIA-Gal4ts (Jiang et al, 2009) was used to specifically target mature ECs. In adult guts of wild-type unchallenged flies, we observe low-level expression of the Dpt-lacZ reporter in ECs, consistent with our observations in the larval fat body (Figure 3C). RNAi of Ras85D using esgGal4ts resulted in induction of Dpt-lacZ in the absence of infection (Figure 5B). Interestingly, Dpt-lacZ expression was observed in almost all the cells in the midgut, including ISCs, EBs and ECs. Depletion of Ras85D using MyoIA-Gal4ts also resulted in induction of Dpt-lacZ, although the phenotype was weaker than that seen with esgGal4ts and induction of Dpt-lacZ was only observed in ECs (Supplementary Figure S8B). As RNAi against Ras85D using esgGal4ts is performed for 8 days before flies are infected, we hypothesize that RNAi in the ISCs and EBs also results in depletion of Ras85D in any differentiated daughter cells during the time course of this experiment. Upon infection with Ecc15, Dpt-lacZ is expressed in the mature ECs of wild-type midguts (Figure 5D). Depletion of Ras85D in ISCs and EBs results in increased Dpt-lacZ expression in all cell types (Figure 5E). RNAi of Ras85D specifically in ECs results in a weaker phenotype (Supplementary Figure S8E).
Figure 5.
Ras/MAPK signalling is required for intrinsic regulation of IMD signalling in vivo in the adult Drosophila midgut. (A–F) Transgenes were expressed in the adult ISCs and EBs using esgGal4ts (esgts>). ISCs and EBs are marked by GFP expression (green) driven by esgGal4ts and DNA stained with Hoechst (blue). Dpt-lacZ (red) was used as a reporter for IMD activity. (A) Wild-type midgut. (B) In the absence of infection, induction of Dpt-lacZ is observed upon expression of Ras85D-RNAi. (C) Overexpression of Ras85DV12 represses basal Dpt-lacZ expression. (D, E) Upon oral infection with Ecc15 for 18 h, Dpt-lacZ is upregulated in Ras85D-depleted guts, (F) while ectopic Ras signalling suppresses IMD activity. Insets show high magnification images of ISCs.
Conversely, overexpression of Ras85DV12 using esgGal4ts resulted in repression of basal Dpt-lacZ expression in unchallenged flies (Figure 5C). In Ecc15-infected guts, high Dpt-lacZ expression is lost in most ECs and the staining pattern is more similar to that of unchallenged flies (Figure 5F). This repression is also observed using MyoIA-Gal4ts after infection (Supplementary Figure S8F). These results provide further evidence of the role of Ras/MAPK in modulating IMD signalling in adult Drosophila immune tissues.
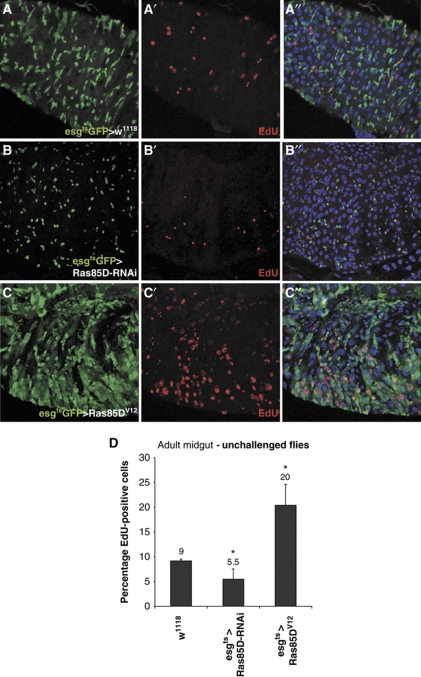
MAPK signalling has recently been shown to be required for proliferation of midgut progenitor cells in the developing larvae (Jiang and Edgar, 2009). As shown in Figure 5, we observe a decrease in the number of GFP-positive cells (ISCs and EBs) upon Ras85D-RNAi using esgGal4ts (Figure 5A and B). Conversely, upon overexpression of Ras85DV12 GFP-positive cell counts increase, as previously observed (Apidianakis et al, 2009) (Figure 5A and C). To examine whether Ras signalling is also required for ISC proliferation in the adult midgut, we monitored EdU incorporation in flies depleted for Ras85D by RNAi or overexpressing Ras85DV12, using esgGal4ts, in the absence of infection (Figure 6). ISCs and EBs were visualized by co-expression of UAS-GFP. As shown in Figure 6A, in wild-type guts, 9% of cells were positive for EdU. Depletion of Ras85D resulted in fewer proliferating cells (5.5%) compared with wild-type guts (Figure 6B and D). Upon overactivation of the Ras/MAPK pathway, using UAS-Ras85DV12, an increase in the number of EdU incorporating cells is seen (Figure 6C and D). This suggests that Ras/MAPK signalling is also required for cell proliferation in the adult midgut as well as in the midgut progenitor population during larval and pupal stages (Jiang and Edgar, 2009).
Figure 6.
Ras/MAPK signalling is required for proliferation of the ISCs. (A–C) Transgenes were expressed in the adult ISCs using esgGal4ts (esgts>). ISCs and EBs are marked by GFP expression (green) driven by esgGal4ts and DNA is stained with Hoechst (blue). EdU incorporation (red) was used to identify proliferating cells. (B) Expression of UAS-Ras85D-RNAi results in reduced numbers of EBs and ISCs, while expression of UAS-Ras85DV12 (C) results in increased proliferation. (D) Quantitation of EdU incorporation in the Drosophila gut upon modulation of Ras signalling. Data are shown as percentage of EdU-positive nuclei per midgut section (*P<0.05).
Ras/MAPK activity affects resistance to infection
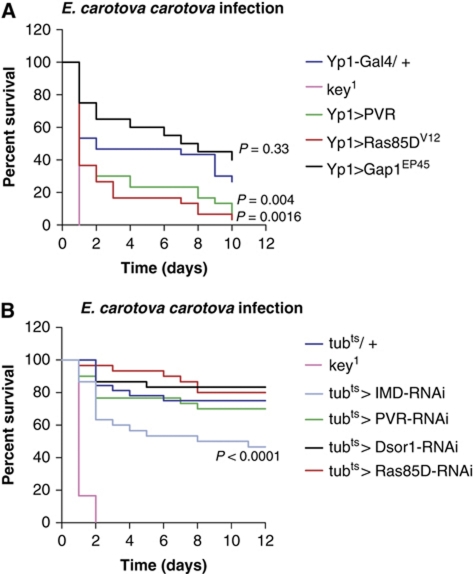
We next asked whether Ras/MAPK pathway modulation would also affect resistance of Drosophila to infection with Ecc15. We therefore compared the survival of flies with altered Ras/MAPK activity upon infection by septic injury with Ecc15. As shown in Figure 7A, flies overexpressing Ras85DV12 or PVR displayed decreased survival rates compared with the control (Yp1-Gal4/+). Gap1 expression resulted in initial increased survival but did not offer significant long-term protection against infection. Similarly, depletion of Ras85D and Dsor1 using tubGal4ts results in initial increased survival but not significant long-term resistance to infection (Figure 7B).
Figure 7.
Ras/MAPK signalling affects resistance to Gram-negative bacterial infection. (A) Flies overexpressing Ras/MAPK components display decreased survival rates upon infection with Ecc15. (B) Flies depleted for Ras pathway components display partial resistance to infection with Ecc15. Data are presented in Kaplan–Meier plots and significance was analysed by log-rank test. Data are representative of three independent experiments.
We also infected flies with the fungus Beauveria bassiana, which elicits a mainly Toll pathway-dependent immune response. As shown in Supplementary Figure S9A, overexpression of Ras pathway components did not significantly alter survival rates compared with control, upon fungal infection, while Dif1 flies, which are defective in Toll-mediated immune signalling, are highly sensitive. Similarly, depletion of Ras pathway components by tubGal4ts did not significantly alter survival rates upon fungal infection (Supplementary Figure S9B). However, the genetic background of tubGal4ts renders flies more sensitive to fungal infection than Yp1-Gal4 flies (compare Supplementary Figure S9A and B). As a control, pricking flies with a sterile needle did not result in altered survival rates, demonstrating that altered Ras/MAPK signalling does not render the flies more sensitive to injury alone (see Supplementary Figure S9C and D).
Repression of IMD signalling by Ras/MAPK signalling occurs upstream or at the level of PGRP-LC
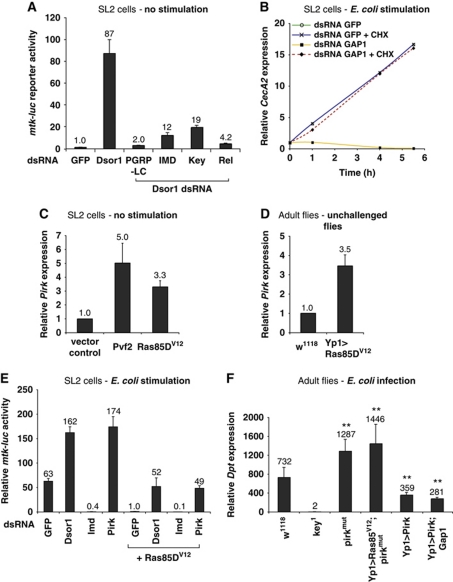
To determine at which level in the cascade Ras/MAPK activity suppresses IMD signalling, we performed an epistasis analysis in SL2 cells. We anticipated that knockdown of an IMD signalling component upstream of the point of intersection should not influence IMD pathway induction by Ras/MAPK knockdown. IMD signalling components were depleted by RNAi, while Ras/MAPK activity was decreased by RNAi of Dsor1. IMD pathway activity was monitored with the mtk-luc reporter. Co-depletion of Dsor1 and GFP resulted in a strong induction of the reporter (Figure 8A). Depletion of PGRP-LC or any downstream component of the IMD pathway abolished the constitutive activation of mtk-luc induced by Dsor1 knockdown. This result indicates that Ras/MAPK mediated suppression of IMD signalling acts upstream of or at the level of the PGRP-LC receptor.
Figure 8.
Mechanism of Ras/MAPK signalling mediated repression of IMD signalling. (A) Ras/MAPK mediated suppression of IMD signalling acts upstream or at level of IMD receptor PGRP-LC. Depletion of PGRP-LC or downstream factors efficiently abolishes the induction of the IMD pathway upon loss of Ras/MAPK signalling (Dsor1 RNAi). (B) Ras/MAPK mediated repression of IMD signalling requires de novo production of a repressor protein. Drosophila SL2 cells depleted for Gap1 were induced with E. coli in the presence or absence of cycloheximide. Depletion of Gap1 represses CecA2 induction. In the presence of cycloheximide, the repressive phenotype caused by Gap1 RNAi is reverted. (C) Ectopic activation of Ras/MAPK signalling results in increased pirk expression in Drosophila SL2 cells. pirk expression was monitored by qRT–PCR in SL2 cells upon Pvf2 and Ras85DV12 overexpression. (D) Expression of UAS-Ras85DV12 using Yp1-Gal4 (Yp1>) results in increased expression of pirk in vivo as monitored by qRT–PCR. (E) Depletion of pirk rescues repression of IMD signalling mediated by Ras/MAPK activation. Mtk-luc activity was monitored upon Ras85DV12 overexpression in SL2 cells stimulated with E. coli. (F) Pirk acts downstream of Ras/MAPK signalling in vivo. Overexpression of UAS-Ras85V12 using Yp1-Gal4 (Yp1>) in a pirkmut (pirkEY00723) mutant background does not result in reduced Dpt expression compared with pirkmut alone. Similarly, overexpression of Gap1 (Gap1EP45) is not able to overcome Dpt repression mediated by overexpression of Pirk (UAS-Pirk). Data are shown as mean (shown above the bars) and s.d. of at least four replicates, and are representative of at least two independent experiments. **P⩽0.005.
We have shown earlier that all components of the Ras/MAPK cascade from PVR to pnt are required for the suppression of IMD signalling. To elucidate if this suppression is mediated through direct cross talk, between a Ras/MAPK pathway component and PGRP-LC, or by a gene target of the Ras/MAPK pathway we modulated Ras/MAPK activity in the presence of cycloheximide to block de novo protein translation. IMD pathway activity was monitored by qRT–PCR of Mtk and CecA2 expression (Figure 8B; Supplementary Figure S10). Drosophila SL2 cells were incubated with dsRNAs against GFP or Gap1 for 5 days and then pretreated with cycloheximide before stimulation with E. coli. As shown in Figure 8B, RNAi of GFP resulted in normal CecA2 expression kinetics in the presence or absence of cycloheximide. As previously shown, activation of Ras/MAPK signalling through depletion of Gap1 resulted in the repression of CecA2 expression. However, in the presence of cycloheximide, this repressive activity was lost, resulting in a near normal CecA2 expression profile. This result was also observed with Mtk expression (Supplementary Figure S10). The expression of sprouty in cycloheximide-treated cells was comparable to untreated cells indicating that cycloheximide did not affect Ras/MAPK pathway activity (data not shown). We therefore conclude that Ras/MAPK signalling controls IMD signalling through the expression of a repressor that inhibits IMD signalling at the level of PGRP-LC.
Ras/MAPK signalling activates the IMD negative regulator Pirk/Rudra/PIMS
Recent studies have identified Pirk/PIMS/Rudra as a negative regulator of IMD signalling. Pirk interacts directly with PGRP-LC and PGRP-LE, and disrupts the interaction of PGRP-LC and IMD, thus interfering with IMD signalling (Aggarwal et al, 2008; Kleino et al, 2008; Lhocine et al, 2008).
To determine if Pirk could be the repressor induced by Ras/MAPK signalling, we examined the induction of pirk upon Ras/MAPK pathway modulation by qRT–PCR. As shown in Figure 8C, overexpression of Pvf2 or Ras85DV12 in SL2 cells results in increased pirk expression. A similar phenotype is also observed in adult flies (Figure 8D). We therefore examined whether depletion of pirk in SL2 cells or loss of pirk in vivo could rescue Ras/MAPK mediated repression of the IMD pathway (Figure 8E and F). SL2 cells were transfected with the mtk-luc reporter and Ras85DV12 overexpression construct and stimulated with E. coli. Depletion of Dsor1 or pirk is able to rescue the repression of the mtk-luc reporter (Figure 8E). In flies, overexpression of Ras85DV12 in a pirk mutant background was unable to silence Dpt induction upon infection (Figure 8F). Finally, disrupting Ras/MAPK signalling by overexpression of Gap1 did not rescue the repression mediated by overexpression of Pirk. These results indicate that Pirk acts downstream of the Ras/MAPK pathway to repress signalling through PGRP-LC. Taken together, the epistasis analysis and the correlation of pirk expression with Ras/MAPK activation suggest that the Ras/MAPK pathway regulates the expression of Pirk, which then suppresses IMD signalling by disrupting the interaction of PGRP-LC and IMD.
Discussion
In this study, we have characterized a novel role for the Ras/MAPK pathway as a regulator of immune responses in Drosophila. Our results show that the Ras/MAPK pathway represses IMD pathway activity in either the presence or absence of an immune stimulus. Furthermore, the Ras/MAPK pathway represses IMD signalling both in Drosophila cell culture and in vivo. The in vivo requirement for Ras/MAPK in immunity was seen in developing larvae as well as adult fat body and midgut. The Ras/MAPK pathway acts to limit the maximum intensity of IMD pathway induction, rather than the kinetics of the response, by controlling transcription of the key IMD inhibitor Pirk. We propose a model where the role of Ras/MAPK signalling in immunity is to limit the strength of IMD signalling during an infection and to prevent spurious immune induction in the absence of infection.
Recent work has shown that the intensity and duration of immune responses in Drosophila are under strict control (Aggarwal and Silverman, 2008). As in mammals, hyperactivated immune responses can be detrimental and efficient downregulation of signalling is critical for proper immune function, overall health and fecundity (Bischoff et al, 2006; Kim et al, 2007; Maillet et al, 2008). To insure effective control of the immune response, the innate immune pathways are modulated at multiple levels by negative regulators. Several of these factors are themselves target genes of these signalling pathways and thus mediate negative feedback regulation.
Pirk/Rudra/PIMS is an IMD pathway target that acts to mediate negative feedback during infection. While many negative feedback regulators are transcriptionally induced by the pathway they regulate, our results show that Pirk is also able to be induced by a different pathway, the Ras/MAPK pathway. Our results also show that Pirk is essential for the immune suppressive effect of Ras/MAPK activation, indicating that Pirk is the key point of integration of these two pathways. In adult flies, loss of pirk does not result in IMD induction in the absence of infection. Similarly, RNAi of Ras components in adult flies does not result in AMP induction as measured by qRT–PCR (data not shown). This is in contrast to our observations in the adult midgut.
Why the Ras/MAPK pathway is deployed in immune suppression remains unclear, but an intriguing possibility is that immune suppression may be particularly important in proliferating cells with high Ras/MAPK signalling. For example, our results show that, in the adult midgut, Ras/MAPK signalling has dual roles, promoting cell proliferation and also repressing IMD signalling. Aberrant activation of IMD signalling in ISCs may therefore be detrimental to gut homeostasis. In mammals, aberrant regulation of immune and inflammatory pathways are thought to be important contributors to inflammatory disease and cancer (Radtke et al, 2006; Fre et al, 2008; Milne et al, 2009).
In haemocytes, Ras/MAPK signalling elicited by PVR and its ligands, Pvf1–3, is involved in cell proliferation and migration during embryogenesis (Duchek et al, 2001; Cho et al, 2002; Munier et al, 2002; Bruckner et al, 2004; Ishimaru et al, 2004; Olofsson and Page, 2005). Pvf2 has a key role in haemocyte proliferation in larvae and PVR has been shown to control plasmatocyte differentiation in the lymph gland (Munier et al, 2002; Jung et al, 2005). PVR has been found as a candidate negative regulator of IMD signalling in several independent RNAi screens for IMD signalling (Foley and O’Farrell, 2004; Kleino et al, 2005).
PVR has also recently been found in an RNAi screen for JNK signalling, and found to regulate AMP expression in vivo (Bond and Foley, 2009). In addition, it has been shown that Pvf2 and Pvf3 expression is induced in S2 cells upon stimulation with peptidoglycan, and that this induction was dependent on active JNK signalling (Bond and Foley, 2009). However, we did not observe significant upregulation of Pvf2 expression upon infection in haemocytes or the larval fat body, nor in whole adult flies in vivo (data not shown). It is therefore possible that induction of Pvf1–3 upon infection can act as a negative feedback loop on IMD signalling solely in the Drosophila gut in vivo and in S2 cells in vitro. In support of this, infection of the Drosophila gut induces expression of Pvf1–2 and the EGFR ligands Vein and Keren by approximately two- to three-fold (Buchon et al, 2009b). Pvf2 has also been shown to be upregulated in the ISCs of ageing flies and correlates with age-associated increases in ISCs and EB proliferation (Choi et al, 2008). Further work is required to show whether upregulation of Pvf2 has an impact on the observation of immune senescence in ageing flies (Zerofsky et al, 2005; Ramsden et al, 2008).
Our results show that Ras/MAPK signalling mediates IMD repression in haemocytes and the adult midgut. Thus, it also has a dual role in promoting developmental proliferation and preventing spurious immune activation in immature immune cells of the fly. Aberrant immune signalling in the lymph gland has been shown to interfere with normal development (Schmidt et al, 2007) and could thereby destroy haemocytes needed in a larval immune response and later during metamorphosis and adulthood. This raises the possibility that differential regulation of Ras/MAPK signalling may allow a degree of developmental control over immune responsiveness.
Immune suppression by Ras/MAPK signalling may also be important for the oncogenic effects of this pathway. Recently, it has been shown that overexpression of oncogenic Ras in the Drosophila ISCs causes intestinal dysplasia when flies are orally infected with virulent Pseudomonas aeruginosa, an opportunistic human gram-negative bacterium (Apidianakis et al, 2009). In agreement with this finding, we also observe increased disorder of the gut epithelium in Ras85DV12 overexpressing flies infected with Ecc15 (see Figure 5). We speculate that Ras/MAPK mediated immune repression could be a contributing factor to the dyplasia seen in guts expressing oncogenic Ras through persistence of the infection coupled with the hyperproliferation of ISCs.
Concluding remarks
The IMD pathway is subject to multiple mechanisms of negative feedback to precisely control signalling activity in response to infection. We have shown that the magnitude of IMD responses can be further modulated by a second signalling pathway, the Ras/MAPK pathway, by having this pathway target transcription of an agent of negative feedback, Pirk. Ras/MAPK signalling could provide constitutive repression of IMD signalling in most tissues, and may do so more strongly in those tissues with the highest levels of signalling. Our observations provide an example of a signalling pathway that regulates cell proliferation that has simultaneously been utilized to limit the response of an innate immune pathway.
Materials and methods
Plasmid constructs
The mtk-luc firefly luciferase reporter and Rp128-RL Renilla luciferase reporter constructs have been described previously (Gesellchen et al, 2005; Bartscherer et al, 2006). To generate the Pvf2 expression plasmid, Pvf2 cDNA (clone RH40211) was subcloned into pAc5.1/V5-HisA (Invitrogen). pUAST-Pvf1 and pUAST-Pvf2 constructs were a gift from B Baum. The pMet-Ras85.V12 construct (a gift from S Katzav) consists of Ras85D.V12 under the control of the metallothionein promoter.
Cell culture and RNAi experiments
SL2 cells were maintained in Schneider's Medium (Invitrogen) (10% fetal bovine serum (PAA) and 1% penicillin/streptomycin (Invitrogen)) at 25°C. dsRNA synthesis, RNAi treatment and luciferase experiments were performed essentially as previously described (Muller et al, 2005). Complete primer and amplicon-sequence information can be found at http://rnai.dkfz.de. SL2 cells were transfected 24 h after dsRNA treatment using Effectene (Qiagen) and incubated at 25°C for 4 days. Heat inactivated E. coli was added to a final concentration of 200 μg/ml. Sixteen hours after stimulation, mtk-luc activity was measured with a Mithras LB940 plate reader (Berthold Technologies). To monitor Pirk expression, SL2 cells were seeded in 24-well plates and transfected with 1 μg pMET-Ras85D.V12 or pAc-Pvf2. Ras85.V12 expression was induced with CuSO4 (500 μM) 6 h post-transfection and cells were harvested for RNA extraction 48 h later.
RNA Extraction and Quantitative RT–PCR
Total RNA was isolated using Trizol (Life Technologies) and reverse transcribed using Superscript III (Invitrogen). Quantitative RT–PCR was performed on a LightCycler (Roche), and transcript levels monitored using Universal Probe Library probes (Roche). Relative expression levels of target genes were calculated according to Pfaffl (2001), using rp49 as an internal control. Primer sequences and UPL probes in Supplementary Table II.
Time course analysis and cycloheximide treatment
SL2 cells were seeded in 24-well plates containing 10 μg dsRNA, induced with heat inactivated E. coli, and collected at appropriate time points by lysis in TRIzol. Isolation of total RNA, reverse transcription and qRT–PCR analysis were performed as described above. For cycloheximide treatment, SL2 cells were incubated with 50 μM cycloheximide for 30 min before E. coli stimulation.
Expression profiling
For expression profiling on Drosophila Gene Array Affymetrix GeneChip arrays (Affymetrix) SL2 cells were treated with dsRNAs targeting GFP, Dsor1 or Gap1 for 4 days, induced with E. coli for 1 h, and RNA isolated as described above. cDNA synthesis was performed with the SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen). Generation and Biotin labelling of antisense cRNA was done using the High Yield RNA Transcript labeling Kit (Enzo Life Sciences). Image data were quantified using dChip software (http://www.dChip.org) (Li and Wong, 2001). For hierarchical clustering, the correlation distance metric and the centroid linkage method were used. Raw and normalized data, including detailed descriptions of the analysis methods used are available at ArrayExpress (http://www.ebi.ac.uk/microarray-as/ae/), Accession: E-MEXP-2145.
Drosophila strains
All stocks were maintained on standard medium at 25°C. Mutations are described in Flybase (http://flybase.org) and UAS-RNAi lines obtained from the Vienna Drosophila RNAi Center (VDRC; http://stockcenter.vdrc.at/). Mutants: w1118, key1, Dif1, pirkmut : pirkEY00723. Gal4 lines used: Cg-Gal4, Yp1-Gal4, w; esgGal4ts: w; esgGal4 tubGal80ts UAS-GFP, MyoIAGal4ts: w; MyoIAGal4 tubGal80tsUAS-GFP (Jiang et al, 2009), w; tubGal4ts: w; tubGal4 tubGal80ts. UAS lines used: UAS-Ras85DV12, UAS-PVR, UAS-Pvf2, Gap1EP45, UAS-Dsor1-RNAi (VDRC transformant ID 107276), UAS-PVR-RNAi (ID 105353). FRT: AFG-Gal4: hsFLP; actin<stop>Gal4 UAS-GFP. Reporters: Diptericin-lacZ (Reichhart et al, 1992). Transgenic RNAi lines UAS-Ras85D-RNAi (BKN28029), UAS-IMD-RNAi (BKN29863) and UAS-Pelle-RNAi (BKN22690) were kindly generated by Krystyna Keleman and Barry Dickson (landing site LS27, chromosome 2).
Larval infection
UAS transgenes were crossed to Cg-Gal4 and progeny raised at 25°C. Third instar larvae were injected with a mixture of E. coli and M. luteus and incubated on agar plates for 6 h before fat body dissection and haemocyte collection. Haemocytes were collected on ice from at least 60 larvae per sample in PBS containing 1 × protease inhibitor cocktail (Roche). Fat body was collected from at least five larvae per sample. Actin flipout Gal4 clones, in the larval fat body, were generated by crossing UAS transgenes to hsFLP; actin<stop>Gal4 UAS-GFP flies and progeny raised at 18°C. Second instar larvae (72 h after egg deposition) were heat shocked for 30 min at 38°C. Fat bodies collected from wandering third instar larvae were then dissected in Schneider's medium and incubated with E. coli for 3 h to induce Dpt-lacZ expression before fixation.
Adult infection
UAS transgenes were crossed to Yp1-Gal4 and progeny raised at 25°C. For infection experiments using tubGal4ts, UAS-RNAi constructs were crossed to w; tubGal4 tubGal80ts and progeny raised at 18°C. Flies were shifted to 29°C (3 days after eclosion) for 10 days to deplete mRNA targets. For septic injury, the thorax of flies was pricked with a needle dipped into a concentrated culture of E. coli, or M. luteus. Flies pricked with E. coli were incubated at 25°C for 6 h before collection; flies infected with M. luteus were incubated for 24 h at 25°C before collection.
Midgut infection
Adult gut infections were performed as previously described (Jiang et al, 2009). MyoIAGalts or esgGalts flies were crossed to UAS transgenes and progeny were raised at 18°C, and then shifted to 29°C (3 days after eclosion). For Ras85DV12 overexpression, flies were incubated at 29°C for 24 h while UAS-Ras85D-RNAi flies were incubated at 29°C for 10 days before infection. Oral infection with E. carotova carotova (Ecc15) was performed as previously described (Buchon et al, 2009b) and flies collected 18 h after transfer to vials with contaminated food.
Antibodies and EdU incorporation
Diptericin-lacZ (Dpt-lacZ) was visualized using a rabbit anti-lacZ antibody (1:1000, ICL) and Alexa Fluor 594-conjugated secondary (1:1000, Invitrogen). GFP was visualized using a mouse anti-GFP-FITC-conjugated antibody (1:200, Biozol Diagnostics). DNA was visualized using Hoechst. For EdU incorporation experiments, the Click-iT EdU Alexa Fluor 594 Imaging Kit (Invitrogen) was used according to the manufacturer's instructions. Images were acquired on a Leica TCS SP5 confocal microscope.
Survival experiments
Flies were pricked with a needle dipped in a concentrated culture of E. carotova carotova, or with B. bassiana by shaking anaesthetized flies for 30 s in a Petri dish containing a sporulating fungal culture and incubated at 29°C. Thirty flies were infected per genotype and surviving flies counted every 24 h. Data are presented in Kaplan–Meier plots and P-values are calculated by log-rank test using GraphPad Prism.
Supplementary Material
Acknowledgments
We thank Pernille Rørth, Shulamit Katzav, Buzz Baum, Pascal Meier, Bruce Edgar, Krystyna Keleman, Barry Dickson and the Bloomington Stock Center for Drosophila strains and reagents. We are grateful to Renato Paro for providing reagents to establish the genome-wide RNAi library. We thank Nadège Pelte, Barry Thompson, Aurelio Teleman, Lennart Zabeau and Sam Lievend for critical discussions and helpful comments on the manuscript. Support by DKFZ Light Microscopy Facility is gratefully acknowledged. Work in MB laboratory is supported by the Helmholtz Alliance for Systems Biology, FP7 CancerPathways and the Emmy Noether Program of the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aggarwal K, Rus F, Vriesema-Magnuson C, Ertürk-Hasdemir D, Paquette N, Silverman N (2008) Rudra interrupts receptor signaling complexes to negatively regulate the IMD pathway. PLoS Pathog 4: e1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal K, Silverman N (2008) Positive and negative regulation of the Drosophila immune response. BMB Rep 41: 267–277 [DOI] [PubMed] [Google Scholar]
- Apidianakis Y, Pitsouli C, Perrimon N, Rahme L (2009) Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci USA 106: 20883–20888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR (2003) Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163: 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125: 523–533 [DOI] [PubMed] [Google Scholar]
- Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J (2006) Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog 2: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H (2008) JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3: 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond D, Foley E (2009) A quantitative RNAi screen for JNK modifiers identifies Pvr as a novel regulator of Drosophila immune signaling. PLoS Pathog 5: e1000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Ahringer J (2008) The art and design of genetic screens: RNA interference. Nat Rev Genet 9: 554–566 [DOI] [PubMed] [Google Scholar]
- Bruckner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N (2004) The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell 7: 73–84 [DOI] [PubMed] [Google Scholar]
- Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C (1994a) The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature 370: 386–389 [DOI] [PubMed] [Google Scholar]
- Brunner D, Oellers N, Szabad J, Biggs WH III, Zipursky SL, Hafen E (1994b) A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76: 875–888 [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, Lemaitre B (2009a) Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev 23: 2333–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B (2009b) Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5: 200–211 [DOI] [PubMed] [Google Scholar]
- Cho NK, Keyes L, Johnson E, Heller J, Ryner L, Karim F, Krasnow MA (2002) Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell 108: 865–876 [DOI] [PubMed] [Google Scholar]
- Choe KM, Werner T, Stöven S, Hultmark D, Anderson KV (2002) Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 296: 359–362 [DOI] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA (2008) Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell 7: 318–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes Rde M, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM (2009) Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325: 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P (2001) Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107: 17–26 [DOI] [PubMed] [Google Scholar]
- Dushay MS, Asling B, Hultmark D (1996) Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc Natl Acad Sci USA 93: 10343–10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erturk-Hasdemir D, Broemer M, Leulier F, Lane WS, Paquette N, Hwang D, Kim CH, Stoven S, Meier P, Silverman N (2009) Two roles for the Drosophila IKK complex in the activation of Relish and the induction of antimicrobial peptide genes. Proc Natl Acad Sci USA 106: 9779–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E, O’Farrell PH (2004) Functional dissection of an innate immune response by a genome-wide RNAi screen. PLoS Biol 2: E203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ID, Germain RN (2009) Navigating the network: signaling cross-talk in hematopoietic cells. Nat Immunol 10: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Vignjevic D, Schoumacher M, Duffy SL, Janssen KP, Robine S, Louvard D (2008) Epithelial morphogenesis and intestinal cancer: new insights in signaling mechanisms. Adv Cancer Res 100: 85–111 [DOI] [PubMed] [Google Scholar]
- Gaul U, Mardon G, Rubin GM (1992) A putative Ras GTPase activating protein acts as a negative regulator of signaling by the Sevenless receptor tyrosine kinase. Cell 68: 1007–1019 [DOI] [PubMed] [Google Scholar]
- Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, Kopczynski C, Duyk G, Reichhart JM, Hoffmann JA (2001) Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell 1: 503–514 [DOI] [PubMed] [Google Scholar]
- Gesellchen V, Kuttenkeuler D, Steckel M, Pelte N, Boutros M (2005) An RNA interference screen identifies inhibitor of apoptosis protein 2 as a regulator of innate immune signalling in Drosophila. EMBO Rep 6: 979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, Ferrandon D, Royet J (2002) The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416: 640–644 [DOI] [PubMed] [Google Scholar]
- Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D (1999) Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell 4: 827–837 [DOI] [PubMed] [Google Scholar]
- Ishimaru S, Ueda R, Hinohara Y, Ohtani M, Hanafusa H (2004) PVR plays a critical role via JNK activation in thorax closure during Drosophila metamorphosis. EMBO J 23: 3984–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Edgar BA (2009) EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136: 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA (2009) Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137: 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U (2005) The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132: 2521–2533 [DOI] [PubMed] [Google Scholar]
- Kim LK, Choi UY, Cho HS, Lee JS, Lee WB, Kim J, Jeong K, Shim J, Kim-Ha J, Kim YJ (2007) Down-regulation of NF-kappaB target genes by the AP-1 and STAT complex during the innate immune response in Drosophila. PLoS Biol 5: e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleino A, Myllymäki H, Kallio J, Vanha-aho LM, Oksanen K, Ulvila J, Hultmark D, Valanne S, Rämet M (2008) Pirk is a negative regulator of the Drosophila Imd pathway. J Immunol 180: 5413–5422 [DOI] [PubMed] [Google Scholar]
- Kleino A, Valanne S, Ulvila J, Kallio J, Myllymäki H, Enwald H, Stöven S, Poidevin M, Ueda R, Hultmark D, Lemaitre B, Rämet M (2005) Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J 24: 3423–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697–743 [DOI] [PubMed] [Google Scholar]
- Leulier F, Vidal S, Saigo K, Ueda R, Lemaitre B (2002) Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr Biol 12: 996–1000 [DOI] [PubMed] [Google Scholar]
- Lhocine N, Ribeiro PS, Buchon N, Wepf A, Wilson R, Tenev T, Lemaitre B, Gstaiger M, Meier P, Leulier F (2008) PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe 4: 147–158 [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH (2001) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98: 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B (2006) Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog 2: e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wu LP, Anderson KV (2001) The antibacterial arm of the drosophila innate immune response requires an IkappaB kinase. Genes Dev 15: 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet F, Bischoff V, Vignal C, Hoffmann J, Royet J (2008) The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation. Cell Host Microbe 3: 293–303 [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N (2006) Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439: 475–479 [DOI] [PubMed] [Google Scholar]
- Milne AN, Carneiro F, O’Morain C, Offerhaus GJ (2009) Nature meets nurture: molecular genetics of gastric cancer. Hum Genet 126: 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Kuttenkeuler D, Gesellchen V, Zeidler MP, Boutros M (2005) Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436: 871–875 [DOI] [PubMed] [Google Scholar]
- Munier AI, Doucet D, Perrodou E, Zachary D, Meister M, Hoffmann JA, Janeway CA Jr, Lagueux M (2002) PVF2, a PDGF/VEGF-like growth factor, induces hemocyte proliferation in Drosophila larvae. EMBO Rep 3: 1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naitza S, Rosse C, Kappler C, Georgel P, Belvin M, Gubb D, Camonis J, Hoffmann JA, Reichhart JM (2002) The Drosophila immune defense against gram-negative infection requires the death protein dFADD. Immunity 17: 575–581 [DOI] [PubMed] [Google Scholar]
- Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, Ewbank JJ, Ferrandon D (2007) A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog 3: e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y, Hata M, Ayaki T, Ryo H, Yamagata M, Shimizu K, Nishizuka Y (1988) Proliferation of both somatic and germ cells is affected in the Drosophila mutants of raf proto-oncogene. EMBO J 7: 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill EM, Rebay I, Tjian R, Rubin GM (1994) The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell 78: 137–147 [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A (2006) The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439: 470–474 [DOI] [PubMed] [Google Scholar]
- Olofsson B, Page DT (2005) Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol 279: 233–243 [DOI] [PubMed] [Google Scholar]
- Perrimon N (1994) Signalling pathways initiated by receptor protein tyrosine kinases in Drosophila. Curr Opin Cell Biol 6: 260–266 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Clevers H, Riccio O (2006) From gut homeostasis to cancer. Curr Mol Med 6: 275–289 [DOI] [PubMed] [Google Scholar]
- Ramsden S, Cheung YY, Seroude L (2008) Functional analysis of the Drosophila immune response during aging. Aging Cell 7: 225–236 [DOI] [PubMed] [Google Scholar]
- Reichhart JM, Meister M, Dimarcq JL, Zachary D, Hoffmann D, Ruiz C, Richards G, Hoffmann JA (1992) Insect immunity: developmental and inducible activity of the Drosophila diptericin promoter. EMBO J 11: 1469–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P (1996) A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc Natl Acad Sci U S A 93: 12418–12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Zhou R, Silverman N, Hoffmann JA, Ferrandon D (2000) Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nat Immunol 1: 342–347 [DOI] [PubMed] [Google Scholar]
- Ryu JH, Ha EM, Oh CT, Seol JH, Brey PT, Jin I, Lee DG, Kim J, Lee D, Lee WJ (2006) An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J 25: 3693–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RL, Trejo TR, Plummer TB, Platt JL, Tang AH (2007) Infection-induced proteolysis of PGRP-LC controls the IMD activation and melanization cascades in Drosophila. FASEB J 22: 918–929 [DOI] [PubMed] [Google Scholar]
- Senger K, Armstrong GW, Rowell WJ, Kwan JM, Markstein M, Levine M (2004) Immunity regulatory DNAs share common organizational features in Drosophila. Mol Cell 13: 19–32 [DOI] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Stoven S, Pandey N, Hultmark D, Maniatis T (2000) A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev 14: 2461–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM (1991) Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell 67: 701–716 [DOI] [PubMed] [Google Scholar]
- Takehana A, Yano T, Mita S, Kotani A, Oshima Y, Kurata S (2004) Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J 23: 4690–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda L, Inoue YH, Yoo MA, Mizuno M, Hata M, Lim YM, Adachi-Yamada T, Ryo H, Masamune Y, Nishida Y (1993) A protein kinase similar to MAP kinase activator acts downstream of the raf kinase in Drosophila. Cell 72: 407–414 [DOI] [PubMed] [Google Scholar]
- Zerofsky M, Harel E, Silverman N, Tatar M (2005) Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4: 103–108 [DOI] [PubMed] [Google Scholar]
- Zhuang ZH, Sun L, Kong L, Hu JH, Yu MC, Reinach P, Zang JW, Ge BX (2006) Drosophila TAB2 is required for the immune activation of JNK and NF-kappaB. Cell Signal 18: 964–970 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.