Abstract
The discovery of a simple protocol capable of generating pluripotent stem cells from terminally differentiated cells has been one of the most promising breakthroughs in recent biomedical research. Since their discovery, manuscripts characterizing properties of induced Pluripotent Stem (iPS) have flooded the literature. Among others, the analysis of the transcriptome and epigenome of iPS is now a recurrent theme that is helping to understand the molecular mechanisms behind reprogramming. Recent works have revealed that transcriptional and epigenetic reprogramming is often incomplete, which has raised some concerns on the nature of iPS. Inevitably, now the genome itself of iPS has been scrutinized; and the reports come with an unexpected twist: the presence of mutations in the genome of iPS.
Differentiated cells are not supposed to change fates. A B lymphocyte will not be found weeks later doing the job of an intestinal cell. However, regardless of whether this occurs in nature, humans have found their way to make it happen (see Yamanaka and Blau (2010) for a recent review). The most revolutionary findings came in 1960s. First, a series of experiments revealed that somewhat committed embryonic cells could change their fate when transplanted to an ectopic location. Second, nuclear transfer from intestinal cells into oocytes was shown to be sufficient to ‘reprogram’ the nucleus and to produce frog clones. Two decades later, a second way to enable nuclear reprogramming was developed based on cell fusion experiments. All these experiments were indicating that trans-acting factors had to be responsible for the reprogramming of a differentiated nucleus. However, the nature of these factors remained elusive.
Based on a combinatorial transduction of genes thought to be associated to ‘stemness’, the group of Shinya Yamanaka identified a minimal set of four factors that were sufficient to convert mouse embryonic fibroblasts into pluripotent stem cells (Takahashi and Yamanaka, 2006). The term iPS was officially born, and has arguably become one of the fastest moving fields in biomedical research. However, a careful look at the original protocol raised the concern that one of the four factors included in the reprogramming cocktail was a well-known oncogene (Myc). In addition, reprogramming can also be stimulated by the presence of other oncogenes such as SV40 large T antigen (Mali et al, 2008) or by the loss of tumour suppressors like p53 or Arf (Menendez et al, 2010). To further fuel the concerns, developmental problems and tumours were reported in mice derived from iPS (Okita et al, 2007; Zhao et al, 2010). As a consequence, much of the recent works on iPS have been dedicated to the development of safer protocols such as defining an even more minimal set of factors that do not include Myc or the transient delivery of the reprogramming factors by non-integrating methods. Now, four independent works report on genomic analyses of iPS and reveal a worrisome presence of mutations in these cells.
The first work from Pasi et al (2011) looks at the problem from a cancer-angle. Given that oncogenes are known to generate a type of DNA damage known as replicative stress (RS) (Halazonetis et al, 2008), and that some of the reprogramming factors like c-Myc or Klf4 are known proto-oncogenes, they explored whether the reprogramming protocol could generate RS. In fact, a previous report had already shown that cells undergoing reprogramming presented a pan-nuclear phosphorylation pattern of histone H2AX, which is reminiscent of RS (Marion et al, 2009). In addition, DNA repair deficient cells show a poor reprogramming efficiency again, suggesting that some form of DNA damage could be generated during reprogramming. To evaluate this hypothesis, Pasi et al performed comparative genomic hybridization (cGH) analyses of iPS genomes. Their data show a significant number of chromosomal aberrations on iPS, which the authors suggest was in part influenced by the use of Myc. In fact, the authors report that whereas Myc is sufficient for the reprogramming of mammary progenitors into mammary stem cells, this protocol is accompanied by chromosomal abnormalities. Interestingly, this work revealed that the chromosomal rearrangements that occur during reprogramming frequently involved deletions mapping closely to known fragile sites, or to very large genes, supporting the concept that reprogramming could be accompanied by significant amounts of RS.
A second paper from Hussein et al (2011) which examined copy number variations (CNVs) agrees on the presence of genomic instability on iPS, reinforces the view that this is linked to RS and that the reprogramming process is mutagenic. However, in their data set this is not influenced by Myc. In addition, this work introduces an important aspect, which is that the genome of iPS is also very dynamic during time in culture. In the authors view, reprogramming will generate a significant load of CNVs, which will be selected for or against during in vitro growth.
Going deeper than the chromosome level, an independent work from Gore et al (2011) sequenced the exomes of 22 human iPS (hiPS) clones obtained by five independent methods. Regardless of the ‘zoom’ the take-home message is somewhat similar to that of the above-mentioned works, and an average of six coding mutations were found on iPS clones. As additional evidence for a link between reprogramming and cancer, some of the mutations identified fall on known cancer-associated genes, one of them being the DNA damage sensor ATM. Interestingly, almost half of all the mutations discovered on the iPS were already present in a small fraction of the original differentiated parental cells, which would suggest that selection for these mutations—rather than reprogramming driven mutagenesis—would be to blame.
In contrast to the previous views, a fourth work of Laurent et al (2011) would argue that genomic instability is not only a feature of iPS, but rather a general property of pluripotent SC. By performing a very comprehensive high-resolution SNP analysis of 189 pluripotent (iPS and ES) and 119 non-pluripotent samples, the authors found that the genomes of pluripotent cells are amazingly plastic, with frequent CNVs in pluripotency-related genes and pseudogenes. Noteworthy, the pattern of genomic aberrations was different in iPS or ES, again suggesting some intrinsic changes linked to the reprogramming process. The process of reprogramming led to small deletions, which included tumour suppressors, and which could be consistent with the idea of reprogramming-induced RS. However, time in culture led to the accumulation and selection of novel genomic aberrations in both iPS and ES, which were quantitatively of the same magnitude as those inflicted during reprogramming. This work illustrates the remarkable plasticity of pluripotent genomes and strongly suggests that the use of early passage lines should be an important factor to consider when working with pluripotent cells.
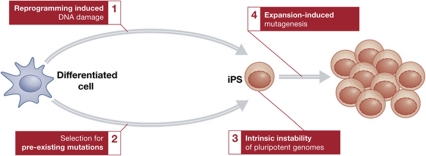
Whereas the process of reprogramming is mutagenic per se or it just selects for previously existing mutations in the donor cells remains to be fully addressed. Moreover, the incredible plasticity of pluripotent genomes is a notable discovery, and reveals the view of an unexpectedly dynamic mammalian genome for many of us. To what extent this is influenced by human manipulation is also to be settled. These different mechanisms to explain the chromosomal abnormalities observed on iPS are illustrated in Figure 1. In any case, there is something in which these reports agree. These four initial pictures of the iPS genomes have revealed the presence of worrisome abnormalities in these cells, which should be seriously considered for the future. With the available technologies, and on the basis of these works, verifying the integrity of the iPS genome should be included as a quality control before iPS are used in actual therapies.
Figure 1.
The roads to genomic instability in iPS. The figure illustrates the different mechanisms that have been postulated as being the drivers of genomic instability on iPS. (1) In an analogy to the oncogene-induced DNA damage theory, it is possible that reprogramming factors (some of which are actually bona fide proto-oncogenes) fuel ‘reprogramming-induced DNA damage’. (2) In contrast, it is possible that the observed mutations (particularly for the case of point coding mutations) might be already present in a small fraction of the differentiated cells. In this model, these mutations would be selected for, rather than generated, during reprogramming. (3) Finally, pluripotent genomes (both iPS and ES) might be intrinsically unstable, (4) a phenomenon that would be further influenced by time in culture and in vitro manipulation. We would like to suggest that this intrinsic instability might be influenced by the ‘open’ chromatin configuration of pluripotent cells, and which might have been behind the selection of pluripotency as a very transient state in multicellular organisms.
Acknowledgments
OFs laboratory is supported by grants from the Spanish Ministry of Science (CSD2007-00017 and SAF2008-01596), EMBO Young Investigator Programme and the European Research Council (ERC-210520). MS is supported by the Spanish Ministry of Science (CSD2007-00017 and SAF2008-02959) and the European Research Council (233270). MAB is funded by the Spanish Ministry of Innovation and Science, the European Union (FP7-Genica), the European Research Council (ERC Advance Grants), the Spanish Association Against Cancer (AECC), the Lilly Foundation Basic Research Award and the Körber European Science Award. MAB and MS are funded by the Botin Foundation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Gore A, Li Z, Fung H-L, Young J, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel M, Kiskinis E, Lee J-H, Loh Y-H, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert M, Yu J, Kirkness EF, Belmonte JCI et al. (2011) Somatic coding mutations in human induced pluripotent stem cells. Nature 629: 205–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halazonetis TD, Gorgoulis VG, Bartek J (2008) An oncogene-induced DNA damage model for cancer development. Science 319: 1352–1355 [DOI] [PubMed] [Google Scholar]
- Hussein SMI, Batada N, Vuoristo S, Ching RW, Autio R, Närvä E, Ng S, Sourour M, Hämäläinen R, Olsson C, Lundin K, Mikkola M, Trokovic R, Peitz M, Brüstle O, Bazett-Jones DP, Alitalo K, Lahesmaa R, Nagy A, Otonkoski T (2011) Copy number variation and selection during reprogramming to pluripotency. Nature [DOI] [PubMed] [Google Scholar]
- Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, Lynch C, Harness JV, Lee S, Barrero MJ, Ku S, Martynova M, Semechkin R, Galat V, Gottesfeld J, Izpisua Belmonte JC, Murry C, Keirstead HS, Park HS, Schmidt U et al. (2011) Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8: 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Ye Z, Hommond HH, Yu X, Lin J, Chen G, Zou J, Cheng L (2008) Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells 26: 1998–2005 [DOI] [PubMed] [Google Scholar]
- Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA (2009) A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460: 1149–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez S, Camus S, Izpisua Belmonte JC (2010) p53: guardian of reprogramming. Cell Cycle 9: 3887–3891 [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448: 313–317 [DOI] [PubMed] [Google Scholar]
- Pasi CE, Dereli-Öz Al, Negrini S, Friedli M, Fragola G, Lombardo A, Houwe GV, Naldini L, Casola S, Testa G, Trono D, Pelicci PG, Halazonetis TD (2011) Genomic instability in induced stem cells. Cell Death Differ (advance online publication 11 February 2011; doi:10.1038/cdd.2011.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Yamanaka S, Blau HM (2010) Nuclear reprogramming to a pluripotent state by three approaches. Nature 465: 704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Wang X, Wang L, Zeng F, Zhou Q (2010) Viable fertile mice generated from fully pluripotent iPS cells derived from adult somatic cells. Stem Cell Rev 6: 390–397 [DOI] [PubMed] [Google Scholar]