Abstract
EMBO J 30 6, 1104–1109 (2011); published online February 04 2011
Adult intestinal stem cells (ISCs) reside at the crypt base, where they continuously proliferate to maintain homeostasis of the intestinal epithelium. As a result of a lifetime of cell division, these LGR5+ ISCs face the combined risk of genomic mutations and telomere attrition. In this issue of EMBOJ, Schepers et al (2011) examine whether ISCs employ telomerase expression and asymmetric chromosome segregation to protect their genome.
The intestinal epithelium sheds hundreds of millions of cells every day, placing considerable replicative strain on the stem cells located at the crypt base. Elegant lineage-tracing experiments have established the ability of LGR5-expressing cells to stably contribute to all epithelial lineages over long chase periods, thus firmly establishing them as ISCs (Barker et al, 2007). These LGR5+ ISCs receive self-renewing signals from Paneth cells, a terminally differentiated epithelial cell found intercalated between the ISCs (Sato et al, 2011). In contrast to most other adult stem cells, LGR5+ cells are in a chronically activated state, cycling once a day on average. This puts ISCs at risk of accumulating genetic lesions and suffering from telomere-induced senescence.
Telomeres are nucleoprotein structures that protect chromosome ends from recombination and degradation. Telomeres shorten with cell division due to the end-replication problem, but this shortening is countered by telomerase, an enzyme consisting minimally of a catalytic subunit, TERT and an RNA-template component, TERC. Telomere attrition causes replicative senescence or apoptosis in cultured human cells, and in telomerase knockout mouse tissues telomere shortening promotes cell death of progenitor cells and impairs self-renewal of stem cells. Telomerase is regulated in part at the level of TERT transcription, and there is increasing evidence that telomerase is restricted in its expression pattern to a subset of stem cells or progenitor cells. A longstanding goal in the field has been to determine precisely the identity of telomerase-positive cells in normal adult tissues. To address this question, Schepers et al (2011) FACS-isolated LGR5+ ISCs, and directly measured telomerase activity by the telomere repeat amplification protocol (TRAP) assay. ISCs expressed the highest level of telomerase activity, whereas telomerase expression was reduced in the transit-amplifying cells of the crypt and was absent in the differentiated epithelial cells of the villi (Figure 1). A recent study using a transgenic TERT-GFP reporter also localized TERT expression to the crypts, but found that TERT was expressed only in very rare crypt cells, perhaps reflecting the relative weakness of the TERT promoter compared with the sensitivity of the TRAP assay (Montgomery et al, 2011). The pathways that lead to specific telomerase expression in LGR5+ ISCs and the progenitor cells they give rise to it are unknown, and it will be important to understand this regulation in depth. Additionally, it remains unclear whether the reduced telomerase expression in progenitor cells reflects downregulation on a per-cell basis, and/or a restriction of expression to a subset of committed progenitor subtypes, as in the haematopoietic system (Morrison et al, 1996).
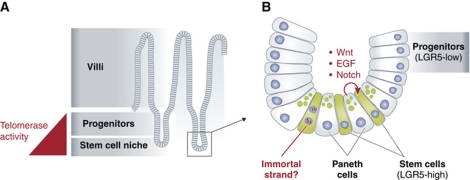
Figure 1.
(A) The epithelium of the small intestine consists of a series of crypts and villi. Stem and progenitor cells proliferate in the crypts, with their differentiated progeny moving upwards into the villi, where they are eventually shed into the intestinal lumen. In this issue of EMBOJ, Schepers et al (2011) find that LGR5+ stem and progenitor cells express telomerase, whereas their differentiated progeny in the villi do not. (B) The stem cell niche is found at the base of the crypt. Stem cells express high levels of LGR5, and are found intercalated between Paneth cells, a specialized epithelial cell. Paneth cells are a source of self-renewal signals for the neighbouring stem cells, helping to promote their daily proliferation. During these cell divisions, Schepers et al (2011) find no evidence that ISCs asymmetrically distribute their chromosomes, a strategy hypothesized to minimize DNA mutation accumulation in stem cells.
Despite telomerase expression, Schepers et al (2011) observed telomere shortening with age in ISCs, suggesting that telomerase levels were insufficient to fully prevent telomere shortening during division of these stem cells. Telomerase knockout experiments have supported the idea that the enzyme serves a critical function in the intestinal crypt. Intercrossing telomerase knockout mice (to deplete the very long telomere reserves of laboratory mice) results in high rates of apoptosis in crypt progenitor cells, as dysfunctional telomeres initiate a cell death response (Wong et al, 2000). An intriguing possibility is that TERT is expressed in ISCs, not only for its role in maintaining telomere reserves, but also for its non-telomeric functions, such as in supporting Wnt signalling activation (Park et al, 2009). Regardless, these experiments highlight LGR5+ ISCs as a powerful system for studying telomerase biology in an endogenous in vivo setting.
In a second set of experiments, Schepers et al (2011) re-examined the crypt base for signs of asymmetric segregation of all chromosomes (ASAC) during cell division. Such events would be consistent with the ‘immortal strand hypothesis’, the idea that stem cells shunt newly synthesized DNA strands to non-stem daughter cells to avoid DNA mutations caused by replication errors (Cairns, 1975). Within the limited number of events examined, Schepers et al (2011) never saw ASAC at the crypt base. This is consistent with a recent report by Falconer et al (2010), who used a sophisticated DNA-labelling method to answer the same question in the mouse colon. They also never observed ASAC, but did measure a pattern of sister chromatid segregation that was non-random, implying that there may indeed be regulation controlling how sister chromatids are apportioned as ISCs divide. Both of these reports contrast with the findings of Quyn et al (2010), who did observe ASAC in roughly half of the cell divisions at the crypt base. However, these cells had recently recovered from irradiation-induced stem cell depletion, a context that could change the homeostatic patterns of cell division and DNA segregation. Especially useful would be a means of tracking the fates of cells receiving new and old chromosomes, to impart biological significance to the patterns observed. A definitive resolution to the immortal strand controversy awaits further experimentation, as does the discovery of the full repertoire of tools that ISCs use to protect their genome.
Footnotes
The authors declare that they have no conflict of interest.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Cairns J (1975) Mutation selection and the natural history of cancer. Nature 255: 197–200 [DOI] [PubMed] [Google Scholar]
- Falconer E, Chavez EA, Henderson A, Poon SS, McKinney S, Brown L, Huntsman DG, Lansdorp PM (2010) Identification of sister chromatids by DNA template strand sequences. Nature 463: 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT (2011) Mouse telomerase reverse transcriptase (mTERT) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 408: 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Prowse KR, Ho P, Weissman IL (1996) Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity 5: 207–216 [DOI] [PubMed] [Google Scholar]
- Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, McLaughlin M, Veenstra TD, Nusse R, McCrea PD, Artandi SE (2009) Telomerase modulates Wnt signaling by association with target gene chromatin. Nature 460: 66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyn AJ, Appleton PL, Carey FA, Steele RJ, Barker N, Clevers H, Ridgway RA, Sansom OJ, Näthke IS (2010) Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 6: 175–181 [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H (2011) Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J 30: 1104–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, Chang S, Weiler SR, Ganesan S, Chaudhuri J, Zhu C, Artandi SE, Rudolph KL, Gottlieb GJ, Chin L, Alt FW, DePinho RA (2000) Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat Genet 26: 85–88 [DOI] [PubMed] [Google Scholar]