Abstract
The tRNAHis guanylyltransferase (Thg1) family comprises a set of unique 3′–5′ nucleotide addition enzymes found ubiquitously in Eukaryotes, where they function in the critical G−1 addition reaction required for tRNAHis maturation. However, in most Bacteria and Archaea, G−1 is genomically encoded; thus post-transcriptional addition of G−1 to tRNAHis is not necessarily required. The presence of highly conserved Thg1-like proteins (TLPs) in more than 40 bacteria and archaea therefore suggests unappreciated roles for TLP-catalyzed 3′–5′ nucleotide addition. Here, we report that TLPs from Bacillus thuringiensis (BtTLP) and Methanosarcina acetivorans (MaTLP) display biochemical properties consistent with a prominent role in tRNA 5′-end repair. Unlike yeast Thg1, BtTLP strongly prefers addition of missing N+1 nucleotides to 5′-truncated tRNAs over analogous additions to full-length tRNA (kcat/KM enhanced 5–160-fold). Moreover, unlike for −1 addition, BtTLP-catalyzed additions to truncated tRNAs are not biased toward addition of G, and occur with tRNAs other than tRNAHis. Based on these distinct biochemical properties, we propose that rather than functioning solely in tRNAHis maturation, bacterial and archaeal TLPs are well-suited to participate in tRNA quality control pathways. These data support more widespread roles for 3′–5′ nucleotide addition reactions in biology than previously expected.
INTRODUCTION
The tRNAHis guanylyltransferase (Thg1), originally identified in yeast, adds a single essential G residue (G−1) to the 5′-end of tRNAHis in eukaryotes (1). The presence of G−1 is a nearly universal feature of tRNAHis in all three domains of life, since G−1 is an important recognition element for aminoacylation of tRNAHis by its cognate histidyl-tRNA synthetase (HisRS) (2–5). In Escherichia coli and chloroplast, G−1 is incorporated into tRNAHis by an alternative pathway; the G−1 residue is genomically encoded, incorporated into the precursor tRNA during transcription, and retained in the mature tRNAHis following processing by ribonuclease P (RNase P) (6,7). A G−1 residue is similarly encoded in the genome of some archaea, and all bacteria, with the exception of 20 α-proteobacteria that are the only species known to lack a requirement for G−1 on tRNAHis (8). Thus G−1 could be incorporated during transcription in these species, as in E. coli (5). In other archaea and in metazoan mitochondria, a G residue is not present at the −1 position of tRNAHis genes, and G−1 is presumably added post-transcriptionally by Thg1 family members present in these species, consistent with the recent demonstration that archaeal Thg1 enzymes catalyze a G−1 addition reaction similar to yeast Thg1 (9,10). Recent results suggest that, even in organisms that contain a genomically encoded G−1, the post-transcriptional pathway for incorporation of G−1 into tRNAHis may be used, since RNase P-catalyzed removal of a genomically encoded G−1 from tRNAHis in plant mitochondria has been reported (11).
Yeast Thg1 adds G−1 to tRNAHis using an unusual 3′–5′ nucleotide (nt) addition reaction, employing a three-step chemical mechanism for nucleotidyltransfer (1) that proceeds via formation of a 5′-adenylylated tRNA intermediate (Figure 1A). The first crystal structure of a Thg1 family enzyme revealed unexpected structural similarity between Thg1 and DNA polymerases, suggesting that Thg1 uses a two-metal ion active site for catalysis, albeit to add nucleotides in the opposite (3′–5′) direction to canonical 5′–3′ nt polymerases (12). In eukaryotes, G−1 addition to cytoplasmic tRNAHis occurs opposite a universally conserved A73 residue, however yeast Thg1 also catalyzes Watson–Crick template-dependent 3′–5′ polymerization of nucleotides in vitro and in vivo (13,14). While archaeal Thg1 family members share the ability to catalyze 3′–5′ nt addition, they do not efficiently catalyze the non-templated addition reaction observed in yeast (addition of G−1 to A73-containing tRNAHis), but preferentially add Watson–Crick base paired nucleotides to tRNAHis substrates (9). Thus, the template-dependent reaction is a shared property of eukaryal and archaeal enzymes, and is likely to represent an ancestral activity of the earliest Thg1 family enzymes. In contrast, addition of non-templated G−1 appears to be a specialized evolution of Thg1 activity that is so far unique to Eukarya.
Figure 1.
BtTLP catalyzes templated, but not non-templated addition of G-1 to tRNAHis. (A) Schematic of p*tRNAHis G−1 addition assay (24); products expected from RNase A/CIP treatment are indicated below each tRNA. The site of RNase A cleavage is indicated on each tRNA. (B) G−1 addition to A73- or C73-containing 5′-32P-tRNAHis substrates was tested using the phosphatase protection assay with serial dilutions of enzymes, as indicated, in the presence of 0.1 mM ATP and 1.0 mM GTP.
The Thg1 enzyme family is comprised of related protein sequences (Pfam PF04446/InterPro IPR007537) whose members, as expected due to the requirement for post-transcriptional G−1 addition, are widely distributed throughout eukarya, and are also present in archaeal species that lack a genomically encoded G−1 residue (1). However, Thg1 family members are also found in bacteria and archaea that contain G−1 in their tRNAHis genes, and thus a role for these proteins in tRNAHis maturation is not necessarily required. The overall similarity between diverse Thg1 family members is relatively high (∼40–45% pairwise sequence similarity between yeast Thg1 and archaeal/bacterial family members), including many highly conserved residues that are required for yeast Thg1-catalyzed 3′–5′ addition activity (15). Nonetheless, phylogenetic analysis indicates a distinct lineage for the archaeal/bacterial genes in the Thg1 enzyme family (16), and this, combined with the uncertainty regarding physiological function of at least some of the prokaryotic enzymes has led us to employ the designation Thg1-like proteins (TLPs) to distinguish the archaeal and bacterial enzymes from the eukaryal Thg1 enzymes that were the founding members of the Thg1/TLP superfamily.
The occurrence of highly conserved TLPs in bacterial and archaeal species that do not inherently require Thg1 activity for tRNAHis maturation suggests the possibility of alternative roles for 3′–5′ addition. To uncover such functions for Thg1/TLP family members, we have investigated the biochemical activities of a bacterial TLP from the Gram-positive soil bacterium Bacillus thuringiensis (BtTLP). Like archaeal TLPs investigated previously, BtTLP preferentially catalyzes template-dependent 3′–5′ addition of nucleotides at the −1 position of various tRNAHis substrates. Surprisingly, we also find that BtTLP exhibits substantial activity with truncated tRNA substrates lacking their mature 5′-end. In each case, the kcat/KM for templated N+1 addition is dramatically greater than for the analogous addition at the −1 position of tRNAHis. Since BtTLP catalyzes the same reaction with 5′-truncated tRNAPhe, the ability to add nucleotides to restore a complete aminoacyl-acceptor stem and thus repair the 5′-end of the tRNA is not restricted to tRNAHis. In addition, we find that archaeal TLPs catalyze similar reactions. Taken together, our data suggest an alternative role for bacterial and archaeal TLPs in tRNA 5′-end repair. This activity bears striking similarities to the 5′-tRNA repair component of a mitochondrial 5′-tRNA editing activity that occurs in several lower eukaryotes (17–23), although the enzyme(s) that catalyze the 5′-tRNA editing reaction remain unknown.
MATERIALS AND METHODS
TLP and tRNA plasmid constructs
The B. thuringiensis TLP was cloned following PCR from B. thuringiensis serovar israelensis genomic DNA (kindly provided by Dr Don Dean, Ohio State University) into a pET15-derived vector for the expression of an N-terminal His6-tagged protein in E. coli. tRNA constructs were derived from previously described yeast tRNAHis and yeast tRNAPhe plasmids for T7 RNA polymerase-dependent in vitro transcription (24); alterations to N73 or N72 and/or removal of the G+1 residue were accomplished by Quik-Change Mutagenesis (Stratagene) according to the manufacturer’s instructions. All NTPs and dNTPs for cloning, substrate preparation and assays were obtained from Roche.
Protein expression and purification
Plasmids encoding yeast Thg1 (1), BtTLP (this work) or MaTLP (9) were transformed into E. coli strain BL21(DE3) pLysS and cultures were grown and proteins were purified using immobilized metal-ion affinity chromatography (IMAC), as previously described (9). All proteins were >95% pure as judged by SDS-PAGE and stored at −20°C. Purified protein concentrations were determined by BioRad protein assay.
3′–5′ nt addition assays
Nucleotide addition assays were performed using tRNA substrates prepared by in vitro transcription followed by 5′-end labeling with 32P using T4 polynucleotide kinase and [γ-32P]-ATP (24). Activity assays contained ∼10–30 nM 5′-32P-tRNA (specific activity 6000 Ci/mmol) in Thg1 assay buffer [25 mM HEPES pH 7.5, 10 mM MgCl2, 3 mM DTT, 125 mM NaCl, 0.2 mg/ml bovine serum albumin (BSA)]. Reactions to test G, U or C addition contained 0.1 mM ATP in addition to 1 mM NTP; A addition reactions contained only 1 mM ATP. For GTP competition assays, 1 mM GTP was added along with 1 mM NTP, as indicated.
Reactions (5 µl each) were initiated using 1 µl enzyme (undiluted or serial dilutions, ∼0.01-15 µg of each purified protein) and were incubated at room temperature for 2-3 h. ATP and GTP addition reactions were quenched by adding 1 mg/ml RNase A (Ambion) and 50 mM EDTA and incubating at 50°C for 10–20 min, whereas UTP and CTP addition reactions were quenched with 1 U RNase T1 (Ambion) in 20 mM NaOAc pH 5.2, 1 mM EDTA, 2 µg Yeast RNA (Ambion), followed by incubation at 37°C for 30 min. RNase digested samples were treated with 0.5 U calf intestinal alkaline phosphatase (CIP) (Invitrogen) and incubated at 37°C for 30 min; reactions were resolved using silica thin-layer chromatography (TLC) in an 1-propanol:NH4OH:H2O (55:35:10) solvent system. TLC plates were visualized using a Typhoon Trio and results quantified using ImageQuant software (GE Healthcare).
Steady-state kinetic parameters for N−1 and N+1 addition were measured as described previously, using triphosphorylated tRNA transcripts (24). To improve resolution of the labeled pyrophosphate product (which is released from the 5′-end of the tRNA following 3′–5′ nt addition) from unreacted labeled substrate tRNA, samples taken at each time point were first treated with 1 mg/ml RNase A and 50 mM EDTA for 10 min at 50°C, and precipitated with 10% (v/v) trichloro-acetic acid (TCA) for 10 min on ice prior to spotting on the PEI-cellulose TLC plates.
Primer extension analysis
tRNAPhe substrates lacking G+1 only, or lacking both G+1 and G+2, were generated by in vitro transcription, and used as the substrate for TLP-catalyzed 3′-5′ nt addition, followed by 5′-end analysis using primer extension, according to (13). Addition reactions contained 2–4 µM unlabeled tRNA, 0.1 mM ATP, 1 mM GTP and 48 µM BtTLP or 25 µM MaTLP in Thg1 assay buffer, and were carried out at room temperature for 2–3 h. The resulting tRNAs (3–4 pmol) were purified by phenol extraction followed by ethanol precipitation and used as the template for primer extension with ∼1 pmol 5′-32P-labeled tRNAPhe-specific DNA primer (5′-GCTCTCCCAACTGAGCTAAA-3′).
Bulk tRNA was isolated from yeast to test the presence of a −1 nt on tRNAHis using hot phenol extraction and ethanol precipitation (1). 5′-32P-labeled tRNAHis-specific DNA primer (5′- ACTAACCACTATACTAAGA-3′) was used for the primer extension assays.
In vivo genetic complementation of Thg1 function by BtTLP
In vivo complementation was tested using the previously described yeast strain (JJY20: relevant genotype, Matα thg1Δ::kanMX his3-1 leu2Δ met15Δ ura3 [CEN URA3 PTHG1-THG1]) (9). Drop tests were performed with strains transformed with plasmids for galactose-inducible expression of yeast THG1 or BtTLP [CEN LEU2 PGAL- THG1/TLP], or with empty vector. To test the effect of tRNAs on complementation, drop tests were also performed with strains containing a second plasmid [CEN HIS3] expressing either yeast wild-type A73-tRNAHis, C73-tRNAHis, or empty vector (14).
RESULTS
BtTLP catalyzes template dependent N-1 addition to tRNAHis
The recombinantly expressed and purified TLP from the bacterium B. thuringiensis serovar israelensis (BtTLP) was tested for its ability to catalyze the prototypical Thg1 reaction, G-1 addition to yeast tRNAHis (24). Addition to the 5′-end of 5′-32P labeled monophosphorylated yeast tRNAHis (p*A73-tRNAHis) results in protection of the labeled phosphate from removal by phosphatase, and reaction products, such as G−1p*GpC (Figure 1A), can be resolved from 32Pi generated from unreacted substrate using TLC. BtTLP only weakly catalyzes addition of a non-templated G−1 to A73-tRNAHis, as evidenced by the relatively small amount of G-1p*GpC product (the G−1 product spot migrates only slightly higher than the major product, described below, and is apparent only in the reactions with the highest concentration of BtTLP) (Figure 1B). However, BtTLP efficiently adds a Watson–Crick base paired G−1 residue to C73-tRNAHis (Figure 1B). The preferential addition of the Watson–Crick paired G−1 over non-templated G−1 to yeast tRNAHis is the same pattern of reactivity previously observed with archaeal TLPs (9).
In assays with A73-tRNAHis substrate in the presence of ATP and GTP, BtTLP accumulates two different lower migrating products, both of which correspond to activated tRNAHis intermediates (Figure 1). The first of these two products (App*GpC) migrates slightly below the G-1 addition product and corresponds to 5′-adenylylated tRNAHis, which is also produced by yeast Thg1 when GTP is omitted from the reaction (9). The second, more slowly migrating product corresponds to 5′-guanylylated tRNAHis (Gpp*GpC) resulting from activation of the 5′-monophosphorylated tRNA with GTP instead of ATP, as evidenced by resistance of this isolated product to RNase T2 digestion and sensitivity to snake venom pyrophosphatase treatment (data not shown). The observation of roughly equivalent amounts of these two activated tRNAHis species suggests that BtTLP exhibits greater flexibility than yeast Thg1 with respect to the identity of the nucleotide (ATP or GTP) used for the activation step at the 5′ end of the tRNA substrate. The direct observation of activated 5′-tRNA intermediates in these assays indicates that BtTLP, like archaeal TLPs (9), uses the same basic mechanism for catalysis of 3′–5′ nt addition as yeast Thg1 (1).
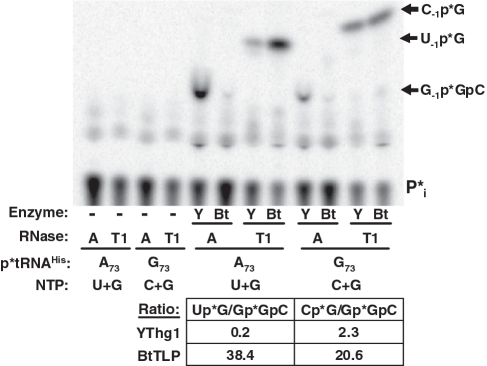
To further probe the preference of BtTLP for templated versus non-templated nucleotide addition, we constructed tRNAHis variant substrates with each of the four possible nucleotides at position 73 (N73-tRNAHis). Using 5′-32P-labeled tRNAs, we developed assays to test addition of each of the four possible NTPs that form Watson–Crick base pairs with the indicated N73 residue (Figure 2). For these assays, the identity of the nuclease used to treat the reactions was altered; to detect purine addition, RNase A was used to generate R−1p*GpC products (where R = A or G) and to detect pyrimidine addition, RNase T1 was used to generate Y−1p*G products (where Y = U or C). In each case, the identities of products were further confirmed by RNase T2 digestion to yield the expected N−1p* nt (data not shown).
Figure 2.
BtTLP catalyzes template-dependent 3′–5′ nucleotide additions to N73-tRNAHis variants. Assays for N-1 nucleotide additions contained tRNAHis variants with each of the four possible N73 discriminator nucleotides, as indicated; the tRNA diagram shows the expected positions of RNase A or RNase T1 cleavage to yield the various labeled oligonucleotide products, as indicated to the right of the figure. Reactions contained 5′-32P-labeled tRNA, 1 mM NTP (either G, A, U or C as indicated) and 0.1 mM ATP (unless ATP was already present in the assay) for activation of the 5′-monophosphorylated tRNA, and were initiated by addition of 1 µl (∼15 µg) yeast Thg1 or BtTLP. Gpp*GpC, formed by BtTLP with A73 or C73-tRNAHis, and G-2pGp*GpC, formed by yeast Thg1 with C73-tRNAHis are not resolved from each other using this TLC solvent system, but have each been verified by further digestion. Lanes dash: buffer control reactions for each N73-tRNAHis variant.
BtTLP, like yeast Thg1, can add any of the 4 nts at the −1 position of tRNAHis (Figure 2). However, BtTLP is distinct from yeast Thg1 in its selective preference for Watson–Crick templated N−1 addition, as demonstrated using a competition experiment. For the competition assay, equimolar amounts of GTP and a competing Watson–Crick pairing nucleotide were provided simultaneously, and then nuclease digestions were performed separately in parallel, to compare the relative amounts of G−1 addition products (RNase A) versus U−1 or C−1 addition products (RNase T1) from the same assay (Figure 3). While yeast Thg1 added ∼5-fold higher amounts of G−1 than U−1 to A73-tRNAHis in the presence of equimolar GTP and UTP, the nucleotide preference for BtTLP was reversed, with ∼40-fold higher amounts of U−1 added over G−1. A similarly enhanced preference of BtTLP for templated C−1 addition was observed (Figure 3).
Figure 3.
GTP does not compete effectively with Watson–Crick base pair forming nucleotides for N−1 addition catalyzed by BtTLP. GTP competition assays were conducted using 5′-32P labeled A73-tRNAHis or G73-tRNAHis substrates in the presence of equimolar amounts (1 mM each) of GTP and the correct Watson–Crick base pairing NTP (either UTP or CTP, as indicated). ATP (0.1 mM) was present in all reactions for 5′-monophosphate activation. Reactions were initiated with 1 µl enzyme and digested as indicated, to separately visualize purine and pyrimidine nucleotide addition products derived from the same assay, so that the ratio of templated addition to non-templated addition could be calculated for each enzyme/substrate combination.
To quantify these biochemical differences, steady-state kinetic parameters were determined. In agreement with the competition assay results, the catalytic efficiency of BtTLP-catalyzed G−1 addition to C73-tRNAHis was ∼50-fold greater than for addition of G−1 to the A73-tRNAHis substrate, whereas the kcat/KM values exhibited by yeast Thg1 for G−1 addition these two substrates are nearly identical (Table 1). While kcat/KM values measured for templated G−1 and C−1 addition were similar, rates of U−1 and A−1 addition were significantly lower. The competition assays and kinetic data demonstrate that BtTLP preferentially catalyzes templated, but not non-templated, N−1 addition reactions. Template-dependent 3′–5′ nt addition, previously shown to be a property of archaeal and eukaryal Thg1/TLP enzymes (9), is therefore an enzymatic activity common to family members from all three domains of life.
Table 1.
Steady-state kinetic parameters for N−1 addition to tRNAHis
| Enzyme | tRNAHis | N−1 | kcat (h−1) | KM (µM) | kcat/KM (M−1s−1) |
|---|---|---|---|---|---|
| yThg1 | A73 | G | 8.4 ± 0.9a | 0.42 ± 0.13a | 5500 ± 1200a |
| yThg1 | C73 | G | 20.4 ± 2.4a | 0.99 ± 0.29a | 5670 ± 1200a |
| BtTLP | A73 | G | ≥3.9b | ≥10b | 108b |
| BtTLP | C73 | G | 23 ± 2 | 1.2 ± 0.3 | 5500 ± 1260 |
| BtTLP | G73 | C | 2.9 ± 0.3 | 0.6 ± 0.2 | 1400 ± 400 |
| BtTLP | U73 | A | 4.2 ± 0.7 | 12 ± 4 | 94 ± 13 |
| BtTLP | A73 | U | 1–2c | ∼1c | 230c |
aValues reproduced from ref. (9).
bkcat/KM was obtained from the linear slope of the initial rate versus [tRNA] plot, which did not reach saturation even at the highest concentration of tRNA achievable in the assays (10 µM). The lower limit for kcat and KM were extrapolated from this value.
cDue to slow rates of U−1 addition observed in the assays, estimates for kcat and KM were made based on the apparent saturation of the initial rate of the reaction at >1 µM A73-tRNAHis and average observed rates of reactions performed at 2, 5 and 10 µM tRNA (ranging from 1 to 2 h−1). The estimate for kcat/KM was subsequently calculated using these values.
BtTLP catalyzes template dependent N+1 addition to 5′-truncated tRNAHis
Although BtTLP adds N−1 nucleotides to tRNAHis, albeit with varying catalytic efficiencies (Table 1), a role for the enzyme in tRNAHis maturation in B. thuringiensis is not necessarily required. Thus, we hypothesized that the biochemical characteristics of BtTLP could be exploited for an alternate function in vivo.
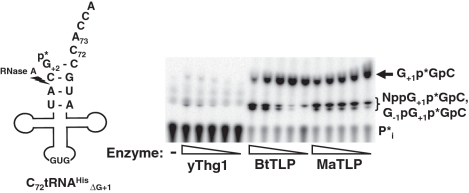
Based on a previously described mitochondrial tRNA editing activity catalyzed by unknown enzymes (17–23,25), we tested whether BtTLP could add nucleotides to 5′-truncated tRNA substrates, thus restoring a completely base paired aminoacyl acceptor stem. We used a tRNAHis substrate previously constructed to test 5′-end repair activity (13); the G+1 nucleotide has been removed from this tRNA leaving an unpaired C72 residue in the aminoacyl acceptor stem (C72tRNAHisΔG+1, Figure 4). Yeast Thg1 has little detectable ability to add the missing G+1 nucleotide to the monophosphorylated 5′-truncated tRNA substrate.
Figure 4.
BtTLP catalyzes robust G+1 addition to 5′-truncated C72-tRNAHisΔG+1. G+1 addition to 5′-32P labeled-C72tRNAHisΔG+1 was performed as described for G−1 addition assay above, using serial dilutions of yeast Thg1 (yThg1), BtTLP or MaTLP, as indicated, in the presence of 0.1 mM ATP and 1.0 mM GTP. The identity of the G+1p*GpC product was verified by migration with standards and RNase T2 digestion to release 3′-GMP (data not shown). The lower migrating products indicated by the bracket cannot be unequivocally identified due to the remote position of the labeled phosphate from the added nucleotide, but further digestions and comparison to known standards suggests that these are a mixture of further activation and addition products following G+1 addition.
Using the phosphatase protection assay with 5′-32P labeled monophosphorylated C72tRNAHisΔG+1, BtTLP, unlike yeast Thg1, displayed robust G+1 addition even at the lowest concentration of enzyme in the assay (Figure 4). Since addition of the missing G+1 restores a full-length tRNAHis, which is essentially the same molecule as the A73-tRNAHis tested previously (Figure 1), we observed additional reaction products at high concentrations of BtTLP (Figure 4). The identity of the lower migrating products can not be unambiguously assigned due to the position of the labeled phosphate between G+1 and G+2, outside of the bond linking the additional nucleotides to the tRNA. Nonetheless, digestions with RNase T2 and snake venom pyrophosphatase (data not shown) suggest that these lower migrating products include a mixture of species derived from the G+1-containing tRNA. These products likely include both activated (NppG+1p*GpC) and G−1-containing (G−1pG+1p*GpC) species, consistent with products seen previously (Figure 1B).
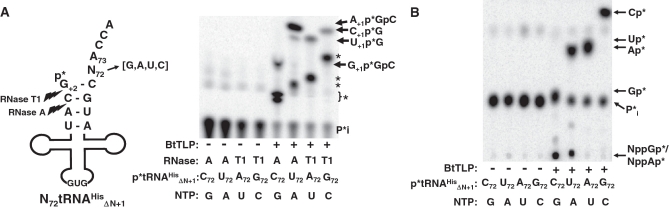
We constructed a set of truncated tRNAHis variants with various N72 residues (N72-tRNAHisΔN+1), similar to the set of N73-tRNAHis variants, to examine each of the four possible templated N+1 addition reactions. Using 5′-end labeled tRNA substrates and varied nuclease digestions to detect each of the four N+1 addition products, we observed addition of each of the four N+1 nucleotides (Figure 5A), as evidenced by further digestion of the reactions with RNase T2 to generate each Np*, as expected (Figure 5B). As with N−1-addition, BtTLP prefers to add the correct Watson–Crick base pairing N+1 nucleotide over adding a non-templated G+1 (Supplementary Figure S1). In the competition assay (Supplementary Figure S1), only RNase T1-dependent C+1 and U+1 addition products were detected, and little, if any, G+1 addition was detected in the parallel RNase A digestions.
Figure 5.
BtTLP adds all four possible templated N+1 nucleotides to 5′-truncated tRNAHis variants (N72-tRNAHisΔN+1). (A) N+1 addition assays were performed using the same assay described in Figure 2, but with 5′-32P labeled tRNAHis variants missing a +1 nt and containing each of the four possible N72 nucleotides to serve as the template for +1 nt addition (see tRNA diagram). The single N+1 addition products produced by the relevant nuclease treatment are indicated by arrows. The full-length tRNAs generated following N+1 addition are each substrates for further activation and/or N−1 addition reactions; products of these reactions are indicated by asterisks to the right of the image, but these products are not further identified since the remote position of the labeled phosphate (between N+1 and G+2 nucleotides) does not readily permit identification by RNase T2 digestion. (B) RNase T2 digestion of reactions from (A) confirms each of the four N+1 nucleotides added to 5′-truncated tRNAHisΔN+1 substrates. RNase T2 products were resolved by PEI-cellulose TLC in 0.5 M formate, pH 3.5; positions of each 3′-32P labeled mononucleotide products (Cp*, Up*, Ap* and Gp*) were identified based on the migration of cold NMP standards. 5′-activated N+1 addition products generated from G+1 and A+1 addition reactions are indicated by NppGp* and NppAp*, respectively.
As seen with G+1 addition above, restoration of the +1–72 base pair allowed formation of additional products with each substrate (starred products, Figure 5A). Although the exact identity of these lower migrating products cannot be unequivocally assigned due to the absence of an appropriately labeled phosphate, RNase T2 digestion of the same reactions shown in Figure 5A revealed that these are a mixture of activation and/or addition products, depending on the substrate used in each assay (Figure 5B).
3′–5′ addition of nucleotides to truncated tRNA substrates is kinetically preferred
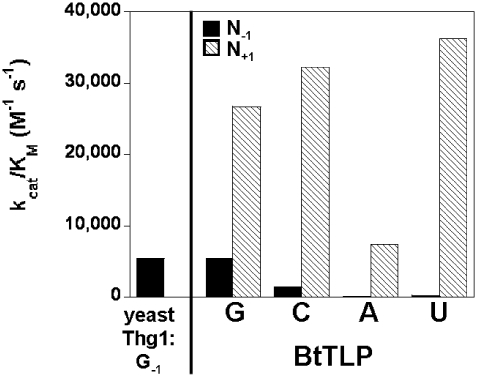
To determine the efficiency with which BtTLP adds missing nucleotides to 5′-truncated tRNAs, we measured steady-state kinetic parameters for N+1 addition to each of the tRNAHisΔN+1 substrates. These assays revealed significant (from 5- to 160- fold) enhancements of kcat/KM for addition of each missing N+1 nucleotide over the analogous N−1 addition reactions measured with full-length tRNAHis (Supplementary Table S1, Figure 6). Moreover, kcat/KM values measured for each of the four templated N+1 additions are quite similar, particularly for G+1, C+1 and U+1, with only 5-fold lower efficiency observed for A+1 (Supplementary Table S1), as compared with the more than 50-fold variation observed in kcat/KM for the corresponding −1 additions (Table 1). These results suggest that 5′-truncated tRNAs are more optimal substrates than full-length tRNAs for 3′–5′ nt addition catalyzed by BtTLP, and suggest that BtTLP is well-suited to function in 5′-end repair of tRNA.
Figure 6.
BtTLP catalyzes N+1 nucleotide addition to 5′-truncated tRNAHis with enhanced catalytic efficiency over N-1 addition reactions. kcat/KM values are shown for BtTLP-catalyzed addition of each of the four possible Watson–Crick templated N−1 (solid bars) or N+1 (hatched bars) nucleotides to full-length tRNAHis or 5′-truncated tRNAHisΔN+1 substrates, respectively. For each of the four nucleotides (G, C, A or U, as indicated below the figure), kinetic parameters were measured using a tRNA substrate with the appropriate N73 or N72 residue to allow Watson–Crick base paired 3′–5′ addition, as in Table 1 and Supplementary Table S1. For comparison, the kcat/KM value measured previously for yeast Thg1-catalyzed G−1 addition to C73-tRNAHis is also shown (9).
5′-end repair of truncated tRNAHis is also catalyzed by archaeal TLPs
Members of the Thg1/TLP enzyme family are found in some Archaea that, as with B. thuringiensis, do not necessarily require post-transcriptional addition of G−1 to tRNAHis. We tested the TLP from Methanosarcina acetivorans, a methanogenic archaeon in which G−1 is genomically encoded, for its ability to add nucleotides to truncated tRNAHisΔN+1 variants, using the same assays described above. The M. acetivorans TLP (MaTLP) catalyzed robust addition of G+1 to C72-tRNAHisΔG+1 (Figure 4), exhibited the same pattern of all four N+1 additions to the various N72-containing truncated tRNA substrates that we observed previously with BtTLP (Supplementary Figure S2), and is similarly selective for addition of the Watson–Crick base pairing nucleotide over non-templated G-addition (Supplementary Figure S3). Finally, as with BtTLP, addition of G+1 to C72-tRNAHisΔG+1 occurs more efficiently than the corresponding G−1 addition reaction (Supplementary Table S1). Thus, the tRNA 5′-end repair reaction is also catalyzed with high efficiency by archaeal members of the Thg1/TLP enzyme family.
TLP-catalyzed N+1 addition is not limited to tRNAHis
Although eukaryal Thg1 enzymes that function in G−1 addition exhibit rigorous specificity for tRNAHis (24), 5′-tRNA repair could be a more generalized process. We tested whether BtTLP could add nucleotides to the 5′-ends of other truncated tRNA substrates. To this end, we generated a 5′-truncated variant of yeast tRNAPhe lacking G+1, and tested G+1 addition using a 5′-32P monophosphorylated substrate. Both BtTLP and MaTLP produce a prominent phosphatase resistant product indicative of addition of the missing G+1 to this substrate, whereas yeast Thg1 exhibits little or no detectable formation of this product (Figure 7). In the absence of a bona fide hexanucleotide standard for G+1 addition to this substrate, we used a primer extension assay (13) to confirm the addition of missing nucleotides to the 5′-end of tRNAPheΔG+1, and to a second tRNAPhe substrate missing both G+1 and G+2 residues (tRNAPheΔG+2) (Supplementary Figure S4). Reactions with either of the 5′-truncated tRNAPhe substrates yielded longer primer extension products than for control untreated tRNAs by 1 or 2 nt, indicating that missing 5′-nt were added to restore a fully base paired aminoacyl acceptor stem (Supplementary Figure S4). A similar kinetic preference was observed for the 5′-end repair reaction over the analogous G−1 addition reaction to full-length C73-tRNAPhe (Supplementary Table S2). Notably, in contrast to assays with 5′-truncated tRNAHis (Figure 5A), we did not observe evidence for further activation/addition reactions beyond the +1 position of full-length tRNAPhe.
Figure 7.
BtTLP catalyzes robust repair of 5′-truncated tRNAPhe substrates. The phosphatase protection assay for G+1 addition was conducted using 5′-32P-labeled C72-tRNAPheΔG+1 (see tRNA diagram) with serial dilutions of BtTLP, MaTLP or yeast Thg1 (yThg1). All reactions contained 1.0 mM GTP and 0.1 mM ATP for activation. The migration of the phosphatase-protected species is consistent with the predicted 6-nt reaction product (see diagram), also confirmed by the addition of a single nucleotide to the 5′-truncated tRNAPheΔG+1 substrate observed using primer extension (see Supplementary Figure S4).
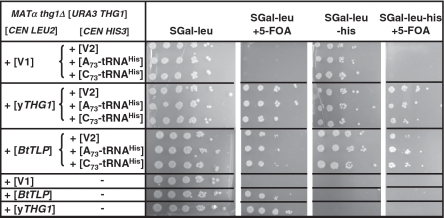
BtTLP weakly complements wild type Yeast Thg1 function in vivo
In yeast, THG1 is essential for optimal growth and the requirement for THG1 can only be bypassed by providing additional copies of both tRNAHis and HisRS to the cells (14). Therefore, the ability of Thg1 homologs to add G−1 to tRNAHis in vivo in yeast can be assessed using a plasmid shuffle assay (9). A yeast thg1Δ strain, made viable by the presence of a wild-type yeast THG1 URA3 plasmid, is transformed with a CEN LEU2 plasmid containing any Thg1/TLP gene of interest, expressed under the control of a galactose inducible promoter. If the Thg1/TLP complements the essential function of yeast THG1 in vivo, the resulting strains are able to grow on media containing 5-fluoroorotic acid (FOA), which causes loss of the URA3 THG1 covering plasmid. Using this assay, we previously showed that four different archaeal TLPs individually supported growth of the yeast thg1Δ strain, but did so only in the presence C73-tRNAHis (9), mirroring the ability of these archaeal Thg1/TLP family members to add only templated, but not non-templated, G−1 to tRNAHis.
However, BtTLP supports growth of the yeast thg1Δ strain even in the presence of only A73-tRNAHis and addition of a plasmid expressing C73-tRNAHis confers no additional growth advantage to the BtTLP-complemented strain (Figure 8). This result was surprising, given the relatively weak levels of G−1 addition activity exhibited by BtTLP in the in vitro assays with A73-tRNAHis (Table 1). A primer extension assay was used to assess the 5′-end status of tRNA isolated from the complemented strains, confirming the presence of a −1 nt on tRNAHis (Supplementary Figure S5).
Figure 8.
Expression of BtTLP in yeast complements the growth defect of the yeast thg1Δ strain. Plasmid shuffle assays were performed with a yeast thg1Δ strain (9) transformed with CEN LEU2 plasmids containing either BtTLP [BtTLP] or yeast Thg1 [yTHG1], or no Thg1 [V1]. The top three panels also contained a second CEN HIS3 plasmid encoding [A73- tRNAHis], [C73-tRNAHis] or no tRNA [V2], as indicated. Positive transformants were grown overnight in selective media, diluted to OD600 = 1 and used to make 10-fold serial dilutions; 2 µl of each dilution was spotted to media (as indicated) and images were taken after 3–4 days of growth at 30°C.
The relatively similar kcat/KM values observed for G−1 and U−1 addition to wild-type (A73) yeast tRNAHis catalyzed by BtTLP (Table 1) suggest that either of these nucleotides may be present at the −1 position of the mature tRNA. The effect of U−1 on histidylation by HisRS in yeast has not been specifically investigated, but A−1- or C−1-containing tRNAHis variants are substrates for HisRS, albeit with decreased catalytic efficiencies, consistent with a predominant role for the 5′-terminal monophosphate in recognition by HisRS (3,26).
DISCUSSION
We have revealed distinct biochemical features of bacterial and archaeal TLPs consistent with a novel physiological function for these enzymes in tRNA 5′-end repair. Initial characterization of the TLP from the bacterium B. thuringiensis (BtTLP) demonstrated a biochemical preference for Watson–Crick template-dependent 3′–5′ nt addition (Figures 1 and 2, Table 1), similar to that observed previously with TLPs from several archaea (9). Upon further investigation, we identified four distinct features of bacterial TLP activity that could be exploited for an alternative function. First, unlike for yeast Thg1, GTP does not effectively compete with other Watson–Crick base pair-forming NTPs for addition by BtTLP (Figure 3 and Supplementary Figure S1). Second, BtTLP adds any of the 4 nts to 5′-truncated tRNAHis substrates with significantly enhanced catalytic efficiency over that observed for nucleotide addition to full-length tRNAHis (Figures 4–6, Supplementary Table S1). Third, while BtTLP adds N−1 nucleotides to tRNAHis with widely varied catalytic efficiencies, with 5′-truncated tRNAHis all four +1 nts are added with similarly high kcat/KM values (Table 1 and Supplementary Table S1, Figure 6). Fourth, BtTLP adds missing nucleotides to a tRNA species other than tRNAHis (Figure 7 and Supplementary Figure S4, Supplementary Table S2). We propose that these distinct biochemical features are well-suited for a physiological role for BtTLP in tRNA 5′-end repair. Similar properties of the archaeal TLP from M. acetivorans (Figures 4 and 7, Supplementary Figures S2 and S3, Supplementary Table S1) suggest a parallel biological function in Archaea, thus greatly expanding the potential scope of 3′–5′ nt addition reactions beyond a simple role for Thg1/TLP family members in tRNAHis maturation.
Identification of bona fide physiological substrates for the 5′-end repair activity is an important future goal that can not be addressed by in vitro characterization alone. In recent years, an increasing number of tRNA quality control mechanisms have been identified, allowing cells to maintain a high-quality cellular pool of tRNAs and thus ensuring optimal fidelity and efficiency of translation (27–34). The TLP-catalyzed tRNA 5′-end repair activity we have identified is well-suited to participating in tRNA quality control. tRNA 5′-end repair mechanisms have not yet been demonstrated in any organism, but several mechanisms for production of 5′-truncated tRNA species provide potential substrates for the 5′-end repair activity. 5′-processing of tRNAs typically generates mature tRNAs initiating at the +1 position [with the notable exception of tRNAHis from certain bacteria and organelles (6,7,11,35)], since removal of the precursor tRNA 5′-leader sequence catalyzed by RNase P occurs for the most part with high fidelity. Nonetheless, miscleavage events occur with significant frequency in bacteria, generating aberrent tRNA 5′-ends, including those that lack one or more nucleotides from the 5′-end (36,37). TLP-catalyzed 5′-end repair of such mis-processed tRNA species would rescue a pool of tRNAs that would otherwise be unusable for translation. In this respect, the 5′-end repair function we propose may be similar to the well-known mechanisms for repair of tRNA 3′-ends catalyzed by the CCA-adding enzyme, which functions to add the 3′-CCA to tRNAs for which this sequence is not genomically encoded, but also functions to repair 3′-ends of tRNA species damaged by cellular nucleases (38,39).
5′-truncated tRNA species could also be generated by the action of 5′–3′ exonucleases that act on tRNA; 5′–3′ exonucleolytic degradation of tRNA has been recently identified in yeast, where the XRN1/RAT1 enzymes act to degrade several hypomodified tRNA species via the rapid tRNA decay pathway (27,40). XRN1/RAT1 family members with unknown functions are widely distributed throughout the bacterial and archaeal domains, including organisms that contain TLPs, and moreover a role for some of these family enzymes in tRNA or rRNA processing or degradation has been proposed (41). Finally, in Archaea, a growing number of alternative tRNA processing/generation pathways have been identified, including production of at least some tRNA species as leaderless transcripts, where it remains unclear how uniformity of 5′-ends is accomplished (42). It is an important future direction to determine the essentiality of TLPs in archaea and bacteria. However, such tests might face the same caveats encountered with tRNA 3′-end repair pathways, which are not inherently essential for viability, but may be particularly required under conditions of stress (39).
Interestingly, the tRNA 5′-end repair reaction identified here is not the first biochemical process proposed to use 3′–5′ nt addition to restore a fully base-paired aminoacyl acceptor stem in tRNA. Previously, a 5′-tRNA editing activity was identified that occurs in the mitochondria of lower eukaryotes, including organisms such as S. punctatus, A. castellani and P. polycephalum (17,19,21,25), and which requires as one of its components an analogous tRNA 5′-end repair activity to the activity described here. 5′-tRNA editing exists to correct genomically encoded mismatches present at the 5′-end of certain mitochondrial tRNAs by first excising the incorrect nucleotides, and then using a 3′–5′ nt addition activity to add the correct nucleotides to the 5′-truncated tRNA, thus creating a fully base paired aminoacyl acceptor stem (17,25). The identity of the protein(s) that catalyze either the nuclease or 5′-end repair components of this activity are not known. The archaeal/bacterial TLP 5′-end repair activity is not likely to function in 5′-tRNA editing in vivo, since sequenced archaeal/bacterial tRNA genes do not contain 5′-mismatched nucleotides that would require editing to generate a functional tRNA. Nonetheless, the existence of the protozoan 5′-tRNA editing activity reinforces the idea that pathways exist for generation of the type of 5′-truncated tRNA substrates that we have associated with bacterial and archaeal TLP function.
The ability of BtTLP to complement the growth defect of the yeast thg1Δ strain was somewhat surprising, given the lack of complementation observed with archaeal TLPs tested previously (9), all of which exhibit similar biochemical activities to BtTLP, including the kinetic preference for 5′-end repair activities over N−1 addition reactions. Interestingly, the reproducibly weaker growth observed in the BtTLP-complemented strain compared to the yeast THG1 control strain (Figure 8) is unlikely to be directly limited by the slower kinetics of G−1 addition to A73-tRNAHis catalyzed by BtTLP, since providing the C73-tRNAHis that is the kinetically preferred substrate for BtTLP activity (Table 1) did not enhance growth (Figure 8). This suggests the interesting possibility that the weaker growth of the BtTLP-complemented strain may reflect alternative activities catalyzed by BtTLP when it is expressed in yeast, perhaps related to the ability of the enzyme to use other substrates for 3′–5′ nt addition (Figure 7).
Alternative 5′-end repair activities of bacterial and archaeal TLPs would resolve the mystery surrounding the presence of TLPs in many organisms that do not inherently require post-transcriptional addition of G−1 to tRNAHis. Nonetheless, these data do not preclude additional roles for bacterial or archaeal TLPs in addition of G−1 to tRNAHis, even in organisms that already contain a genomically encoded G−1. This activity would be required if the encoded G−1 is removed by RNase P-catalyzed processing (11), or by 5′-end degradation pathways such as those described above. A recent independent report of G−1-addition activity catalyzed by two bacterial TLPs (including BtTLP) (16) is consistent with this possibility, and with the various N−1 addition activities demonstrated with tRNAHis substrates in this work (Figures 1 and 2). Moreover, TLPs derived from Archaea that lack a genomically encoded G−1 and thus predictably function in tRNAHis maturation (9), such as M. thermoautotrophicus, also catalyze 5′-end repair with the tRNA substrates tested here (data not shown). Thus prokaryotic TLPs may catalyze both tRNAHis-specific G−1 addition and tRNA 5′-end repair reactions, and further study of these enzymes may yield important insights into the evolution of 3′–5′ addition activities and their varied uses in biology.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: National Institutes of Health (GM087543 to J.E.J.).
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors thank Juan Alfonzo, Venkat Gopalan, Jonatha Gott, Mike Gray, Eric Phizicky and Brian Smith for valuable discussions and help with the manuscript.
REFERENCES
- 1.Gu W, Jackman JE, Lohan AJ, Gray MW, Phizicky EM. tRNAHis maturation: an essential yeast protein catalyzes addition of a guanine nucleotide to the 5′ end of tRNAHis. Genes Dev. 2003;17:2889–2901. doi: 10.1101/gad.1148603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Himeno H, Hasegawa T, Ueda T, Watanabe K, Miura K, Shimizu M. Role of the extra G-C pair at the end of the acceptor stem of tRNA(His) in aminoacylation. Nucleic Acids Res. 1989;17:7855–7863. doi: 10.1093/nar/17.19.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nameki N, Asahara H, Shimizu M, Okada N, Himeno H. Identity elements of Saccharomyces cerevisiae tRNA(His) Nucleic Acids Res. 1995;23:389–394. doi: 10.1093/nar/23.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudinger J, Florentz C, Giege R. Histidylation by yeast HisRS of tRNA or tRNA-like structure relies on residues -1 and 73 but is dependent on the RNA context. Nucleic Acids Res. 1994;22:5031–5037. doi: 10.1093/nar/22.23.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orellana O, Cooley L, Soll D. The additional guanylate at the 5′ terminus of Escherichia coli tRNAHis is the result of unusual processing by RNase P. Mol. Cell. Biol. 1986;6:525–529. doi: 10.1128/mcb.6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkard U, Soll D. The 5′-terminal guanylate of chloroplast histidine tRNA is encoded in its gene. J. Biol. Chem. 1988;263:9578–9581. [PubMed] [Google Scholar]
- 8.Wang C, Sobral BW, Williams KP. Loss of a universal tRNA feature. J. Bacteriol. 2007;189:1954–1962. doi: 10.1128/JB.01203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abad MG, Rao BS, Jackman JE. Template-dependent 3′-5′ nucleotide addition is a shared feature of tRNAHis guanylyltransferase enzymes from multiple domains of life. Proc. Natl Acad. Sci. USA. 2010;107:674–679. doi: 10.1073/pnas.0910961107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L'Abbe D, Lang BF, Desjardins P, Morais R. Histidine tRNA from chicken mitochondria has an uncoded 5′-terminal guanylate residue. J. Biol. Chem. 1990;265:2988–2992. [PubMed] [Google Scholar]
- 11.Placido A, Sieber F, Gobert A, Gallerani R, Giege P, Marechal-Drouard L. Plant mitochondria use two pathways for the biogenesis of tRNAHis. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq646. doi:10.1093/nar/gkq646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyde SJ, Eckenroth BE, Smith BA, Eberley WA, Heintz NH, Jackman JE, Doublie S. THG1, a unique 3′-5′ nucleotidyltransferase, shares unexpected structural homology with canonical 5′-3′ DNA polymerases. Proc. Natl Acad. Sci. USA. doi: 10.1073/pnas.1010436107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackman JE, Phizicky EM. tRNAHis guanylyltransferase catalyzes a 3′-5′ polymerization reaction that is distinct from G-1 addition. Proc. Natl Acad. Sci. USA. 2006;103:8640–8645. doi: 10.1073/pnas.0603068103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preston MA, Phizicky EM. The requirement for the highly conserved G-1 residue of Saccharomyces cerevisiae tRNAHis can be circumvented by overexpression of tRNAHis and its synthetase. RNA. 2010;16:1068–1077. doi: 10.1261/rna.2087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackman JE, Phizicky EM. Identification of critical residues for G-1 addition and substrate recognition by tRNA(His) guanylyltransferase. Biochemistry. 2008;47:4817–4825. doi: 10.1021/bi702517q. [DOI] [PubMed] [Google Scholar]
- 16.Heinemann I, Randau L, Tomko RJ, Jr, Soll D. 3′-5′ tRNA(His) guanylyltransferase in bacteria. FEBS Lett. 2010;584:3567–3572. doi: 10.1016/j.febslet.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bullerwell CE, Gray MW. In vitro characterization of a tRNA editing activity in the mitochondria of Spizellomyces punctatus, a Chytridiomycete fungus. J. Biol. Chem. 2005;280:2463–2470. doi: 10.1074/jbc.M411273200. [DOI] [PubMed] [Google Scholar]
- 18.Laforest MJ, Bullerwell CE, Forget L, Lang BF. Origin, evolution, and mechanism of 5′ tRNA editing in chytridiomycete fungi. RNA. 2004;10:1191–1199. doi: 10.1261/rna.7330504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonergan KM, Gray MW. Editing of transfer RNAs in Acanthamoeba castellanii mitochondria. Science. 1993;259:812–816. doi: 10.1126/science.8430334. [DOI] [PubMed] [Google Scholar]
- 20.Lonergan KM, Gray MW. Predicted editing of additional transfer RNAs in Acanthamoeba castellanii mitochondria. Nucleic Acids Res. 1993;21:4402. doi: 10.1093/nar/21.18.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gott JM, Somerlot BH, Gray MW. Two forms of RNA editing are required for tRNA maturation in Physarum mitochondria. RNA. 2010;16:482–488. doi: 10.1261/rna.1958810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price DH, Gray MW. Confirmation of predicted edits and demonstration of unpredicted edits in Acanthamoeba castellanii mitochondrial tRNAs. Curr. Genet. 1999;35:23–29. doi: 10.1007/s002940050428. [DOI] [PubMed] [Google Scholar]
- 23.Laforest MJ, Roewer I, Lang BF. Mitochondrial tRNAs in the lower fungus Spizellomyces punctatus: tRNA editing and UAG 'stop' codons recognized as leucine. Nucleic Acids Res. 1997;25:626–632. doi: 10.1093/nar/25.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackman JE, Phizicky EM. tRNAHis guanylyltransferase adds G-1 to the 5′ end of tRNAHis by recognition of the anticodon, one of several features unexpectedly shared with tRNA synthetases. RNA. 2006;12:1007–1014. doi: 10.1261/rna.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price DH, Gray MW. A novel nucleotide incorporation activity implicated in the editing of mitochondrial transfer RNAs in Acanthamoeba castellanii. RNA. 1999;5:302–317. doi: 10.1017/s1355838299981840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen AE, Brooks BS, Guth E, Francklyn CS, Musier-Forsyth K. Evolutionary conservation of a functionally important backbone phosphate group critical for aminoacylation of histidine tRNAs. RNA. 2006;12:1315–1322. doi: 10.1261/rna.78606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 28.Dupasquier M, Kim S, Halkidis K, Gamper H, Hou YM. tRNA integrity is a prerequisite for rapid CCA addition: implication for quality control. J. Mol. Biol. 2008;379:579–588. doi: 10.1016/j.jmb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004;18:1227–1240. doi: 10.1101/gad.1183804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotelawala L, Grayhack EJ, Phizicky EM. Identification of yeast tRNA Um(44) 2′-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNA(Ser) species. RNA. 2008;14:158–169. doi: 10.1261/rna.811008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain R, Shuman S. Bacterial Hen1 is a 3′ terminal RNA ribose 2′-O-methyltransferase component of a bacterial RNA repair cassette. RNA. 2009;16:316–323. doi: 10.1261/rna.1926510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichert AS, Morl M. Repair of tRNAs in metazoan mitochondria. Nucleic Acids Res. 2000;28:2043–2048. doi: 10.1093/nar/28.10.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schurer H, Schiffer S, Marchfelder A, Morl M. This is the end: processing, editing and repair at the tRNA 3′-terminus. Biol. Chem. 2001;382:1147–1156. doi: 10.1515/BC.2001.144. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Reimers S, Pandit S, Deutscher MP. RNA quality control: degradation of defective transfer RNA. Embo J. 2002;21:1132–1138. doi: 10.1093/emboj/21.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burkard U, Willis I, Soll D. Processing of histidine transfer RNA precursors. Abnormal cleavage site for RNase P. J. Biol. Chem. 1988;263:2447–2451. [PubMed] [Google Scholar]
- 36.Kikovska E, Brannvall M, Kufel J, Kirsebom LA. Substrate discrimination in RNase P RNA-mediated cleavage: importance of the structural environment of the RNase P cleavage site. Nucleic Acids Res. 2005;33:2012–2021. doi: 10.1093/nar/gki344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kufel J, Kirsebom LA. Different cleavage sites are aligned differently in the active site of M1 RNA, the catalytic subunit of Escherichia coli RNase P. Proc. Natl Acad. Sci. USA. 1996;93:6085–6090. doi: 10.1073/pnas.93.12.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deutscher MP. Ribonucleases, tRNA nucleotidyltransferase, and the 3′ processing of tRNA. Prog. Nucleic Acid Res. Mol. Biol. 1990;39:209–240. doi: 10.1016/s0079-6603(08)60628-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L, Deutscher MP. tRNA nucleotidyltransferase is not essential for Escherichia coli viability. EMBO J. 1987;6:2473–2477. doi: 10.1002/j.1460-2075.1987.tb02528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 2008;22:1369–1380. doi: 10.1101/gad.1654308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anantharaman V, Aravind L. The NYN domains: novel predicted RNAses with a PIN domain-like fold. RNA Biol. 2006;3:18–27. doi: 10.4161/rna.3.1.2548. [DOI] [PubMed] [Google Scholar]
- 42.Heinemann IU, Soll D, Randau L. Transfer RNA processing in archaea: unusual pathways and enzymes. FEBS Lett. 2010;584:303–309. doi: 10.1016/j.febslet.2009.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.