Abstract
The lens was the first tissue in which the concept of embryonic induction was demonstrated. For many years lens induction was thought to occur at the time the optic vesicle and lens placode came in contact. Since then, studies have revealed that lens placodal progenitor cells are specified already at gastrula stages, much earlier than previously believed, and independent of optic vesicle interactions. In this review, I will focus on how individual signalling molecules, in particular BMP, FGF, Wnt and Shh, regulate the initial specification of lens placodal cells and the progressive development of lens cells. I will discuss recent work that has shed light on the combination of signalling molecules and the molecular interactions that affect lens specification and proper lens formation. I will also discuss proposed tissue interactions important for lens development. A greater knowledge of the molecular interactions during lens induction is likely to have practical benefits in understanding the causes and consequences of lens diseases. Moreover, knowledge regarding lens induction is providing fundamental important insights into inductive processes in development in general.
Keywords: lens, induction, BMP, FGF, Shh, Wnt
1. Introduction
One of the fundamental goals in developmental biology is to understand the molecular mechanisms that regulate the induction and patterning of different tissues and organs. In the beginning of the twentieth century Hans Spemann introduced the concept of inductive interactions by studying lens development [1], in which induction is a process by which one group of cells or tissue regulates the development of another group of cells or tissue. Since then several studies have used the lens as a developmental model system to better understand the role of specific signalling molecules, the interplay of different signals and tissue interactions in regulating lens induction and patterning events. The acquired knowledge is not only important for understanding normal lens development, but also key to defining general mechanisms in cell specification, as well as to better understanding of lens function and lens diseases.
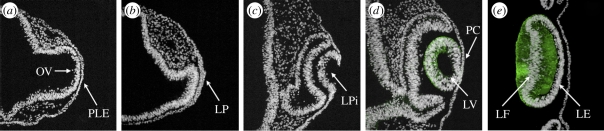
The lens is a component of the peripheral nervous system, which arises from the neural plate border. Lens development is morphologically first visualized by the thickening of the ectoderm into the lens placode, in the vicinity of the prospective optic vesicle (figure 1a,b) [3]. In higher vertebrates, including chick, mouse and humans, the lens placode invaginates and forms the lens pit (figure 1c). Subsequently, the lens pit deepens and the connection with the overlying surface ectoderm, the prospective cornea, is abolished resulting in the formation of the lens vesicle (figure 1d). In lower vertebrates, such as fish and frog, lens formation proceeds via delamination of the lens placodal cells [4,5]. After formation of the lens vesicle, cells at the centre of the posterior side of the lens will elongate and differentiate into primary lens fibre cells, whereas cells of the lens epithelium at the anterior side of the lens retain their ability to proliferate and will generate fibre cells throughout life (figure 1e) [3].
Figure 1.
Morphological changes during early lens development. Immunohistochemistry performed on sections of developing chick lens. (a) The prospective lens ectoderm (PLE) lies close to the optic vesicle (OV), and (b) will subsequently thicken and form the lens placode (LP). (c) Next the lens placode invaginates, leading to the formation of the lens pit (LPi). (d) The lens pit deepens and the connection with the overlying surface ectoderm, the prospective cornea (PC), is lost, shaping the lens vesicle (LV). (e) Lens cells in the posterior part of the lens will elongate and differentiate into primary lens fibre cells (LF), whereas cells at the anterior side consist of lens epithelial cells (LE). (d,e) Differentiated primary lens fibre cells upregulate crystallin proteins, here detected by δ-crystallin [2] shown in green. Nuclei in white are detected with DAPI staining.
Induction of a tissue or organ seldom occurs in one signalling step, but rather as a result of multi-step processes. Subsequently, it can be difficult to point out a single developmental stage for the initiation of induction of a tissue or organ, including lens induction. Thus, it is often more informative to describe ‘specification’ of cell fates, which denotes the step whereby cells have received a signal that will instruct the cells to become a specific cell type unless exposed to signals that divert them to alternative fates. In this review, I will highlight known signalling events that control the initial specification of lens placodal cells and progressive development of lens cells between late blastula to lens vesicle stages, and also discuss proposed tissue interactions important for lens development during these stages. I will not address the role of various transcription factors in early lens specification, since this topic has been reviewed in detail elsewhere [6–9].
2. Lens cells are initially specified at gastrula stages
Specification can be defined under experimental settings (explants assays), such that specified cells will maintain their fate if removed from the embryo and cultured in vitro in the absence of exogenous factors. After specification to a particular cell fate, cells most often have the ability to respond to external signals to acquire another cell identity, before cell fate commitment. Thus, it is important that these experiments are conducted in serum-free conditions in the absence of surrounding tissues to avoid uncontrolled addition of signalling molecules or other components that may affect cell fate.
Early in development, neural plate border cells develop at the junction between the neural and epidermal ectoderm and give rise to placodal and neural crest cells [10–13]. Besides the lens placode, the hypophyseal and olfactory placodes are generated in the rostral part of the neural plate border, whereas the otic, trigeminal and epibranchial placodes, as well as neural crest cells, are formed at a more caudal position of the neural plate border region [14,15]. The well-known rotation transplantation experiment performed by Jacobson in amphibians, in which the neural plate border region was rotated along its rostrocaudal axis at neural plate and late neurula stages, analysed the competence and commitment of the olfactory, lens and otic placodes [16]. The main finding in this paper is that at the neural plate stage, prospective placodal cells are competent to acquire an olfactory, lens and otic placode in response to specific external signals, whereas at the late neurula stage, the different placodal cells appear to be committed to their respective fate. In agreement, transplantation studies in Xenopus also suggest that lens cells are committed to a lens fate at the end of neurulation [17]. Moreover, another study in Xenopus embryos suggests that at gastrula stages the head ectoderm has a ‘lens-forming bias’ [18], however, in this study ‘lens bias’ is defined as the ability of the head ectoderm to respond to external signals from the optic vesicle to acquire lens identity, indicating that these studies actually address competence similar to Jacobson's study. Neither of these studies, however, address when lens cells initially are specified.
A recent study using explant assays in chick has provided evidence that the specification of neural plate border cells is initiated at the late blastula stage [12]. However, at this stage independent of rostrocaudal position, prospective neural plate border cells are specified as neural crest cells, but no placodal cells are detected [12], indicating that at the late blastula stage lens cells are not yet specified. Shortly thereafter, at the late gastrula stage, rostral neural plate border explants cultured in vitro generate cells of lens character, providing evidence that the initial specification of lens cells occurs at the late gastrula stage [19]. In contrast, in Xenopus embryos, lens cells are suggested to be specified as the neural tube closes [20], which can be due to differences in experimental settings or a species-specific difference. Using markers of both differentiated lens and olfactory epithelial cells in specification maps, the study of Sjödal et al. has shown that late gastrula stage and head fold stage rostral neural plate border explants generate both lens and olfactory placodal cells in a non-overlapping manner (figure 2a) [19]. This is consistent with fate maps of gastrula stage chick and zebrafish embryos showing that prospective lens and olfactory placodal cells are intermingled in a domain of the rostral neural plate border [10,11], whereas cells in the caudal neural plate border region are fated to give rise to neural crest and caudal placodal cells [13]. At the late gastrula stage to head fold stages caudal neural plate border explants give rise to neural crest, but no placodal cells ([21,22]; M. Sjödal, C. Patthey, L. Gunhaga 2007, unpublished results), indicating that caudal placodal cells are specified at later stages, and strengthen the argument that lens progenitors are situated in the rostral neural plate border. In contrast to these findings, a study of Bailey et al. [23], using explant assays of head fold stage chick embryos, has suggested that lens specification is the ‘default’ state of all sensory placodes and neural crest cells. However, in this study prospective neural plate border explants from different rostrocaudal regions were analysed by using only lens, but no olfactory placodal or neural crest markers, and although cells of non-lens fate were detected they were not further characterized [23].
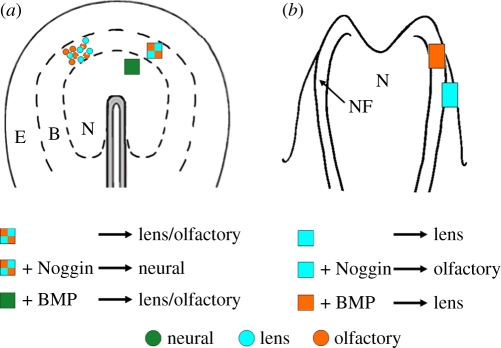
Figure 2.
Sustained BMP activity is critical for lens development. (a) Specification maps of gastrula stage chick embryos; lens (blue circles) and olfactory (orange circles) placodal progenitors are situated in the rostral neural plate border. (a,b) Blue/orange, green, orange and blue boxes indicate regions of explants. (a) Gastrula stage lens/olfactory explants (blue/orange box) generate cells of lens and olfactory character when cultured alone, and cells of neural character if cultured together with Noggin. Gastrula stage forebrain explants (green box) generate cells of lens and olfactory character when cultured together with BMP4. (b) Neural fold stage lens explants (blue box) generate cells of lens character when cultured alone, and cells of olfactory character if cultured together with Noggin. Neural fold stage olfactory explants (orange box) generate cells of lens character when cultured together with BMP4. Modified from [19]. E, epidermal; B, neural plate border; N, neural plate; NF, neural fold. See text for more details.
3. Directed or random migration
Since prospective lens and olfactory placodal cells are intermingled at gastrula stages [10], it still remains unclear whether the differential specification of lens and olfactory cells occurs in the rostral neural plate border or at their final spatial destinations. Fate and specification maps have shown that at neural fold stages, olfactory and lens placodal progenitor cells are spatially separated (figure 2b) [10,19,24]. This process strongly resembles the development of the eye and the odour-detecting antenna in Drosophila, where the visual and olfactory cells arise from a common imaginal disc, and at later stages separate and acquire their distinct identities [25]. In Drosophila, it has been suggested that the transcription factors Eyeless (Ey) (Pax6 homologue) and Distalless (Dll) (Dlx homologue), in which Ey negatively regulates Dll [26], play a role in the differential specification of eye and antennal cells, respectively [25]. In chick, Pax6 and Dlx5 remain co-expressed until the lens and olfactory placodes are morphologically visualized, and the differential expression of Pax6 in the lens and Dlx5 in the olfactory placode occurs only at later stages [10], indicating that these molecular cues cannot be involved in restricted cell migration. However, lens cells forced to maintain Dlx5 expression in chick [10] and Pax6−/− cells in mouse chimeras [27] are expelled from the developing lens, implicating these transcription factors in regulation of cell sorting during lens development.
It is possible that cells in the rostral neural plate border are specified as rostral placodal progenitor cells, and retain the capacity to adopt either lens or olfactory placodal fate until exposed to additional molecular signals from the surrounding environment. At neural fold stages, Pax6, Dlx5, Six1 and Sox2, are expressed in both lens and olfactory placodal precursor cells [10,19,28]. In addition, at this stage no molecular marker has been shown to distinguish the morphologically separated lens and olfactory placodal precursors, supporting the idea that rostral placodal precursors may initially comprise a common fate. Nevertheless, at the neural fold stage, presumptive lens placodal cells cultured in vitro acquire lens but not olfactory placodal character [19,23], providing evidence that at this stage lens placodal precursors have been exposed to signals that direct them towards a lens fate. Thus, the question of whether the differential specification of lens and olfactory placodal cells occurs in the rostral neural plate border followed by a cell restricted migration, or at their final spatial destinations remains to be determined.
4. Tissue interactions
Without doubt the development of mature lens cells from presumptive lens ectodermal cells requires tissue interactions. When and how specific tissues might regulate lens development has also been discussed elsewhere [29–31]. Spemann's classical studies in 1901 suggested that the optic vesicle, which will give rise to the retina, is required for the development of the lens [1]. This statement was later challenged by findings that a lens or lens-like structures can form even in the absence of retinal tissue [32,33]. These first studies concerning lens induction were based exclusively on morphology. Since then, accumulating results using cytological criteria and molecular markers indicate that structures resembling a lens develop independent of interactions with the neural retina [19,23,34]. However, at later developmental stages the optic vesicle appears to play an important role for further maturation of the lens [35–38].
Explant assays in chick have provided evidence that gastrula stage prospective lens placodal cells cultured alone, without interactions of the neuroectoderm, epidermal ectoderm or underlying mesoderm, generate cells of lens character [19,22,23], suggesting that the initial specification of lens cells is independent of tissue interactions. In Xenopus the situation is somewhat different, since both rostral neural tissue and mesoderm are suggested to be required and/or enhance lens induction [20], once again pointing towards a difference in experimental settings or species-specific difference in lens induction. In chick, it has been shown that in the absence of the underlying mesoderm prospective adenohypophyseal placodal cells generate cells of lens character ([23]; D. Gustavsson and L. Gunhaga 2007, unpublished results), suggesting that the mesoderm underlying adenohypophyseal placodal progenitors restricts lens fate in prospective hypophyseal placodal ectoderm. Consistently, prospective hypophyseal placodal explants cultured in contact with the underlying head mesoderm generate cells of adenohypophyseal character, and under these conditions no lens cells are detected (D. Gustavsson and L. Gunhaga 2007, unpublished results). Taken together, in chick, the initial specification of lens cells does not require interactions with the underlying head mesoderm, but rather the reverse; the most rostro-medial part of the head mesoderm appears to restrict specification of lens fate.
The restriction of lens fate in the caudal part of the embryo is regulated by neural crest cells [23]. At neural fold stage, prospective lens ectodermal explants cultured together with prospective, pre-migratory or migratory neural crest cells all fail to generate cells of lens character, analysed by Pax6 and δ-crystallin expression [23]. Moreover, partial ablation of prospective neural crest cells from fore- and midbrain levels at neural fold stage in chick [23] and amphibians [39], resulted in ectopic lens formation in a fraction of the studied embryos. Why all embryos did not generate ectopic lenses could be due to the fact that cells are exposed to other lens-repressing signals together with lack of lens-promoting signals in the caudal region of the embryo. Taken together, the initial specification of lens cells appears to depend on planar signals within the ectoderm, and one major challenge has been to define when and how different signals regulate this process.
5. Bone morphogenetic protein signals play a key role in the specification of lens placodal cells
Bone morphogenetic protein (BMP) signals have been shown to play important roles during lens formation. Already at gastrula stages Bmp2 and Bmp4, as well as the BMP downstream activator, p-Smad-1/5/8, are detected in the rostral neural plate border, where both prospective lens and olfactory placodal cells are positioned [40,41]. Recent results using chick explants and intact embryo assays have provided evidence that at this stage, BMP activity is both required and sufficient to induce lens and olfactory placodal cells, and that sustained exposure of placodal progenitors to BMP signals is required for lens induction [19]. Prospective olfactory/lens placodal explants from gastrula stage chick embryos cultured together with the BMP inhibitor Noggin generate cells of neural character (figure 2a) [19]. Vice versa, prospective forebrain explants cultured in the presence of BMP4 generate cells of lens and olfactory placodal character (figure 2a) [19]. Thus, chick explant assays provide evidence that at gastrula stages, BMP activity inhibits neural fate and regulates the initial specification of lens placodal cells (figure 3a). Consistently, Bmp4 knockout mouse embryos lack morphological lens placodes, but the expression of Pax6 and Six3 is detected in prospective lens ectoderm [42]. These observations indicate that lens placodal progenitor cells are induced in Bmp4 mutant mice, which might reflect functional redundancy between Bmp members, as Bmp2 is also expressed in the neural plate border region at gastrula stages [40].
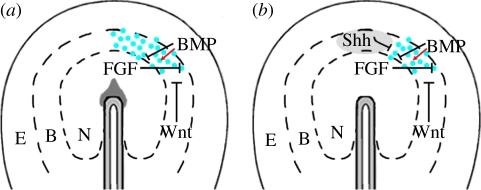
Figure 3.
Model of the initial specification of lens cells. Proposed signalling events at gastrula to head fold stages in the rostral neural plate border during the initial specification of lens cells. (a) From gastrula stages, FGF activity represses the generation of epidermal cells. BMP signals inhibit neural formation, and in the context of FGF activity induce lens/olfactory placodal progenitor cells. Wnt activity restricts caudal expansion. (b) From head fold stages, Shh emanating from the underlying mesoderm (grey domain) restricts rostral expansion of lens formation. (a,b) The broken lines indicate that there is not a strict boundary, but actually an overlap between prospective neural, rostral placodal and epidermal cells [10,13]. E, epidermal; B, neural plate border; N, neural plate; blue circles, prospective lens placodal cells. See text for more details.
A useful tool to study the roles of signalling molecules in early lens development in mice is the Lens-Cre construct. In this construct the Cre recombinase is driven by a lens-specific enhancer of Pax6, expressed in the prospective lens ectoderm and the surface ectoderm near the presumptive lens, generating lens-specific transgenes [43]. By using the Lens-Cre construct to delete either the two type I BMP receptors Alk2/Alk3, or Smad1/Smad5 or Smad4, a recent study has shown that BMP activity regulates lens formation in mice [44], supporting previous findings in chick [19]. This study suggests that lens placodal invagination and upregulation of the lens markers FoxE3 and αA-crystallin is mediated by BMP signalling in a Smad-independent manner, while cell proliferation in the lens is mediated by the Smad pathway [44]. However, in the study of Rajagopal et al., no data for an alternative downstream BMP pathway regulating lens specification was presented, and it is unclear why the significant increase in cell death in Smad1/Smad5 deficient lens cells does not prevent or cause disturbed lens formation. Thus, further studies have to be performed to define in detail the downstream pathway(s) of BMP receptor activation that regulate the formation and invagination of the lens placode, and the initial upregulation of lens markers.
At the neural fold stages in chick, pSmad-1/5/8 is preferentially detected in the prospective lens ectoderm compared to the prospective olfactory placodal region [19]. At this stage using prospective lens and olfactory placodal explants, cells can switch between lens and olfactory placodal fate in response to changes in BMP activity, providing evidence that at neural fold stages BMP signals promote the generation of lens cells at the expense of olfactory placodal cells (figure 2b) [19]. Furthermore, in intact neural fold stage chick embryos, inhibition of BMP signals in prospective lens cells completely abolishes lens placodal formation and inhibits the onset of L-Maf and δ-crystallin expression [19]. Consistently in Bmp4 mutant embryos, where rostral placodal progenitor cells are generated, although lens placodes fail to develop the olfactory placodes appear normal [42]. At later stages, Bmp7 is expressed in the lens ectoderm and optic vesicle, while Bmp4 is expressed in the optic vesicle [45,46]. Since both Bmp7 − /− and Bmp4 − /− mice embryos exhibit disturbed lens formation [42,46], it appears that both Bmp4 and Bmp7 are required for lens development, and that these Bmp family members cannot subsidize for one another. Collectively, these results indicate that sustained BMP activity regulates the specification and formation of the lens placode. What specific role, if any, BMP signals play in the differentiation of lens fibre cells has, however, not been determined.
6. The role of fibroblast growth factor signalling in lens placodal cell fate
Many studies have shown that fibroblast growth factor (FGF) signals play an important role in lens development, primarily in secondary lens fibre cell differentiation [6,47,48]. In support of this, several members of the FGF family are expressed in the eye region and all four FGF receptors (FGFR1–4) are expressed in the developing vertebrate lens [48]. Thus, the requirement of FGF signals during lens formation is apparent, but how does FGF activity regulate early lens specification?
A recent study has provided evidence that at the late gastrula stage, when lens placodal cells are initially specified, FGF activity prevents prospective lens/olfactory placodal cells in the rostral neural plate border from acquiring epidermal fate [19]. However, at this stage, FGF8 is not sufficient to induce cells of lens character in either prospective neural or epidermal cells [19]. The above results, taken together with the role BMP signals play at gastrula stages, suggest a possible model of early lens specification (figure 3a), in which FGF and BMP signals act in the neural plate border region in an opposing manner, to restrict neural and epidermal cell fate, respectively. Thus, in the context of FGF signals, which prevent the generation of epidermal cell fate, BMP activity specifies lens/olfactory placodal progenitor cells in the rostral neural plate border (figure 3). In the light of this model previous results can be interpreted in new ways. Mis-expression of Fgf8 in the chick head ectoderm at early neural tube stages ectopically induces expression of the early lens marker L-Maf [49]. Rather than a direct lens-inducing role of FGF signals, these results indicate that mis-expression of Fgf8 in the ectoderm inhibits the generation of epidermal cells, thereby enabling BMP signals to ectopically induce cells of lens character. Similar results are observed in Xenopus embryos, where placodal Six1 expression is induced by a combination of FGF8 and low levels of BMP activity, but not by FGF8 alone [50].
Neither at the gastrula stage nor at the neural fold stage do FGF signals contribute to the differential specification of lens and olfactory placodal cells [19,23]. Although Bailey et al. proposed that FGF signalling represses lens specification and induces olfactory fate, both their studies and another study reveal that at the neural fold stage, lens progenitor cells do not upregulate olfactory markers when exposed to FGF8 [19,23]. In addition, in the presence of FGF activity, presumptive lens cells still generate L-Maf and δ-crystallin positive cells [19]. Moreover, at this stage inhibition of FGF signalling does not induce lens character in prospective olfactory cells [19,23]. Thus, at early stages of development changes in FGF activity are not sufficient to switch between a lens and olfactory placodal fate.
In embryonic day (E) 8.5–E9.5 mouse eye explants, inhibition of FGF signals reduces Pax6 expression in the lens placode and the size of the lens pits formed in culture [51]. Moreover, blocking FGF activity in the presumptive lens by expressing a dominant-negative FgfR1 using the Lens-Cre construct results in reduced Pax6 expression, decrease in placodal thickness and delayed placodal invagination, but nevertheless a lens, although smaller, develops [51]. This phenotype resembles the disrupted lens formation in Frs2α2F/2F mice mutants [52]. Frs2α2F is a docking protein mediating FGF signalling via the ERK pathway, and in Frs2α2F/2F mice mutants Pax6 and Six3 expression are decreased in the presumptive lens ectoderm, the placodal thickness is reduced and approximately 70 per cent of the mutants have smaller lenses [52]. At lens pit stages, both α- and β-crystallins are induced in the lens in Lens-Cre;FgfR1/2/3-deficient mouse embryos [53]. Consistently, in chick, the induction of δ-crystallin expression is not directly regulated by FGF signals, whereas the induction of Caprin2 expression and further differentiation of lens fibre cells requires FGF activity [36]. In summary, although FGF activity is required at early stages for preventing lens progenitor cells from acquiring an epidermal fate and for proper lens placodal formation, the initial differentiation of primary lens fibre cells and onset of early lens-specific markers are not dependent on FGF signals.
7. Caudal restriction of lens cells by wnt activity
Though, at gastrula stages, the importance of BMP and FGF signalling in ensuring a correct medial–lateral restriction of lens placodal cell character is clear, other signals provide rostral and caudal suppression of lens fate. Several studies have provided evidence that Wnt signals play a key role in suppressing lens formation in the caudal part of the neural plate border region. As previously described in this review, at the late gastrula stage cells in the rostral neural plate border are specified as lens/olfactory placodal cells, while cells in the caudal border are specified as neural crest cells [19,21,22]. At this stage, Wnt activity imposes caudal character on neural plate border cells, thereby inhibiting lens and olfactory placodal specification, while promoting the generation of neural crest cells (figure 3a) [22,54]. Moreover, gain-and-loss of Wnt activity studies in chick explant assays have provided evidence that cells can switch between a lens/olfactory placodal and neural crest fate in response to changes in Wnt activity [22]. Prospective neural crest cells cultured in the presence of soluble Frizzled receptor, acting as a Wnt inhibitor, acquire lens and olfactory placodal character. On the other hand, prospective lens/olfactory placodal cells cultured together with Wnt3 conditioned medium acquire neural crest fate [22]. Consistently, Masterblind and headless zebrafish mutant embryos, which exhibit exaggerated Wnt signalling in the rostral neural plate border, lack or have reduced lens and olfactory placodes, while trigeminal placodal and neural crest cells have expanded into the rostral part of the embryo [55–57]. Similarly, in the Drosophila eye-antennal imaginal disc, the expression of Ey (the Pax6 homolog) is suppressed by Wingless signals [58].
The findings that Wnt signals inhibit the specification of lens cells indicates that prospective lens cells need to develop in a Wnt-free domain. These results are supported by findings in chick and mouse, showing that the Wnt antagonist, Secreted frizzled-related protein 2 (Sfrp2) is expressed in the lens placode of mice and chick [46,59]. Moreover, in Xenopus, Dickkopf1 (Dkk1), another Wnt inhibitor, is required for the exclusion of neural crest formation in the rostral neural plate border region [60]. In LacZ reporter mouse lines, which express LacZ in response to activation of the canonical Wnt pathway [61,62] there is no indication of X-gal staining, i.e. Wnt activity, in the lens region at E8.5–E15.5 [63,64]. Consistently, even in the presence of Wnt inhibitors prospective lens cells in chick explants still upregulate the lens markers L-Maf and δ-crystallin (Sjödal and Gunhaga 2006, unpublished data). Finally, in mice, individual deletions of two Wnt co-receptors, Lrp5 and Lrp6 required for Wnt canonical signalling, do not perturb lens fate determination [64–66]. Although, a double knock-out of Lrp5 and Lrp6 would determine whether these co-receptors may act redundantly during early lens specification, it seems unlikely that such double mutants would exhibit a lens phenotype, since the above results clearly provide evidence that Wnt signals are not required for lens fate specification.
Also at lens placodal stages, several functional studies in mice have provided evidence that Wnt activity through the canonical pathway represses lens formation. In mice, gain of β-catenin activity through the Lens-Cre system prevents lens formation, suppresses Pax6 expression and upregulates Tuj1 expression [64,67]. Smith and colleagues argue that surface ectodermal cells have acquired a neural fate, but since studies performed at earlier stages have shown that prospective lens cells can switch to neural crest fate in response to Wnt activity [22], it is also possible that Tuj1 positive neural crest derived neurons are generated. Analyses of specific neural crest and neuroectoderm markers would reveal this uncertainty. In agreement, loss of β-catenin activity in the presumptive lens and extraocular ectoderm using the Lens-Cre construct does not perturb lens fate decision, but leads to formation of small ectopic lentoids expressing Pax6 and crystallin proteins in the extraocular ectoderm [63,64]. The overall conclusion is that between gastrula stages and lens placodal stages Wnt activity restricts caudal expansion of lens formation (figure 3).
8. Wnt independent β-catenin signalling affects lens morphogenesis
Although no activation of the canonical Wnt pathway has been detected in the developing lens [63,64], β-catenin is expressed in the lens placode at E9.5 [64], suggesting that Wnt independent β-catenin activity plays a role in lens formation. β-catenin is known to affect cell adhesion and morphogenesis, and is a central component of the cadherin–catenin adhesion complex, which anchors the adhesion complex to the actin cytoskeleton. Conditional deletion of β-catenin, using the Lens-Cre construct, results in breaks in the continuous curve of the epithelium. These breaks corresponds to disruption of cytoskeletal and/or junctional complexes, detected by F-actin and ZO1 labelling, respectively [63,64]. These results indicate that Wnt independent β-catenin activity is required, not for lens fate decision or initial lens placodal invagination, but for further lens morphogenesis.
9. Rostral restriction of lens cells by sonic hedgehog activity
Fate maps in chick and zebrafish at gastrula to neural fold stages have shown that lens precursor cells are located in a more caudal–distal domain compared to adenohypophysis progenitors, which arise from the most rostral–medial region of the neural plate border [10,11,68]. Several studies have provided evidence that Hedgehog (Hh) signals play a major role in the development of the adenohypophysis, and have suggested that Hh activity promotes the generation of adenohypophyseal cells, while inhibiting lens formation [69–71]. In agreement with these results, a recent study has observed that at the head fold stage in chick, Sonic Hedgehog (Shh) is expressed in the mesoderm underlying the prospective hypophyseal placode, which expresses Ptc2, a receptor for Shh signalling [72]. In contrast, at this stage, the mesoderm underlying the prospective lens placode does not express Shh, and prospective lens placodal cells do not express Ptc2 [72]. Thus, the expression patterns of members of the Shh signalling pathway support the idea that Hh signals suppress lens fate and promote the specification of adenohypophyseal cells.
Convincing studies of different vertebrate mutants with disturbed Hh activity have provided evidence that Hh signals direct the choice between adenohypophyseal and lens fate. Talpid3 chicken mutants, which have a defective activation of the Shh pathway, develop ectopic lenses usually located in the midline deriving from or connected to the hypophyseal duct [69,70]. Similarly, several zebrafish mutants with disturbed Hh-signalling have ectopic lenses at the expense of the adenohypophysis [71,73,74]. In these mutants, the head ectoderm that normally forms the hypophyseal placode instead develops into a lens indicated by the repression of adenohypophyseal markers together with upregulation of lens-specific markers [71,73,74]. In addition, transgenic mice that over-express the Shh inhibitor Hip (Huntingtin interacting protein) in the oral ectoderm and in the adenohypophyseal placode only develop a rudimentary Rathke's pouch [75]. In agreement with these studies, in chick explant assays when Shh signals are blocked prospective adenohypophyseal placodal cells generate cells of lens and olfactory epithelial fate (Gustavsson and Gunhaga 2007, unpublished data). Consistently, over-expression of Hh in zebrafish suppresses lens formation [11,76]. In Xenopus, Xhip (Xenopus hip1) is expressed in the prospective lens ectoderm, and loss of Xhip, leading to exaggerated Hh activity, results in the suppression of lens placode formation [77]. These observations indicate that the absence or presence of Shh activity in the rostral part of the surface ectoderm mediates a switch between lens and adenohypophyseal placodal cell identity. In summary, at the head fold stage, Shh signals from the underlying mesoderm act on the most rostral neural plate border cells, thereby inhibiting lens specification and inducing adenohypophyseal placodal cell fate. Thus, from head fold stages, Shh activity in the neural plate border region regulates the rostral restriction of lens specification (figure 3b).
In zebrafish, the Nodal signalling pathway, belonging to the transforming growth factor (TGF)β-family, has been implicated in directly activating transcription of the Shh gene [78]. Subsequently, in zebrafish embryos with mutations in the cyclops gene, encoding for nodal-related protein2, midline expression of Shh is lost in the ventral central nervous system [79,80]. Consistently, mutations affecting components of the Nodal signalling pathway result in severe axial defects with two lens placodes in close proximity or a single median eye and lens formed at the expense of the adenohypophysis [79,81,82]. Taken together, as seen in both Hh and Nodal mutants, Hh signals play a key role in generating two separated lens domains via the formation of a medial located hypophyseal placode.
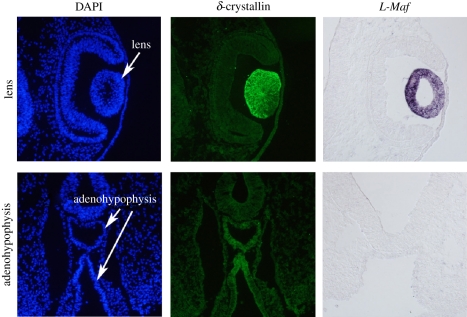
At later stages of development, the characteristic protein of chick lens fibre cells, δ-crystallin, is also transiently expressed in the adenohypophysis, as first demonstrated by Barabanov and Fedtsova [83]. Although the adenohypophysis expresses δ-crystallin, this expression is very weak compared with the δ-crystallin expression in the lens (figure 4), and an estimated concentration of δ-crystallin in the stage 22 chick adenohypophysis is only approximately 1/3000 of the detected amount in the lens [84]. Moreover, lens fibre cells, but not the adenohypophysis, express L-Maf, an early lens marker first expressed in the prospective lens ectoderm (figure 4) [85], and the morphological cell elongation characteristic of lens fibre cells is not detected in adenohypophyseal cells [84,86]. These results imply that high levels of δ-crystallin expression in cells of chick is characteristic of lens fibre cells.
Figure 4.
Expression of δ-crystallin and L-Maf in lens and adenohypophysis. The expression of δ-crystallin in the lens of a stage 17 chick embryo is strong, while there is barely detectable δ-crystallin expression in the adenohypophysis. L-Maf is expressed in the lens but not in the adenohypophysis. The same immunohistochemistry slide using the same magnification and time of exposure was used.
10. Retinoic acid and notch activity in early lens formation
In the last 10 years, several studies concerning how retinoic acid (RA) regulates lens development have been reported, and recently reviewed [87]. RA activity is first observed in the lens placode around neural fold stage and at later stages in the lens pit and lens vesicle, using in vivo reporter systems to visualize RA signalling [88,89]. The invagination of the lens placode appears to depend on RA activity, while inhibition of RA signals using antisense oligonucleotides against cellular retinal-binding protein (CRBP) in early neural fold to early neural tube stages in mouse results in disturbed invagination of the lens placode [90]. A similar phenotype, with failure of lens placodal invagination, is also observed in Pax6Sey/Sey mice mutants, which interestingly exhibit reduced RA activity in the lens placode [88]. Since, mice mutants suppressed in BMP or FGF activity, also exhibit reduced or completely blocked Pax6 expression [46,52,91], it is possible that several signalling molecules regulate Pax6 expression, which in turn is critical for proper lens placodal invagination and further lens development.
Notch signalling is known to promote proliferation and inhibit cell differentiation in many embryonic tissues. When the intracellular domain of Notch (NotchIC) translocates to the nucleus it acts in a transcriptional complex with the DNA-binding transcription factor Rbpj and Mastermind to promote transcription. Loss of Notch signalling in the lens of mice, by using a Lens-Cre;Rbpj construct, results in premature exit from the cell cycle and subsequently accelerated primary lens fibre cell differentiation [92,93]. Consistently, mice with constitutive Notch activity, through Lens-Cre;Notch1IC, exhibit delayed primary fibre cell differentiation [92]. However, the overall conclusion from studies using the Lens-Cre construct to suppress Notch activity is that Notch signals are not essential for lens formation, but are primarily required during secondary lens fibre cell differentiation [92–95].
To date, studies regarding how Notch signals affect lens development at earlier stages are few. The first direct evidence that Notch activity plays a role in lens induction is that inhibition of the Notch ligand, Delta2, in Xenopus results in loss or severe reduction of FoxE3 expression followed by failure of placodal formation and reduced or absent γ1-crystallin expression [96]. Interestingly, the FoxE3 promoter includes a target sequence for Su(H)-binding motif for Notch signalling and also for Smad1-binding motif for BMP signalling, and both are suggested to be required for proper upregulation of FoxE3 [96]. This finding beautifully highlights the general knowledge that a combination of signalling factors affects lens specification and proper lens formation.
11. Concluding remarks
A model that describes how specific signalling molecules regulate the initial specification of lens cells in vertebrates at gastrula stages has emerged (figure 3). At this stage, planar BMP, FGF and Wnt signal(s) within the ectoderm regulate lens induction. In the context of FGF signals, which repress epidermal ectoderm formation, BMP activity specifies lens/olfactory placodal progenitor cells in the rostral neural plate border region. Wnt activity restricts caudal expansion of lens formation. From head fold stages, Shh activity derived from the underlying mesoderm restricts lens formation in the most rostral part of the neural plate border. Taken together, around gastrula to head fold stages the activities of BMP, FGF, Shh and Wnt appear to choreograph the patterning of the neural plate border and subsequently restrict the ectoderm fated to become the future lens (figure 3).
Acknowledgements
I thank Michael Wride, the referees and group members of L.G. for their comments on the manuscript. L.G. is supported by Umeå University, Sweden.
Footnotes
One contribution of 10 to a Theme Issue ‘The ocular lens: a classic model for development, physiology and disease’.
References
- 1.Spemann H. 1901. Uber Korrelationen in der Entwicklung des Auges. Vehr. Anat. Ges. 15, 61–79 [Google Scholar]
- 2.Beebe D. C., Piatigorsky J. 1981. Translational regulation of delta-crystallin synthesis during lens development in the chicken embryo. Dev. Biol. 84, 96–101 10.1016/0012-1606(81)90374-2 (doi:10.1016/0012-1606(81)90374-2) [DOI] [PubMed] [Google Scholar]
- 3.Ogino H., Yasuda K. 2000. Sequential activation of transcription factors in lens induction. Dev. Growth Differ. 42, 437–448 10.1046/j.1440-169x.2000.00532.x (doi:10.1046/j.1440-169x.2000.00532.x) [DOI] [PubMed] [Google Scholar]
- 4.Greiling T. M., Clark J. I. 2009. Early lens development in the zebrafish: a three-dimensional time-lapse analysis. Dev. Dyn. 238, 2254–2265 10.1002/dvdy.21997 (doi:10.1002/dvdy.21997) [DOI] [PubMed] [Google Scholar]
- 5.McDevitt D. S., Brahma S. K. 1973. Ontogeny and localization of the crystallins during embryonic lens development in Xenopus laevis. J. Exp. Zool. 186, 127–140 10.1002/jez.1401860204 (doi:10.1002/jez.1401860204) [DOI] [PubMed] [Google Scholar]
- 6.Cvekl A., Duncan M. K. 2007. Genetic and epigenetic mechanisms of gene regulation during lens development. Progr. Retin. Eye Res. 26, 555–597 10.1016/j.preteyeres.2007.07.002 (doi:10.1016/j.preteyeres.2007.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang R. A. 2004. Pathways regulating lens induction in the mouse. Int. J. Dev. Biol. 48, 783–791 10.1387/ijdb.041903rl (doi:10.1387/ijdb.041903rl) [DOI] [PubMed] [Google Scholar]
- 8.Medina-Martinez O., Jamrich M. 2007. Foxe view of lens development and disease. Development 134, 1455–1463 10.1242/dev.000117 (doi:10.1242/dev.000117) [DOI] [PubMed] [Google Scholar]
- 9.Reza H. M., Yasuda K. 2004. Lens differentiation and crystallin regulation: a chick model. Int. J. Dev. Biol. 48, 805–817 10.1387/ijdb.041863hr (doi:10.1387/ijdb.041863hr) [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya S., Bailey A. P., Bronner-Fraser M., Streit A. 2004. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev. Biol. 271, 403–414 10.1016/j.ydbio.2004.04.010 (doi:10.1016/j.ydbio.2004.04.010) [DOI] [PubMed] [Google Scholar]
- 11.Dutta S., Dietrich J. E., Aspock G., Burdine R. D., Schier A., Westerfield M., Varga Z. M. 2005. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development 132, 1579–1590 10.1242/dev.01723 (doi:10.1242/dev.01723) [DOI] [PubMed] [Google Scholar]
- 12.Patthey C., Edlund T., Gunhaga L. 2009. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development 136, 73–83 10.1242/dev.025890 (doi:10.1242/dev.025890) [DOI] [PubMed] [Google Scholar]
- 13.Streit A. 2002. Extensive cell movements accompany formation of the otic placode. Dev. Biol. 249, 237–254 10.1006/dbio.2002.0739 (doi:10.1006/dbio.2002.0739) [DOI] [PubMed] [Google Scholar]
- 14.Baker C. V., Bronner-Fraser M. 2001. Vertebrate cranial placodes. I. Embryonic induction. Dev. Biol. 232, 1–61 10.1006/dbio.2001.0156 (doi:10.1006/dbio.2001.0156) [DOI] [PubMed] [Google Scholar]
- 15.McCabe K. L., Bronner-Fraser M. 2009. Molecular and tissue interactions governing induction of cranial ectodermal placodes. Dev. Biol. 332, 189–195 10.1016/j.ydbio.2009.05.572 (doi:10.1016/j.ydbio.2009.05.572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson A. G. 1963. The determination and positioning of the nose, lens and ear. III. Effects of reversing the antero-posterior axis of epidermis, neural plate and neural fold. J. Exp. Zool. 154, 293–303 10.1002/jez.1401540305 (doi:10.1002/jez.1401540305) [DOI] [PubMed] [Google Scholar]
- 17.Henry J. J., Grainger R. M. 1987. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev. Biol. 124, 200–214 10.1016/0012-1606(87)90472-6 (doi:10.1016/0012-1606(87)90472-6) [DOI] [PubMed] [Google Scholar]
- 18.Grainger R. M., Mannion J. E., Cook T. L., Jr, Zygar C. A. 1997. Defining intermediate stages in cell determination: acquisition of a lens-forming bias in head ectoderm during lens determination. Dev. Genet. 20, 246–257 (doi:10.1002/(SICI)1520-6408(1997)20:3<246::AID-DVG7>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 19.Sjodal M., Edlund T., Gunhaga L. 2007. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev. Cell 13, 141–149 10.1016/j.devcel.2007.04.020 (doi:10.1016/j.devcel.2007.04.020) [DOI] [PubMed] [Google Scholar]
- 20.Henry J. J., Grainger R. M. 1990. Early tissue interactions leading to embryonic lens formation in Xenopus laevis. Dev. Biol. 141, 149–163 10.1016/0012-1606(90)90110-5 (doi:10.1016/0012-1606(90)90110-5) [DOI] [PubMed] [Google Scholar]
- 21.Basch M. L., Bronner-Fraser M., Garcia-Castro M. I. 2006. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441, 218–222 10.1038/nature04684 (doi:10.1038/nature04684) [DOI] [PubMed] [Google Scholar]
- 22.Patthey C., Gunhaga L., Edlund T. 2008. Early development of the central and peripheral nervous systems is coordinated by Wnt and BMP signals. PLoS ONE 3, e1625. 10.1371/journal.pone.0001625 (doi:10.1371/journal.pone.0001625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey A. P., Bhattacharyya S., Bronner-Fraser M., Streit A. 2006. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev. Cell 11, 505–517 10.1016/j.devcel.2006.08.009 (doi:10.1016/j.devcel.2006.08.009) [DOI] [PubMed] [Google Scholar]
- 24.Couly G. F., Le Douarin N. M. 1987. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev. Biol. 120, 198–214 10.1016/0012-1606(87)90118-7 (doi:10.1016/0012-1606(87)90118-7) [DOI] [PubMed] [Google Scholar]
- 25.Kumar J. P., Moses K. 2001. Eye specification in Drosophila: perspectives and implications. Semin. Cell Dev. Biol. 12, 469–474 10.1006/scdb.2001.0270 (doi:10.1006/scdb.2001.0270) [DOI] [PubMed] [Google Scholar]
- 26.Kurata S., Go M. J., Artavanis-Tsakonas S., Gehring W. J. 2000. Notch signaling and the determination of appendage identity. Proc. Natl Acad. Sci. USA 97, 2117–2122 10.1073/pnas.040556497 (doi:10.1073/pnas.040556497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collinson J. M., Hill R. E., West J. D. 2000. Different roles for Pax6 in the optic vesicle and facial epithelium mediate early morphogenesis of the murine eye. Development 127, 945–956 [DOI] [PubMed] [Google Scholar]
- 28.Rex M., Orme A., Uwanogho D., Tointon K., Wigmore P. M., Sharpe P. T., Scotting P. J. 1997. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev. Dyn. 209, 323–332 (doi:10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K) [DOI] [PubMed] [Google Scholar]
- 29.Chow R. L., Lang R. A. 2001. Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 17, 255–296 10.1146/annurev.cellbio.17.1.255 (doi:10.1146/annurev.cellbio.17.1.255) [DOI] [PubMed] [Google Scholar]
- 30.Collinson J. M., Hill R. E., West J. D. 2004. Analysis of mouse eye development with chimeras and mosaics. Int. J. Dev. Biol. 48, 793–804 10.1387/ijdb.041885jc (doi:10.1387/ijdb.041885jc) [DOI] [PubMed] [Google Scholar]
- 31.Fisher M., Grainger R. M. 2004. Lens induction and determination. In Development of the ocular lens (eds Lovicu F. J., Robinson M. L.). New York, NY: Cambridge University Press [Google Scholar]
- 32.King H. H. 1905. Experimental studies on the eye of the frog embryo. Arch. Entwicklungsmech. 19, 85–107 10.1007/BF02162203 (doi:10.1007/BF02162203) [DOI] [Google Scholar]
- 33.Mencl E. 1903. Ein fall von beiderseitiger augenlinsenausbildung wahrend der abwesenheit von augenblasen. Arch. Entwicklungsmech. 16, 328–339 10.1007/BF02162834 (doi:10.1007/BF02162834) [DOI] [Google Scholar]
- 34.McAvoy J. W. 1981. The spatial relationship between presumptive lens and optic vesicle/cup during early eye morphogenesis in the rat. Exp. Eye Res. 33, 447–458 10.1016/S0014-4835(81)80095-4 (doi:10.1016/S0014-4835(81)80095-4) [DOI] [PubMed] [Google Scholar]
- 35.Coulombre J. L., Coulombre A. J. 1963. Lens development: fiber elongation and lens orientation. Science 142, 1489–1490 10.1126/science.142.3598.1489 (doi:10.1126/science.142.3598.1489) [DOI] [PubMed] [Google Scholar]
- 36.Loren C. E., Schrader J. W., Ahlgren U., Gunhaga L. 2009. FGF signals induce Caprin2 expression in the vertebrate lens. Differentiation 77, 386–394 10.1016/j.diff.2008.11.003 (doi:10.1016/j.diff.2008.11.003) [DOI] [PubMed] [Google Scholar]
- 37.Lovicu F. J., Chamberlain C. G., McAvoy J. W. 1995. Differential effects of aqueous and vitreous on fiber differentiation and extracellular matrix accumulation in lens epithelial explants. Invest. Ophthalmol. Vis. Sci. 36, 1459–1469 [PubMed] [Google Scholar]
- 38.McAvoy J. W., Fernon V. T. 1984. Neural retinas promote cell division and fibre differentiation in lens epithelial explants. Curr. Eye Res. 3, 827–834 10.3109/02713688409000795 (doi:10.3109/02713688409000795) [DOI] [PubMed] [Google Scholar]
- 39.von Woellwarth C. 1961. Die rolle des neuralleistenmaterials und der temperature bei der determination der augenlinse. Embryologia (Nagoya) 6, 219–242 10.1111/j.1440-169X.1961.tb00126.x (doi:10.1111/j.1440-169X.1961.tb00126.x) [DOI] [Google Scholar]
- 40.Chapman S. C., Schubert F. R., Schoenwolf G. C., Lumsden A. 2002. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev. Biol. 245, 187–199 10.1006/dbio.2002.0641 (doi:10.1006/dbio.2002.0641) [DOI] [PubMed] [Google Scholar]
- 41.Faure S., De Santa Barbara P., Roberts D. J., Whitman M. 2002. Endogenous patterns of BMP signaling during early chick development. Dev. Biol. 244, 44–65 10.1006/dbio.2002.0579 (doi:10.1006/dbio.2002.0579) [DOI] [PubMed] [Google Scholar]
- 42.Furuta Y., Hogan B. L. 1998. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 12, 3764–3775 10.1101/gad.12.23.3764 (doi:10.1101/gad.12.23.3764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashery-Padan R., Marquardt T., Zhou X., Gruss P. 2000. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14, 2701–2711 10.1101/gad.184000 (doi:10.1101/gad.184000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajagopal R., et al. 2009. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev. Biol. 335, 305–316 10.1016/j.ydbio.2009.08.027 (doi:10.1016/j.ydbio.2009.08.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudley A. T., Robertson E. J. 1997. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev. Dyn. 208, 349–362 (doi:10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 46.Wawersik S., Purcell P., Rauchman M., Dudley A. T., Robertson E. J., Maas R. 1999. BMP7 acts in murine lens placode development. Dev. Biol. 207, 176–188 10.1006/dbio.1998.9153 (doi:10.1006/dbio.1998.9153) [DOI] [PubMed] [Google Scholar]
- 47.Lovicu F. J., McAvoy J. W. 2005. Growth factor regulation of lens development. Dev. Biol. 280, 1–14 10.1016/j.ydbio.2005.01.020 (doi:10.1016/j.ydbio.2005.01.020) [DOI] [PubMed] [Google Scholar]
- 48.Robinson M. L. 2006. An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 17, 726–740 10.1016/j.semcdb.2006.10.002 (doi:10.1016/j.semcdb.2006.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurose H., Okamoto M., Shimizu M., Bito T., Marcelle C., Noji S., Ohuchi H. 2005. FGF19-FGFR4 signaling elaborates lens induction with the FGF8-L-Maf cascade in the chick embryo. Dev. Growth Differ. 47, 213–223 10.1111/j.1440-169X.2005.00795.x (doi:10.1111/j.1440-169X.2005.00795.x) [DOI] [PubMed] [Google Scholar]
- 50.Ahrens K., Schlosser G. 2005. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev. Biol. 288, 40–59 10.1016/j.ydbio.2005.07.022 (doi:10.1016/j.ydbio.2005.07.022) [DOI] [PubMed] [Google Scholar]
- 51.Faber S. C., Dimanlig P., Makarenkova H. P., Shirke S., Ko K., Lang R. A. 2001. Fgf receptor signaling plays a role in lens induction. Development 128, 4425–4438 [DOI] [PubMed] [Google Scholar]
- 52.Gotoh N., et al. 2004. Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc. Natl Acad. Sci. USA 101, 17 144–17 149 10.1073/pnas.0407577101 (doi:10.1073/pnas.0407577101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao H., et al. 2008. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276–288 10.1016/j.ydbio.2008.03.028 (doi:10.1016/j.ydbio.2008.03.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Litsiou A., Hanson S., Streit A. 2005. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development 132, 4051–4062 10.1242/dev.01964 (doi:10.1242/dev.01964) [DOI] [PubMed] [Google Scholar]
- 55.Itoh M., Kudoh T., Dedekian M., Kim C. H., Chitnis A. B. 2002. A role for iro1 and iro7 in the establishment of an anteroposterior compartment of the ectoderm adjacent to the midbrain–hindbrain boundary. Development 129, 2317–2327 [DOI] [PubMed] [Google Scholar]
- 56.Kim C. H., Oda T., Itoh M., Jiang D., Artinger K. B., Chandrasekharappa S. C., Driever W., Chitnis A. B. 2000. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407, 913–916 10.1038/35038097 (doi:10.1038/35038097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van de Water S., Van de Wetering M., Joore J., Esseling J., Bink R., Clevers H., Zivkovic D. 2001. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development 128, 3877–3888 [DOI] [PubMed] [Google Scholar]
- 58.Treisman J. E., Rubin G. M. 1995. Wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development 121, 3519–3527 [DOI] [PubMed] [Google Scholar]
- 59.Chen Y., Stump R. J., Lovicu F. J., McAvoy J. W. 2004. Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int. J. Dev. Biol. 48, 867–877 10.1387/ijdb.041882yc (doi:10.1387/ijdb.041882yc) [DOI] [PubMed] [Google Scholar]
- 60.Carmona-Fontaine C., Acuna G., Ellwanger K., Niehrs C., Mayor R. 2007. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev. Biol. 309, 208–221 10.1016/j.ydbio.2007.07.006 (doi:10.1016/j.ydbio.2007.07.006) [DOI] [PubMed] [Google Scholar]
- 61.Dasgupta R., Fuchs E. 1999. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 [DOI] [PubMed] [Google Scholar]
- 62.Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M., Piccolo S. 2003. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl Acad. Sci. USA 100, 3299–3304 10.1073/pnas.0434590100 (doi:10.1073/pnas.0434590100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kreslova J., Machon I O., Ruzickova I J., Lachova I J., Wawrousek E. F., Kemler R., Krauss S., Piatigorsky J., Kozmik1 Z. 2007. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis 45, 157–168 10.1002/dvg.20277 (doi:10.1002/dvg.20277) [DOI] [PubMed] [Google Scholar]
- 64.Smith A. N., Miller L. A., Song N., Taketo M. M., Lang R. A. 2005. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev. Biol. 285, 477–489 10.1016/j.ydbio.2005.07.019 (doi:10.1016/j.ydbio.2005.07.019) [DOI] [PubMed] [Google Scholar]
- 65.Gong Y., et al. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 10.1016/S0092-8674(01)00571-2 (doi:10.1016/S0092-8674(01)00571-2) [DOI] [PubMed] [Google Scholar]
- 66.Stump R. J., et al. 2003. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev. Biol. 259, 48–61 10.1016/S0012-1606(03)00179-9 (doi:10.1016/S0012-1606(03)00179-9) [DOI] [PubMed] [Google Scholar]
- 67.Miller L. A., Smith A. N., Taketo M. M., Lang R. A. 2006. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev. Biol. 6, 14. 10.1186/1471-213X-6-14 (doi:10.1186/1471-213X-6-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Couly G. F., Le Douarin N. M. 1985. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev. Biol. 110, 422–439 10.1016/0012-1606(85)90101-0 (doi:10.1016/0012-1606(85)90101-0) [DOI] [PubMed] [Google Scholar]
- 69.Buxton P., Davey M. G., Paton I. R., Morrice D. R., Francis-West P. H., Burt D. W., Tickle C. 2004. Craniofacial development in the talpid3 chicken mutant. Differentiation 72, 348–362 10.1111/j.1432-0436.2004.07207006.x (doi:10.1111/j.1432-0436.2004.07207006.x) [DOI] [PubMed] [Google Scholar]
- 70.Davey M. G., et al. 2006. The chicken talpid3 gene encodes a novel protein essential for Hedgehog signaling. Genes Dev. 20, 1365–1377 10.1101/gad.369106 (doi:10.1101/gad.369106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kondoh H., Uchikawa M., Yoda H., Takeda H., Furutani-Seiki M., Karlstrom R. O. 2000. Zebrafish mutations in Gli-mediated hedgehog signaling lead to lens transdifferentiation from the adenohypophysis anlage. Mech. Dev. 96, 165–174 10.1016/S0925-4773(00)00387-7 (doi:10.1016/S0925-4773(00)00387-7) [DOI] [PubMed] [Google Scholar]
- 72.Sjodal M., Gunhaga L. 2008. Expression patterns of Shh, Ptc2, Raldh3, Pitx2, Isl1, Lim3 and Pax6 in the developing chick hypophyseal placode and Rathke's pouch. Gene Exp. Patterns 8, 481–485 10.1016/j.gep.2008.06.007 (doi:10.1016/j.gep.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 73.Swindell E. C., Zilinski C. A., Hashimoto R., Shah R., Lane M. E., Jamrich M. 2008. Regulation and function of foxe3 during early zebrafish development. Genesis 46, 177–183 10.1002/dvg.20380 (doi:10.1002/dvg.20380) [DOI] [PubMed] [Google Scholar]
- 74.Varga Z. M., Amores A., Lewis K. E., Yan Y. L., Postlethwait J. H., Eisen J. S., Westerfield M. 2001. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development 128, 3497–3509 [DOI] [PubMed] [Google Scholar]
- 75.Treier M., O'connell S., Gleiberman A., Price J., Szeto D. P., Burgess R., Chuang P. T., McMahon A. P., Rosenfeld M. G. 2001. Hedgehog signaling is required for pituitary gland development. Development 128, 377–386 [DOI] [PubMed] [Google Scholar]
- 76.Barth K. A., Wilson S. W. 1995. Expression of zebrafish nk2.2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development 121, 1755–1768 [DOI] [PubMed] [Google Scholar]
- 77.Cornesse Y., Pieler T., Hollemann T. 2005. Olfactory and lens placode formation is controlled by the hedgehog-interacting protein (Xhip) in Xenopus. Dev. Biol. 277, 296–315 10.1016/j.ydbio.2004.09.016 (doi:10.1016/j.ydbio.2004.09.016) [DOI] [PubMed] [Google Scholar]
- 78.Muller F., Albert S., Blader P., Fischer N., Hallonet M., Strahle U. 2000. Direct action of the nodal-related signal cyclops in induction of sonic hedgehog in the ventral midline of the CNS. Development 127, 3889–3897 [DOI] [PubMed] [Google Scholar]
- 79.Hatta K., Kimmel C. B., Ho R. K., Walker C. 1991. The cyclops mutation blocks specification of the floor plate of the zebrafish central nervous system. Nature 350, 339–341 10.1038/350339a0 (doi:10.1038/350339a0) [DOI] [PubMed] [Google Scholar]
- 80.Krauss S., Concordet J. P., Ingham P. W. 1993. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75, 1431–1444 10.1016/0092-8674(93)90628-4 (doi:10.1016/0092-8674(93)90628-4) [DOI] [PubMed] [Google Scholar]
- 81.Gritsman K., Zhang J., Cheng S., Heckscher E., Talbot W. S., Schier A. F. 1999. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97, 121–132 10.1016/S0092-8674(00)80720-5 (doi:10.1016/S0092-8674(00)80720-5) [DOI] [PubMed] [Google Scholar]
- 82.Rebagliati M. R., Toyama R., Haffter P., Dawid I. B. 1998. Cyclops encodes a nodal-related factor involved in midline signaling. Proc. Natl Acad. Sci. USA 95, 9932–9937 10.1073/pnas.95.17.9932 (doi:10.1073/pnas.95.17.9932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barabanov V. M., Fedtsova N. G. 1982. The distribution of lens differentiation capacity in the head ectoderm of chick embryos. Differentiation 21, 183–190 10.1111/j.1432-0436.1982.tb01212.x (doi:10.1111/j.1432-0436.1982.tb01212.x) [DOI] [PubMed] [Google Scholar]
- 84.Ueda Y., Okada T. S. 1986. Transient expression of a ‘lens-specific’ gene, delta-crystallin, in the embryonic chicken adenohypophysis. Cell Differ. 19, 179–185 10.1016/0045-6039(86)90094-1 (doi:10.1016/0045-6039(86)90094-1) [DOI] [PubMed] [Google Scholar]
- 85.Ogino H., Yasuda K. 1998. Induction of lens differentiation by activation of a bZIP transcription factor, L-Maf. Science 280, 115–118 10.1126/science.280.5360.115 (doi:10.1126/science.280.5360.115) [DOI] [PubMed] [Google Scholar]
- 86.Sullivan C. H., Marker P. C., Thorn J. M., Brown J. D. 1998. Reliability of delta-crystallin as a marker for studies of chick lens induction. Differentiation 64, 1–9 10.1046/j.1432-0436.1998.6410001.x (doi:10.1046/j.1432-0436.1998.6410001.x) [DOI] [PubMed] [Google Scholar]
- 87.Cvekl A., Wang W. L. 2009. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 89, 280–291 10.1016/j.exer.2009.04.012 (doi:10.1016/j.exer.2009.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Enwright J. F., III, Grainger R. M. 2000. Altered retinoid signaling in the heads of small eye mouse embryos. Dev. Biol. 221, 10–22 10.1006/dbio.2000.9652 (doi:10.1006/dbio.2000.9652) [DOI] [PubMed] [Google Scholar]
- 89.Molotkov A., Molotkova N., Duester G. 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901–1910 10.1242/dev.02328 (doi:10.1242/dev.02328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bavik C., Ward S. J., Chambon P. 1996. Developmental abnormalities in cultured mouse embryos deprived of retinoic by inhibition of yolk-sac retinol binding protein synthesis. Proc. Natl Acad. Sci. USA 93, 3110–3114 10.1073/pnas.93.7.3110 (doi:10.1073/pnas.93.7.3110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faber S. C., Robinson M. L., Makarenkova H. P., Lang R. A. 2002. Bmp signaling is required for development of primary lens fiber cells. Development 129, 3727–3737 [DOI] [PubMed] [Google Scholar]
- 92.Rowan S., Conley K. W., Le T. T., Donner A. L., Maas R. L., Brown N. L. 2008. Notch signaling regulates growth and differentiation in the mammalian lens. Dev. Biol. 321, 111–122 10.1016/j.ydbio.2008.06.002 (doi:10.1016/j.ydbio.2008.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saravanamuthu S. S., Gao C. Y., Zelenka P. S. 2009. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev. Biol. 332, 166–176 10.1016/j.ydbio.2009.05.566 (doi:10.1016/j.ydbio.2009.05.566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jia J., Lin M., Zhang L., York J. P., Zhang P. 2007. The Notch signaling pathway controls the size of the ocular lens by directly suppressing p57Kip2 expression. Mol. Cell Biol. 27, 7236–7247 10.1128/MCB.00780-07 (doi:10.1128/MCB.00780-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le T. T., Conley K. W., Brown N. L. 2009. Jagged 1 is necessary for normal mouse lens formation. Dev. Biol. 328, 118–126 10.1016/j.ydbio.2009.01.015 (doi:10.1016/j.ydbio.2009.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogino H., Fisher M., Grainger R. M. 2008. Convergence of a head-field selector Otx2 and notch signaling: a mechanism for lens specification. Development 135, 249–258 10.1242/dev.009548 (doi:10.1242/dev.009548) [DOI] [PMC free article] [PubMed] [Google Scholar]