Abstract
The eye lens is avascular, deriving nutrients from the aqueous and vitreous humours. It is, however, unclear which mechanisms mediate the transfer of solutes between these humours and the lens' fibre cells (FCs). In this review, we integrate the published data with the previously unpublished ultrastructural, dye loading and magnetic resonance imaging results. The picture emerging is that solute transfer between the humours and the fibre mass is determined by four processes: (i) paracellular transport of ions, water and small molecules along the intercellular spaces between epithelial and FCs, driven by Na+-leak conductance; (ii) membrane transport of such solutes from the intercellular spaces into the fibre cytoplasm by specific carriers and transporters; (iii) gap-junctional coupling mediating solute flux between superficial and deeper fibres, Na+/K+-ATPase-driven efflux of waste products in the equator, and electrical coupling of fibres; and (iv) transcellular transfer via caveoli and coated vesicles for the uptake of macromolecules and cholesterol. There is evidence that the Na+-driven influx of solutes occurs via paracellular and membrane transport and the Na+/K+-ATPase-driven efflux of waste products via gap junctions. This micro-circulation is likely restricted to the superficial cortex and nearly absent beyond the zone of organelle loss, forming a solute exchange barrier in the lens.
Keywords: vertebrate eye lens, solute flux, ultrastructure, gap junctions, MRI, dye loading
1. Introduction
Unlike the majority of tissues, the post-natal lens has no direct blood supply. Instead, it obtains its nutrients mainly from the aqueous humour, which bathes the anterior face of the lens. Oxygen is derived from the iris' and retina's vasculature and across the cornea by diffusion through the aqueous and vitreous humours, respectively [1]. The aqueous humour is secreted by the ciliary body and contains all the ingredients needed to sustain the lens. It flows from the equatorial region of the lens and through the narrow space between the anterior lens surface and the iris, before moving through the pupil into the anterior chamber and finally leaving the eye through the trabecular meshwork at the periphery of the anterior chamber. This allows the lens to take up anabolites, such as glucose and amino acids, from the aqueous humour and to discharge catabolites, most notably lactate, into it. The flow of the aqueous humour towards the posterior lens pole will be limited as the anterior hyaloid of the vitreous is closely attached to the posterior surface of the lens leaving only the small space of Berger for solute transfer.
The equatorial and anterior lens epithelium forms a barrier between the aqueous humour and the lens fibre cells (FCs), which constitute the bulk of the lens. The well-established correlation between impaired lens epithelial function and the development of cataracts suggests that this cellular barrier has an important regulatory role in solute and water influx and outflux in the lens FC [2–11]. The prevailing theory for the supply of the lens FCs is that the lens epithelium actively takes up ions and anabolic molecules, which subsequently diffuse across the epithelial–fibre cell interface (EFI) into the outermost FCs and into their neighbours via an extensive network of gap junctions (GJs), which interconnects lens FCs [12–14]. The tight metabolic coupling of lens epithelial and FCs has, however, been challenged and therefore whether GJ and transcellular transports are the main mechanisms of solute transfer is now under discussion. We have re-evaluated the relevant literature in an attempt to assess the contributions of different routes for solutes to be exchanged between the aqueous humour and the lens FCs across the lens epithelium. This re-evaluation suggests that the lens has developed multiple transport mechanisms to compensate for the lack of a direct blood supply: (i) GJ-based transport, (ii) paracellular transport, (iii) FC membrane-based transport, and (iv) transcellular transport.
2. Gap-junctional transport
In the lens, solutes are actively taken up by the epithelium from the aqueous humour and are thought to diffuse across the EFI into and within the lens fibre mass via an extensive network of GJs, which interconnect the lens FCs [12–14]. Furthermore, the high degree of electrical coupling between anterior lens epithelial cells (LECs) [15], and between LECs and the underlying FCs [16] seemed to indicate a role for GJ-mediated transport across the EFI to FCs. Dye transfer was observed only in 10 per cent of LECs, but this was proposed to support the observed GJ-based transport across the EFI [17].
GJs are essential for lens development and function. The targeted knockout of, or mutations in, any of the three lens connexins causes cataracts in mice (α1-connexin (Cx43) [18,19]; α3-connexin (Cx46) [20]; α8-connexin (Cx50/MP70) [21–27]). Similarly, connexin mutations in humans cause cataract (α3-connexin [28–31]; α8-connexin [32–38]). Recent studies in mice suggest that FCs of the lens cortex are also partially connected by a Lim2-mediated large molecule diffusion pathway (LMDP) and form a cytoplasmic syncytium. In contrast to the radially oriented GJ pathway, this pathway is predominantly circumferential (i.e. within a tissue stratum). It has been suggested that LMDP is mainly involved in the short-range diffusion of large molecules within a lens FC stratum and is of minor relevance for the transfer of ions, water and small molecules and thus outside the focus of this paper [39,40].
On account of this evidence, it was widely believed that all cells in the mammalian lens were well-coupled, both electrically and metabolically, via an extensive network of GJs and by membrane fusions mediated by LIM2. This coupling was thought to include the EFI, but recent data do not support this conclusion. GJ-coupling across the EFI was apparently confirmed by studies reporting the transfer of both small and soluble tracer molecules between LECs and underlying FCs [17,41]. Also, morphologically identified GJs were found at the EFI [17,41–44]. Other reports [45,46] have, however, failed to detect significant dye-coupling between LECs and the underlying FCs or significant numbers of morphologically identifiable GJs at the EFI in the chicken lens.
In a preliminary confocal microscopy study on whole rat lenses, which were biochemically loaded with 6-carboxyfluorescein diacetate, the carboxyfluorescein signal was found to be restricted to the epithelium (figure 1a–d), with the exception of the very early elongating FCs in the equatorial region (figure 1d); no signal was observed in the underlying fibres (figure 1a–d; for details on the materials and methods used, see the electronic supplementary material). This observation of low but reproducible levels of apparent dye transfer between LECs and FCs near the rat lens equator is supported by data obtained for the annular pad in the equatorial region of the embryonic chicken lens [46]. While this signal might reflect residual esterase activity in early elongating cells, there is evidence for the presence of GJs at the EFI in this region. Extensive freeze-fracture EM analyses of large, contiguous areas of the anterior EFI of adult chicken lenses revealed very few GJs: less than one of 100 cells with a size of less than 0.1 µm2 each [47]. Subsequent analyses of embryonic chicken [46], adult primate and human [48,49], rat [50], bovine [51], frog (Rana pipiens) [50] and zebrafish lenses [52] (J. van Marle 2010, unpublished observations) confirmed the lack of GJs at the anterior EFI. These results explain the absence of dye transfer between LECs and FCs along the majority of the EFI. However, in the embryonic chicken lens [45] and in adult rat, primate and human lenses numerous GJs were detected in the equatorial region [48,49,53] (figures 2 and 3). This suggests that the reproducibly low levels of dye transfer in this region might be due to GJ coupling. The restriction of the dye signal to these equatorial LECs and FCs and its failure to spread to other FC neighbours indicate that there is limited GJ connectivity between these youngest FCs and that these connections are yet to mature.
Figure 1.

Deacetylated 6-carboxyfluorescein is retained in the lens epithelium. Confocal laser scanning microscopy images of sections of 6-carboxyfluorescein-diacetate-loaded adult rat lenses (a,c) counterstained with propidium iodide (b,d) to detect nuclear DNA. (a) Low magnification of the equatorial epithelium and bow region of an adult rat lens. While the epithelial cells (ECs) display strong 6-carboxyfluorescein fluorescence, the signal in the fibre cells (FCs) is not above background levels. A low level of 6-carboxyfluorescein fluorescence signal can, however, be detected in the very early elongating FCs, albeit at much lower levels than in the lens epithelium. Note that there appears to be a relatively steep decline in the level of 6-carboxyfluorescein fluorescence from ECs to the first differentiating FCs (arrow). Higher magnification images show (c) 6-carboxyfluorescein and (d) propidium iodide fluorescence in the equatorial lens epithelium and early differentiating lens cells. While there still is 6-carboxyfluorescein fluorescence (c) in the early elongating FCs, the signal intensities are significantly below those observed in the epithelium (a). In all micrographs, the anterior pole of the lens is towards the top of the image, the posterior pole towards the bottom. Scale bars, (a,b) 50 µm; (c,d) 100 µm.
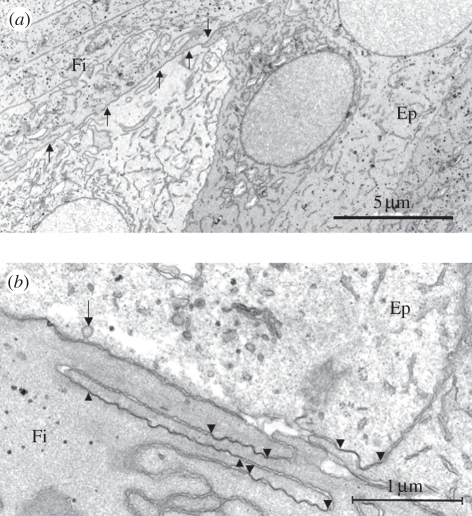
Figure 2.
Gap junctions (GJs) connecting differentiating FCs (Fi) and ECs (Ep) at the lens equator. (a) Medium and (b) high-power transmission electron microscopy micrographs of the epithelial–fibre cell interface (EFI) in the equatorial region of a young human lens. Numerous GJs are evident between the apical ends of the early differentiating FCs and between the equatorial ECs and the underlying fibres (arrows in (a) and arrowheads in (b)). Note the presence of a coated endocytotic–exocytotic vesicle (arrow in (b)).
Figure 3.
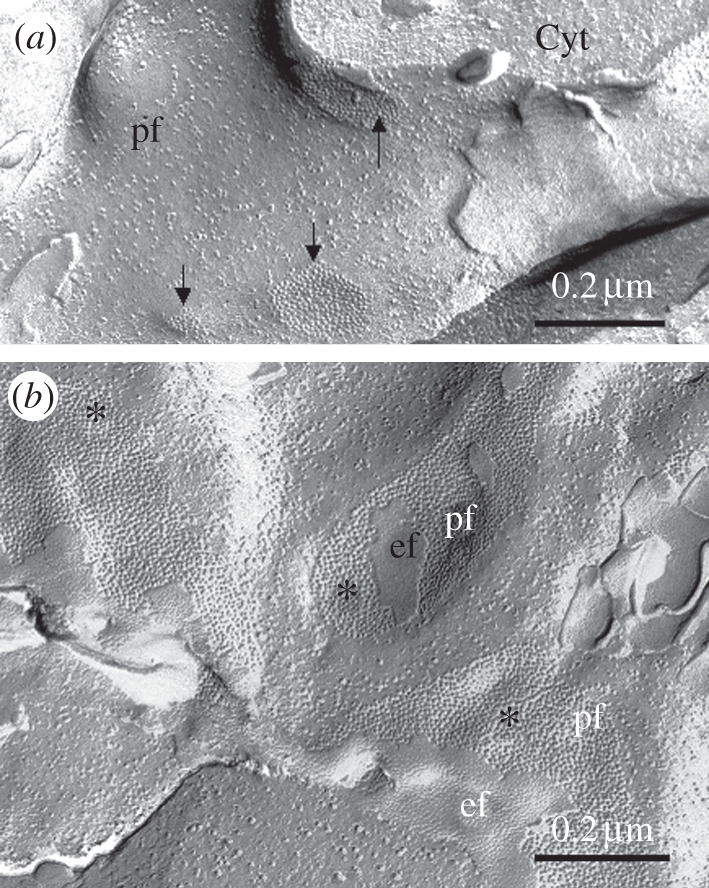
Morphology of GJs between LECs. Freeze-fracture micrographs taken from a human lens show (a) moderate, both in number and size, GJs (arrows) at the lateral surfaces between LECs and (b) large and numerous GJs at the interface between early elongating FCs (asterisks). ef and pf, e and p-face, respectively, of the fractured membranes; Cyt, cytoplasm.
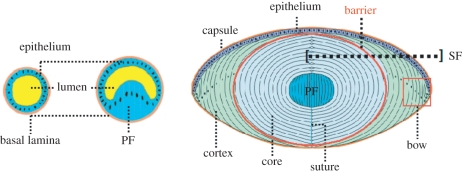
The absence of GJs along the vast majority of the EFI is a surprising phenomenon. It has to be noted, however, that the lens epithelium is functionally inverted when compared with other absorptive epithelia, such as the intestinal epithelium, with the LECs' apical membranes facing the lens vesicle's lumen during early lens development and the fibre mass at later stages. The LECs' basal membrane, by contrast, faces the lens capsule or basal lamina (figure 4a). Cell-to-cell junctions are restricted to specific membrane regions and in most epithelia GJs and tight junctions (TJs) are restricted to the apical lateral membranes. Re-assessment of scanning, ultrathin section and freeze fracture electron microscopy images provides a view of the differentiation of post-mitotic epithelial cells (ECs) supporting the conclusion that GJs are unlikely to occur at the EFI on account of the way lens fibres elongate. The transition from LECs to lens FCs, in addition to changes in gene expression, involves substantial morphological alterations.
Figure 4.
Lens development. The left and middle cartoon illustrate the inverted character of the embryonic lens vesicle with the basal side of the epithelium—lined by the basal lamina—facing outwards and the apical side facing the vesicle lumen. The right cartoon shows that this inverted polarity is maintained in the adult lens. Note that the posterior LECs grow as primary lens fibres (PFs) in anterior direction filling-up the vesicle, forming the embryonic lens nucleus (EN) in the adult lens and that the post-mitotic equatorial LECs differentiate to secondary lens fibres (SFs) forming the main mass (cortex and core or nucleus) of the lens. As discussed, the lens fibre membranes beyond the zone of nuclear degradation and organelle loss have different physiological properties and form a solute transfer barrier between the lens cortex and the lens core or nucleus.
As shown in figure 5b,c, the basal (capsule-facing) plasma membranes of the sigmoid-shaped differentiating LECs extend in a posterior direction. The fibre-to-fibre membranes in the posterior region originate from the lateral plasma membranes of adjacent LECs and remain linked during the FC elongation process. This explains why they are studded with numerous GJs (figure 3). At their leading edges, they form structures reminiscent of neuronal growth cones and have a rich complement of organelles (figure 6b), including centrosomes [54].
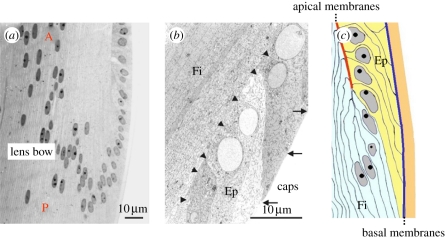
Figure 5.
Morphology of early differentiating lens FCs. As illustrated in the light microscope image shown in (a) taken from a mouse lens, the nuclei of the post-mitotic LECs change their shape from round to ellipsoid and apparently move first in a posterior (P) and subsequently in an anterior (A) direction as fibre differentiation proceeds. The low magnification TEM micrograph (b), taken from a young human lens, demonstrates (boxed in the right cartoon of figure 4) that the LECs in a very early phase of differentiation assume an asymmetric sigmoid-like shape with protruding edges at the interface with the capsule (arrows) and at the interface with the preceding elongating FCs (arrowheads). The posterior outgrowing edge seems more advanced relative to the nuclear position than the fibre-facing edge (compare the length of the apical (arrows) and basal (arrowheads) membranes of individual elongating LECs). The cartoon in (c) emphasizes the point that the epithelial–fibre interface (EFI) is formed by the apical membranes of the LECs and the FC apical membranes. It additionally shows that the basal membranes of LECs are covering the whole surface of the lens capsule. caps, capsule; Ep, differentiating epithelial cells; Fi, lens FCs.
Figure 6.
Ultrastructure of the cytoplasm at the apical/basal ends of lens FC apical membranes. TEM micrographs of the growth cone-like structure (GC) with their leading edges (asterisks) at the (a) anterior and (b) posterior ends show them to be rich in organelles, such as mitochondria (m) and cisternae of the endoplasmic reticulum as well as an accumulation of unidentified electron-dense bodies (arrowheads). Micrographs taken from a young human lens.
As shown in figure 5b,c and also in figure 2a,b, the apical (fibre-facing) plasma membranes of the elongating FCs grow in an anterior direction and remain apposed to the apical surface of the LECs. The lateral membranes of adjacent growing FCs remain in contact and form new GJs while continuing to extend (figures 2b and 3b). They are, however, absent from the EFI where the apical membranes of the undifferentiated LECs and apical membranes of lens FCs contact each other (figure 7b,c). The GJs between the anterior lateral plasma membranes of the growing FCs are large and numerous (figure 3b), as observed by others [53]. This contrasts with the relatively few and small GJs observed between neighbouring undifferentiated LECs (figure 3a). It is an open question whether the switch in expression from Cx43, which is the predominant GJ protein in LECs, to Cx46 and Cx50, which are the only connexins expressed in FCs, occurs at this point during differentiation. The anterior growth cone-like structures (figure 6a) are rich in organelles. So it seems likely that the differentiation and elongation of LECs is by appositional growth, both anteriorly and posteriorly. This appositional growth is confirmed, at least for the anterior part of the lens, by the scanning electron microscopy images shown in figure 8 (see legend for details). There is a difference in texture between the plasma membrane interface of LECs and FCs compared with that which exists between neighbouring FCs (figure 8b,d). This might represent a particular proteinaceous coating as previously suggested [55], representing a remnant of the apical glycocalyx normal for an epithelial lining of lumens. This surface does express different carbohydrate antigens in support of this possibility [56]. Although GJs are dynamic connections between cells, which can be formed and broken down when required, the apposition of apical-to-apical membranes and the presence of a glycocalyx-like coating at the EFI makes GJ formation an unlikely event for the fibres and the anterior LECs.
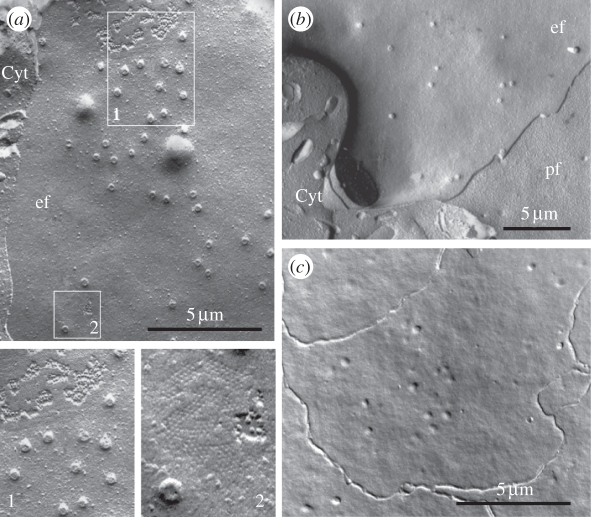
Figure 7.
Evidence for vesicular events between LECs and at the EFI in the human lens. (a) Freeze-fracture electron micrograph of the equatorial region. The lateral plasma membranes of the differentiating fibres show numerous necks of endocytotic–exocytotic vesicles (see also boxed region no. 1) and gap junctions (gj) interconnecting adjacent FCs (boxed region no. 2). (b) The plasma membranes of the apical ends of the differentiating FCs show numerous necks of endocytotic–exocytotic vesicles, but GJs are absent. (c) This micrograph shows the plasma membrane associations of four cells in the lens epithelium near the anterior lens pole. At low magnification, the p-face of the apical membranes of these LECs, parallel to the EFI, shows the presence of numerous vesicle necks and the absence of GJs. ef, pf, e-face and p-face, respectively, of the fractured plasma membrane; Cyt, cytoplasm.
Figure 8.
Growth cone-like structures of new FCs migrating along the EFI. The SEM images in this figure were taken from a (a,b) rabbit and (c,d) a human lens. In lateral views (a,b), cohorts of FCs are seen growing along the EFI towards the anterior lens pole (Ant). En face observations (c,d) show cohorts of FCs growing together along the EFI. The high magnification SEM micrograph of (d) also shows that the epithelial lining of the EFI has a texture different from that of the FC apical membranes. Moreover, this micrograph shows the presence of numerous ball-and-socket joints at the apices of the shorter FC plasma membranes, which differ morphologically from the interdigitating protrusions along the edges of the FCs. Note also the protrusions (arrowheads) at the very ends of the FCs. Ep, epithelium; Ca, capsule; EFI, epithelial–fibre interface.
The picture emerging from this re-evaluation of the early differentiation and elongation of fibres concurs with previous studies in mammals (see for instance [53,57,58]). As also indicated by Zampighi et al. [53] in their ‘simple folded epithelium model’, the original polarity of the ECs of the embryonic lens vesicle is maintained throughout life (figure 4). The lateral membranes elongate by appositional growth at their basal (posterior) and apical (anterior) regions and GJs are largely confined to these membranes. This means that GJ solute exchange and electrical coupling remains fundamentally restricted to the fibres. In agreement with the model proposed by Zampighi et al. [53], it can also be noted that the basal plasma membranes of the LECs and the basal extensions of the growing lens fibres cover the whole anterior, equatorial and posterior lens surface, and are therefore only separated from the aqueous and vitreous humours by the capsule. Moreover, the posterior and anterior sutures are formed by the convergence of the basal and apical plasma membranes of FCs from neighbouring lens sectors, respectively, which means that the suture lines are specific meeting points of membranes which most likely lack GJs and TJs [57]. This concurs with a recent suggestion [53,59] that the sutures are in some way involved specifically in solute entry.
(a). Conclusions
FC lateral membranes are extensions of the lateral membranes of the post-mitotic differentiating and elongating LECs. Connexins are concentrated on the lateral membranes of LECs and FCs. There are few, if any on the basal or apical domains of both the LECs and FCs. There are notable regional differences—for instance at the equator—where some GJ structures are seen at the apico-lateral surfaces of cells. This explains the dye transfer observed in this region of the lens. By reason of location of connexins, GJs do not seem to be a major factor in the uptake of solutes via the apical membranes of the LECs. The general absence of GJs at the EFI further challenges the view that solute transfer from the aqueous to the FCs by transcellular epithelial transport and direct transfer to the underlying fibres by GJ-coupling is an important route of solute transfer into (or out of) the lens. Nevertheless, it is abundantly clear from the numerous studies on GJ function in the lens that GJs are essential to lens development, lens growth and lens transparency. We propose that this function of GJs is largely limited to either ECs or FCs and for inter-cellular communication within these cell populations, and only very occasionally between these two cell populations in specific lens regions such as the equatorial region.
3. Paracellular transport
As there is little evidence to support a combined transcellular epithelial transport and GJ transfer across the EFI in the transfer of solutes between the aqueous humour and the lens fibre mass, paracellular transport from the aqueous into the intercellular space of the fibre mass needs to be examined. In general, TJs function to seal off the space between adjacent cells, thus establishing a barrier to uncontrolled diffusion of water-soluble molecules and macromolecules across EC sheets. Ultrastructural analyses of TJs between LECs suggested that they are not abundant or well-developed. Measurements of transepithelial resistance indicated that the bovine lens epithelium is a low-resistance epithelium for ions and small molecules [60]. In fact, the TJs of LECs exist within an apical junction complex where the distinction between discrete adherens junctions and TJs is poor. TJ markers were not concentrated into a clearly defined region, indicating immature apical junction complexes between bovine LECs [61]. In the human lens epithelium, morphologically recognizable TJs are also sparse (J. van Marle 2010, unpublished observations). Nevertheless, the TJs between LECs have been shown to be sufficient to prevent paracellular uptake of colloidal lanthanum and horseradish peroxidase (HRP; MW 44 kDa) into the lenses of frogs [62] and rats [53,62]. When these data are combined with the poor transepithelial resistance observed for the bovine lens epithelium [60], we conclude that LEC TJs probably belong to the type of TJs that have a high permeability for inorganic ions and also allow the paracellular flow of glucose and amino acids [63].
Paracellular transport of ions and small molecules crucially depends on the kinetics of water transport into and out of the lens. This has been carefully analysed for isolated human lenses using diffusion tensor nuclear magnetic resonance micro-imaging [64] and by magnetic resonance microscopy after replacement of H2O by deuterium oxide (D2O) [65]. The outcome of these detailed and technically high-standard studies allows the conclusion that the transport of water is spatially inhomogeneous and anisotropic. Diffusion parallel to the long axis of the lens FCs is largely unrestricted with a higher diffusivity in the equatorial region. The radial inward diffusion towards the lens nucleus is substantially inhibited, especially in the inner cortex of the lens, suggesting a diffusion barrier coincident with the lens nucleus. Furthermore, the diffusion from the lens cortex to its nucleus becomes reduced with age.
Preliminary experiments by our groups on lenses in intact bovine eyes (summarized in electronic supplementary material, figure S1 and material S1) show that after 8 h the water-soluble magnetic resonance imaging (MRI) marker Gd3+-DTPA appeared to have entered the lens evenly across its anterior surface up to a depth of approximately 2 mm. These in situ observations corroborate the observations on isolated human lenses [64,65] and support the existence of a diffusion barrier in the lens outer cortex. In contrast, however, to the results by Moffat et al. [64,65], who found nearly identical diffusion rates in the anterior and posterior poles of isolated human lenses, we observed lower signals in the posterior lens pole. The high MRI signals in the iris and ciliary body in our studies suggest, however, that the low signals at the posterior pole are probably not due to a lack of availability of Gd3+-DTPA, but instead could be caused by restricted diffusion of the Gd3+-DTPA from the aqueous humour to the posterior pole.
It is noteworthy that the space of Berger between the posterior surface of the lens and the anterior hyaloid membrane of the vitreous is very narrow and could therefore represent a diffusion barrier between the anterior and posterior segments of the eye, including the lens posterior pole [66]. Another possibility might be that there are ‘non-leaky’ TJs at the posterior pole of the lens, which comprises the basal membranes of the elongating FCs. Recently, we investigated the junctions between the lens fibres in the posterior pole of bovine and human lenses. In addition to GJs (figure 9a), we observed a series of focal connections or ‘membrane kisses’ (figure 9b). Although these latter structures are often considered as TJs, higher magnifications did not reveal the typical melting of the outer leaflets of the membranes and freeze-fracture analyses failed to show regularly arranged ridges of transmembrane particles [67]. These structures thus more likely represent as yet unspecified adherens junctions [63].
Figure 9.

Plasma membrane connections between FCs in the posterior region of the human lens. Freeze-fracture (a) micrographs of the posterior region of a human lens reveal GJs between adjacent FCs (a, arrow). In freeze fracture images (b) and ultrathin sections (c), closely apposed plasma membranes (‘membrane kisses’) (c, arrowheads) between adjacent fibres at the cells' most capsular sides are observed. Although these structures are often considered as tight junctions, they do not reveal the typical melting of the outer leaflets of the membranes and more likely represent as yet unspecified adherens junctions. pf, p-face of fractured membrane.
(a). Conclusions
The evidence summarized above suggests that paracellular transport is more important for the transfer of ions, small molecules and water between the aqueous humour and the fibre intercellular space than transepithelial transport at least for the anterior half of the lens [68]. This conclusion recognizes the low transepithelial resistance that was measured and corroborates the recently described Na+-driven internal micro-circulatory system [12,69]. Nevertheless, the presence of TJs within the immature apical junction complex of LECs [61] and the sparse TJs observed by Lo [62] are capable of preventing the diffusion of large molecules into the extracellular space of the lens but not the diffusion of ions and small molecules, such as amino acids and glucose. Assessing the relative balance of para- and transcellular solute flow into the intercellular space of the lens, however, is difficult.
4. Specific fibre membrane protein carriers and transporters
If we accept that paracellular transport is indeed an important route for solute transfer between the aqueous and the FC mass of the lens, then the next question to be addressed is how these solutes enter the FC cytoplasm. The strong GJ-coupling between FCs suggests that uptake via the outermost superficial FCs and subsequent passive diffusion through GJs to deeper FCs in the lens is an option. Einstein's law on diffusion predicts this to be a very slow process, especially when the distance exceeds several diameters of FC. It is unlikely that GJ-mediated diffusion could account for sufficient supply of glucose and essential amino acids necessary for the rapid growth of new fibres in the lens cortex and the maintenance of lens integrity and transparency in the lens core [68]. A more attractive hypothesis is that at least the superficial, rapidly growing cortical fibres take up solutes from the circulating intercellular fluid by membrane-based active transport.
Numerous studies have shown that lens FC membranes are rich in ion channels (K+, Na+, Ca2+), pumps (Na/K ATPase), water channels (aquaporins) (reviewed by [69]) and specific transporters for small molecules such as glucose and amino acids [70–74]. Freeze-fracture studies have shown that fibre membranes are densely populated with intra-membranous particles (IMPs) and there is good ultrastructural evidence to suggest that some of these IMPs represent AQ-0 (previously called MIP26) [53]. Confocal microscopy studies have further demonstrated that transporters for several amino acids and for glucose are preferentially located on FC membranes [59,70,71,74,75]. This leads us to conclude that transporters, water and ion channels and pumps located in the FC membranes may play an important role in the transport of solute from the intercellular space via the EFI and equator of the lens into FC cytoplasm.
5. Transcellular transport by endocytosis
Vesicular events have been reported to occur at the surfaces of LECs and FCs [46,47,76–79]. A study on the distribution of endocytotic events in the adult rat lens has demonstrated clathrin-coated pits and caveoli in the epithelium and the superficial FCs [76,80]. Additionally, evidence for receptor-mediated endocytosis at the anterior EFI has been presented for the chicken [46,47]. In line with these observations, we commonly observed endocytotic–exocytotic vesicles on the lateral plasma membranes between LECs (figure 7a), the membranes between adjacent equatorial fibres (figure 7b) and on the apical membranes of anterior LECs (figure 7c) in the human lens. As shown in the enlarged insets of figure 7a, the vesicles are occurring in the vicinity of GJs, suggesting that GJ-coupling and endocytotic–exocytotic events are not mutually exclusive processes, but can occur within close spatial proximity on the same membrane. In the post-equatorial region of the posterior lens pole in the bovine and human lens [67], the basal ends of FC plasma membranes adjacent to the lens capsule often show endocytotic–exocytotic vesicles, and at higher magnification these are seen to be coated vesicles (figure 10). The presence of clathrin in both LECs and FCs was confirmed biochemically [47,81]. A recent study examining the zebrafish lens by freeze-fracture transmission electron microscopy (TEM) also detected numerous vesicles at the EFI and in cortical fibres [52]. The extent of the contribution of this mode of transport to the overall movement of material into and within the lens remains to be elucidated. Based on the assessment of numerous random micrographs, the low incidence at the EFI, however, suggests vesicular transport is unlikely to be the main transcellular pathway for ion and small molecule transport between the aqueous humour and lens FCs.
Figure 10.
Evidence for endocytotic–exocytotic events in posterior FCs of the human lens. TEM micrographs showing the lens capsule bordering the plasma membrane of a superficial mid-posterior lens FC of a young human lens. Various stages of coated endocytotic–exocytotic vesicles can be seen at the FC's plasma membrane (arrowheads). At higher magnification, these vesicles are seen to be coated (lower row of images).
Vesicle traffic is, however, important in growth factor uptake and receptor recycling in many tissues. Lens cell proliferation and differentiation is governed by numerous external aqueous and vitreous-derived growth factors and regulators (e.g. EGF: [82], FGF: [83–88], EGFR: [89], TGFβ: [90], BMP: [86,90–92], PDGF: [82]). The lens capsule itself is a reservoir of growth factors that can be released by the action of matrix metalloproteinases [93]. As detailed above, the TJ-containing apical junctional complex between LECs prevents the uptake of colloidal lanthanum and macromolecules (HRP: MW 44 kDa) into isolated lenses of frog [62] and rat [53,62]. There is strong evidence that growth factors are needed not only for the differentiation of LECs into FCs but also for the maintenance of FCs [94–96], and it has been shown that growth factor receptors are present on the differentiated fibres [85]. In order to reach differentiating lens FCs, growth factors have to be taken up from the aqueous and vitreous humours by a transepithelial, endocytotic transport mechanism.
Receptor-mediated transport may also play a role in the uptake of cholesterol and lipoproteins into lens cells. Plasma membrane synthesis to support the elongation of LECs into FCs demands significant transport of lipids into these cells. It has been shown by Cenedella [97] that in adult rat lenses, intrinsic de novo synthesis of cholesterol is insufficient to furnish the newly formed membranes and that an extrinsic source is necessary. El-Sayed & Cenedella [98] further showed that an inhibition of cholesterol synthesis by mevinolin stopped the proliferation of LECs, which is completely reversed when cholesterol is added to the culture medium in the form of low-density lipoproteins. Furthermore, Lo et al. [76] showed that cholesterol depletion of LECs by methyl-β-cyclodextrin drastically reduced the number of caveolae.
(a). Conclusions
It is generally accepted that caveolae are involved in receptor-mediated endocytosis of macromolecules and clathrin-coated vesicles especially in the uptake of low-density lipoproteins and cholesterol. This short review highlights the potential importance of vesicle-mediated transport to the lens especially for the uptake of growth factors, cholesterol and lipoproteins into lens FCs.
6. Summary and discussion
The overall picture emerging from the re-assessment of the available physiological and morphological literature, combined with our preliminary MRI and dye-coupling studies, is that the solute transfer from the aqueous and vitreous humours towards the lens fibres is governed by four mechanisms. In order of relevance to solute transfer, these mechanisms are:
— Paracellular transport via the intercellular spaces between the LECs and the underlying lens fibres driven by Na+ leak conductance as outlined by Mathias et al. [12]. This mechanism accounts for the influx of ions, water, and small molecules such as glucose, amino acids and ascorbic acid.
— Plasma membrane-based transport of small molecules from the lens' extracellular space into the fibre cytoplasm by ion and water channels and specific transporters for glucose, amino acids and other small molecules.
— Gap-junctional transport accounting for the flux of ions, water, and small molecules such as glucose, amino acids and ascorbic acid from superficial fibres towards the deep fibres, and also accounting for the efflux of waste products at the EFI in the equatorial regions as outlined by Mathias et al. [12] and Paterson & Delamere [99]. In view of the absence of GJs at the EFI, GJ transport is unlikely to play an important role in the solute transfer at the anterior pole of the lens.
— Vesicle-mediated active transport by caveoli accounting for the uptake of macromolecules such as growth factors and other regulatory factors necessary for the normal development and maintenance of lens fibres. Coated vesicles are involved in the uptake of lipoproteins and cholesterol by superficial fibres needed for the biogenesis of new membranes as lens FCs increase their length by up to 1000-fold.
The proposed role of paracellular transport presents us with a paradox. On the one hand, disruption of the lens epithelium disturbs the integrity of the underlying FCs indicating that an intact epithelium is a prerequisite for controlling the water and ion homeostasis of the fibre mass by regulating the water and ion content of FCs [2–9]. On the other hand, there are good arguments for a free Na+-driven paracellular transport of water and nutrients across the lens epithelium into the intercellular space between the FCs under physiological conditions. A crucial observation of the in vivo studies of UV-exposed lenses by Michael et al. [7] was the appearance of opacities in the lens equator, and not the central region of the lens, through the accumulation of water-filled vacuoles. The recently proposed micro-circulatory model of Mathias et al. [12] might offer an explanation for this apparent paradox. The efflux of water, ions and waste products, particularly lactate, is an active, Na+/K+-ATPase-driven process regulated by the equatorial LECs [12,99]. This model is based on and extends the much earlier observations on diffusion of ions and amino acids in the extracellular space [100–102]. Challenging this active process might lead to peripheral swelling of fibres and their opacification. This might mean that disruption of the epithelial layer leads to a dysregulation of ions, water and solute entry into the lens from the aqueous humour. The equatorial opacity could then be due to an accumulation of water at the equatorial region, where these should normally exit the lens, resulting in an imbalance of the influx and efflux processes.
Michael et al. [8] also demonstrated that repair of the epithelium leads to the formation of normal new lens FCs and the disappearance of the swollen fibres, indicating that the balance between influx and efflux can be dynamically restored. This also indicates that despite the unhindered Na+-driven influx of ions, water and solutes, the relatively free access is somehow regulated by the ECs. Perhaps this is a function of the sparse and ‘leaky’ TJs.
Another point of concern is whether efficient paracellular, GJ and membrane-based transport mechanisms, even when working in harmony and facilitated by a microcirculatory flow, are sufficient to supply the needs of all FCs in adult lenses. Both paracellular and GJ transport rely on diffusion, which is a limiting factor, as predicted by Einstein's law on diffusion, certainly for the deep cortical and nuclear entry of solutes. As summarized by Truscott [103], there is good evidence that in adult human lenses a deep cortical barrier exists, beyond which the fibres can be considered as metabolically quiescent or inert. This means that for the integrity and transparency of the deep cortex and nucleus, the limited diffusion might not be a serious problem. MRI studies on isolated human lenses [64,65] and preliminary in situ observations in bovine lenses (electronic supplementary material, figure S1) support a restricted entry of water and ions into the deep cortex and nucleus of the lens. Two recent studies [104,105] have shown that early age-related opacities and cortical cataracts have their origin in and stay restricted to the lens cortex, while the lens nucleus remains unaffected, supporting the independence of the lens nucleus behind the barrier compared with the more peripheral regions beyond the barrier. This also suggests that the cortical–nuclear interface forms a barrier preventing or delaying noxious substances from entering the nuclear region and thus postponing occurrence of nuclear lens opacities early in life.
With respect to the morphological and biochemical nature of this barrier, it must be noted that FC membranes are not uniform throughout the lens, but vary and change quite dramatically with respect to their protein and lipid components. As described by Vrensen et al. [106], human FC membranes gradually lose their IMPs, and their GJs become disordered upon maturation. In the transitional zone [107,108], numerous so-called orthogonal lattices of repeating subunits or square arrays (SAs) and disordered GJs are observed. Because SAs show no complementarity of pits and particles on apposing membrane surfaces, this might reflect uncoupling of these deeper FCs. The changes in fibre membrane architecture upon maturation coincide with dramatic changes in the phospholipid composition of the lens fibre membranes [109,110] and in the cholesterol-to-lipid ratio, changing from 0.8–1.0 in the superficial fibres to 4–5 in the deep cortex and nucleus [111–113]. Increased cholesterol content passively reduces both water and ion transports across the membranes and inactivates the transmembrane proteins that govern membrane permeability [114]. These changes in phospholipid composition and cholesterol content as well as the paucity of IMPs (and also of ion-channels, transporters and aquaporins) corroborate the electrophysiological characterization of deep cortical lens fibres as ‘non-leaky’ [115] or ‘degenerate’ [102]. Moreover, AQP0 (MP20) is inserted into the lens membranes at the time of organelle loss in lens FC [116]. These deeper fibres can thus be considered as excellently insulated cells with minimal fluxes of ions, water and small molecules, and may form the morphological, biochemical and electrophysiological counterpart of the lens barrier recently advocated by Truscott [103]. Based on the appearance of an age-related discoloration and hardening of the lens nucleus in humans [117], Truscott emphasizes that the barrier forms at middle age. However, insertion of MP20 into lens cortical membranes occurs in the transitional zone of organelle degradation [108,118], and coincides with a restrictive diffusion of molecules in young (21 days) rats [116]. Moreover, the cholesterol content of cortical fibre membranes rapidly increases to their value in nuclear fibres within 10–20% below the lens surface in both young (9 years) and old (65 years) human lenses [113]. Because high cholesterol content can be considered as a marker of reduced solute entrance, this means that already in young lenses the deeper cortical membranes will have a restricted solute influx. Finally, the changes in membrane architecture of human lens fibres upon maturation (see above) are equally present in young (>22 years) and old lenses [106]. This evidence strongly suggests that the cortical–nuclear diffusion barrier is a consistent characteristic of both young and old lenses. This barrier will certainly limit the solute transfer to these deeper regions. As FCs beyond the transitional zone have no mitochondria [107,118,119], their aerobic metabolic activity will be lower than that of LECs and glycolytic demand for substrates is equally low. Lens FCs actually do express glucose transporters to allow diffusion throughout the lens as evidenced by the impact of non-enzymatic glycation events during cataractogenesis. The barrier may also be responsible for the limited availability of glutathione (GSH) and other anti-oxidative molecules in the lens nucleus. As outlined by Truscott [103], this reduced GSH may be the main cause of age-related nuclear coloration that coincides with middle age and pre-empts nuclear cataract.
Acknowledgements
This work was supported by a BBSRC CASE Award fellowship (RD) and the Leverhulme Trust (RAQ).
Footnotes
One contribution of 10 to a Theme Issue ‘The ocular lens: a classic model for development, physiology and disease’.
Glossary
- AQ-0
aquaporin-0
- BMP
bone morphogenetic proteins
- Cx
connexin
- EFI
epithelial fibre interface
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FC
fibre cell
- FGF
fibroblast growth factor
- Gd3+-DTPA
trivalent gadolinium chelated to diethylene triamine pentaacetic acid
- GJ
gap junction
- GSH
glutathione
- HRP
horseradish peroxidase
- LEC
lens epithelial cells
- LM
light microscopy
- LMDP
large molecule diffusion pathway
- MRI
magnetic resonance imaging
- SEM
scanning electron microscopy
- PDGF
platelet derived growth factor
- TEM
transmission electron microscopy
- TGF
transforming growth factor
- TJ
tight junctions
References
- 1.Shui Y. B., et al. 2006. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest. Ophthalmol. Vis. Sci. 47, 1571–1580 10.1167/iovs.05-1475 (doi:10.1167/iovs.05-1475) [DOI] [PubMed] [Google Scholar]
- 2.Hightower K. R. 1995. The role of the lens epithelium in development of UV cataract. Curr. Eye Res. 14, 71–78 10.3109/02713689508999916 (doi:10.3109/02713689508999916) [DOI] [PubMed] [Google Scholar]
- 3.Li W. C., et al. 1995. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J. Cell. Biol. 130, 169–181 10.1083/jcb.130.1.169 (doi:10.1083/jcb.130.1.169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W. C., Kuszak J. R., Wang G. M., Wu Z. Q., Spector A. 1995. Calcimycin-induced lens epithelial cell apoptosis contributes to cataract formation. Exp. Eye Res. 61, 91–98 10.1016/S0014-4835(95)80062-X (doi:10.1016/S0014-4835(95)80062-X) [DOI] [PubMed] [Google Scholar]
- 5.Spector A., Wang G. M., Wang R. R., Li W. C., Kleiman N. J. 1995. A brief photochemically induced oxidative insult causes irreversible lens damage and cataract. II. Mechanism of action. Exp. Eye Res. 60, 483–493 10.1016/S0014-4835(05)80063-6 (doi:10.1016/S0014-4835(05)80063-6) [DOI] [PubMed] [Google Scholar]
- 6.Spector A., Wang G. M., Wang R. R., Li W. C., Kuszak J. R. 1995. A brief photochemically induced oxidative insult causes irreversible lens damage and cataract. I. Transparency and epithelial cell layer. Exp. Eye Res. 60, 471–481 10.1016/S0014-4835(05)80062-4 (doi:10.1016/S0014-4835(05)80062-4) [DOI] [PubMed] [Google Scholar]
- 7.Michael R., Vrensen G. F., van Marle J., Gan L., Soderberg P. G. 1998. Apoptosis in the rat lens after in vivo threshold dose ultraviolet irradiation. Invest. Ophthalmol. Vis. Sci. 39, 2681–2687 [PubMed] [Google Scholar]
- 8.Michael R., Vrensen G. F., van Marle J., Lofgren S., Soderberg P. G. 2000. Repair in the rat lens after threshold ultraviolet radiation injury. Invest. Ophthalmol. Vis. Sci. 41, 204–212 [PubMed] [Google Scholar]
- 9.Yamada Y., Kojima M., Vrensen G. F., Takahashi N., Sasaki K. 2001. Acute ultraviolet B induced lens epithelial cell photo-damage and its repair process. Nippon Ganka Gakkai Zasshi 105, 102–110 [PubMed] [Google Scholar]
- 10.Dahm R. 1999. Lens fibre cell differentiation: a link with apoptosis? Ophthalmic Res. 31, 163–183 10.1159/000055530 (doi:10.1159/000055530) [DOI] [PubMed] [Google Scholar]
- 11.Wride M. A. 2000. Minireview: apoptosis as seen through a lens. Apoptosis 5, 203–209 10.1023/A:1009653326511 (doi:10.1023/A:1009653326511) [DOI] [PubMed] [Google Scholar]
- 12.Mathias R. T., Kistler J., Donaldson P. 2007. The lens circulation. J. Membr. Biol. 216, 1–16 10.1007/s00232-007-9019-y (doi:10.1007/s00232-007-9019-y) [DOI] [PubMed] [Google Scholar]
- 13.De Rosa A. M., Martinez-Wittinghan M. J., Mathias R. T., White T. W. 2005. Intracellular communication in lens development and disease. In Gap junctions in development and disease (ed. Winterharger E.), pp. 173–195 Berlin, Germany: Springer [Google Scholar]
- 14.Goodenough D. A. 1992. The crystalline lens. A system networked by gap junctional intercellular communication. Semin. Cell Biol. 3, 49–58 10.1016/S1043-4682(10)80007-8 (doi:10.1016/S1043-4682(10)80007-8) [DOI] [PubMed] [Google Scholar]
- 15.Duncan G., Stewart S., Prescott A. R., Warn R. M. 1988. Membrane and junctional properties of the isolated frog lens epithelium. J. Membr. Biol. 102, 195–204 10.1007/BF01925713 (doi:10.1007/BF01925713) [DOI] [PubMed] [Google Scholar]
- 16.Rae J. L., Kuszak J. R. 1983. The electrical coupling of epithelium and fibers in the frog lens. Exp. Eye Res. 36, 317–320 10.1016/0014-4835(83)90114-8 (doi:10.1016/0014-4835(83)90114-8) [DOI] [PubMed] [Google Scholar]
- 17.Rae J. L., Bartling C., Rae J., Mathias R. T. 1996. Dye transfer between cells of the lens. J. Membr. Biol. 150, 89–103 10.1007/s002329900033 (doi:10.1007/s002329900033) [DOI] [PubMed] [Google Scholar]
- 18.White T. W., Sellitto C., Paul D. L., Goodenough D. A. 2001. Prenatal lens development in connexin43 and connexin50 double knockout mice. Invest. Ophthalmol. Vis. Sci. 42, 2916–2923 [PubMed] [Google Scholar]
- 19.Gao Y., Spray D. C. 1998. Structural changes in lenses of mice lacking the gap junction protein connexin43. Invest. Ophthalmol. Vis. Sci. 39, 1198–1209 [PubMed] [Google Scholar]
- 20.Gong X., Li E., Klier G., Huang Q., Wu Y., Lei H., Kumar N. M., Horwitz J., Gilula N. B. 1997. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91, 833–843 10.1016/S0092-8674(00)80471-7 (doi:10.1016/S0092-8674(00)80471-7) [DOI] [PubMed] [Google Scholar]
- 21.Xia C. H., Liu H., Cheung D., Cheng C., Wang E., Du X., Beutler B., Lo W. K., Gong X. 2006. Diverse gap junctions modulate distinct mechanisms for fiber cell formation during lens development and cataractogenesis. Development 133, 2033–2040 10.1242/dev.02361 (doi:10.1242/dev.02361) [DOI] [PubMed] [Google Scholar]
- 22.Xia C. H., Cheung D., DeRosa A. M., Chang B., Lo W. K., White T. W., Gong X. 2006. Knock-in of alpha3 connexin prevents severe cataracts caused by an alpha8 point mutation. J. Cell Sci. 119, 2138–2144 10.1242/jcs.02940 (doi:10.1242/jcs.02940) [DOI] [PubMed] [Google Scholar]
- 23.Xia C. H., Cheng C., Huang Q., Cheung D., Li L., Dunia I., Benedetti L. E., Horwitz J., Gong X. 2006. Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Exp. Eye Res. 83, 688–696 10.1016/j.exer.2006.03.013 (doi:10.1016/j.exer.2006.03.013) [DOI] [PubMed] [Google Scholar]
- 24.Chang B., Wang X., Hawes N. L., Ojakian R., Davisson M. T., Lo W. K., Gong X. 2002. A Gja8 (Cx50) point mutation causes an alteration of alpha 3 connexin (Cx46) in semi-dominant cataracts of Lop10 mice. Hum. Mol. Genet. 11, 507–513 10.1093/hmg/11.5.507 (doi:10.1093/hmg/11.5.507) [DOI] [PubMed] [Google Scholar]
- 25.Graw J., Loster J., Soewarto D., Fuchs H., Meyer B., Reis A., Wolf E., Balling R., Hrabe de Angelis M. 2001. Characterization of a mutation in the lens-specific MP70 encoding gene of the mouse leading to a dominant cataract. Exp. Eye Res. 73, 867–876 10.1006/exer.2001.1096 (doi:10.1006/exer.2001.1096) [DOI] [PubMed] [Google Scholar]
- 26.Steele E. C., Jr, Lyon M. F., Favor J., Guillot P. V., Boyd Y., Church R. L. 1998. A mutation in the connexin 50 (Cx50) gene is a candidate for the No2 mouse cataract. Curr. Eye Res. 17, 883–889 10.1076/ceyr.17.9.883.5144 (doi:10.1076/ceyr.17.9.883.5144) [DOI] [PubMed] [Google Scholar]
- 27.White T. W., Goodenough D. A., Paul D. L. 1998. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J. Cell Biol. 143, 815–825 10.1083/jcb.143.3.815 (doi:10.1083/jcb.143.3.815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Addison P. K., Berry V., Holden K. R., Espinal D., Rivera B., Su H., Srivastava A. K., Bhattacharya S. S. 2006. A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol. Vis. 12, 791–795 [PubMed] [Google Scholar]
- 29.Bennett T. M., Mackay D. S., Knopf H. L., Shiels A. 2004. A novel missense mutation in the gene for gap-junction protein alpha3 (GJA3) associated with autosomal dominant ‘nuclear punctate’ cataracts linked to chromosome 13q. Mol. Vis. 10, 376–382 [PubMed] [Google Scholar]
- 30.Rees M. I., Watts P., Fenton I., Clarke A., Snell R. G., Owen M. J., Gray J. 2000. Further evidence of autosomal dominant congenital zonular pulverulent cataracts linked to 13q11 (CZP3) and a novel mutation in connexin 46 (GJA3). Hum. Genet. 106, 206–209 10.1007/s004390051029 (doi:10.1007/s004390051029) [DOI] [PubMed] [Google Scholar]
- 31.Mackay D., Ionides A., Kibar Z., Rouleau G., Berry V., Moore A., Shiels A., Bhattacharya S. 1999. Connexin46 mutations in autosomal dominant congenital cataract. Am. J. Hum. Genet. 64, 1357–1364 10.1086/302383 (doi:10.1086/302383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponnam S. P., Ramesha K., Tejwani S., Ramamurthy B., Kannabiran C. 2007. Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. J. Med. Genet. 44, e85. 10.1136/jmg.2007.050138 (doi:10.1136/jmg.2007.050138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polyakov A. V., Shagina I. A., Khlebnikova O. V., Evgrafov O. V. 2001. Mutation in the connexin 50 gene (GJA8) in a Russian family with zonular pulverulent cataract. Clin. Genet. 60, 476–478 10.1034/j.1399-0004.2001.600614.x (doi:10.1034/j.1399-0004.2001.600614.x) [DOI] [PubMed] [Google Scholar]
- 34.Berry V., et al. 1999. Connexin 50 mutation in a family with congenital ‘zonular nuclear’ pulverulent cataract of Pakistani origin. Hum. Genet. 105, 168–170 10.1007/s004390051082 (doi:10.1007/s004390051082) [DOI] [PubMed] [Google Scholar]
- 35.Shiels A., Mackay D., Ionides A., Berry V., Moore A., Bhattacharya S. 1998. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant ‘zonular pulverulent’ cataract, on chromosome 1q. Am. J. Hum. Genet. 62, 526–532 10.1086/301762 (doi:10.1086/301762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong X., Cheng C., Xia C. H. 2007. Connexins in lens development and cataractogenesis. J. Membr. Biol. 218, 9–12 10.1007/s00232-007-9033-0 (doi:10.1007/s00232-007-9033-0) [DOI] [PubMed] [Google Scholar]
- 37.Gao J., Sun X., Martinez-Wittinghan F. J., Gong X., White T. W., Mathias R. T. 2004. Connections between connexins, calcium, and cataracts in the lens. J. Gen. Physiol. 124, 289–300 10.1085/jgp.200409121 (doi:10.1085/jgp.200409121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerido D. A., White T. W. 2004. Connexin disorders of the ear, skin, and lens. Biochim. Biophys. Acta 1662, 159–170 10.1016/j.bbamem.2003.10.017 (doi:10.1016/j.bbamem.2003.10.017) [DOI] [PubMed] [Google Scholar]
- 39.Shestopalov V. I., Bassnett S. 2000. Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. J. Cell Sci. 113, 1913–1921 [DOI] [PubMed] [Google Scholar]
- 40.Shi Y., Barton K., De Maria A., Petrash J. M., Shiels A., Bassnett S. 2009. The stratified syncytium of the vertebrate lens. J. Cell Sci. 122, 1607–1615 10.1242/jcs.045203 (doi:10.1242/jcs.045203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller T. M., Goodenough D. A. 1986. Evidence for two physiologically distinct gap junctions expressed by the chick lens epithelial cell. J. Cell Biol. 102, 194–199 10.1083/jcb.102.1.194 (doi:10.1083/jcb.102.1.194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo W. K., Harding C. V. 1986. Structure and distribution of gap junctions in lens epithelium and fiber cells. Cell Tissue Res. 244, 253–263 10.1007/BF00219200 (doi:10.1007/BF00219200) [DOI] [PubMed] [Google Scholar]
- 43.Lo W. K., Reese T. S. 1993. Multiple structural types of gap junctions in mouse lens. J. Cell Sci. 106, 227–235 [DOI] [PubMed] [Google Scholar]
- 44.Goodenough D. A., Dick J. S., II, Lyons J. E. 1980. Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J. Cell Biol. 86, 576–589 10.1083/jcb.86.2.576 (doi:10.1083/jcb.86.2.576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller A. G., Hall J. E. 1996. Junctional permeability measurements in the embryonic chick lens. Exp. Eye Res. 62, 339–349 10.1006/exer.1996.0039 (doi:10.1006/exer.1996.0039) [DOI] [PubMed] [Google Scholar]
- 46.Bassnett S., Kuszak J. R., Reinisch L., Brown H. G., Beebe D. C. 1994. Intercellular communication between epithelial and fiber cells of the eye lens. J. Cell Sci. 107, 799–811 [DOI] [PubMed] [Google Scholar]
- 47.Brown H. G., Pappas G. D., Ireland M. E., Kuszak J. R. 1990. Ultrastructural, biochemical, and immunologic evidence of receptor-mediated endocytosis in the crystalline lens. Invest. Ophthalmol. Vis. Sci. 31, 2579–2592 [PubMed] [Google Scholar]
- 48.van Marle J., Vrensen G. F. 1996. Epithelial cell-fibre coupling in the equator of the human lens. Ophthalmic Res. 28(Suppl. 1), 77–80 10.1159/000267976 (doi:10.1159/000267976) [DOI] [PubMed] [Google Scholar]
- 49.Kuszak J. R., Novak L. A., Brown H. G. 1995. An ultrastructural analysis of the epithelial–fiber interface (EFI) in primate lenses. Exp. Eye Res. 61, 579–597 10.1016/S0014-4835(05)800521 (doi:10.1016/S0014-4835(05)800521) [DOI] [PubMed] [Google Scholar]
- 50.Prescott A., Duncan G., Van Marle J., Vrensen G. 1994. A correlated study of metabolic cell communication and gap junction distribution in the adult frog lens. Exp. Eye Res. 58, 737–746 10.1006/exer.1994.1071 (doi:10.1006/exer.1994.1071) [DOI] [PubMed] [Google Scholar]
- 51.Dahm R., van Marle J., Prescott A. R., Quinlan R. A. 1999. Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp. Eye Res. 69, 45–56 10.1006/exer.1999.0670 (doi:10.1006/exer.1999.0670) [DOI] [PubMed] [Google Scholar]
- 52.Dahm R., Schonthaler H. B., Soehn A. S., van Marle J., Vrensen G. F. 2007. Development and adult morphology of the eye lens in the zebrafish. Exp. Eye Res. 85, 74–89 10.1016/j.exer.2007.02.015 (doi:10.1016/j.exer.2007.02.015) [DOI] [PubMed] [Google Scholar]
- 53.Zampighi G. A., Eskandari S., Kreman M. 2000. Epithelial organization of the mammalian lens. Exp. Eye Res. 71, 415–435 10.1006/exer.2000.0895 (doi:10.1006/exer.2000.0895) [DOI] [PubMed] [Google Scholar]
- 54.Dahm R., Procter J. E., Ireland M. E., Lo W.-K., Mogensen M. M., Quinlan R. A., Prescott A. R. 2007. Reorganization of centrosomal marker proteins coincides with epithelial cell differentiation in the vertebrate lens. Exp. Eye Res. 85, 696–713 10.1016/j.exer.2007.07.022 (doi:10.1016/j.exer.2007.07.022) [DOI] [PubMed] [Google Scholar]
- 55.Denk P. O., Breipohl W., Naib-Majani W., Knorr M. 1997. Regional glycoprotein expression in the chicken lens. Curr. Eye Res. 16, 527–533 10.1076/ceyr.16.6.527.5083 (doi:10.1076/ceyr.16.6.527.5083) [DOI] [PubMed] [Google Scholar]
- 56.Ogiso M., Saito N., Hoshi M., Komoto M. 1997. Developmental changes in carbohydrate antigens in embryonic rat lens. Glycobiology 7, 605–615 10.1093/glycob/7.5.605 (doi:10.1093/glycob/7.5.605) [DOI] [PubMed] [Google Scholar]
- 57.Kuwabara T. 1975. The maturation of the lens cell: a morphologic study. Exp. Eye Res. 20, 427–443 10.1016/0014-4835(75)900858 (doi:10.1016/0014-4835(75)900858) [DOI] [PubMed] [Google Scholar]
- 58.Hogan M. J., Alvarado J. A., Weddell J. 1971. Histology of the human eye: an atlas and textbook. Philadelphia, PA: W. B. Saunders Company [Google Scholar]
- 59.Li L., Lim J., Jacobs M. D., Kistler J., Donaldson P. J. 2007. Regional differences in cystine accumulation point to a sutural delivery pathway to the lens core. Invest. Ophthalmol. Vis. Sci. 48, 1253–1260 10.1167/iovs.06-0861 (doi:10.1167/iovs.06-0861) [DOI] [PubMed] [Google Scholar]
- 60.Zhang J. J., Jacob T. J. 1994. A new approach to measuring transepithelial potentials in the bovine lens reveals a chloride-dependent component. Exp. Physiol. 79, 741–753 [DOI] [PubMed] [Google Scholar]
- 61.Sugiyama Y., Prescott A. R., Tholozan F. M., Ohno S., Quinlan R. A. 2008. Expression and localisation of apical junctional complex proteins in lens epithelial cells. Exp. Eye Res. 87, 64–70 10.1016/j.exer.2008.03.017 (doi:10.1016/j.exer.2008.03.017) [DOI] [PubMed] [Google Scholar]
- 62.Lo W. K. 1987. In vivo and in vitro observations on permeability and diffusion pathways of tracers in rat and frog lenses. Exp. Eye Res. 45, 393–406 10.1016/S0014-4835(87)80126-4 (doi:10.1016/S0014-4835(87)80126-4) [DOI] [PubMed] [Google Scholar]
- 63.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. 2008. Molecular biology of the cell. New York, NY: Garland Science [Google Scholar]
- 64.Moffat B. A., Pope J. M. 2002. Anisotropic water transport in the human eye lens studied by diffusion tensor NMR micro-imaging. Exp. Eye Res. 74, 677–687 10.1006/exer.2001.1164 (doi:10.1006/exer.2001.1164) [DOI] [PubMed] [Google Scholar]
- 65.Moffat B. A., Landman K. A., Truscott R. J., Sweeney M. H., Pope J. M. 1999. Age-related changes in the kinetics of water transport in normal human lenses. Exp. Eye Res. 69, 663–669 10.1006/exer.1999.0747 (doi:10.1006/exer.1999.0747) [DOI] [PubMed] [Google Scholar]
- 66.De Groot V., Hubert M., Van Best J. A., Engelen S., Van Aelst S., Tassignon M. J. 2003. Lack of fluorophotometric evidence of aqueous–vitreous barrier disruption after posterior capsulorhexis. J. Cataract Refract. Surg. 29, 2330–2338 10.1016/S0886-3350(03)00341-9 (doi:10.1016/S0886-3350(03)00341-9) [DOI] [PubMed] [Google Scholar]
- 67.Van Marle J. V. G., Hoeben K. 2009. Cell conections between lenses fibres in the posterior pole of human and bovine lens. Acta Ophthalmol. 87, s244. 10.1111/j.1755-3768.2009.01614.x (doi:10.1111/j.1755-3768.2009.01614.x) [DOI] [Google Scholar]
- 68.Fischbarg J., Diecke F. P., Kuang K., Yu B., Kang F., Iserovich P., Li Y., Rosskothen H., Koniarek J. P. 1999. Transport of fluid by lens epithelium. Am. J. Physiol. 276, C548–C557 [DOI] [PubMed] [Google Scholar]
- 69.Mathias R. T., Rae J. L. 2004. The lens: local transport and global transparency. Exp. Eye Res. 78, 689–698 10.1016/j.exer.2003.07.001 (doi:10.1016/j.exer.2003.07.001) [DOI] [PubMed] [Google Scholar]
- 70.Lim J., Li L., Jacobs M. D., Kistler J., Donaldson P. J. 2007. Mapping of glutathione and its precursor amino acids reveals a role for GLYT2 in glycine uptake in the lens core. Invest. Ophthalmol. Vis. Sci. 48, 5142–5151 10.1167/iovs.07-0649 (doi:10.1167/iovs.07-0649) [DOI] [PubMed] [Google Scholar]
- 71.Lim J., Lorentzen K. A., Kistler J., Donaldson P. J. 2006. Molecular identification and characterisation of the glycine transporter (GLYT1) and the glutamine/glutamate transporter (ASCT2) in the rat lens. Exp. Eye Res. 83, 447–455 10.1016/j.exer.2006.01.028 (doi:10.1016/j.exer.2006.01.028) [DOI] [PubMed] [Google Scholar]
- 72.Marcantonio J. M., Duncan G. 1983. Amino acid transport and crystallin synthesis in the bovine lens. Exp. Eye Res. 36, 429–440 10.1016/0014-4835(83)90124-0 (doi:10.1016/0014-4835(83)90124-0) [DOI] [PubMed] [Google Scholar]
- 73.Marcantonio J., Duncan G. 1987. Amino acid transport and protein synthesis in human normal and cataractous lenses. Curr. Eye Res. 6, 1299–1308 10.3109/02713688708997555 (doi:10.3109/02713688708997555) [DOI] [PubMed] [Google Scholar]
- 74.Merriman-Smith R., Donaldson P., Kistler J. 1999. Differential expression of facilitative glucose transporters GLUT1 and GLUT3 in the lens. Invest. Ophthalmol. Vis. Sci. 40, 3224–3230 [PubMed] [Google Scholar]
- 75.Lucas V. A., Zigler J. S., Jr 1988. Identification of the monkey lens glucose transporter by photoaffinity labelling with cytochalasin B. Invest. Ophthalmol. Vis. Sci. 29, 630–635 [PubMed] [Google Scholar]
- 76.Lo W. K., Zhou C. J., Reddan J. 2004. Identification of caveolae and their signature proteins caveolin 1 and 2 in the lens. Exp. Eye Res. 79, 487–498 10.1016/j.exer.2004.06.019 (doi:10.1016/j.exer.2004.06.019) [DOI] [PubMed] [Google Scholar]
- 77.Mellman I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625 10.1146/annurev.cellbio.12.1.575 (doi:10.1146/annurev.cellbio.12.1.575) [DOI] [PubMed] [Google Scholar]
- 78.Kuszak J. R., Sivak J. G., Weerheim J. A. 1991. Lens optical quality is a direct function of lens sutural architecture. Invest. Ophthalmol. Vis. Sci. 32, 2119–2129 [PubMed] [Google Scholar]
- 79.Cenedella R. J., Sexton P. S., Brako L., Lo W. K., Jacob R. F. 2007. Status of caveolin-1 in various membrane domains of the bovine lens. Exp. Eye Res. 85, 473–481 10.1016/j.exer.2007.05.01 (doi:10.1016/j.exer.2007.05.01) [DOI] [PubMed] [Google Scholar]
- 80.Lo W. K., Mills A., Zhang W., Zhu H. 1991. Polarized distribution of coated pits and coated vesicles in the rat lens: an electron microscopy and WGA-HRP tracer study. Curr. Eye Res. 10, 1151–1163 10.3109/02713689109024133 (doi:10.3109/02713689109024133) [DOI] [PubMed] [Google Scholar]
- 81.Zhou C. J., Lo W. K. 2003. Association of clathrin, AP-2 adaptor and actin cytoskeleton with developing interlocking membrane domains of lens fibre cells. Exp. Eye Res. 77, 423–432 10.1016/S0014-4835(03)00171-4 (doi:10.1016/S0014-4835(03)00171-4) [DOI] [PubMed] [Google Scholar]
- 82.Collison D. J., Duncan G. 2001. Regional differences in functional receptor distribution and calcium mobilization in the intact human lens. Invest. Ophthalmol. Vis. Sci. 42, 2355–2363 [PubMed] [Google Scholar]
- 83.Schulz M. W., Chamberlain C. G., de Iongh R. U., McAvoy J. W. 1993. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development 118, 117–126 [DOI] [PubMed] [Google Scholar]
- 84.Zhao H., et al. 2008. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276–288 10.1016/j.ydbio.2008.03.028 (doi:10.1016/j.ydbio.2008.03.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Iongh R. U., Lovicu F. J., Hanneken A., Baird A., McAvoy J. W. 1996. FGF receptor-1 (flg) expression is correlated with fibre differentiation during rat lens morphogenesis and growth. Dev. Dyn. 206, 412–426 10.1002/(SICI)1097-0177199608 (doi:10.1002/(SICI)1097-0177199608) [DOI] [PubMed] [Google Scholar]
- 86.Boswell B. A., Lein P. J., Musil L. S. 2008. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol. Biol. Cell. 19, 2631–2641 10.1091/mbc.E08-02-0124 (doi:10.1091/mbc.E08-02-0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le A. C., Musil L. S. 2001. FGF signaling in chick lens development. Dev. Biol. 233, 394–411 10.1006/dbio.2001.0194 (doi:10.1006/dbio.2001.0194) [DOI] [PubMed] [Google Scholar]
- 88.Le A. C., Musil L. S. 2001. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J. Cell Biol. 154, 197–216 10.1083/jcb.200101057 (doi:10.1083/jcb.200101057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L., Wormstone I. M., Reddan J. R., Duncan G. 2005. Growth factor receptor signalling in human lens cells: role of the calcium store. Exp. Eye Res. 80, 885–895 10.1016/j.exer.2005.01.002 (doi:10.1016/j.exer.2005.01.002) [DOI] [PubMed] [Google Scholar]
- 90.Rajagopal R., Ishii S., Beebe D. C. 2007. Intracellular mediators of transforming growth factor beta superfamily signaling localize to endosomes in chicken embryo and mouse lenses in vivo. BMC Cell. Biol. 8, 25. 10.1186/1471-2121-8-25 (doi:10.1186/1471-2121-8-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faber S. C., Robinson M. L., Makarenkova H. P., Lang R. A. 2002. Bmp signaling is required for development of primary lens fiber cells. Development 129, 3727–3737 [DOI] [PubMed] [Google Scholar]
- 92.Belecky-Adams T. L., Adler R., Beebe D. C. 2002. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development 129, 3795–3802 [DOI] [PubMed] [Google Scholar]
- 93.Tholozan F. M., Gribbon C., Li Z., Goldberg M. W., Prescott A. R., McKie N., Quinlan R. A. 2007. FGF-2 release from the lens capsule by MMP-2 maintains lens epithelial cell viability. Mol. Biol. Cell. 18, 4222–4231 10.1091/mbc.E06-05-0416 (doi:10.1091/mbc.E06-05-0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chamberlain C. G., McAvoy J. W. 1997. Fibre differentiation and polarity in the mammalian lens: a key role for FGF. Progr. Ret. Eye Res. 16, 443–478 10.1016/S1350-9462(96)00034-1 (doi:10.1016/S1350-9462(96)00034-1) [DOI] [Google Scholar]
- 95.Wang Q., Stump R., McAvoy J. W., Lovicu F. J. 2009. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp. Eye Res. 88, 293–306 10.1016/j.exer.2008.08.023 (doi:10.1016/j.exer.2008.08.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iyengar L., Patkunanathan B., McAvoy J. W., Lovicu F. J. 2009. Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors 27, 50–62 10.1080/08977190802610916 (doi:10.1080/08977190802610916) [DOI] [PubMed] [Google Scholar]
- 97.Cenedella R. J. 1982. Sterol synthesis by the ocular lens of the rat during postnatal development. J. Lipid Res. 23, 619–626 [PubMed] [Google Scholar]
- 98.El-Sayed G. N., Cenedella R. J. 1987. Relationship of cholesterolgenesis to DNA synthesis and proliferation by lens epithelial cells in culture. Exp. Eye Res. 45, 443–451 10.1016/S0014-4835(87)80129-X (doi:10.1016/S0014-4835(87)80129-X) [DOI] [PubMed] [Google Scholar]
- 99.Paterson C. A., Delamere N. A. 2004. ATPases and lens ion balance. Exp. Eye Res. 78, 699–703 10.1016/j.exer.2003.09.018 (doi:10.1016/j.exer.2003.09.018) [DOI] [PubMed] [Google Scholar]
- 100.Kinsey V. E., Reddy D. V. 1965. Studies on the crystalline lens. XI. The relative role of the epithelium and capsule in transport. Invest. Ophthalmol. 4, 104–116 [PubMed] [Google Scholar]
- 101.Paterson C. A., Maurice D. M. 1971. Diffusion of sodium in extracellular space of the crystalline lens. Am. J. Physiol. 220, 256–263 [DOI] [PubMed] [Google Scholar]
- 102.Duncan G., Jacob T. J. C. 1984. The lens as a physicochemical system. In The eye (ed. Davson H.), pp. 159–204 Orlando, FL: Academic Press [Google Scholar]
- 103.Truscott R. J. 2005. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. 80, 709–725 10.1016/j.exer.2004.12.007 (doi:10.1016/j.exer.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 104.Michael R., Barraquer R. I., Willekens B., van Marle J., Vrensen G. F. 2008. Morphology of age-related cuneiform cortical cataracts: the case for mechanical stress. Vision Res. 48, 626–634 10.1016/j.visres.2007.12.005 (doi:10.1016/j.visres.2007.12.005) [DOI] [PubMed] [Google Scholar]
- 105.Vrensen G. F. 2009. Early cortical lens opacities: a short overview. Acta Ophthalmol. 87, 602–610 10.1111/j.1755-3768.2009.01674.x (doi:10.1111/j.1755-3768.2009.01674.x) [DOI] [PubMed] [Google Scholar]
- 106.Vrensen G., Van Marle J., Van Veen H., Willekens B. 1992. Membrane architecture as a function of lens fibre maturation: a freeze fracture and scanning electron microscopic study in the human lens. Exp. Eye Res. 54, 433–446 10.1016/0014-4835(92)90055-W (doi:10.1016/0014-4835(92)90055-W) [DOI] [PubMed] [Google Scholar]
- 107.Bassnett S. 1992. Mitochondrial dynamics in differentiating fiber cells of the mammalian lens. Curr. Eye Res. 11, 1227–1232 10.3109/02713689208999548 (doi:10.3109/02713689208999548) [DOI] [PubMed] [Google Scholar]
- 108.Bassnett S. 2002. Lens organelle degradation. Exp. Eye Res. 74, 1–6 10.1006/exer.2001.1111 (doi:10.1006/exer.2001.1111) [DOI] [PubMed] [Google Scholar]
- 109.Broekhuyse R. M., Roelfzema H., Breimer M. E., Karlsson K. A. 1974. Lipids in tissues of the eye. X. Molecular species of sphingomyelins from different parts of calf lens in relation to differentiation and aging. Exp. Eye Res. 19, 477–484 10.1016/0014-4835(74)90054-2 (doi:10.1016/0014-4835(74)90054-2) [DOI] [PubMed] [Google Scholar]
- 110.Borchman D., Byrdwell W. C., Yappert M. C. 1994. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Invest. Ophthalmol. Vis. Sci. 35, 3938–3942 [PubMed] [Google Scholar]
- 111.Li L. K., So L., Spector A. 1985. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J. Lipid Res. 26, 600–609 [PubMed] [Google Scholar]
- 112.Vrensen G. F., Duindam H. J. 1995. Maturation of fiber membranes in the human eye lens. Ultrastructural and Raman microspectroscopic observations. Ophthalmic Res. 27, 78–85 10.1159/000267846 (doi:10.1159/000267846) [DOI] [PubMed] [Google Scholar]
- 113.Duindam J. J., Vrensen G. F., Otto C., Greve J. 1996. Aging affects the conformation of cholesterol in the human eye lens. Ophthalmic Res. 28, 86–91 10.1159/000267978 (doi:10.1159/000267978) [DOI] [PubMed] [Google Scholar]
- 114.Van Marle J., Vrensen G., Van Veen H. 1991. Maturing human eye lens fibre membranes and flipin cytochemistry. Top Aging Res. Eur. 15, 123–124 [Google Scholar]
- 115.Mathias R. T., Rae J. L., Eisenberg R. S. 1979. Electrical properties of structural components of the crystalline lens. Biophys. J. 25, 181–201 10.1016/S0006-3495(79)85284-4 (doi:10.1016/S0006-3495(79)85284-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grey A. C., Jacobs M. D., Gonen T., Kistler J., Donaldson P. J. 2003. Insertion of MP20 into lens fibre cell plasma membranes correlates with the formation of an extracellular diffusion barrier. Exp. Eye Res. 77, 567–574 10.1016/S0014-4835(03)00192-1 (doi:10.1016/S0014-4835(03)00192-1) [DOI] [PubMed] [Google Scholar]
- 117.Heys K. R., Cram S. L., Truscott R. J. 2004. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol. Vis. 10, 956–963 [PubMed] [Google Scholar]
- 118.Bassnett S., Beebe D. C. 1992. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Dev. Dyn. 194, 85–93 10.1002/aja.1001940202 (doi:10.1002/aja.1001940202) [DOI] [PubMed] [Google Scholar]
- 119.Dahm R., Gribbon C., Quinlan R. A., Prescott A. R. 1998. Changes in the nucleolar and coiled body compartments precede lamina and chromatin reorganization during fibre cell denucleation in the bovine lens. Eur. J. Cell. Biol. 75, 237–246 [DOI] [PubMed] [Google Scholar]