Abstract
The purpose of the lens is to project a sharply focused, undistorted image of the visual surround onto the neural retina. The first pre-requisite, therefore, is that the tissue should be transparent. Despite the presence of remarkably high levels of protein, the lens cytosol remains transparent as a result of short-range-order interactions between the proteins. At a cellular level, the programmed elimination of nuclei and other light-scattering organelles from cells located within the pupillary space contributes directly to tissue transparency. Scattering at the cell borders is minimized by the close apposition of lens fibre cells facilitated by a plethora of adhesive proteins, some expressed only in the lens. Similarly, refractive index matching between lens membranes and cytosol is believed to minimize scatter. Refractive index matching between the cytoplasm of adjacent cells is achieved through the formation of cellular fusions that allow the intermingling of proteins. Together, these structural adaptations serve to minimize light scatter and enable this living, cellular structure to function as ‘biological glass’.
Keywords: eye lens, transparency, cataract, electron microscopy, confocal microscopy
1. Introduction
Structures are opaque due to absorption and/or elastic scattering of light, both of which remove energy from the incident beam. The biomolecular constituents of the cornea, lens and vitreous body absorb only weakly in the visible spectrum. As a result, light absorption by ocular tissues is minimal, at least in young, non-diseased eyes. With age, chromophores may accumulate, especially in the nucleus of the lens. This leads to increased absorption and coloration of the older (more than 50 years) lens. Age-related changes in coloration and the mechanisms that underlie them are dealt with in the paper by Michael & Bron [1] and will not be discussed here. Elastic light scattering in tissues is mainly due to differences in refractive index between the main cellular constituents: proteins, nucleic acids, cell and organelle membranes, and water. These components are distributed randomly in most cells and tissues. That is why tissues are usually opaque. The intrinsic transparency of the cornea, lens and vitreous implies that their internal organization must differ in some fundamental way from that of other tissues.
Cornea, lens and vitreous are unusual tissues, insofar as all three are transparent and allow, at least in the young, healthy eye, the passage of light to the retina with minimal scatter, distortion or absorption. The vitreous, in the human eye the largest of the three tissues, is a viscoelastic gel with a high (99%) water content and few resident cells. The vitreous is an optically neutral tissue. The task of focusing light falls, therefore, to the cornea and lens. The cornea is the more refractive of the two tissues, a consequence of its location at the ocular surface. The large refractive index (n) difference between air and tear film (n = 1.000 versus 1.376, respectively) ensures that, in humans, the cornea contributes two-thirds of the focusing power of the eye. The transparency of the cornea is secured by the regular and rigid organization of collagen fibrils [2] and the uniform distribution of keratocytes in the corneal stroma [3], which accounts for 90 per cent of the corneal thickness. The cornea and its non-transparent extension, the sclera, are inelastic structures that also function to provide mechanical support to the eye globe. One consequence of its relative rigidity is that the refractive power of the cornea (approx. 43 Dioptres (D) in humans) is fixed. Variable focusing (accommodation) is a task that, in humans at least, falls exclusively to the lens.
Unlike the cornea, the lens is located within the eye and its refractive surfaces are bathed by ocular humours. To focus light, the refractive index of the lens substance must significantly exceed that of the surrounding media (n = 1.336). In fact, the refractive index of the human lens fibre cytoplasm varies from 1.380 near the lens surface to 1.409 in the centre of the tissue [4]. These high values reflect the extraordinary concentration (sometimes exceeding 450 mg ml−1) of crystallin proteins in the cytoplasm of lens fibre cells [5]. Given that light scatter in protein solutions is usually proportional to protein concentration, the transparency of the lens substance is perhaps unexpected. The explanation for this apparent paradox is that at very high protein concentrations, short-range-order interactions between lens proteins virtually eliminate light scatter through the process of destructive interference [6].
Light scattering in biological tissue occurs at boundaries between compartments of differing refractive index, such as at the cell border or within the cell, where cellular organelles tend to have a different (usually higher) refractive index than the cytoplasm in which they are located. A priori, it might be expected that the lens, a complex three-dimensional cellular structure, would be no more transparent than any other tissue type. Yet the lens is exquisitely transparent. How can we account for this property? In this article, we examine structural and ultrastructural specializations that contribute to lens transparency by minimizing spatial fluctuations in refractive index. Together, these features ensure that light rays pass through a uniform medium and enable this living, cellular structure to function as ‘biological glass’. In some regards, the optical performance of the living lens exceeds that of a simple glass lens. The biological lens is flexible and in many species (including humans) the curvature of its surfaces can be adjusted, allowing objects at various distances to be brought into focus. Furthermore, the refractive index is not uniform within the living lens; values in the nucleus are markedly higher than at the periphery. The refractive index gradient effectively corrects the spherical aberration of the lens, leading to enhanced optical performance.
2. Growth and organization of the lens
The lens is positioned in the eye behind the iris. Its anterior surface is bathed by aqueous humour, while its posterior face contacts the vitreous body. The lens is connected at its equator to the adjacent ciliary body by rings of suspensory ligaments, the zonules of Zinn. In humans, the zonules are subdivided into anterior, equatorial/meridional, and posterior groupings according to their insertion point on the lens capsule. The zonules, which are composed of inelastic material, transfer forces from the ciliary body muscle to the lens during accommodation.
Although the embryonic human lens is nourished by an external blood supply, the tunica vasculosa lentis, this regresses in the course of development and the late foetal, juvenile and adult lens is completely avascular. The absence of blood vessels ensures that light is not absorbed by haem pigments.
In humans, the lens forms during the 5th week of gestation [7], when an invagination of head ectoderm pinches off to form a hollow ball of epithelial cells known as the lens vesicle (see [8]). The vesicle is bounded by a collagenous basement membrane, the lens capsule. Cells of the posterior vesicle wall elongate, in the process obliterating the lumen of the vesicle and forming the first lens fibre cells, known as the primary fibre cells, which constitute the embryonic nucleus in the adult lens (figure 1a). Thus, early in development, the fundamental polarized organization of the lens is established, with an epithelial layer covering the anterior surface of the fibre cell mass. Subsequent growth of the tissue is facilitated by the division of cells in the germinative zone (GZ, figure 1a), a band of epithelial cells located anterior to the lens equator. Progeny of epithelial mitoses withdraw from the cell cycle, become aligned in meridional rows (figure 1c), migrate posteriorly (figure 1b,c), and begin to differentiate into lens fibre cells (figure 1b), in response to fibroblast growth factor (FGF) or other growth factors secreted by the retina [10]. The meridional rows of fibre cells are the first manifestation of the radial cell column arrangement found throughout the fibre cell mass (figure 1d,e). Fibre cell terminal differentiation involves a plethora of morphological and biochemical transformations including: cellular elongation (figure 1f), elaboration of adhesion complexes, syncytial formation and, ultimately, degradation of nuclei and other cytoplasmic organelles. Fibre cell formation continues throughout life, albeit at a much reduced rate in adulthood [11]. There is no cell turnover or shedding. Therefore, each lens retains a complete cellular history. The age of a fibre cell can be inferred from its radial position, the oldest cells being located in the centre of the lens and the youngest cells nearest the surface. A diagram illustrating the cellular organization of the lens based on microscopic observations is shown in figure 1a.
Figure 1.
Cellular organization of the vertebrate lens. The lens is bounded by the lens capsule (Cap). (a,b) Its anterior surface is covered by a monolayer of epithelial cells (Ep). The primary lens fibre cells (pLF) are formed early in embryonic development and constitute the embryonic nucleus in the adult lens. Secondary lens fibre cells are formed continuously by mitosis of cells in the germinative zone (GZ) at the equatorial margin of the epithelium. The fibres are stacked one upon another in meridional rows (c). These secondary lens fibre cells differentiate (b, dLF) and subsequently elongate (b, eLF) beneath the apical surface of the epithelium (f, aEp) and along the posterior capsule until their tips reach the anterior (aS) and posterior (pS) sutures. Mature fibre cells in the organelle-free zone (OFZ) of the lens lack nuclei and other organelles. Most fibre cells are hexagonal in cross-section and very regularly organized in closed sheets as shown in the light and scanning electron microscope images of (d) and (e). Diagram adapted from Shi et al. [9]. GC, growth cone.
3. Microanatomy of the lens
Fibre cells are the main elements in all vertebrate lenses, comprising more than 95 per cent of the tissue volume. As shown in scanning electron microscope (SEM) images of frontal (figure 2a,c) and tangential (figure 2b,d) fracture planes through a zebrafish (figure 2a,b), a rabbit (figure 2c) and a human lens (figure 2d), fibre cells are tightly packed and arranged in a series of concentric shells, like the layers in an onion. An exception is the small embryonic nucleus located in the centre of the tissue (depicted in figure 1a). In the embryonic nucleus, fibre cells are much more irregular in shape and organization.
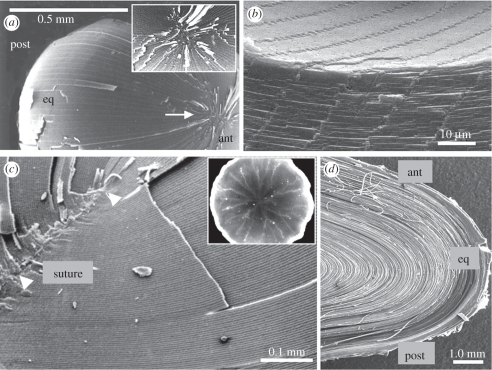
Figure 2.
Cellular architecture of the vertebrate lens. Scanning electron micrographs of the lenses of zebrafish (a,b), rabbit and (c,d), human lenses. The inset in (c) shows the complex branching pattern of sutures in the adult human lens. ant, anterior pole; post, posterior pole; eq, equator.
Elongating fibre cells arc around the lens equator (figure 2d), their tips converging at the lens poles (figure 2a, arrow). At the poles, fibre tips interlock in a region called the lens suture (figure 2a, arrow; figure 2c, arrowhead). Sutures may be umbilical (or point-like) in fish and birds, as shown for the zebrafish (figure 2a and inset), line-like, as in the rabbit lens (figure 2c), or Y-shaped, as in mice, rats, guinea pigs, cats, dogs, cows and sheep [12]. In the human lens, the suture is initially Y-shaped but by adulthood becomes a complex multi-branched star suture (figure 2c inset).
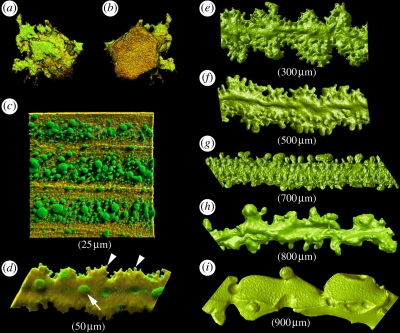
When viewed en face in conventional flat mounts, lens epithelial cells have a regular polygonal appearance (figure 1f, apical surface of the epithelium (aEP)). This impression is based entirely on the shape of the apical membrane. In experiments in which green fluorescent protein (GFP) expression is induced in individual epithelial cells, it is possible to visualize the complex three-dimensional shape of an individual living epithelial cell. Such studies demonstrate that while the apical membrane (i.e. the region of the cell in contact with the underlying fibre mass) has a smooth polygonal profile (figure 1f, aEP and figure 3b), the basal membrane (i.e. the region of the cell adjacent to the overlying lens capsule) has a variable and complex morphology (figure 3a). The three-dimensional morphology of the fibre cells is similarly complex and varies, depending on the location of the cell (figure 3c–i).
Figure 3.
Three-dimensional structure of mouse lens cells at various stages of differentiation as revealed by confocal microscopy. (a,b). Lens epithelial cells showing (a) basal or (b) apical surfaces. (c) Young elongating fibre cells located near the surface of a two-month-old mouse lens. The fibres are initially smooth and ribbon-like. Their membrane surface features a large number of gap junction plaques (green) visualized here by immunofluorescence with anti-connexin (Cx) 50. (d) At this stage, the fibre cell is in the process of losing its organelles. At the membrane surface, ball-and-socket processes (enriched with Cx50) are formed on the broad face of the lateral membrane (arrow). Smaller, finger-like structures protrude from the narrow membrane faces (arrowheads). (e) With the disappearance of organelles the fibres take on an undulating appearance. (f–i). Fibre cells dissected from progressively deeper cell layers. The primary fibre cells from the centre of the lens (i) are characterized by a very irregular structure. The approximate location (distance beneath the lens equatorial surface) of the fibre cells is indicated in parentheses. Mouse lenses of this age (two months) are approximately 1800 µm in diameter.
As exemplified in the light micrograph (figure 1d) and tangential SEM fracture (figure 1e) through the cortical region of a rabbit lens, lens fibre cells have a hexagonal cross-sectional profile and extend from the anterior to the posterior suture (see also figure 2). This allows for tight packing with little extracellular space between the cells. The hexagonal profile consists of two broad faces (parallel to the lens surface) and four narrow faces. The width and thickness of the fibre cells vary considerably between species. Fibre cells can be 5–15 µm in width, with a thickness of 1–5 µm (compare the thickness of the fibre cells in zebrafish lenses and rabbit lenses: figures 1e versus 2b).
In all lenses studied to date, finger-like membrane protrusions have been found to project from the six vertices of the fibre cell lateral membrane. These projections appear primarily to interconnect cells with their lateral neighbours in adjoining radial cell columns (figures 3d–h and 4a–d). Early in fibre cell elongation the cells have a smooth, ribbon-shaped form with very small protrusions [13] but, over time, they become increasingly elaborate (figure 4c,d, see also [14]). A seemingly distinct structure, the ball-and-socket process, is often present on the broad face of differentiating fibre cells, where it connects fibre cells with neighbouring cells located immediately above or below them in a radial cell column (figures 3d and 4c,d). Ball-and-socket processes are common in differentiating fibre cells, especially in the distal regions of the cells, near the migrating cell tips (figure 1f). In animals with longer life spans, the membrane surfaces of fibre cells in the deep cortical and nuclear regions are gradually remodelled. The oldest cells are characterized by the presence of microplicae (sometimes referred to as ridge-and-groove processes) (figure 4i,j) and complex protrusions (figure 3d–g). The membranes of microplicae have few intramembrane particles (IMPs). Gap junctions are relatively sparse in this region and those that are present have a fuzzy, degenerated appearance [15].
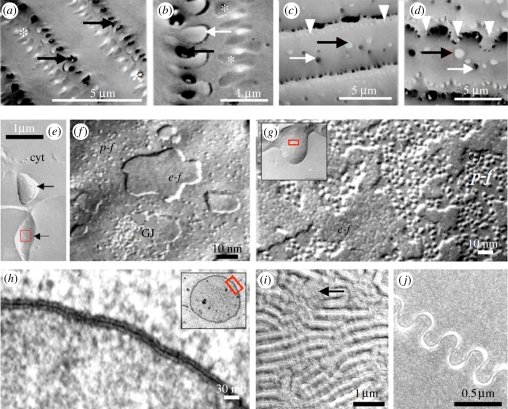
Figure 4.
Membrane associations between fibre cells. (a,b) Scanning EM images of interlocking edge protrusions (asterisks and white and black arrows) between superficial lens fibres. In deeper cortical regions (c,d) the edge protrusions (arrowheads) become tortuous and ball and sockets junctions (black and white arrows) appear. Freeze fracture images at low (e) magnification support the close interlocking of neighbouring fibres by edge protrusions (arrows) as seen in scanning EM images (a,b). High magnification images (f) reveal the relatively small number of particles on the protoplasmic (p–f) and external (e–f) faces of the fibre membranes. Incidentally a gap junction is found (GJ). High magnification freeze fracture images (g) show the high density of membrane particles on ball-and-socket junctions (inset). This is corroborated by low (inset) and high magnification TEM observations (h) suggesting that these junctions are giant gap junctions with the typical close apposition of adjacent membranes. In freeze fracture images of deep cortical regions (i), lens fibre membranes show grooves and ridges (microplicae) on their surface which correspond to undulating membranes of neighbouring fibres as shown in TEM images (j). Gap junctions (arrowed in i) are found occasionally on these membranes. Note the high spatial order of the cytoplasm in (j). Figure (a–d) are taken from a rabbit lens; (e–l) from a human lens. cyt, fibre cytoplasm.
The edge protrusions and ball-and-socket processes interconnect different populations of cells. They also appear to be structurally distinct. Freeze fracture observations reveal that the membranes of the edge protrusions (figure 4e) are studded with relatively few IMPs, which are most evident on the protoplasmic faces of the fractured membranes (figure 4f). Edge protrusions also have relatively few gap junctions (as can also be judged from the lack of connexin 50 in the edge protrusions shown in figure 3d). In contrast, the membranes of ball-and-socket processes (figure 4g) are densely populated with IMPs, which are observed on both their protoplasmic and external faces, suggesting that they form part of a single large gap junctional complex (figure 4h inset). This is verified by transmission electron microscopy (TEM) observations showing that the entire circumference of ball-and-socket processes consists of uninterrupted gap junction plaques (figure 4h). The presence of gap junctions on the ball-and-socket processes is corroborated by the connexin 50 immunofluorescence in figure 3d. In view of this difference in ultrastructure, it can be speculated that edge protrusions, which are present in lens fibre cells of all ages, function primarily to physically interlock cells and thus contribute to tissue cohesion. This is probably especially important in the lenses of species that accommodate, where substantial shear forces generated during deformation of the tissue must be resisted. UV radiation [16] and incubation with p-chloromercuri-phenylsulphonate (pCMPS) [17] of rat lenses both lead to dysregulation of Ca2+ and lens water homeostasis and consequently to osmotic stress. Initially this causes opacification of the equatorial region. TEM analysis revealed that this is due to swelling of the fibre cells and to formation of small intercellular vacuoles in this region. However, the reciprocal interlocking of fibres remains largely intact. Similar morphology is found in diabetic and galactosemic cataracts [18]. These observations may indicate that the edge protrusion may resist disorganization of the regular fibre pattern under physiological and stress conditions. Ball-and-socket junctions may also have a structural role but the concentration of gap junctions in the ball-and-socket membranes suggests that they are involved in fibre-to-fibre radial transfer of small solutes (see [8]). Such transfer serves the influx of metabolically important nutrients and efflux of waste products and thus, indirectly, to maintenance of lens clarity.
4. Lens organelle degradation
First noted by Meyer in 1851 [19] and described later in detail by Rabl [20], the abrupt disappearance of nuclei from maturing lens fibre cells is a striking aspect of the differentiation process and one that contributes directly to lens transparency. Light is scattered (through various angles, depending on the size and shape of the scattering particle) each time it traverses the boundary between structures of differing refractive indices. The refractive index (n) of cytoplasm is approximately 1.37 for a typical cell. Generally, the refractive index of organelles and other sub-cellular components is substantially higher than this. For example, the index values for nuclei, mitochondria and lysosomes are 1.39, 1.4 and 1.6, respectively (see [21] and references therein). As a consequence, organelles are responsible for a significant fraction of the light scattered by living cells and a major reason that most biological tissues are opaque.
Organelles are absent from the innermost cells of the adult vertebrate lens as a consequence of an organelle degradation process that begins during embryonic development and continues throughout life. In the embryonic chicken lens, all fibre cells contain a usual complement of intracellular organelles until the twelfth day of development (E12), at which time organelles in the primary fibre cells abruptly disappear [22]. In mice, the degradation of nuclei and organelles takes place between E15 and E18 [23]. The region of the lens core in which cells have degraded their organelles has been termed the organelle-free zone (OFZ) of the lens [22]. The border of the OFZ is surprisingly sharp, indicating that organelle degradation occurs in a rapid and coordinated fashion within the space of a few cell layers. The border of the OFZ represents an abrupt transition from one state of fibre cell physiology to another. Outside the OFZ, fibre cells have mitochondria and generate ATP from oxidative phosphorylation; within the OFZ, fibres must rely exclusively on glycolysis. Similarly, with the disappearance of nuclei and ribosomes from the cytoplasm (and the accompanying decay of extant rRNA and mRNA [24]), opportunities for transcription and translation of new cellular components cease. The endomembrane system is degraded concomitantly with the other organelles. Thus, the ability to actively remodel the cell surface through targeted membrane trafficking is lost. Proteins and other cellular components are subject to oxidation, glycation, deamidation and a host of other post-translational modifications within the OFZ, with no prospect for repair or replacement of damaged components. The border of the OFZ can be thought to represent the end of cellular development and the onset of cellular ageing.
Although the phenomenon of organelle breakdown has been recognized for more than 150 years [19], much about the underlying mechanisms remains mysterious. Any model of this process has to account for how the myriad individual components of the various organelle systems are rapidly degraded while the cytoplasm (most notably the assembly of crystallin proteins), the membrane, and critical components of the lens fibre cytoskeleton (including actin and the beaded filament proteins CP49 and filensin) are spared. Attention has focused on the events preceding DNA/chromatin breakdown [25] and the identity of the nuclease(s) responsible for degrading fibre cell nuclear DNA.
In vertebrate lenses the process of denucleation is rapid, making it difficult to study the stages of nuclear breakdown. However, as first described by McAvoy [26] and, more recently, by Vrensen et al. [25], raising rats on a tryptophan-deficient diet appears to delay or arrest the process. In tryptophan-deficient animals, the lens bow (the region of nucleated fibre cells) extends much further into the lens than usual (figure 5a,b). Intermittently reintroducing tryptophan into the diet results in the absence of nuclei in the cell layers formed while tryptophan was present (figure 5c). Ultrastructural analysis has revealed the sequence of events preceding denucleation [25]. Following the breakdown of the nuclear envelope, chromatin is gradually condensed and segregated from other fibrillar nuclear material (figure 5d). Condensed chromatin with a similar structure is also observed during the denucleation process in normal human (figure 5f) and rat lenses, suggesting that the condensation process is not due to absence of tryptophan. X-ray microanalysis reveals that the condensed chromatin material contains DNA, co-localized with proteins, especially γ-crystallin as shown immunohistochemically. Electron tomographic reconstruction of the condensed chromatin in an early stage revealed deconvolved strands of chromatin with dimensions of 30 nm [25]. In late stages, the chromatin had dimensions of 10 nm and a ‘beads-on-a-string’ appearance. We hypothesize that the final degradation of nuclear DNA (mediated by DNase 2β, see below) may occur when the DNA is in this beads-on-a-string configuration. In between the condensed chromatin material, large numbers of 10 nm diameter particles have been observed (figure 5e). These particles are the size of proteasomes. In view of the recently described role for the ubiquitin proteasome pathway (UPP) in organelle degradation [27], it is tempting to speculate that the UPP could also play a role in the final breakdown of chromatin and DNA.
Figure 5.
Fibre cell denucleation in rodent lenses. (a) Programmed degradation of nuclei occurs in cortical lens fibre cells of rats raised under normal conditions. (b) Chromatin breakdown is inhibited in rats fed a tryptophan-deficient diet. (c) Transient reintroduction of tryptophan causes chromatin breakdown in fibres that underwent denucleation during the period tryptophan was present. (d) Electron micrograph of a nucleus from the cortex of a tryptophan-deficient lens. Note the segregation of chromatin/DNA (electron dense) from other nuclear constituents. Inset shows a light micrograph of the same nucleus stained with Hoechst 33258 dye to visualize DNA. (e) At higher magnification, small (approx. 10 nm) particles (putative proteasomes) are visible in the matrix of disintegrating nuclei tryptophan-deficient rats. (f) Similar condensed chromatin/DNA is also found in normal rat lenses and in young human lenses. (g) Denucleation also occurs in the cortex of wild-type mouse lenses but is blocked in lenses from DNase-IIβ-null mice (h). Ep, epithelium; OFZ, organelle-free zone.
The molecular identity of the nuclease responsible for degradation of lens fibre cell DNA was a contentious issue for several years (reviewed in [28]) but recently deoxyribonuclease II beta (DNase-IIβ) has been shown to play a critical role in lens fibre nuclear degradation. Microarray studies have indicated that transcripts for DNase-IIβ (a.k.a. DNase II-like acid nuclease; DLAD) are found at higher levels in the mouse lens than in any other of 96 murine tissues and cell lines examined [29] and PCR measurements suggest that DNase-IIβ mRNA may be similarly abundant in the human lens [30]. Both DNase-IIβ mRNA [31] and protein levels [32] are increased specifically in fibre cells bordering the OFZ. In lenses of mice deficient in DNase-IIβ, nuclear breakdown is blocked and chromatin persists in the normally anucleate central fibre cells (see [30] and figure 6g,h). The measured acidic pH optimum of DNase-IIβ, in conjunction with immuno-localization studies [32], suggests that this nuclease is a lysosomal enzyme. It is not clear, however, how the nuclease is transferred from the lumen of the lysosomes to the nuclear compartment. Immuno-electron microscopic studies have indicated that lysosomes may fuse with the nuclear membrane shortly before the chromatin is degraded [32]. Dual-labelling studies in our laboratory have also revealed that only a subset of lens fibre lysosomes contain DNase-IIβ (unpublished observation). This raises the possibility that fibre cells may contain a specialized lysosomal population with a dedicated role in organelle degradation.
Figure 6.
Calpain activation in cells bordering the OFZ in a lens from a three-day-old mouse. The distribution of calpain-cleaved spectrin (green) is used as a surrogate for calpain activation. Calpain-cleaved spectrin (arrow) first appears in fibre cells undergoing nuclear degeneration. Lens cell nuclei are visualized with propidium iodide (red). Image adapted from De Maria et al. [34].
Progress in identifying the proteolytic systems underlying organelle breakdown has been slow. Early studies focused on the possible involvement of caspase proteases in this process, based largely on the view that lens organelle breakdown might represent an attenuated form of apoptosis [33]. More recently, a role for the UPP in organelle degradation was demonstrated [27]. Injection of the proteasome inhibitor, lactacystin into the embryonic chicken eye immediately prior to the onset of organelle degradation blocked the programmed elimination of a mitochondrial marker enzyme, succinate-ubiquinone oxidoreductase. These results implicated the UPP, at least in the final stages of organelle breakdown.
Although studies have failed to show that caspase activation accompanies organelle breakdown, another class of cysteine proteases, calpains, are activated [34]. Calpains have long been known to be activated in the lens under pathological conditions of calcium overload and have been suspected of playing a role in human cataract formation. Recently, it has emerged that calpains also play a role in the normal process of fibre cell differentiation. Antibodies raised against calpain-cleaved spectrin label specifically lens fibre cells adjacent to the OFZ [34]. The calpain-dependent fragmentation of spectrin in this cohort of cells is direct evidence that one or more calpains are activated in this region of the lens. What is less certain is whether calpain activation is a cause or consequence of organelle degradation. Mitochondria and endoplasmic reticulum are two major calcium stores in cells. With their disappearance, free calcium is released into the cytoplasm, where it could activate calcium-dependent enzymes, such as calpains [17,35]. Calcium imaging experiments [36] have shown that free calcium is elevated in mature fibre cells compared with young fibre cells (700 versus 300 nM, respectively). With the availability of calpain knockout mice it should be possible to test the role of these enzymes in organelle breakdown explicitly.
5. Intercellular junctions and adhesion proteins
To focus light, the refractive index of the lens must exceed that of the humours in which it is immersed. While a high cytoplasmic refractive index is a prerequisite for focusing, it poses an optical dilemma for a tissue composed of many cell layers. Aqueous humour, the fluid that fills the spaces between lens cells, has a relatively low refractive index (n = 1.336). As light rays pass through the lens, it might be expected that light would be scattered at every interface between cellular and extracellular compartments and that, consequently, the lens would be opaque. That this evidently is not the case is due primarily to adhesive interactions between fibre cells, which minimize the dimensions of the extracellular space. The physical nature of these interactions and the molecular identity of the adhesive proteins is an emerging area of lens research facilitated by recent technological developments in fields such as atomic force microscopy and proteomics.
Adhesive junctions between cells can be divided into four categories: desmosomes, tight junctions, adherens junctions and gap junctions. Desmosomes are present in the epithelium [37] but are rare or absent from lens fibres. Morphological [38], physiological [39] and molecular evidence [40] supports the existence of lens epithelial tight junctions. However, the archetypical tight junction proteins claudin and occludin were not detected in the fibre membrane proteome [41], suggesting that tight junctions are absent from fibres, a finding thus far corroborated by electron microscopy [42]. The two remaining types of junction, adherens junctions and gap junctions, are well represented in the lens and discussed in detail below. An additional adhesion complex, probably unique to the lens and using Mip and possibly Lim2 (two abundant integral membrane proteins), is assembled late in fibre cell differentiation. This (presumably) durable adhesion complex may supplant the more labile adherens junctions in the central regions of the lens where, due to organelle degradation, protein turnover is no longer possible.
Lens adhesion proteins have been studied for years in a piecemeal fashion, but recently shotgun proteomic techniques have provided a more comprehensive picture of the lens membrane proteome [41,43]. Table 1 shows adhesion proteins identified by MudPIT analysis of mouse lens fibre cell membranes (data from [41]).
Table 1.
Adhesion proteins identified in the lens fibre cell membrane proteome [41].
| protein | spectral counts | family |
|---|---|---|
| Mip (major intrinsic protein) | 2095 | aquaporin |
| Lim (lens intrinsic membrane protein, a.k.a. MP20) | 360 | Pmp22_claudin |
| Tmem47 (transmembrane protein 47) | 23 | |
| PMP22 (peripheral myelin protein 22) | 14 | |
| PERP (p53 apoptosis effecter related to PMP-22) | 4 | |
| N-Cad (N-cadherin, cadherin 2) | 528 | cadherin |
| R-Cad (R-cadherin, retinal cadherin, cadherin 4) | 14 | |
| Fat4 (fat tumour suppressor homolog 4) | 8 | |
| Celsr (EGF LAG seven-pass G-type receptor 1) | 3 | |
| E-Cad (E-cadherin, epithelial cadherin, cadherin 1) | 2 | |
| Tjp1 (tight junction protein 1, ZO-1) | 13 | zonular occludens |
| Cadm1 (cell adhesion molecule-1, SynCAM, Necl2, Tslc1) | 138 | immunoglobulin superfamily (IgSF) |
| Cxadr (coxsackievirus and adenovirus receptor) | 92 | |
| Lsamp (limbic system associated membrane protein) | 38 | |
| Jam3 (junction adhesion molecule 3) | 33 | |
| Nr-Cam (neuron-glia-CAM-related cell adhesion molecule) | 8 | |
| AVRCF (armadillo repeat gene deleted in velo-cardio-facial syndrome) | 63 | |
| NCam1 (neural cell adhesion molecule 1) | 48 | |
| Sdk2 (sidekick homologue 2) | 76 | |
| Sdk1 (sidekick homologue 1) | 45 | |
| Ceacam2 (CEA-related cell adhesion molecule 2) | 9 | |
| Negr1 (neuronal growth regulator 1) | 3 | |
| IgSF9 (immunoglobulin superfamily member 9) | 5 | |
| Emb (embigin) | 2 | |
| Kirrel (Kin of IRRE like) | 4 |
Functionally, lens adhesion proteins can be organized into several groups. The first (Nr-Cam, Cadm1 and N-Cam) includes a subset of the immunoglobulin super family (IgSF). These proteins facilitate calcium-independent homotypic and heterotypic adhesion. In other contexts, they are implicated in dynamic interactions, such as axon guidance or synapse remodelling. Immunofluorescence studies indicate that Nr-Cam, Cadm1 and N-Cam are present in lens epithelial membranes and the plasma membrane of elongating fibres [44,45]. Targeted deletion of Nr-Cam results in cellular disruption in differentiating fibres [44], and elongating fibre cells have aberrant morphologies in Cadm1-null mice (Bassnett, unpublished observation). IgSF proteins are not usually incorporated into junctional complexes but their expression can facilitate the assembly of such complexes [46].
Cadherins are single-pass, integral membrane glycoproteins that mediate calcium-dependent cell adhesion and constitute the major intercellular link at adherens junctions. E-cadherin (Cdh1) expression is restricted to the lens epithelium [47]. N-cadherin is expressed at modest levels in the lens epithelium but is by far the most abundant cadherin in the fibre membrane (table 1). In lens epithelial cells, cadherin-based junctions form a continuous ring (classically referred to as a zonula adherens) around the apico-lateral membrane [40]. Zonulae adherens may also be present at the apical tips of elongating fibre cells [48].
It has been suggested that the lateral membrane of the fibre cells represents a single adhesive domain, the cortex adhaerens [42], composed of microdomains of N-cadherin-based complexes (containing N-cadherin, α- and β-catenin, plakoglobin, p120ctn and vinculin) and the so-called EPPD complex (composed of ezrin, periplakin, periaxin and desmoyokin). At the electron microscope level, morphologically identifiable adherens junctions are detected in the membranes of superficial cortical fibre cells, where they are present as small, plaque-like structures 100–200 nm in diameter [37,48]. The junctional membranes are relatively flat and separated by an intermembrane space of 15–20 nm, filled with electron-dense material. Occasional cross-bridges (composed, presumably, of N-cadherin) are observed within the junctions. An electron-dense plaque of actin microfilaments is associated with the cytoplasmic face of lens adherens junctions [37]. Adherens junctions are particularly abundant at the intersections where three fibre cells meet and on the narrow sides of the fibre cells (in which location they conjoin fibre cells located in neighbouring radial cell columns). Thus, electron microscopic observations of adherens junction distribution correlate well with the distribution of N-cadherin as visualized by immunofluorescence microscopy, which also places N-cadherin predominantly at the narrow faces of the fibre cells [42]. Although prominent in differentiating fibre cells, adherens junctions are not usually observed in the anucleated fibre cells of the lens core. The disassembly of adherens junction structures is paralleled by the degradation of N-cadherin protein which, in the human lens, becomes undetectable in the lens core soon after birth [49].
Several IgSF proteins identified in the fibre cell membrane proteome (table 1) function as accessory adhesion proteins that regulate the assembly or stability of adherens junctions. For example, coxsakievirus and adenovirus receptor (Cxadr) and the structurally related protein junctional adhesion molecule (Jam3) both localize to adherens junctions in other tissue [50,51], although their distribution in the lens is not known. Avrcf is a prominent component of adherens junctions [52] and a member of the p120-catenin sub-family that functions in stabilization of cadherin-based junctions [53]. ZO-1 acts as a cytosolic scaffold, regulating the assembly of both adherens and tight junctions [54] and associating with connexin proteins in gap junction plaques. The distribution of ZO-1 in lens fibre cells (on the narrow sides of young fibre cells and on the broad sides of mature fibres) indicates that it may interact with adherens junctions in the outer fibre cells and gap junctions in inner fibres [55].
Adherens junctions are not common on the broad faces of the fibre cell membrane (i.e. the surface which connects one layer of lens fibre cells to the next). Instead, gap junctions dominate this membrane domain, in some species accounting for 50 per cent of the membrane surface [56]. In the lens, two connexins (Cx46 and Cx50) are expressed at extraordinarily high levels in the fibre cell membrane. In non-primate lenses, a third connexin (Cx23) is also abundant [57]. The principal role of gap junctions is to permit the intercellular diffusion of small molecules. However, gap junctions have adhesive qualities distinct and separable from this physiological role [58,59]. Thus, fibre cell layers in the lens cortex may adhere to each other, at least in part, through gap junction-mediated contacts.
Two proteins with suspected adhesive functions, Mip and Lim2, are abundant members of the lens membrane proteome. Mip is the founder member of the aquaporin family of water channels but the long-standing suspicion that it also acts as an adhesive molecule in the lens was lent credence by recent experiments in which Mip was shown to induce L-cell aggregation [60]. Mip and Lim2 are both synthesized by elongating fibre cells and, unlike cadherin or IgSF proteins, they do not disappear during fibre cell maturation. In fact, knockout of either protein results in cataracts in which the outer cell layers are largely spared [61,62], suggesting that it is in the mature fibre cells that Mip and Lim2 have irreplaceable functions (figure 7).
Figure 7.
Disaggregation of lens fibre cells in the Lim2-null mouse lens. Partially-fixed lenses were teased apart with fine forceps. Note that in the absence of Lim2, fibre cells readily separate into individual cells. These data support the notion that Lim2 may have an adhesive function in the lens.
As fibre cells age, the plasma membrane surface begins to take on a ‘corrugated’ appearance due to the formation of microplicae (see figure 4). In the oldest region of the lens, three types of cell–cell junction can be discerned by electron microscopy [12]. Gap junctions (16 nm thick) are present but at lower density than in the cortical fibre cells. Much more numerous are 14 nm and 11 nm thick junctions. The 14 nm thick junctions are enriched in flat regions of the cell surface or, conversely, where membrane undulations are of particularly high amplitude. The 11 nm thick junctions are enriched in regions of low, amplitude membrane undulations. Freeze fracture electron microscopy [63,64] and, more recently, atomic force microscopy (AFM) [65,66] have revealed that large areas of fibre plasma membrane contain two-dimensional arrays of intramembrane particles. The arrays are composed of regularly arranged tetrameric proteins with lattice dimensions of a = b = 6.5 nm, γ = 90° [67]. SDS-fracture immunolabelling [68] has demonstrated that the orthogonal arrays consist of Mip protein. AFM images further suggest that each Mip lattice is cordoned by densely packed connexin channels. Most studies have shown that Mip exists as square arrays in one fibre cell membrane but the molecular composition of the adjoining membrane is not clear. According to some models, Mip is distributed asymmetrically [12]. Thus, a square array in one membrane may be apposed to an essentially protein-free region of the neighbouring membrane, resulting in asymmetric protein/lipid junctions. This could correspond to the 11 nm thick junctions detected by TEM. Further, it has been hypothesized that the offset in Mip distribution may cause the membrane to flex and might drive the formation of the undulating membranes that characterize the central fibre cells. Electron diffraction studies have indicated that Mip has a propensity to form bi-layered crystals, especially when prepared from the nuclear region of the lens [69]. Several authors have suggested that similar crystals may by present in vivo and constitute adhesive junctions. Such bilayered crystals might represent the 14 nm thick symmetric junctions observed in the TEM studies.
The notion that Lim2 could also be an adhesive protein is based on the recognition that Lim2 is a member of a family of adhesion-related proteins that includes claudins and peripheral myelin protein 22 [62]. Lim2 is present throughout the lens fibre membrane [70,71], where it is believed to interact with galectin 3 [72], a known facilitator and modulator of cellular adhesion [73]. Finally, in mice deficient in Lim2, the fibre mass is readily dissociated into individual cells [62], suggesting a defect in intercellular adhesion (see also figure 8).
Figure 8.
Refractive index matching of lens membranes and cytoplasm.
The overall picture emerging from these studies is one in which fibre cells can be divided into two distinct populations according to the type of adhesive structures that connect them. Near the surface, in young, actively differentiating fibre cells, a range of adhesion molecules are expressed. Members of the IgSF family (Cadm1, N-Cam, Nr-Cam) are widely distributed throughout the fibre membranes. Cadherin-based junctions are common, particularly between cells in neighbouring radial cell columns. On the broad faces of the fibre cells, high densities of gap junction plaques are present. With the disappearance of fibre cell organelles the ability to synthesize new components is lost. Effective cell adhesion between anucleate fibre cells in the lens will, of necessity, depend on durable molecular interactions. Square array junctions are seen to decorate the undulating plasma membrane surfaces of the older cells. These junctions are composed of Mip in a near-crystalline state. It is probable that Lim2, another abundant lens-specific membrane protein, contributes to intercellular adhesion, although so far its role is poorly defined.
6. Refractive index matching of lens membranes and cytoplasm
In most cells, the refractive index of the plasma membrane exceeds that of the cytoplasm it encapsulates. Typical phospholipid membranes have a refractive index of about 1.48, whereas cytoplasm usually has a value of about 1.37. The mismatch in refractive index between the surface membrane and the cytoplasm is one reason why cells are usually not transparent. In the lens, the situation is complex because the cytoplasmic refractive index is not uniform. The concentration of cytoplasmic proteins may be twofold or threefold higher in cells located in the centre of the lens than near the surface. Consequently, the cytoplasmic refractive index in central fibre cells is significantly higher than in those near the surface. This gradient in refractive index serves to correct the lens for longitudinal spherical aberration. In the young human lens, the refractive index gradient varies parabolically, from 1.380 near the surface to 1.409 near the centre [4]. Michael et al. used immersion refractometry to measure the refractive index of plasma membranes in cells located at varying depths in the human lens [74]. Perhaps surprisingly, the measurements indicate that membrane refractive index is highest near the surface and lowest in the lens core. The surface values (n = 1.42–1.47 for cells located less than 500 µm below the tissue surface) exceed those of the cytoplasm whereas values for the central cells (1.38–1.42 for cells located more than 500 µm below the surface) closely match the index of the cytoplasm. The change in phospholipid composition, with a preponderance of sphingomyelins, and the high cholesterol content (cholesterol : phospholipid ratio of 3 : 4) may be responsible for this change in membrane refractive index in the deeper fibres [75–77]. The mismatch between membrane and cytoplasm in surface cells would be expected to increase light scatter and, in fact, the outermost cells scatter more light than the inner cells [78,79]. This could be due to membrane effects but may also reflect the presence of light-scattering organelles in this population of cells. It has been suggested that light scattering from the membranes is minimized by the singular morphology and packing arrangement of superficial fibre cells. Near the surface, the fibres have relatively smooth membranes (figure 8, upper panel) and are arranged in a precise hexagonal lattice (figure 1d,e). The lattice acts as a diffraction grating, where the regular spacing of the individual scattering centres leads to constructive interference in the forward direction and destructive interference at other angles. Conversely, in the nucleus, the cells have irregular surfaces and are not organized in a strict lattice (figure 8, lower panel). As a result, it might be expected that this region would scatter a disproportionate amount of light. However, the close matching of membrane and cytoplasmic indices means that the lack of spatial order in the lens core is tolerated and, in healthy lens, this is one of the most transparent regions.
7. Cell fusion and the notion of the lens syncytium
The intercellular electrical resistance in the lens is negligible [80], due to the presence of large numbers of gap junctions (figure 5). Consequently, the lens is usually treated as an electrical syncytium within which ions and other small molecules freely diffuse from cell to cell [81]. That proteins might be similarly diffusible was not appreciated until recently. Working with amphibian lenses, Kuszak et al. noted that the hexagonal cross-sectional packing of fibre cells was disturbed on occasion by the presence of large pentagonal cells [82]. On closer examination, it was apparent that these represented zones of cellular fusion. Fusions involved only a small portion of the fibre cell length; outside the fusion zone fibre cells were again separated by a pair of intact plasma membranes. Due to the length of lens fibre cells and the tortuosity of their lateral membranes it was difficult to map precisely the distribution of fusions within the lens or to estimate their density accurately. Using published data [82], Mathias & Rae estimated a fusion rate of one fusion per ten fibre cells [83]. Such a low density suggested that fusions were unlikely to contribute significantly to the physiological properties of the lens and most electrical models have since neglected to include fusions as a significant intercellular pathway. Instead, it was suggested that fusions might play a purely architectural role in the lens [84].
With the introduction of fluorescent protein technology, it is possible to monitor the cell–cell diffusion of labelled proteins in living, undisturbed lenses. In a series of experiments on embryonic lenses, Bassnett and colleagues microinjected expression plasmids (encoding GFP) into individual fibre cells in the centre of the embryonic lens in ovo [85]. GFP was expressed initially by injected cells but subsequently the GFP fluorescence spread throughout the lens core. Injections into superficial fibre cells resulted in a different pattern. In that location, GFP was restricted to the cytoplasm of expressing cells. These data suggested that during embryonic development, cells in the centre of the lens became connected by a pathway that permitted the intercellular diffusion of proteins. Expression of a Mip–GFP fusion construct revealed that membrane proteins diffused from plasma membrane to plasma membrane in the centre of the lens. Again, this effect was not seen in the superficial fibre cells. The diffusion of Mip–GFP indicated that fibre cell membranes in the lens core form a continuous system. This was consistent with the notion that the protein permeable pathway might consist of regions of cell–cell fusion as described earlier [82]. Volumetric reconstructions of confocal images were used to identify cell fusions in the lens core. Fusions were not observed in the uncoupled outer cell layers. The fact that GFP diffused throughout the lens core and was not excluded from the cytoplasm of any fibre cells strongly suggested that the number of cell fusions in the core region must be significantly higher than the value of 1 fusion per 10 cells calculated previously [83]. If fusions are the only path by which GFP diffuses from cell to cell then there must be at least two fusions per fibre cell.
Experiments with mouse lenses in which GFP is expressed in a mosaic pattern confirmed the results from chicken lenses and demonstrated that the core syncytium forms during embryonic development [86]. In the adult mouse lens, most fibre cells are incorporated into the syncytial region; only a thin (less than 100 µm) layer of cells at the lens surface is excluded. Shi et al. introduced gene induction techniques to the lens field to label individual differentiating fibre cells with GFP in vivo and to follow the fate of the GFP as fibre cells were incorporated into the lens [13]. Curiously, intercellular GFP diffusion was not isotropic (figure 9a–c). Rather, there was a greater tendency for GFP to diffuse between cells located in the same layer of the lens than there was for GFP to diffuse between layers [9]. The intercellular diffusion of protein within a stratum of the lens allows proteins in neighbouring cells to mingle, matching the refractive index of the two cytoplasms and minimizing scatter. The fact that proteins move more readily between cells located within one layer of the lens than between cell layers may ensure that cells of the same age have the same refractive index while differences in index may be maintained between layers. The latter property may be relevant for the establishment and maintenance of the radial gradient in refractive index observed in many lenses (including human lenses) which serves to correct longitudinal spherical aberration of the tissue.
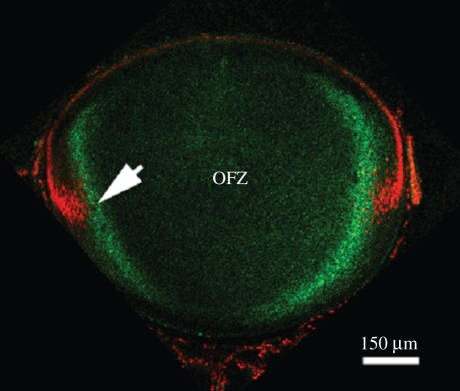
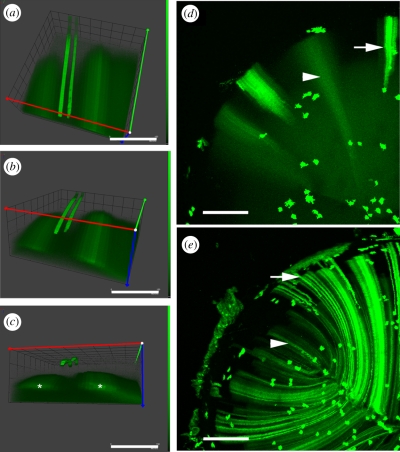
Figure 9.
Lim2-dependent cell fusion during mouse lens fibre cell differentiation. (a–c) A rotational series of volumetric reconstructions of a region of the outer cortex of a wild-type lens. GFP expression was induced in scattered lens cells. In the outer region (depths less than 50 µm) GFP is retained in the cytoplasm of expressing cells. Three such cells are shown. At greater depths, the cytoplasm of neighbouring cells becomes continuously linked through regions of partial membrane fusion. As a result, discretely labelled cells are not observed in the deeper cell layers. Rather, GFP diffuses among a cluster of neighbouring fibre cells (asterisks). The formation of cellular fusions is dependent on the presence of an intrinsic membrane protein, Lim2 (a.k.a. MP20). In wild-type mouse lenses (d) GFP is restricted to the cytoplasm of fibre cells located near the lens surface (arrow) but diffuses from expressing cells when those cells are buried to a depth of more than 50 µm (arrowhead). In the absence of Lim2 (e), fusions are not formed and GFP is retained by both superficial fibre cells (arrow) and fibre cells located deep below the lens surface (arrowhead). Scale bars, (a,b,c) 150 µm; (d,e) 250 µm.
The trigger for fusion formation is unknown but experiments with Lim2-null mice have established that Lim2 is required for fusion formation [9]. Based on its predicted molecular structure, it seems unlikely that Lim2 acts as a fusogen directly, in a manner analogous to the influenza haemagglutinin protein, for example. Rather, it may permit an intimate intercellular contact, a prerequisite for membrane fusion in many systems [87]. In the absence of Lim2, proteins do not diffuse between lens cells (figure 9c,d) and the resulting lens phenotype is characterized by diffuse cataract and internal refractive defects [62]. Formation of the lens syncytium also depends on expression of the gap junction proteins Cx46 and Cx50 [88].
In summary, the lens is usually modelled as an electrical syncytium where the flow of ions between cells is facilitated by large numbers of gap junctions. Recent studies suggest the existence of a parallel pathway consisting of cell membrane fusions and permeable to macromolecules. Fusions require the expression of gap junction proteins and Lim2 and probably have a role in refractive index matching. Most of the cells in the lens are fused, with the exception of a thin layer of cells at the surface of the tissue.
8. Summary
Here, we have reviewed structural adaptations that are believed to contribute to the exquisite transparency of the ocular lens. Beyond simple intellectual curiosity, it is important to better understand the cellular basis of transparency because loss of transparency, i.e. increased light scattering and absorption and, ultimately, cataract, is a global health problem that adversely impacts the lives of millions of people. In cataractous lenses we find evidence that the mechanisms discussed here are each impaired to some degree (see [1]). Thus, cataractous lenses may show incomplete denucleation, enlargement of extracellular space, disarrangement of cell packing, and so forth. Future studies in this area should provide a firm foundation for rational development of agents to delay or prevent cataract, a condition which is currently treatable only by surgery.
Acknowledgements
This work was supported by NEI Grants R01EY018 185 and EY09 852 (to S. B.), Core Grants for Vision Research P30EY02 687, and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness (RPB). S.B. is the recipient of an RPB Wasserman award.
Footnotes
One contribution of 10 to a Theme Issue ‘The ocular lens: a classic model for development, physiology and disease’.
References
- 1.Michael R., Bron A. J. 2011. The ageing lens and cataract: a model of normal and pathological ageing. Phil. Trans. R. Soc. B 366, 1278–1292 10.1098/rstb.2010.0300 (doi:10.1098/rstb.2010.0300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller L. J., Pels E., Schurmans L. R. H. M., Vrensen G. F. J. M. 2004. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp. Eye Res. 78, 493–501 10.1016/S0014-4835(03)00206-9 (doi:10.1016/S0014-4835(03)00206-9) [DOI] [PubMed] [Google Scholar]
- 3.Muller L. J., Pels L., Vrensen G. F. 1995. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest. Ophthalmol. Vis. Sci. 36, 2557–2567 [PubMed] [Google Scholar]
- 4.Kasthurirangan S., Markwell E. L., Atchison D. A., Pope J. M. 2008. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest. Ophthalmol. Vis. Sci. 49, 2531–2540 10.1167/iovs.07-1443 (doi:10.1167/iovs.07-1443) [DOI] [PubMed] [Google Scholar]
- 5.Duncan M. K., Cvekl A., Kantorow M., Piatigorsky J. 2004. Lens crystallins. In Development of the ocular lens (eds Lovicu F. J., Robinson M. L.), pp. 119–150 Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Delaye M., Tardieu A. 1983. Short-range order of crystallin proteins accounts for eye lens transparency. Nature 302, 415–417 10.1038/302415a0 (doi:10.1038/302415a0) [DOI] [PubMed] [Google Scholar]
- 7.Barishak Y. 2001. Embryology of the eye and its adnexa. Basel, Switzerland: Karger; [PubMed] [Google Scholar]
- 8.Dahm R., van Marle J., Quinlan R. A., Prescott A. R., Vrensen G. F. J. M. 2011. Homeostasis in the vertebrate lens: mechanisms of solute exchange. Phil. Trans. R. Soc. B 366, 1265–1277 10.1098/rstb.2010.0299 (doi:10.1098/rstb.2010.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y., Barton K., De Maria A., Petrash J. M., Shiels A., Bassnett S. 2009. The stratified syncytium of the vertebrate lens. J. Cell Sci. 122, 1607–1615 10.1242/jcs.045203 (doi:10.1242/jcs.045203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovicu F. J., McAvoy J. W. 2005. Growth factor regulation of lens development. Dev. Biol. 280, 1–14 10.1016/j.ydbio.2005.01.020 (doi:10.1016/j.ydbio.2005.01.020) [DOI] [PubMed] [Google Scholar]
- 11.Augusteyn R. C. 2008. Growth of the lens: in vitro observations. Clin. Exp. Optom. 91, 226–239 10.1111/j.1444-0938.2008.00255.x (doi:10.1111/j.1444-0938.2008.00255.x) [DOI] [PubMed] [Google Scholar]
- 12.Kuszak J. R., Costello M. 2004. The structure of the vertebrate lens. In Development of the ocular lens (eds Lovicu F. J., Robinson M. L.), pp. 71–118 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Shi Y., Bassnett S. 2007. Inducible gene expression in the lens using tamoxifen and a GFP reporter. Exp. Eye Res. 85, 732–737 10.1016/j.exer.2007.08.008 (doi:10.1016/j.exer.2007.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blankenship T., Bradshaw L., Shibata B., FitzGerald P. 2007. Structural specializations emerging late in mouse lens fiber cell differentiation. Invest. Ophthalmol. Vis. Sci. 48, 3269–3276 10.1167/iovs.07-0109 (doi:10.1167/iovs.07-0109) [DOI] [PubMed] [Google Scholar]
- 15.Vrensen G., Van Marle J., Van Veen H., Willekens B. 1992. Membrane architecture as a function of lens fibre maturation: a freeze fracture and scanning electron microscopic study in the human lens. Exp. Eye Res. 54, 433–446 10.1016/0014-4835(92)90055-W (doi:10.1016/0014-4835(92)90055-W) [DOI] [PubMed] [Google Scholar]
- 16.Michael R., Vrensen G., van Marle J., Lofgren S., Soderberg P. G. 2000. Repair in the rat lens after threshold ultraviolet radiation injury. Invest. Ophthalmol. Vis. Sci. 41, 204–212 [PubMed] [Google Scholar]
- 17.Vrensen G. F., Sanderson J., Willekens B., Duncan G. 1995. Calcium localization and ultrastructure of clear and pCMPS-treated rat lenses. Invest. Ophthalmol. Vis. Sci. 36, 2287–2295 [PubMed] [Google Scholar]
- 18.Bond J., Green C., Donaldson P., Kistler J. 1996. Liquefaction of cortical tissue in diabetic and galactosemic rat lenses defined by confocal laser scanning microscopy. Invest. Ophthalmol. Vis. Sci. 37, 1557–1565 [PubMed] [Google Scholar]
- 19.Dahm R. 2009. Nuclear degradation in the lens, circa 1897–1899. The Scientist 23, 84 [Google Scholar]
- 20.Rabl C. 1899. Uber den Bau und die Entwicklung der Linse. III. Die Linse der Saugetiere: Ruckblick und Schluss. Z. Wiss. Zool. 67, 1–138 [Google Scholar]
- 21.Bassnett S. 2009. On the mechanism of organelle degradation in the vertebrate lens. Exp. Eye Res. 88, 133–139 10.1016/j.exer.2008.08.017 (doi:10.1016/j.exer.2008.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassnett S., Beebe D. C. 1992. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Dev. Dyn. 194, 85–93 [DOI] [PubMed] [Google Scholar]
- 23.Vrensen G. F., Graw J., De Wolf A. 1991. Nuclear breakdown during terminal differentiation of primary lens fibres in mice: a transmission electron microscopic study. Exp. Eye Res. 52, 647–659 10.1016/0014-4835(91)90017-9 (doi:10.1016/0014-4835(91)90017-9) [DOI] [PubMed] [Google Scholar]
- 24.Faulkner-Jones B., Zandy A. J., Bassnett S. 2003. RNA stability in terminally differentiating fibre cells of the ocular lens. Exp. Eye Res. 77, 463–476 10.1016/S0014-4835(03)00172-6 (doi:10.1016/S0014-4835(03)00172-6) [DOI] [PubMed] [Google Scholar]
- 25.Vrensen G. F., van Marle J., Jonges R., Voorhout W., Breipohl W., Wegener A. R. 2004. Tryptophan deficiency arrests chromatin breakdown in secondary lens fibers of rats. Exp. Eye Res. 78, 661–672 10.1016/j.exer.2003.07.004 (doi:10.1016/j.exer.2003.07.004) [DOI] [PubMed] [Google Scholar]
- 26.McAvoy J. W., Palfrey L. J., van Heyningen R. 1979. A light microscope study of the lens of the tryptophan deficient rat. Exp. Eye Res. 28, 533–538 10.1016/0014-4835(79)90041-1 (doi:10.1016/0014-4835(79)90041-1) [DOI] [PubMed] [Google Scholar]
- 27.Zandy A. J., Bassnett S. 2007. Proteolytic mechanisms underlying mitochondrial degradation in the ocular lens. Invest. Ophthalmol. Vis. Sci. 48, 293–302 10.1167/iovs.06-0656 (doi:10.1167/iovs.06-0656) [DOI] [PubMed] [Google Scholar]
- 28.Bassnett S., Mataic D. 1997. Chromatin degradation in differentiating fiber cells of the eye lens. J. Cell Biol. 137, 37–49 10.1083/jcb.137.1.37 (doi:10.1083/jcb.137.1.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lattin J. E., et al. 2008. Expression analysis of G protein-coupled receptors in mouse macrophages. Immunome Res. 4, 5. 10.1186/1745-7580-4-5 (doi:10.1186/1745-7580-4-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimoto S., et al. 2003. Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature 424, 1071–1074 10.1038/nature01895 (doi:10.1038/nature01895) [DOI] [PubMed] [Google Scholar]
- 31.Ivanov D., Dvoriantchikova G., Pestova A., Nathanson L., Shestopalov V. 2005. Microarray analysis of fiber cell maturation in the lens. FEBS Lett. 579, 1213–1219 10.1016/j.febslet.2005.01.016 (doi:10.1016/j.febslet.2005.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakahara M., Nagasaka A., Koike M., Uchida K., Kawane K., Uchiyama Y., Nagata S. 2007. Degradation of nuclear DNA by DNase II-like acid DNase in cortical fiber cells of mouse eye lens. FEBS J. 274, 3055–3064 10.1111/j.1742-4658.2007.05836.x (doi:10.1111/j.1742-4658.2007.05836.x) [DOI] [PubMed] [Google Scholar]
- 33.Wride M. A. 2000. Minireview: apoptosis as seen through a lens. Apoptosis 5, 203–209 10.1023/A:1009653326511 (doi:10.1023/A:1009653326511) [DOI] [PubMed] [Google Scholar]
- 34.De Maria A., Shi Y., Kumar N. M., Bassnett S. 2009. Calpain expression and activity during lens fiber cell differentiation. J. Biol. Chem. 284, 13 542–13 550 10.1074/jbc.M900561200 (doi:10.1074/jbc.M900561200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrensen G. F., de Wolf A. 1996. Calcium distribution in the human eye lens. Ophthal. Res. 28(Suppl. 2), 78–85 10.1159/000267960 (doi:10.1159/000267960) [DOI] [PubMed] [Google Scholar]
- 36.Gao J., Sun X., Martinez-Wittinghan F. J., Gong X., White T. W., Mathias R. T. 2004. Connections between connexins, calcium, and cataracts in the lens. J. Gen. Physiol. 124, 289–300 10.1085/jgp.200409121 (doi:10.1085/jgp.200409121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo W. K. 1988. Adherens junctions in the ocular lens of various species: ultrastructural analysis with an improved fixation. Cell Tissue Res. 254, 31–40 10.1007/BF00220014 (doi:10.1007/BF00220014) [DOI] [PubMed] [Google Scholar]
- 38.Rafferty N. S., Goossens W. 1977. Ultrastructure of traumatic cataractogenesis in the frog: a comparison with mouse and human lens. Am. J. Anat. 148, 385–407 10.1002/aja.1001480307 (doi:10.1002/aja.1001480307) [DOI] [PubMed] [Google Scholar]
- 39.Lo W. K., Harding C. V. 1983. Tight junctions in the lens epithelia of human and frog: freeze-fracture and protein tracer studies. Invest. Ophthalmol. Vis. Sci. 24, 396–402 [PubMed] [Google Scholar]
- 40.Sugiyama Y., Prescott A. R., Tholozan F., Ohno S., Quinlan R. 2008. Expression and localisation of apical junctional complex proteins in lens epithelial cells. Exp. Eye Res. 87, 64–70 10.1016/j.exer.2008.03.017 (doi:10.1016/j.exer.2008.03.017) [DOI] [PubMed] [Google Scholar]
- 41.Bassnett S., Wilmarth P. A., David L. L. 2009. The membrane proteome of the mouse lens fiber cell. Mol. Vis. 15, 2448–2463 [PMC free article] [PubMed] [Google Scholar]
- 42.Straub B. K., Boda J., Kuhn C., Schnoelzer M., Korf U., Kempf T., Spring H., Hatzfeld M., Franke W. W. 2003. A novel cell–cell junction system: the cortex adhaerens mosaic of lens fiber cells. J. Cell Sci. 116, 4985–4995 10.1242/jcs.00815 (doi:10.1242/jcs.00815) [DOI] [PubMed] [Google Scholar]
- 43.Wang Z., Han J., Schey K. L. 2008. Spatial differences in an integral membrane proteome detected in laser capture microdissected samples. J. Proteome Res. 7, 2696–2702 10.1021/pr700737h (doi:10.1021/pr700737h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.More M. I., Kirsch F. P., Rathjen F. G. 2001. Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation. J. Cell Biol. 154, 187–196 10.1083/jcb.200104038 (doi:10.1083/jcb.200104038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe M., Kobayashi H., Rutishauser U., Katar M., Alcala J., Maisel H. 1989. NCAM in the differentiation of embryonic lens tissue. Dev. Biol. 135, 414–423 10.1016/0012-1606(89)90190-5 (doi:10.1016/0012-1606(89)90190-5) [DOI] [PubMed] [Google Scholar]
- 46.Sakurai-Yageta M., Masuda M., Tsuboi Y., Ito A., Murakami Y. 2009. Tumor suppressor CADM1 is involved in epithelial cell structure. Biochem. Biophys. Res. Commun. 390, 977–982 10.1016/j.bbrc.2009.10.088 (doi:10.1016/j.bbrc.2009.10.088) [DOI] [PubMed] [Google Scholar]
- 47.Xu L., Overbeek P. A., Reneker L. W. 2002. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp. Eye Res. 74, 753–760 10.1006/exer.2002.1175 (doi:10.1006/exer.2002.1175) [DOI] [PubMed] [Google Scholar]
- 48.Lo W. K., Shaw A. P., Paulsen D. F., Mills A. 2000. Spatiotemporal distribution of zonulae adherens and associated actin bundles in both epithelium and fiber cells during chicken lens development. Exp. Eye Res. 71, 45–55 10.1006/exer.2000.0848 (doi:10.1006/exer.2000.0848) [DOI] [PubMed] [Google Scholar]
- 49.Atreya P. L., Barnes J., Katar M., Alcala J., Maisel H. 1989. N-cadherin of the human lens. Curr. Eye Res. 8, 947–956 [PubMed] [Google Scholar]
- 50.Morris A. P., Tawil A., Berkova Z., Wible L., Smith C. W., Cunningham S. A. 2006. Junctional adhesion molecules (JAMs) are differentially expressed in fibroblasts and co-localize with ZO-1 to adherens-like junctions. Cell Commun. Adhes. 13, 233–247 10.1080/15419060600877978 (doi:10.1080/15419060600877978) [DOI] [PubMed] [Google Scholar]
- 51.Walters R. W., Freimuth P., Moninger T. O., Ganske I., Zabner J., Welsh M. J. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110, 789–799 10.1016/S0092-8674(02)00912-1 (doi:10.1016/S0092-8674(02)00912-1) [DOI] [PubMed] [Google Scholar]
- 52.Borrmann C. M., Mertens C., Schmidt A., Langbein L., Kuhn C., Franke W. W. 2000. Molecular diversity of plaques of epithelial-adhering junctions. Ann. NY Acad. Sci. 915, 144–150 10.1111/j.1749-6632.2000.tb05237.x (doi:10.1111/j.1749-6632.2000.tb05237.x) [DOI] [PubMed] [Google Scholar]
- 53.Hatzfeld M. 2005. The p120 family of cell adhesion molecules. Eur. J. Cell. Biol. 84, 205–214 10.1016/j.ejcb.2004.12.016 (doi:10.1016/j.ejcb.2004.12.016) [DOI] [PubMed] [Google Scholar]
- 54.Fanning A. S., Anderson J. M. 2009. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. NY. Acad. Sci. 1165, 113–120 10.1111/j.1749-6632.2009.04440.x (doi:10.1111/j.1749-6632.2009.04440.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen P. A., Baruch A., Shestopalov V. I., Giepmans B. N. G., Dunia I., Lucio Benedetti E., Kumar N. M. 2003. Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1). Mol. Biol. Cell 14, 2470–2481 10.1091/mbc.E02-10-0637 (doi:10.1091/mbc.E02-10-0637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuszak J., Maisel H., Harding C. V. 1978. Gap junctions of chick lens fiber cells. Exp. Eye Res. 27, 495–498 10.1016/0014-4835(78)90026-X (doi:10.1016/0014-4835(78)90026-X) [DOI] [PubMed] [Google Scholar]
- 57.Sonntag S., Sohl G., Dobrowolski R., Zhang J., Theis M., Winterhager E., Bukauskas F., Willecke K. 2009. Mouse lens connexin23 (Gje1) does not form functional gap junction channels but causes enhanced ATP release from HeLa cells. Eur. J. Cell Biol. 88, 65–77 10.1016/j.ejcb.2008.08.004 (doi:10.1016/j.ejcb.2008.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cotrina M. L., Lin J. H., Nedergaard M. 2008. Adhesive properties of connexin hemichannels. Glia 56, 1791–1798 10.1002/glia.20728 (doi:10.1002/glia.20728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin J. H., et al. 2002. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J. Neurosci. 22, 4302–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumari S. S., Varadaraj K. 2009. Intact AQP0 performs cell-to-cell adhesion. Biochem. Biophys. Res. Commun. 390, 1034–1039 10.1016/j.bbrc.2009.10.103 (doi:10.1016/j.bbrc.2009.10.103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Ghoul K. J., Kirk T., Kuszak A. J., Zoltoski R. K., Shiels A., Kuszak J. R. 2003. Lens structure in MIP-deficient mice. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 273, 714–730 10.1002/ar.a.10080 (doi:10.1002/ar.a.10080) [DOI] [PubMed] [Google Scholar]
- 62.Shiels A., King J. M., Mackay D. S., Bassnett S. 2007. Refractive defects and cataracts in mice lacking lens intrinsic membrane protein-2. Invest. Ophthalmol. Vis. Sci. 48, 500–508 10.1167/iovs.06-0947 (doi:10.1167/iovs.06-0947) [DOI] [PubMed] [Google Scholar]
- 63.Costello M. J., McIntosh T. J., Robertson J. D. 1989. Distribution of gap junctions and square array junctions in the mammalian lens. Invest. Ophthalmol. Vis. Sci. 30, 975–989 [PubMed] [Google Scholar]
- 64.Lo W. K., Harding C. V. 1984. Square arrays and their role in ridge formation in human lens fibers. J. Ultrastruct. Res. 86, 228–245 10.1016/S0022-5320(84)90103-5 (doi:10.1016/S0022-5320(84)90103-5) [DOI] [PubMed] [Google Scholar]
- 65.Buzhynskyy N., Girmens J. F., Faigle W., Scheuring S. 2007. Human cataract lens membrane at subnanometer resolution. J. Mol. Biol. 374, 162–169 10.1016/j.jmb.2007.09.022 (doi:10.1016/j.jmb.2007.09.022) [DOI] [PubMed] [Google Scholar]
- 66.Buzhynskyy N., Hite R. K., Walz T., Scheuring S. 2007. The supramolecular architecture of junctional microdomains in native lens membranes. EMBO Rep. 8, 51–55 10.1038/sj.embor.7400858 (doi:10.1038/sj.embor.7400858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mangenot S., Buzhynskyy N., Girmens J.-F., Scheuring S. 2009. Malformation of junctional microdomains in cataract lens membranes from a type II diabetes patient. Pflugers Arch. 457, 1265–1274 10.1007/s00424-008-0604-4 (doi:10.1007/s00424-008-0604-4) [DOI] [PubMed] [Google Scholar]
- 68.Benedetti E. L., Dunia I., Recouvreur M., Nicholas P., Kumar N. M., Bloemendal H. 2000. Structural organization of gap junctions as revealed by freeze-fracture and SDS fracture-labeling. Eur. J. Cell Biol. 79, 575–582 10.1078/0171-9335-00081 (doi:10.1078/0171-9335-00081) [DOI] [PubMed] [Google Scholar]
- 69.Gonen T., Sliz P., Kistler J., Cheng Y., Walz T. 2004. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 429, 193–197 10.1038/nature02503 (doi:10.1038/nature02503) [DOI] [PubMed] [Google Scholar]
- 70.Tenbroek E., Arneson M., Jarvis L., Louis C. 1992. The distribution of the fiber cell intrinsic membrane proteins MP20 and connexin46 in the bovine lens. J. Cell Sci. 103, 245–257 [DOI] [PubMed] [Google Scholar]
- 71.Voorter C. E., Kistler J., Gruijters W. T. M., Mulders J. W. M., Christie D., de Jong W. W. 1989. Distribution of MP17 in isolated lens fibre membranes. Curr. Eye Res. 8, 697–706 10.3109/02713688909025804 (doi:10.3109/02713688909025804) [DOI] [PubMed] [Google Scholar]
- 72.Gonen T., Grey A. C., Jacobs M. D., Donaldson P. J., Kistler J. 2001. MP20, the second most abundant lens membrane protein and member of the tetraspanin superfamily, joins the list of ligands of galectin-3. BMC Cell Biol. 2, 17. 10.1186/1471-2121-2-17 (doi:10.1186/1471-2121-2-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaltner H., Stierstorfer B. 1998. Animal lectins as cell adhesion molecules. Acta Anat. (Basel) 161, 162–179 10.1159/000046456 (doi:10.1159/000046456) [DOI] [PubMed] [Google Scholar]
- 74.Michael R., van Marle J., Vrensen G. F. J. M., van den Berg T. J. T. P. 2003. Changes in the refractive index of lens fibre membranes during maturation–impact on lens transparency. Exp. Eye Res. 77, 93–99 10.1016/S0014-4835(03)00065-4 (doi:10.1016/S0014-4835(03)00065-4) [DOI] [PubMed] [Google Scholar]
- 75.Borchman D., Byrdwell W. C., Yappert M. C. 1994. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Invest. Ophthalmol. Vis. Sci. 35, 3938–3942 [PubMed] [Google Scholar]
- 76.Duindam J. J., Vrensen G. F., Otto C., Greve J. 1996. Aging affects the conformation of cholesterol in the human eye lens. Ophthal. Res. 28, 86–91 10.1159/000267978 (doi:10.1159/000267978) [DOI] [PubMed] [Google Scholar]
- 77.Li L. K., So L., Spector A. 1985. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J. Lipid Res. 26, 600–609 [PubMed] [Google Scholar]
- 78.van den Berg T. J. 1996. Depth-dependent forward light scattering by donor lenses. Invest. Ophthalmol. Vis. Sci. 37, 1157–1166 [PubMed] [Google Scholar]
- 79.van den Berg T. J. 1997. Light scattering by donor lenses as a function of depth and wavelength. Invest. Ophthalmol. Vis. Sci. 38, 1321–1332 [PubMed] [Google Scholar]
- 80.Duncan G. 1969. The site of the ion restricting membranes in the toad lens. Exp. Eye Res. 8, 406–412 10.1016/S0014-4835(69)80006-0 (doi:10.1016/S0014-4835(69)80006-0) [DOI] [PubMed] [Google Scholar]
- 81.Mathias R. T., Rae J. L., Eisenberg R. S. 1981. The lens as a nonuniform spherical syncytium. Biophys. J. 34, 61–83 10.1016/S0006-3495(81)84837-0 (doi:10.1016/S0006-3495(81)84837-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuszak J. R., Macsai M. S., Bloom K., Rae J., Weinstein R. 1985. Cell-to-cell fusion of lens fiber cells in situ: correlative light, scanning electron microscopic, and freeze-fracture studies. J Ultrastruct. Res. 93, 144–160 10.1016/0889-1605(85)90094-1 (doi:10.1016/0889-1605(85)90094-1) [DOI] [PubMed] [Google Scholar]
- 83.Mathias R. T., Rae J. L. 1989. Cell to cell communication in the lens. In Cell interactions and gap junctions. (eds Sperelakis N., Cole W. C.), p. 1 Boca Raton, FL: CRC Press [Google Scholar]
- 84.Kuszak J. R., Ennesser C. A., Bertram B. A., Imherr-McMannis S., Jones-Rufer L. S., Weinstein R. S. 1989. The contribution of cell-to-cell fusion to the ordered structure of the crystalline lens. Lens Eye Toxic Res. 6, 639–673 [PubMed] [Google Scholar]
- 85.Shestopalov V. I., Bassnett S. 2000. Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. J. Cell Sci. 113, 1913–1921 [DOI] [PubMed] [Google Scholar]
- 86.Shestopalov V. I., Bassnett S. 2003. Development of a macromolecular diffusion pathway in the lens. J. Cell Sci. 116, 4191–4199 10.1242/jcs.00738 (doi:10.1242/jcs.00738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Podbilewicz B. 2006. Cell fusion. WormBook, 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng C., Xia C. H., Li L., White T., Niimi J., Gong X. 2008. Gap junction communication influences intercellular protein distribution in the lens. Exp. Eye Res. 86, 966–974 10.1016/j.exer.2008.03.015 (doi:10.1016/j.exer.2008.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]