Abstract
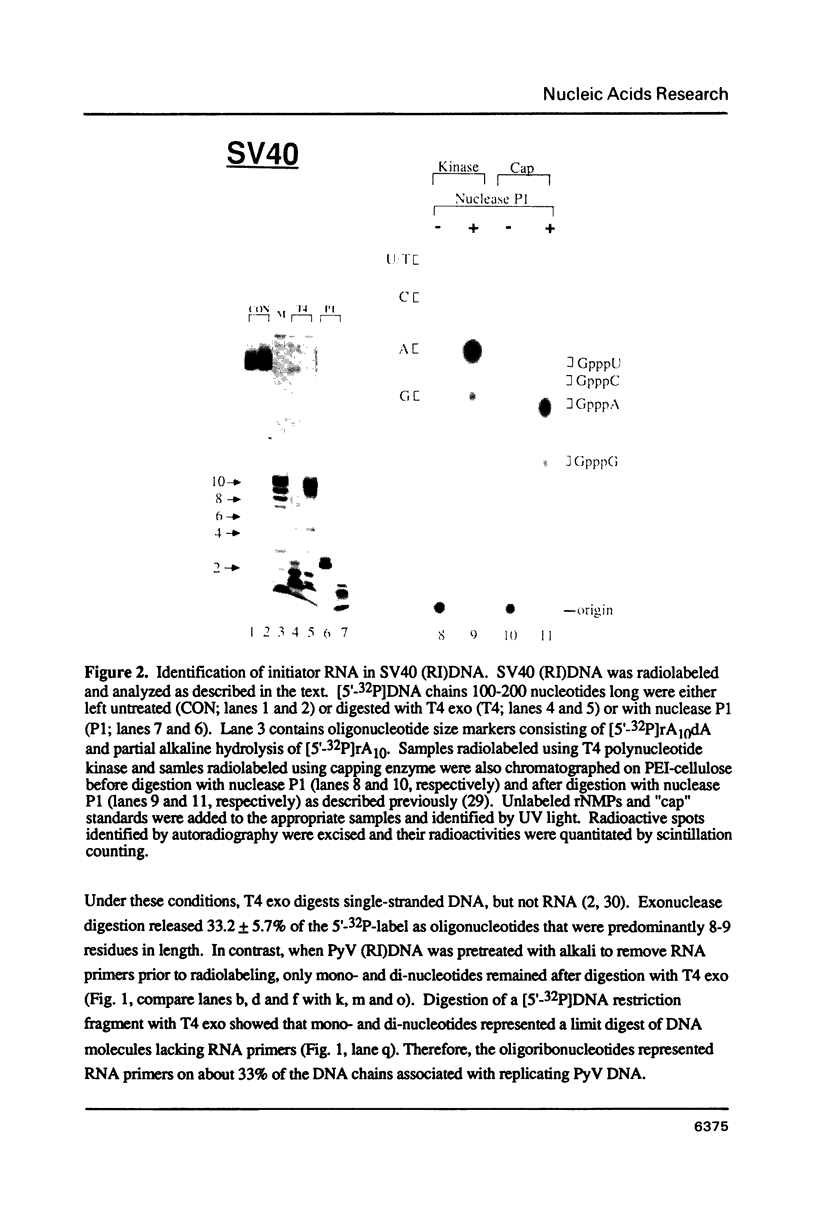
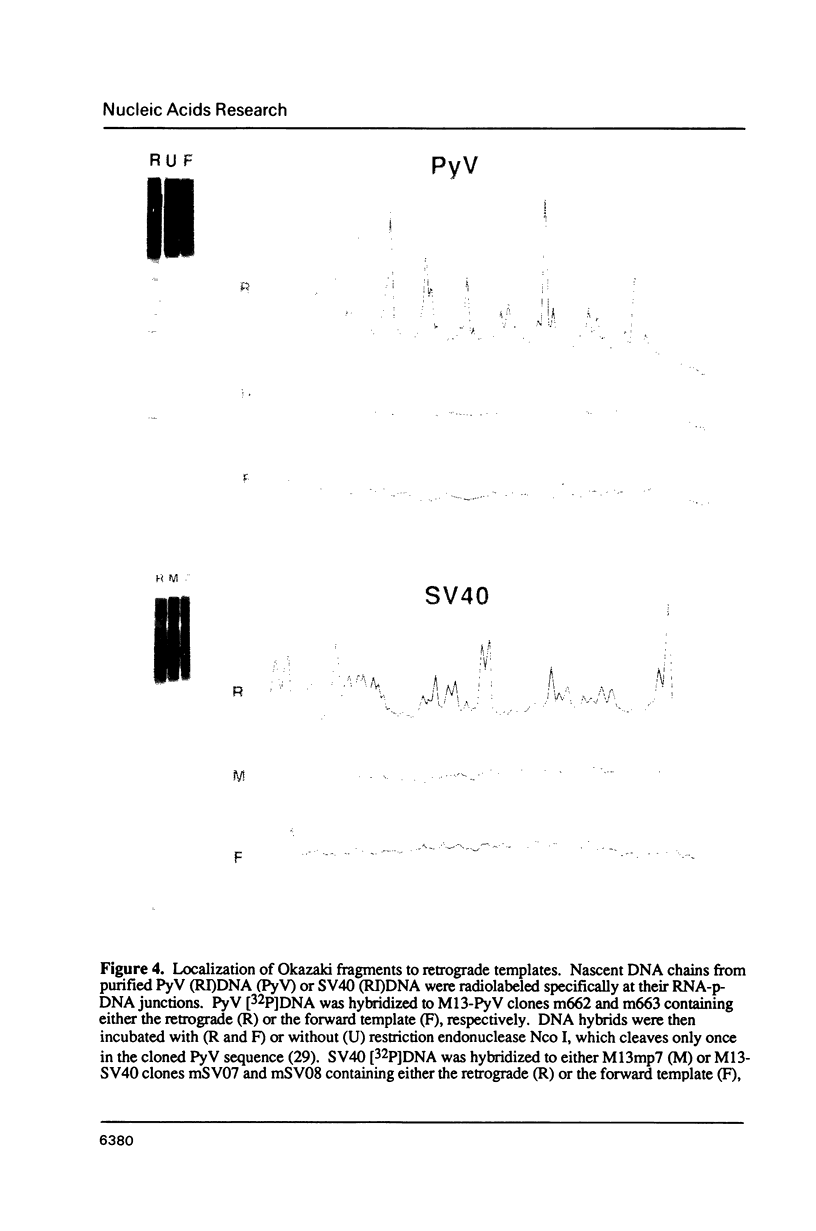
In marked contrast to simian virus 40 (SV40), polyoma virus (PyV) has been reported to replicate discontinuously on both arms of replication forks. In an effort to clarify the relationship between the mechanisms of DNA replication in these closely related viruses, the distribution of RNA-primed DNA chains at replication forks was examined concurrently in PyV and SV40 replicating DNA purified from virus-infected cells. About one third of PyV DNA chains contained 7 to 9 ribonucleotides covalently linked to their 5'-end. A similar fraction of DNA chains from replicating SV40 DNA contained an oligoribonucleotide that was 6 to 9 residues long and began with either (p)ppA or (p)ppG. Greater than 80% of PyV or SV40 RNA-primed DNA chains hybridized specifically to the retrograde template. Moreover, at least 95% of the RNA-primed DNA chains from either PyV or SV40 whose initiation sites could be mapped to unique nucleotide locations originated from the retrograde template. Therefore, PyV and SV40 DNA replication forks are essentially the same; DNA synthesis is discontinuous predominantly, if not exclusively, on the retrograde template.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kornberg A. Unique primed start of phage phi X174 DNA replication and mobility of the primosome in a direction opposite chain synthesis. Proc Natl Acad Sci U S A. 1981 Jan;78(1):69–73. doi: 10.1073/pnas.78.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfalvi G., Sarkar N. Origin and degradation of the RNA primers at the 5' termini of nascent DNA chains in Bacillus subtilis. J Mol Biol. 1985 Nov 20;186(2):275–282. doi: 10.1016/0022-2836(85)90104-4. [DOI] [PubMed] [Google Scholar]
- Buckler-White A. J., Pigiet V. Isolation and characterization of replication forks from discrete regions of the polyoma genome. J Virol. 1982 Nov;44(2):499–508. doi: 10.1128/jvi.44.2.499-508.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V. The adsorption of polyoma virus. Virology. 1962 Oct;18:177–181. doi: 10.1016/0042-6822(62)90003-x. [DOI] [PubMed] [Google Scholar]
- Cusick M. E., Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: prenucleosomal deoxyribonucleic acid is rapidly excised from replicating simian virus 40 chromosomes by micrococcal nuclease. Biochemistry. 1981 Nov 10;20(23):6648–6658. doi: 10.1021/bi00526a020. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Beard P., Berg P. Synthesis of Superhelical Simian Virus 40 Deoxyribonucleic Acid in Cell Lysates*. J Biol Chem. 1975 Jun 10;250(11):4340–4347. [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Deninger P. L., Esty A., LaPorte P., Hsu H., Friedmann T. The nucleotide sequence and restriction enzyme sites of the polyoma genome. Nucleic Acids Res. 1980 Feb 25;8(4):855–860. [PMC free article] [PubMed] [Google Scholar]
- Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. Synthesis and distribution of initiator RNA. J Biol Chem. 1978 Oct 25;253(20):7469–7475. [PubMed] [Google Scholar]
- Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. VII. Initiator RNA synthesis during nucleotide depletion. J Mol Biol. 1979 Apr 15;129(3):393–409. doi: 10.1016/0022-2836(79)90503-5. [DOI] [PubMed] [Google Scholar]
- Flory P. J., Jr Strandedness of newly synthesized short pieces of polyoma DNA from isolated nuclei. Nucleic Acids Res. 1977;4(5):1449–1464. doi: 10.1093/nar/4.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Hunter T. In vitro polyoma DNA synthesis: discontinuous chain growth. J Mol Biol. 1974 Feb 15;83(1):99–121. doi: 10.1016/0022-2836(74)90426-4. [DOI] [PubMed] [Google Scholar]
- Francke B., Vogt M. In vitro polyoma DNA synthesis: self-annealing properties of short DNA chains. Cell. 1975 Jun;5(2):205–211. doi: 10.1016/0092-8674(75)90028-8. [DOI] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Hendrickson E. A., DePamphilis M. L. Sequence specificity for the initiation of RNA-primed simian virus 40 DNA synthesis in vivo. J Mol Biol. 1984 May 15;175(2):131–157. doi: 10.1016/0022-2836(84)90471-6. [DOI] [PubMed] [Google Scholar]
- Hunter T., Francke B., Bacheler L. In vitro polyoma DNA synthesis: asymmetry of short DNA chains. Cell. 1977 Dec;12(4):1021–1028. doi: 10.1016/0092-8674(77)90166-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Bar-Shavit R., DePamphilis M. L. Okazaki pieces grow opposite to the replication fork direction during simian virus 40 DNA replication. Nucleic Acids Res. 1978 Jul;5(7):2535–2545. doi: 10.1093/nar/5.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G. Characterization of initiator RNA from replicating simian virus 40 DNA synthesized in isolated nuclei. J Mol Biol. 1981 Mar 25;147(1):25–39. doi: 10.1016/0022-2836(81)90077-2. [DOI] [PubMed] [Google Scholar]
- Kitani T., Yoda K., Ogawa T., Okazaki T. Evidence that discontinuous DNA replication in Escherichia coli is primed by approximately 10 to 12 residues of RNA starting with a purine. J Mol Biol. 1985 Jul 5;184(1):45–52. doi: 10.1016/0022-2836(85)90042-7. [DOI] [PubMed] [Google Scholar]
- Kitani T., Yoda K., Okazaki T. Discontinuous DNA replication of Drosophila melanogaster is primed by octaribonucleotide primer. Mol Cell Biol. 1984 Aug;4(8):1591–1596. doi: 10.1128/mcb.4.8.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P. The initiation of SV40 DNA synthesis is not unique to the replication origin. Cell. 1980 Jun;20(2):381–391. doi: 10.1016/0092-8674(80)90624-8. [DOI] [PubMed] [Google Scholar]
- Moss B. 5' end labeling of RNA with capping and methylating enzymes. Gene Amplif Anal. 1981;2:253–266. [PubMed] [Google Scholar]
- Närkhammar-Meuth M., Eliasson R., Magnusson G. Discontinuous synthesis of both strands at the growing fork during polyoma DNA replication in vitro. J Virol. 1981 Jul;39(1):11–20. doi: 10.1128/jvi.39.1.11-20.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Närkhammar-Meuth M., Kowalski J., Denhardt D. T. Both strands of polyoma DNA are replicated discontinuously with ribonucleotide primers in vivo. J Virol. 1981 Jul;39(1):21–30. doi: 10.1128/jvi.39.1.21-30.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Hirose S., Okazaki T., Ogawa T., Kurosawa Y. Assay of RNA-linked nascent DNA pieces with polynucleotide kinase. Biochem Biophys Res Commun. 1975 Feb 17;62(4):1018–1024. doi: 10.1016/0006-291x(75)90424-6. [DOI] [PubMed] [Google Scholar]
- Perlman D., Huberman J. A. Asymmetric Okazaki piece synthesis during replication of simian virus 40 DNA in vivo. Cell. 1977 Dec;12(4):1029–1043. doi: 10.1016/0092-8674(77)90167-2. [DOI] [PubMed] [Google Scholar]
- Pigiet V., Winnacker E. L., Eliasson R., Reichard P. Discontinuous elongation of both strands at the replication forks in polyoma DNA replication. Nat New Biol. 1973 Oct 17;245(146):203–205. doi: 10.1038/newbio245203a0. [DOI] [PubMed] [Google Scholar]
- Reichard P., Eliasson R., Söderman G. Initiator RNA in discontinuous polyoma DNA synthesis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4901–4905. doi: 10.1073/pnas.71.12.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell V. M., Folk W. R. Comparison of the DNA sequence of the Crawford small-plaque variant of polyomavirus with those of polyomaviruses A2 and strain 3. J Virol. 1983 Nov;48(2):472–480. doi: 10.1128/jvi.48.2.472-480.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Proctor G. N. Two major replicating simian virus 40 chromosome classes. Synchronous replication fork movement is associated with bound large T antigen during elongation. J Biol Chem. 1987 May 5;262(13):6339–6349. [PubMed] [Google Scholar]
- Triezenberg S. J., Folk W. R. Essential nucleotides in the polyomavirus origin region. J Virol. 1984 Aug;51(2):437–444. doi: 10.1128/jvi.51.2.437-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Erickson J. M., Goulian M. Initiator RNA of nascent DNA from animal cells. J Mol Biol. 1979 Apr 25;129(4):531–545. doi: 10.1016/0022-2836(79)90467-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Hendrickson E. A., DePamphilis M. L. DNA primase-DNA polymerase alpha from simian cells. Modulation of RNA primer synthesis by ribonucleoside triphosphates. J Biol Chem. 1985 May 25;260(10):6254–6263. [PubMed] [Google Scholar]
- Yamaguchi M., Hendrickson E. A., DePamphilis M. L. DNA primase-DNA polymerase alpha from simian cells: sequence specificity of initiation sites on simian virus 40 DNA. Mol Cell Biol. 1985 May;5(5):1170–1183. doi: 10.1128/mcb.5.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]