Abstract
Growth factors play key roles in influencing cell fate and behaviour during development. The epithelial cells and fibre cells that arise from the lens vesicle during lens morphogenesis are bathed by aqueous and vitreous, respectively. Vitreous has been shown to generate a high level of fibroblast growth factor (FGF) signalling that is required for secondary lens fibre differentiation. However, studies also show that FGF signalling is not sufficient and roles have been identified for transforming growth factor-β and Wnt/Frizzled families in regulating aspects of fibre differentiation. In the case of the epithelium, key roles for Wnt/β-catenin and Notch signalling have been demonstrated in embryonic development, but it is not known if other factors are required for its formation and maintenance. This review provides an overview of current knowledge about growth factor regulation of differentiation and maintenance of lens cells. It also highlights areas that warrant future study.
Keywords: growth factors, lens development, MAPK, Akt/PI3K, cell signalling, differentiation
1. Introduction
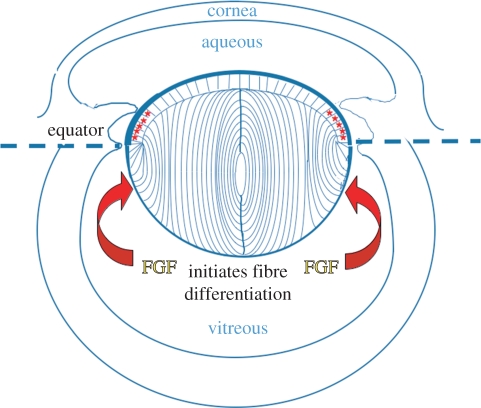
The lens of the eye is an ectodermally derived tissue predominantly made up of a spheroidal mass of regularly packed, elongated lens fibre cells, capped anteriorly by an epithelial monolayer (figure 1). During embryogenesis, the lens arises from the ectoderm situated next to outpocketings of the neural tube, the optic vesicles. Local thickening of ectoderm forms a lens placode that invaginates to form the lens vesicle. Subsequently, posterior lens vesicle cells elongate to form the primary fibres, whereas anterior vesicle cells give rise to a sheet of cuboidal epithelial cells. The divergent fates of these embryonic cells give the lens its distinctive polarity. Once formed, the lens grows rapidly by continued cell proliferation and differentiation. Proliferation becomes restricted to the epithelium, with greatest activity in a region just above the lens equator known as the germinative zone [1]. Progeny of divisions migrate below the equator where they elongate and differentiate into secondary fibre cells. In this way, the lens grows throughout life and maintains its polarity. How the two forms of lens cells differentiate in the two compartments of the lens to give it its distinctive polarity has been a major focus in lens developmental biology.
Figure 1.
Diagram of the rodent eye showing the structure of the fully formed lens. Epithelial cells and fibre cells are contained within a capsule of extracellular matrix (thick blue line). The germinative zone (red asterisks) is situated just above the equator. Cells that shift below the equator are exposed to the relatively high level of fibroblast growth factor (FGF) in vitreous and initiate a signalling cascade that promotes fibre differentiation.
The importance of the ocular environment in determining lens polarity was demonstrated in classical lens inversion experiments [2], where the chicken lens was inverted in the optic cup so that the epithelium, instead of facing the aqueous and cornea, faced the vitreous and neural retina. In this new environment, the epithelial cells elongated and differentiated into a new fibre mass, whereas a new epithelial sheet grew over the original fibre mass facing the cornea. Similar lens inversion experiments with mice confirmed that this phenomenon also occurred in mammals [3]. In short, these experiments showed that the vitreous environment promotes fibre cell differentiation, whereas the aqueous environment promotes epithelial maintenance and growth.
The ocular media are a rich source of growth and regulatory factors. The lens itself expresses members of major growth factor families and a variety of growth factor receptors and molecules involved in a range of signalling pathways. Studies over the last 20 years have mostly concentrated on identifying factor(s) that control fibre differentiation, whereas epithelial cells have been studied mostly in relation to their proliferative function. However, in recent years, largely owing to renewed appreciation for the role of the epithelium in maintaining normal lens structure and function, this cell layer is currently being investigated with renewed vigour with particular attention to identifying factors that influence the epithelial phenotype. In this review, we will deal with the latest findings on growth factor regulation of developmental processes in both epithelial and fibre cell compartments.
2. Lens fibre differentiation
In the quest to identify the ‘lens fibre differentiation factor’, a major advance was the development of lens epithelial explants as an in vitro experimental system [4,5]. This simple in vitro system was instrumental in linking fibroblast growth factors (FGFs) with secondary lens fibre differentiation [6–8]. Essentially, studies showed that FGF1 or FGF2 promoted morphological and molecular changes in lens epithelial explants that are characteristic of secondary fibre differentiation in vivo [7,9]. One of the most significant findings using this explant system was that FGF induced different responses in lens epithelial cells with increased dosage; a low concentration of FGF induced cell proliferation, whereas sequentially higher doses were required to induce cell migration and fibre cell differentiation [8]. This finding, together with the fact that more FGF is recoverable from vitreous than aqueous [10], led to the proposal [4,11] that the distinct polarity of the lens in situ may be determined by an FGF gradient in the eye (figure 1). This also fits well with the fact that the antero-posterior patterns of lens cell behaviour correlate with the distribution of the ocular media, and that vitreous humour (which bathes lens fibre cells in vivo), but not aqueous humour (bathes the lens epithelium), can induce fibre differentiation in rat lens explants [12]. Fractionation of vitreous showed that most of its fibre-differentiating activity was associated with FGF [10].
The ‘FGF Gradient Hypothesis’ is well supported by genetic manipulations in various mouse models. Disturbing normal patterns of FGF distribution by lens-specific over-expression of FGFs led to ectopic fibre differentiation in the epithelial compartment [13,14]. The mammalian lens expresses at least three of the four FGF receptor (FGFR) genes [15,16]. A series of experiments that deleted combinations of these FGFRs showed that there is considerable redundancy among FGFRs but that loss of all receptors resulted in failure of fibre differentiation such that the embryonic lens remains at the vesicle stage [4,17,18]. These studies provide compelling evidence that FGF signalling is necessary for fibre differentiation.
(a). Fibroblast growth factor signalling
While it is clear that FGF receptor signalling is essential for normal lens development and the differentiation of lens fibre cells, the exact nature of this signalling is only now being elucidated. In an attempt to better understand the mechanism(s) underlying the differential responsiveness of lens epithelial cells to different doses of FGF, the signalling pathways mediating FGF-induced lens cell proliferation and fibre differentiation were examined and characterized. Some of the main downstream targets regulating lens cellular processes were the extracellular-regulated kinases 1 and 2 (ERK1/2), members of the mitogen-activated protein kinase (MAPK) family [19,20]. It has been shown by in vitro studies using chick and rat that FGF can induce a robust phosphorylation of ERK1/2 [21,22], and that both FGF-induced lens cell proliferation and fibre differentiation are dependent on ERK1/2 activation. In the presence of UO126 (a selective inhibitor of ERK1/2 phosphorylation), FGF-induced lens cell proliferation in rat lens epithelial explants was blocked [21]. UO126 also effectively blocked FGF-induced lens cell elongation and the accompanying expression of the fibre-specific intermediate filament, filensin [21]. However, surprisingly, blocking ERK1/2 phosphorylation had little to no effect on the accumulation of other fibre-differentiation markers, such as β- and γ-crystallins [21,23]. This uncoupling of the fibre-differentiation process indicated that other signalling pathways, as well as MAPK/ERK1/2, contribute to this cellular response. Support for this in vivo comes from mice that lack Shp2 in the lens [24]. Loss of Shp2 results in the blocking of ERK, but not Akt activation, and in these mutant lenses there is failure of equatorial epithelial cells to undergo fibre differentiation. Instead, they continue to migrate along the posterior capsule as epithelial-like cells. Moreover, the primary fibre cells of mutant lenses still expressed α-, β- and γ-crystallins [24].
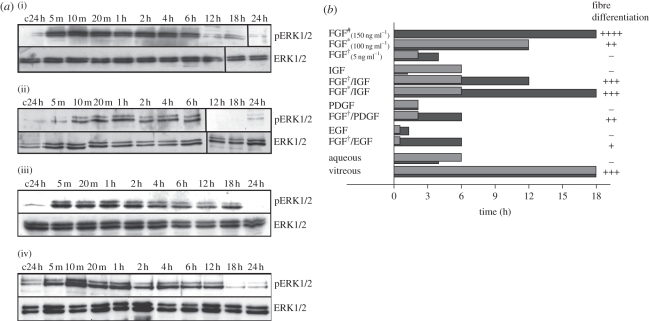
Although a ‘differentiating dose’ of FGF had a more profound effect on the level of phosphorylation of ERK1/2, relative to that induced by a lower, ‘proliferating dose’ of FGF, it was the difference in the duration of ERK1/2 phosphorylation that related to the ability of FGF to induce a differentiation response in rat lens epithelial cells [25]. It was shown that a high dose of FGF, which leads to lens fibre differentiation, prolonged the phosphorylated state of ERK1/2, up to three to four times that induced by a lower dose of FGF (that can only induce epithelial cell proliferation). Consistent with this notion that duration of ERK activity regulates cell behaviour, aqueous humour, which induces lens epithelial cell proliferation but not fibre differentiation, stimulated ERK1/2 phosphorylation for 4–6 h, whereas vitreous humour induced an extended duration of ERK1/2 phosphorylation (up to 18 h) leading to lens fibre differentiation (figure 2). If the duration of vitreous-induced ERK1/2 activation was prematurely blocked at 6 h (using a selective inhibitor for FGFR signalling), the vitreous lost its ability to induce lens fibre differentiation but retained the ability to induce lens cell proliferation [25]. More recent studies in this same in vitro model have shown that while a prolonged ERK1/2 phosphorylation was associated with and necessary for fibre cell differentiation, it was not sufficient for this process to proceed normally [27]. Thus, while these studies underscore the requirement of FGF–MAPK signalling in the regulation of lens cell behaviours by ocular media, they also highlight the importance of other growth factor signalling pathways in refining vitreous-induced lens fibre differentiation.
Figure 2.
Growth factor-induced signalling profiles of ERK1/2 and pAkt phosphorylation in lens epithelial cells. (a). Representative western blots of rat lens epithelial explants treated with either: (i) 50% bovine aqueous; (ii) 5 ng ml−1 FGF-2; (iii) 50% bovine vitreous; or (iv) 100 ng ml−1 FGF-2, and assayed for phosphorylated ERK1/2 (upper panels) or total ERK1/2 (lower panels) over a 24 h period. The first lanes indicate control explants assayed at 24 h (c24 h). (b) Summary of the duration of ERK1/2 (dark grey bars) and Akt (light grey bars) phosphorylation over a 24 h period, in cultured explants of rat lens epithelial cells exposed to different treatments. Each treatment is scored for its ability to induce secondary lens fibre differentiation in vitro: –, no response; +, weak response with little multi-layering and some β-crystallin accumulation; ++, intermediate response with notable multi-layering, some cell elongation and β-crystallin accumulation; +++, strong response with extensive multi-layering, cell elongation and abundant β-crystallin accumulation (hash denotes the study of [26]; asterisk denotes 100 ng ml−1; dagger denotes 5 ng ml−1). Adapted from Wang et al. [27].
(b). Other growth factors involved in fibre differentiation
Indications that other growth factors besides FGF are required to elicit an unabridged fibre-differentiation response have come from two main lines of investigation. Firstly, explant studies comparing the effects of FGF with vitreous led to the conclusion that vitreous provides additional signals that enhance FGF-induced differentiation. Secondly, studies directed at interfering with signalling by the various other growth factor families that are expressed in the eye have identified pathways that also appear to be critical for regulating specific events in the complex process of lens fibre differentiation.
(c). Vitreous studies
Our early studies investigating the effects of ocular media on rat lens epithelial explants showed that vitreous humour can induce all the morphological and molecular changes associated with secondary fibre differentiation in vivo, including the ability to stimulate lens cells to synthesize and deposit extracellular matrix (ECM) that contributes to the remodelling and growth of the lens capsule [12]. However, such ECM accumulation has not been observed in FGF-treated lens explants. Further studies comparing the effects of vitreous and FGF show that while both FGF and vitreous initiate and stimulate many similar differentiation events, there was greater fibre cell organization and integrity, and maintenance of transparency in vitreous humour-treated cultures [28]. Thus, while FGF is essential for initiation of fibre cell differentiation, it is clearly not sufficient to reproduce all the effects induced by vitreous humour. This is perhaps not surprising given that the vitreous is likely to contain numerous other growth factors.
Vitreal factors such as insulin-like growth factors (IGF), platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) have been shown to potentiate the effects of FGF on rat lens epithelial explants, leading to a fibre-differentiation response [27,29–31]. This is consistent with studies on chick lens cells that showed roles for insulin/IGF [32], EGF/transforming growth factor (TGF)-α [33,34] and also bone morphogenetic proteins (BMPs) [35] in promoting the fibre-differentiation process. Although a low dose of IGF, PDGF and EGF alone cannot induce fibre cell differentiation, when combined with a low dose of FGF, a fibre-differentiation response is elicited to varying degrees, depending on the specific growth factor combination [27]. IGF, PDGF and EGF, also potentiated the effects of a low dose of FGF by extending the duration of ERK1/2 phosphorylation in rat lens epithelial explants, similar to that seen in vitreous-treated cells and with high concentrations of FGF, leading to lens fibre differentiation.
More recently, we have shown that vitreous humour stimulates a more robust phosphorylation of Akt in rat lens epithelial cells than FGF alone [27], raising the possibility that these other growth factors may be involved in vitreous-induced effects via the PI3-K/Akt signalling pathway. This signalling pathway is known to promote cell survival and protein synthesis in many cellular systems and has been shown to be essential for fibre differentiation induced in rat lens epithelial explants [23]. Taken together, these studies indicate that various vitreal growth factors may be involved in fine-tuning ERK1/2- and Akt-phosphorylation levels that are required for the initiation and/or maintenance of secondary lens fibre differentiation in vivo.
(d). Growth factor inhibition studies
Further evidence that factors other than FGF are necessary for fibre differentiation comes from studies that have used various strategies to interfere with other growth factor signalling pathways. Most notably, modulation of signalling through the TGF-β superfamily [35–39] or Wnts [40–44] resulted in aberrant fibre differentiation. This has led to the view that a cascade of growth factor signalling is required for fibre differentiation [4]. While FGF appears to be the initiating signal, how such a cascade is regulated and how interconnected signalling pathways regulate specific events in the complex process of fibre differentiation and maturation is a major focus of current research.
(i). Transforming growth factor-β superfamily signalling: bone morphogenetic proteins
The role of BMP signalling in lens differentiation was first indicated by in vitro experiments, culturing ocular rudiments with BMPs or with their natural antagonists such as noggin, chordin and follistatin. Addition of BMP4 (40 ng ml−1) or BMP7 (100 ng ml−1) to organ cultures of 2 day chick embryo optic vesicles and lens placodes from embryos (HH11–12) resulted in increased lens growth and expression of the differentiation marker δ-crystallin [45]. Subsequent studies [35,46] showed that inhibition of BMP signalling, by injecting noggin-expressing retrovirus into HH15–18 chick lens vesicles in ovo, resulted in defective fibre cell elongation, decreased Prox1 expression and apoptosis. Similarly, organotypic cultures of E10.5 mouse ocular primordia treated with noggin also resulted in smaller lenses with compromised elongation of fibre cells [36]. However, these BMP-blocking studies did not examine effects on differentiation markers such as γ- or β-crystallin. Generation of transgenic mice that expressed a truncated BMP receptor (Bmpr1b) resulted in embryonic lenses with asymmetric defects of primary fibre cell elongation and differentiation (delayed and decreased γ-crystallin and MIP26 expression), suggesting that BMP signalling is important for primary fibre differentiation [36]. The unusual asymmetric defect in these lenses may be owing to the transgenic receptor being a form (Bmpr1b/Alk6) not normally expressed in lens [47]. The fact that different BMP receptors have different ligand affinities, coupled with an asymmetric distribution of BMPs in the developing eye may explain why this transgene affected primary fibre cell differentiation on the nasal but not temporal aspect of the lens [36].
More recently, studies using cultured chick lens epithelial cells [39] showed that BMP2, 4 and 7 (≥5 ng ml−1) can induce expression of lens fibre-specific markers (CP49, filensin, Mp28 and δ-crystallin), similar to FGF or vitreous-conditioned medium. BMP signalling also upregulated gap junction-mediated intercellular coupling, a key feature of differentiating cells at the lens equator [38]. Moreover, the fibre-differentiating activity of vitreous humour was inhibited by BMP antagonists (noggin, chordin) or BMP antibodies. Surprisingly, these BMP antagonists also inhibited FGF-induced secondary fibre-differentiation responses in these cell cultures, suggesting that BMP activity is required for FGF-mediated responses. Indeed, these authors also showed that FGF-mediated gap junction coupling is reliant on lens cell-derived BMPs [38]. Further evidence that BMP signals are required for secondary fibre differentiation came from mice that over-express noggin. While embryonic and postnatal lens development appeared normal in these mice, by P11 there was an apparent failure of epithelial cells to elongate and differentiate [39]. The apparent phenotypic delay, compared with the dominant-negative Bmpr1b mice (see above), may be due to the levels of noggin required to inhibit endogenous levels of BMP in these eyes [39]. In contrast over-expression of Bmp7 in the lens from E12.5 did not result in premature differentiation of anterior lens epithelial cells [48], as occurs when FGFs are over-expressed [13,14,49]. Subsequently at E15.5, the lenses of these mice do show an abnormal lens phenotype but this appears to be secondary to the deleterious effects of BMP7 on neural retina [48], a known source of differentiation signals for the lens [4]. These data suggest that BMP signalling is insufficient to initiate fibre cell differentiation in lens epithelial cells, particularly the more quiescent anterior epithelial cells. Indeed, Boswell et al. [38,39] speculated that FGF and BMP signalling needs to be coupled for fibre differentiation and suggested that such coupling occurs in equatorial but not anterior epithelial cells. The mechanism underlying such signalling cross-talk is unclear. FGF signalling does not upregulate Bmp4 or Bmp7 expression in lens cells and inhibition of FGFR signalling or ERK1/2 activation does not appear to affect BMP-mediated effects on fibre differentiation [38,39].
(ii). Transforming growth factor-β superfamily signalling: transforming growth factor-β/activin
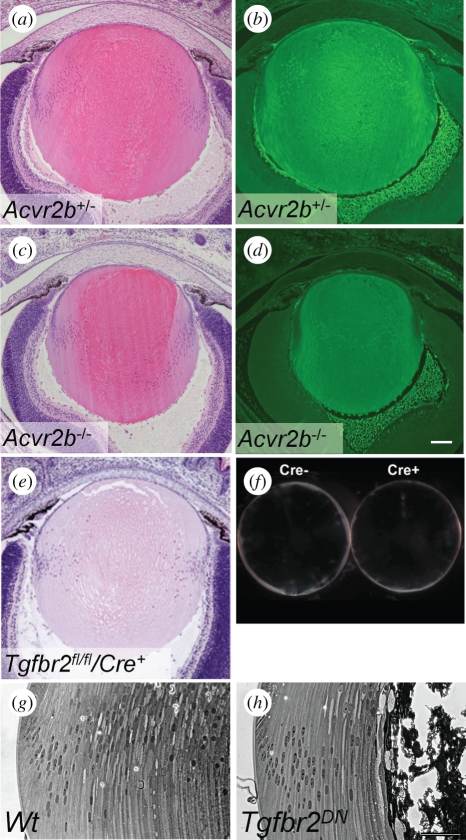
Single null mutations of TGF-β [50–52] or activin [53,54] do not appear to affect lens development, suggesting that these ligands are not required for lens differentiation or that there is functional redundancy. However, experiments with dominant-negative TGF-β receptors (Tgfbr2, Tgfbr1/Alk5), expressed in lens fibres under the control of the αA-crystallin promoter [37], indicate that signalling via TGF-β receptors is required to maintain fibre cell survival (figure 3). The cortical fibres in these lenses showed altered expression of fibre markers (α-crystallin, filensin, phakinin, MIP), attenuated elongation [37], altered focal adhesion kinase (FAK) activity [56] and aberrant expression of Bmpr1a [47]. Consistent with the lack of FAK activity, transgenic cells also showed impaired migration on laminin and reduced F-actin [37]. In contrast, conditional deletion of Tgfbr2 from lens ectoderm at E9.5, using the Le-Cre mice, resulted in normal lenses [55]. However, unlike the dominant-negative transgenic lenses, Tgfbr2CKO lenses did not show abrogation of Smad2 phosphorylation, suggesting that there may be compensatory signalling by other mechanisms such as activin receptors, which can also activate Smad2. Preliminary examination of mice that are null for Acvr2b showed that the lenses of P3 mice are structurally normal and show normal β-crystallin expression (figure 3). To date, there has been no report on the phenotype of Acvr2a null lenses or of lenses null for both activin type II receptors. A transgenic dominant-negative type II activin receptor has also not been employed in lens. Of all the TGF-β receptors, the type II activin receptors appear to be the most promiscuous as they can form signalling complexes with Acvr1A/Alk2, Acvr1B/Alk4, Bmpr1B/Alk6 and Acvr1C/Alk7, and thus can potentially activate both BMP (Smad1/5/8) and TGF-β (Smad2/3) pathways [57].
Figure 3.
Role of activin and TGF-β receptors in fibre differentiation. Null mutations of Acvr2b (a–d) or Tgfbr2 (e, f) do not affect lens development. However, expression of a dominant-negative Tgfbr2 receptor results in the failure of terminal lens fibre differentiation (h). (a–d); Tissues courtesy of Dr S. Paul Oh, de Iongh, 2004, unpublished data, University of Florida, Gainesville, FL, USA. (e,f) Reproduced and adapted with permission from Beebe et al. [55]. (g,h) Adapted from de Iongh et al. [37].
(iii). Wnt signalling
Members of the Wnt family of secreted glycolipoproteins are important regulators of cell proliferation, polarity and fate determination during embryonic development and tissue-specific stem cells in tissue homeostasis [58–61]. There are 19 known Wnt ligands and 10 known Frizzled (Fz) receptors, though complexity of signalling repertoires is increased with the inclusion of other ligands (e.g. Norrin) and receptors (e.g. ROR, Ryk) in the superfamily. Historically, signalling by Wnts and Fzs has been categorized according to activation of downstream ‘canonical’ (Wnt/β-catenin) or ‘non-canonical’ (Wnt/planar-cell polarity (PCP) and Wnt/Ca2+) pathways [62,63].
Wnt/β-catenin signalling is initiated when a Wnt ligand forms a complex with a Fz receptor and a low-density lipoprotein-related protein (Lrp) co-receptor. Once this complex is formed, dishevelled (Dvl) is activated and axin is recruited to the cell membrane. In the absence of Wnt, axin is part of a module containing glycogen synthase kinase-3β (GSK3β) that phosphorylates β-catenin and targets it for destruction [58,64]. Thus, ligand–receptor interaction leads to an increase in stabilized (hypophosphorylated) β-catenin, which can then associate with TCF/Lef transcription factors to regulate target gene expression.
Wnt/β-catenin (canonical signalling). While much of the focus on the Wnt pathway has been on its roles in the lens epithelium (see later), there is also evidence to suggest that Wnt signals play a role in fibre differentiation. Expression studies showed the presence of Wnt5b, 7a, Fz3 and Fz6 [65] in lens fibres. In vitro studies [40] showed that Wnt3a-conditioned medium, LiCl (a Wnt mimetic) and β-catenin over-expression are sufficient to induce the accumulation of β-crystallin in lens explants or in lens cell lines. However, this is not accompanied by lens cell elongation. By contrast, priming of cells with FGF (50 ng ml−1) for 1 h followed by Wnt3a-conditioned medium augmented this response and resulted in cell elongation as well as accumulation of several fibre cell markers (β-crystallin, MIP26, N-cadherin, p57Kip2) and loss of E-cadherin after 5 days, which were not seen in cultures treated with FGF alone. Moreover, these changes were accompanied by nuclear translocation of β-catenin. These data suggest that Wnt/β-catenin signalling functions cooperatively with FGF to regulate fibre cell differentiation, at least in the explant culture model.
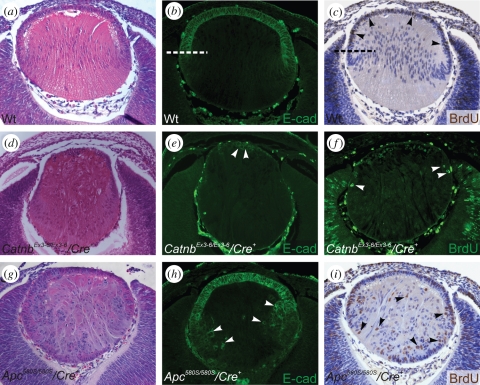
Support for a role in fibre differentiation comes from studies on mice that are deficient in Wnt signalling. Embryonic lenses, lacking β-catenin (Catnb) and thus lacking canonical Wnt signals, show decreased levels of β-crystallin and impaired fibre differentiation [43]. By contrast, deletion of β-catenin from differentiated fibres appeared to have no major effects on fibre cell differentiation. Cain et al. [43] concluded that β-catenin, acting either in canonical Wnt signalling or as a component of adhesion junctions, is required in the epithelium and/or during early fibre differentiation. However, over-activation of the Wnt pathway is also detrimental to fibre differentiation as constitutively activating the Wnt/β-catenin pathway in the lens, by mutating Apc (figure 4) or deleting exon3 of Catnb, leads to excessive epithelial proliferation, inhibition of fibre differentiation, epithelial-to-mesenchymal transition (EMT) and apoptosis [44]. Thus, it appears that levels of Wnt signalling need to be tightly regulated during epithelial cell cycle exit and initiation of fibre cell differentiation in the embryonic lens.
Figure 4.
Wnt/β-catenin signalling regulates epithelial cell phenotype, proliferation and differentiation in embryonic lenses. Tissues were stained with either haematoxylin and eosin (a,d,g), E-cadherin antibody (b,e,h) or for BrdU-incorporation (c,f,i). (a–c) At E13.5, cell proliferation in wild-type lens (Wt) is restricted to the E-cadherin-positive epithelial cells (c, arrowheads). (d–f) Loss of β-catenin (CatnbEx3-6/Ex3-6/Cre) abrogates Wnt signalling and results in the loss of lens epithelial cells (e, arrowheads) and reduction in lens cell proliferation (f, arrowheads). (g–i) Constitutive activation of Wnt/β-catenin signalling by a truncating mutation of Apc (Apc580S/580S/Cre) results in the failure of lens equatorial cells to differentiate into fibres, with aberrant labelling for E-cadherin (h) and cell proliferation (i) extending into the fibre cell mass. Adapted from Cain et al. [43] and Martinez et al. [44].
Wnt/planar cell polarity (non-canonical signalling). Although not as well understood as canonical Wnt signalling, there is a growing awareness that non-canonical signalling, particularly through the Wnt planar cell polarity (PCP) pathway, has a critical role in regulating many key developmental processes that involve intricate cytoskeletal remodelling. Signalling through the PCP pathway involves activation of Dvl, through a different domain(s) than for canonical signalling, and generally involves activation of the small Rho GTPases and Jnk [58,62,63,66]. Interestingly, beyond E14.5, no TCF/Lef–LacZ reporter activity has been detected in any part of the lens in reporter mice [65,67,68]. Similarly, other commonly used TOPgal and BATgal reporter mouse lines do not reveal Wnt/β-catenin signalling activity in lens [69–71]. However, reports of the expression of Wnts, Fzs and Dvls [40,41,65,72,73], as well other Wnt/PCP core signalling components Prickle (Pk) and Van Gogh-like (Vangl) in elongating mammalian fibre cells [74,75], suggest non-canonical Wnt signalling may have a role in lens fibre differentiation [76]. Consistent with this, studies show impairment of the fibre cell cytoskeleton as a consequence of inhibiting the small Rho GTPases [77–80].
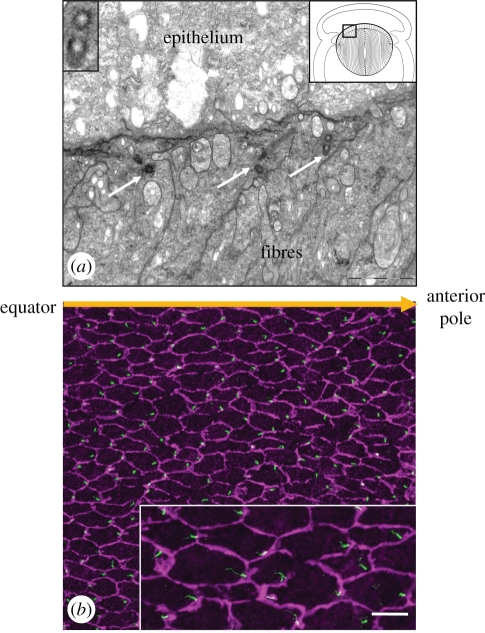
While it is recognized that some cells overtly display PCP by orientation of extensions such as hairs or cilia [81,82], an important development in recent years has been the identification of genetic links between the PCP pathway and ciliogenesis in vertebrates [83]. Direct evidence that the lens is a Wnt/PCP-organized tissue comes from the observation that the centrosome/primary cilium and core PCP proteins in lens cells exhibit a polarized distribution [84]. This is particularly striking in lens fibre cells located in the outer lens cortex; each fibre cell has an apical cilium located on the side of the cell that faces away from the lens equator and towards the anterior pole of the lens (figure 5). Another characteristic manifestation of PCP is partitioning of PCP components to specific cellular domains [81,82,85,86]. In lens fibre cells, core PCP proteins, notably Fz6 and Vangl2, localize to a different cellular domain than Pk1 [84]. This association between Fz and Vangl proteins in lens appears consistent with their co-localization in another planar epithelium, sensory inner ear cells [87,88]. Evidence that PCP signalling has a role in promoting alignment/orientation of lens fibres comes from studies of lenses from mice with mutations in PCP genes, Vangl2 and Celsr1. In lenses of Loop-tail (Vangl2 mutation) and Crash (Celsr1 mutation) mice, the alignment and orientation of fibres is perturbed so that normal Y-shaped sutures do not form [84]. In addition, transgenic mice that overexpress Sfrp2, a known regulator of Wnt signalling, in lens fibres show that their alignment and packing is disrupted [42]. A striking feature of these lenses is that fibres do not develop the convex curvature typically seen in normal lenses (figure 6). This is related to a loss of ability of fibres to re-orient and undergo directed cell migration so that the anterior and posterior tips of fibres from different segments do not meet appropriately to form the characteristic Y-shaped sutures at the lens poles. Consistent with the involvement of Wnt/PCP signalling in regulating this process, components of this pathway are downregulated in these transgenic lenses [42]. Taken together, these studies indicate a role for Wnt/PCP signalling in coordinating the precise alignment and orientation of fibres that is required during their differentiation. The question of whether primary cilia may also be involved in regulating these processes is a tantalizing one that warrants further study.
Figure 5.
Centrosome/cilium polarization in lens cells. (a) Transmission electron microscopy of the boxed area in the diagram (top right inset) shows the location of three adjoining cortical fibres in the lens cortex that display basal bodies (arrows) at their apical ends. The basal body of one fibre cell includes a section through its ciliary axoneme (middle arrow). A transverse section through basal bodies shows that central microtubules are absent (top left inset) and identifies them as primary cilia. (b) Pericentrin (green) immuno-reactivity localizes to the centrosome/cilium, and β-catenin (purple) localization demarcates cell margins in lens whole mounts. The centrosome/cilium is clearly associated with the cell margin proximal to the anterior pole. Scale bar; (a) 2 µm; (a, top left inset) 0.7 µm; (b), 20 µm; (b, inset) 10 µm. Adapted from Sugiyama et al. [84].
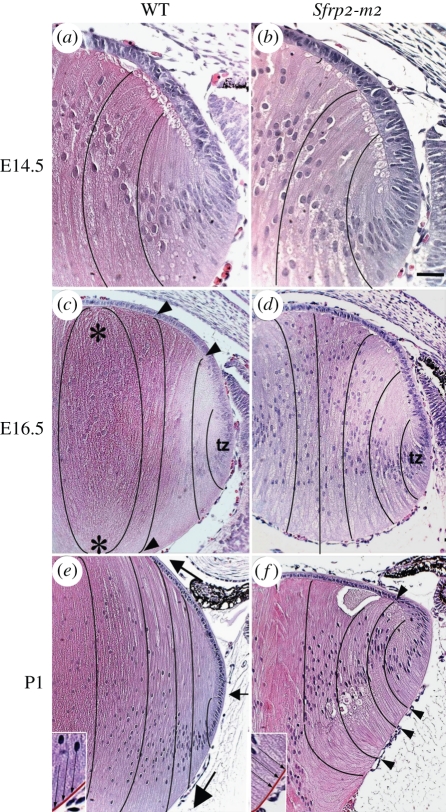
Figure 6.
Histological analysis of embryonic and neonatal Sfrp2 transgenic lenses. Lens sections from E14.5 (a,b), E16.5 (c,d) and P1 (e,f) Sfrp2-m2 transgenic mice (b,d,f) and wild-type (WT) littermates (a,c,e) stained with haematoxylin and eosin. (a,b) At E14.5, compared with the WT lens, the lenses in Sfrp2-m2 mice showed essentially normal features in the epithelial and fibre cell compartments. (c,d) At E16.5 the Sfrp2-m2 lenses were slightly smaller than lenses from WT littermates. The secondary fibres in the Sfrp2-m2 lenses appeared to be less closely packed. Moreover, fibres did not curve towards the developing sutures as they did in WT lenses (black lines indicate the curvature of fibre cells in all figures). In WT lenses, the elongating fibres had a concave curvature in the transitional zone but as they moved centrally they progressively developed a convex curvature (arrowheads in c). When fibres met up with equivalent fibres from another segment of the lens, they formed rudimentary sutures (asterisks in c). In the Sfrp2-m2 lenses, the fibres retained a concave curvature and no sutures were evident. (e,f) At P1 in the transitional zone just below the lens equator (small arrow in e) the elongating fibres had a concave curvature but took on a convex curvature as they migrated at their anterior and posterior tips (large arrows) towards the anterior and posterior poles of the lens, respectively. In the sfrp2-m2 lenses, the concave curvature of the fibres was more pronounced than at earlier stages. The elongating fibres in the Sfrp2-m2 lenses remained predominantly at right angles to the posterior capsule and to the epithelium (arrowheads in f). The insets in (e) and (f) show more details of the alignment of the fibres (arrows) and the capsule (red) in WT and Sfrp2-m2 lenses. No sutures formed in the Sfrp2-m2 lenses. tz, Transitional zone. Adapted from Chen et al. [42].
(e). Cell–cell signalling
(i). Notch signalling
Several recent studies indicate a role for the Notch signalling pathway in regulating fibre differentiation. The Notch ligand Jagged1 (Jag1) is expressed in lens fibre cells and several studies indicate that bi-directional Jag1–Notch signalling conveys a lateral inductive signal that is required for fibre differentiation [89,90]. One function so far identified is the regulation of cadherin expression, specifically the switch to N-cadherin expression during the fibre-differentiation process [90]. Jag1–Notch signalling has also been shown to be important in regulating secondary fibre differentiation through its role in maintaining a proliferative epithelial population in the germinative zone to provide precursors for fibre cells (see later section on epithelial cells; [89–92]). Consistent with FGF being the initiator of fibre differentiation, results from explant studies have shown that FGF induces Jag1 expression and Notch2 signalling. This is accompanied by induction of the Notch effector, Hes5, as well as upregulation of N-cadherin and downregulation of E-cadherin, which are characteristic of fibre differentiation [90].
(ii). Ephrin–eph signalling
Further evidence for cell–cell signalling in fibre differentiation comes from studies of human cataract, which showed that mutations in EPHA2, encoding a receptor tyrosine kinase, are associated with autosomal dominant congenital cataract and age-related cortical cataract [93–95]. Consistent with this, null mutation in mice of EphA2 or one of its ligands, Ephrin-A5 (Efna5), also lead to cataracts [95,96]. Analyses of wild-type and Efna5−/− lenses indicate that Ephrin–A5/EphA2 signalling regulates N-cadherin/β-catenin interactions, and thus adhesion and structure of the fibre cells [96]. However, the mechanisms by which this occurs are unclear.
3. Formation, maintenance and proliferation of the lens epithelium
As noted earlier, it is only relatively recently that attention has turned towards the lens epithelium. This layer has largely been thought of in terms of its role as a source of precursor of secondary fibres. However, the appreciation that aberrant growth of the epithelium is the basis of some forms of cataract (see [97] for review) has ensured renewed attention on factors that regulate its formation, maintenance and proliferation.
(a). Factors that regulate epithelial formation/maintenance
(i). Bone morphogenetic protein/activin signalling
Studies indicate that there is an interaction between FGF and BMP signalling during early lens induction. Mice expressing a dominant-negative FGFR in pre-lens ectoderm have delayed lens pit invagination, decreased lens pit cell proliferation and decreased ectodermal Pax6 expression, phenotypes which are all exacerbated by the presence of a Bmp7 null allele [98]. Further evidence of BMP signals in this process of early lens morphogenesis comes from studies that have conditionally ablated BMP receptors in the lens placode. Conditional deletion of Bmpr1a from the placode lens ectoderm using Le-Cre mice [99] leads to increased apoptosis of placode cells, but no apparent change in cell proliferation [100]. However, the loss of placode cells is insufficient to prevent lens formation and subsequently, at E13.5, the lenses appear smaller with reduced epithelial thickness. With postnatal stages, the epithelium is further thinned, fibre cell differentiation is attenuated and there appears to be abnormal persistence of epithelial cells along the posterior capsule [55]. By contrast, conditional deletion of Acvr1 in the presumptive lens ectoderm results in decreased proliferation of lens placode cells but no apparent induction of cell death, as is observed in Bmpr1a−/− lenses. Similar to Bmpr1a−/− mice, reduced placodal proliferation in Acvr1−/− mice is insufficient to affect lens formation, but results in smaller lenses owing to disrupted patterns of epithelial proliferation and fibre cell differentiation, as well as increased epithelial and fibre cell death [101]. An intriguing finding in the Acvr1−/− lenses was the failure of cells in the transitional zone and cortical fibres to properly exit the cell cycle. As Acvr1 can mediate both activin and BMP signals, Rajagopal et al. [101] examined lenses that were conditionally null for Smads that mediate BMP signals (Smad1, 5) or activin/TGFβ signals (Smad2) or the common Smad (Smad4). They found that mice null for Smad1, Smad5 or the common Smad4, but not Smad2, showed a similar cell cycle defect, suggesting that BMP rather than activin/TGFβ signals regulate epithelial cell cycle exit at the lens equator.
(ii). Wnt/β-catenin signalling
Members of the Wnt growth factor family have been shown in a number of organ systems to be key regulators of epithelial differentiation [102–105]. Wnt13 was the first Wnt to be detected in the chick lens [106]. Fz1, Fz2 and Fz7 were later detected in the chick lens placode [107]. The secreted frizzled-related proteins (sFRPs), which are Wnt signalling regulators, were also detected during mouse lens morphogenesis [108]. We subsequently showed that various Wnts, Fz receptors and Lrp5/6 co-receptors, as well as antagonists (Dkk, Sfrps) and the downstream Dvl proteins, are expressed in the differentiating mammalian lens during development [41,65,72,109].
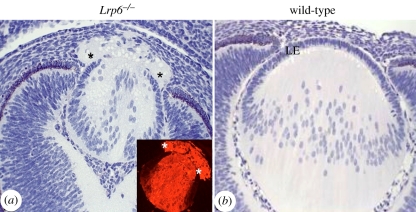
Various studies have shown that regulation of canonical and non-canoncial Wnt signalling pathways are important for eye induction [69,110,111]. Critically, the canonical Wnt/β-catenin pathway must be downregulated in presumptive lens ectoderm for lens induction to occur [112,113], whereas activation of the antagonistic non-canonical Wnt pathways, for example by Fz3 over-expression in early Xenopus embryos, promotes eye induction [114]. Indeed, non-canonical Wnt4 signals appear to be required for eye formation in Xenopus [115]. However, the Wnt/β-catenin pathway does appear to play a role during later stages of embryonic lens differentiation. Impaired Wnt/β-catenin signalling in mouse embryos homozygous for a mutation in the Lrp6 gene results in abnormal lens development [109]. This defect becomes evident at E13.5 when the anterior lens vesicle cells fail to form a normal confluent sheet of polarized epithelial cells. Some cells in this vicinity also show abnormal accumulation of the fibre-specific marker β-crystallin. As a result of the epithelial deficiency, the fibre cells typically extrude into the corneal stroma (figure 7). Similarly, mice conditionally null for β-catenin (Catnb) from E13.5, also have a deficient lens epithelium due to dysregulated cell cycle progression but not cell death [43]. Intriguingly, the epithelial cells precociously exit the cell cycle, indicated by the expression of p57Kip2, a cyclin-dependent kinase inhibitor and early fibre differentiation marker [116], and showed abnormal expression of c-maf, a transcription factor known to regulate β-crystallin [117]. Despite the presence of these fibre cell markers, no aberrant expression of β-crystallin was detected in epithelial cells of these mutant lenses [43]. Both the Lrp6 null and Catnb conditional null mice indicate that Wnt signalling is central to regulating lens epithelial phenotype and prevents precocious cell cycle exit and initiation of fibre differentiation.
Figure 7.
Lrp6−/− mutant mouse: sagittal sections of an eye from an Lrp6−/− mutant embryo (a) and a wild-type embryo (b) at E14.5. Histological sections stained with haematoxylin and phloxine show that the embryonic eye in the Lrp6−/− mutant is smaller than the wild-type. The lens epithelium is incompletely formed and elongating lens fibres have extruded into the overlying cornea (asterisks). The inset in (a) confirms that the extruded material contains fibre-specific β-crystallin. Adapted from Stump et al. [109].
Further evidence that Wnt activity regulates the epithelial phenotype comes from a recent study where the activity of the Wnt/β-catenin pathway was activated in lenses by either truncating the Apc gene or by deleting exon 3 of the Catnb gene [44]. Both mutations prevent proteasomal degradation of β-catenin and thus result in constitutive activation of the pathway from E13.5. In these lenses, there is enhanced epithelial cell cycle progression and a failure of epithelial cells at the equator to exit the cell cycle and initiate fibre differentiation (figure 3). The epithelial cells populate the posterior fibre compartment of the lens and continue to express epithelial markers (E-cadherin, Pax6) and fail to properly initiate the expression of fibre markers (p57Kip2, c-Maf, β-crystallin). Subsequently, the mutant epithelial cells undergo abnormal differentiation (EMT) and apoptotic cell death.
Taken together these data indicate a requirement for Wnt/β-catenin signalling between E11.5 and E14.5 in the formation and maintenance of the anterior lens epithelium, particularly in regulating epithelial progenitors. Wnt/β-catenin signals also appear to play a role in regulating cell cycle exit (p57Kip2) and β-crystallin expression in fibre cells [40,43]. However, uncontrolled activation of this pathway disturbs the transition of epithelial cells into differentiating fibre cells at the equator. Thus, the interplay between Wnt and FGF signals and tight regulation of the level of Wnt/β-catenin signalling at the lens equator may be crucial for determining the timing of epithelial cell cycle exit and fibre cell differentiation. What is not clear is whether Wnt/β-catenin signalling is required beyond these embryonic stages, as known reporter systems indicate no activity beyond E14.5, and mutations of the pathway have not been conducted at later stages.
Once the epithelial sheet has formed, maintenance of its normal structure and function depends on cells remaining attached to the lens capsule. Comparison of mammalian-derived lens epithelial cells maintained in vitro on their capsule (explants) with dissociated cells reveal that in the absence of normal capsule attachment, cells undergo major phenotypic changes and, unlike explants, do not respond appropriately to fibre-differentiation stimuli [118–120]. Consistent with this, loss of integrin signalling by deletion of α3 and α6 integrins [121,122], or deletion of integrin-linked kinase [123] or β1 integrin [124] leads to loss of epithelial cells by decreased proliferation, apoptosis and abnormal differentiation (EMT). Preliminary studies suggest that signalling by integrin-linked kinase is also required for complete activation of MAPK and PI3K/Akt pathways [125]. Various secreted factors in the aqueous also appear to be important for regulating these pathways and epithelial proliferative activity.
(b). Factors that regulate epithelial proliferation
As discussed earlier, a multitude of growth factors in the vitreous humour influence lens fibre differentiation, with FGFs providing the primary and essential inductive cue [4]. While characterization of growth factors in the aqueous humour is incomplete, various growth factors have been identified and shown to play similar or overlapping roles in lens cells. FGF [8,126], PDGF [127,128], IGF [30,129], hepatocyte growth factor (HGF) [130,131], TGF-α [132] and EGF [133] are some of the mitogenic growth factors found in aqueous. Studies in lens epithelial explants have shown that aqueous-induced proliferation is dependent on MAPK/ERK1/2 and PI3-K/Akt signalling [21,22,25]. Moreover, similar to vitreous-induced fibre differentiation, aqueous-induced cell proliferation also appears to be due to a combination [134] of growth factor signals, of which FGF plays a central role. Analysis of aqueous-induced responses in lens epithelial explants typically showed induction of ERK phosphorylation for up to 6 h. This could be mimicked by a ‘low’ dose (5 ng ml−1) of FGF but not by PDGF, IGF or EGF, which only stimulated ERK phosphorylation for up to 1 h. By specifically blocking FGF, PDGF and IGF receptors, we assayed the contribution of each of these growth factors to the aqueous-induced ERK1/2 phosphorylation profile and showed there are at least two phases of ERK1/2 phosphorylation, with the early phase dependent on IGF and/or PDGF and the later phase dependent on FGF [134]. Selective FGFR inhibition reduced the extended 6 h period of aqueous-induced ERK phosphorylation to 20 min. By contrast, selective inhibition of IGFR or PDGFR blocked the early 20 min phase, but not the later, extended phase of ERK phosphorylation. Furthermore, none of the growth factor receptor inhibitors completely blocked aqueous-induced lens cell proliferation on their own. Complete block of aqueous-induced lens cell proliferation required the presence of FGFR inhibitor in combination with one or more of the other inhibitors [134]. These data not only support a role for multiple mitogens in aqueous regulating lens cell proliferation, but also demonstrate that FGFR signalling is necessary for this process in rat lens epithelial explants. Consistent with FGFs being required for lens development in vivo, a recent study has shown that conditional ablation of Shp2, an adaptor protein critical for FGFR signal transduction, resulted in decreased ERK-activation and epithelial cell proliferation in murine embryonic lenses, as well as increased apoptosis in these epithelial cells [24].
In addition to exogenous factors from ocular media, endogenous cell–cell signalling appears to have an important role in regulating the proliferative epithelial cell population in the germinative zone. Recent studies have shown that Notch signalling is required for maintaining a population of proliferative epithelial cells. Conditional knockout of the Notch signalling regulator, the DNA-binding protein RPB-Jκ, results in premature exit from the cell cycle and a diminished supply of epithelial progenitor cells for secondary fibre differentiation [91]. Evidence from several studies now indicates that this regulation is mediated by unidirectional Notch signalling, activated by Jag-1-expressing fibre cells [89,90,92].
4. Fine-tuning of fibroblast growth factor signalling
From the molecular studies described above, it is clear that lens cells are exquisitely sensitive to FGF signals and that the level of activity needs to be finely tuned in the two cellular compartments of the lens. While much of this regulation may be at the level of FGF bioavailability in ocular media and receptor expression, more recent studies have identified important intracellular regulators and particularly selective endogenous antagonists, whose significant function is only starting to be appreciated. Not surprisingly, several such antagonists such as Sef, Sprouty1, Sprouty2 [135] and the Spreds [136] have been shown to be expressed in lens in similar patterns, with greatest expression in the epithelium and declining as fibre cells begin to differentiate [135].
To date, there are no reports of ocular defects in mice deficient for Sef [137,138], suggesting that there may be some functional redundancy between the negative regulatory molecules in the lens (Spry1/2 and the Spreds). However, over-expression studies in transgenic mice show that Sef effectively blocks fibre cell differentiation [138]. Microphthalmia in Sef transgenic mice results from a failure of primary and subsequently secondary lens fibre cells to elongate, leading to retention of a lens vesicle, similar to that observed in mice with impaired FGFR signalling [17], suggesting that Sef antagonizes this pathway. Although lens cell elongation is perturbed, β- and γ-crystallins are still expressed [138], suggesting that Sef over-expression in vivo has similar uncoupling effects on fibre cell elongation and crystallin gene expression as does pharmacological inhibition of ERK activation in vitro [21,23]. This is consistent with Sef's ability to not only block FGFR signalling but also to negatively regulate ERK1/2 signalling [139]. The precise roles played by these antagonists in the lens are still uncertain, but there is increasing evidence that molecules such as Spry2 are potent modulators of receptor tyrosine kinases and MAPK/ERK1/2 signalling [24], and thus lens development. Further mechanistic studies manipulating their expression or activity, either independently, or in combination, will be required to identify how key growth factor signalling pathways promote the maintenance and behaviour of lens cells.
5. Conclusions and perspectives for future studies
The anterior and posterior ocular environments clearly play determining roles in ensuring promotion and maintenance of epithelial and fibre phenotypes, respectively. There is now a wealth of evidence that points to the fibre-differentiating activity of vitreous being due to its ability to promote a high level of FGF signalling. However, studies also show that while FGF signalling is very effective and necessary for initiating and promoting fibre differentiation, it is not sufficient. Evidence is growing for important roles of other growth factor signalling pathways. So far, signals mediated by TGFβ and Wnt/Fz families appear to have roles in regulating aspects of fibre differentiation. Much work needs to be done to elucidate the roles of these different factors and how they cooperate to regulate the required signalling networks. This will not be a simple matter because of the extent and complexity of the processes involved in transforming a relatively small (approx. 10 µm tall) epithelial cell into a highly specialized, millimeter-long, fibre cell that undergoes progressive structural and functional changes as it becomes further displaced from the lens surface during its lifetime.
Apart from roles for canonical Wnt and Notch signalling at embryonic (E11.5–14.5) stages, little is known about other factors or signals required to promote formation of lens epithelium from anterior lens vesicle. However, once established in a confluent sheet, all indications are that the epithelial cells are relatively efficient at maintaining themselves provided they are not disturbed. For this, ECM interactions appear to be essential. This can be readily deduced from analysis of epithelial cell behaviour in the numerous in vitro studies that have been conducted over many years. When lens epithelial cells are dissociated from, and cultured out of contact with, their native substratum, they invariably undergo major phenotypic changes that include an EMT-like process. However, when explanted intact on their capsule, they remain viable and maintain their epithelial phenotype. Much is still to be learned about the interactions between epithelial cells and lens capsule components and the signalling pathways activated in this situation. Such interactions are also central to the directed migration that fibre cells undergo at their basal ends to form posterior sutures.
In addition to the need to know more about lens cell–ECM interactions, the studies on Notch and Ephrin signalling also remind us of the importance of cell–cell interactions, particularly those between epithelial and fibre cells. These reciprocal interactions are likely to be required for promoting and maintaining both lens cell phenotypes. A better understanding of epithelial–fibre interactions will provide foundations for minimizing post-cataract surgery complications and devising strategies for promoting lens regeneration after cataract surgery.
Acknowledgements
F.J.L. and J.Mc.A. acknowledge financial support from NHMRC (Australia) and NIH grant EY03177.
Footnotes
One contribution of 10 to a Theme Issue ‘The ocular lens: a classic model for development, physiology and disease’.
References
- 1.McAvoy J. W. 1978. Cell division, cell elongation and distribution of alpha-, beta- and gamma-crystallins in the rat lens. J. Embryol. Exp. Morphol. 44, 149–165 [PubMed] [Google Scholar]
- 2.Coulombre J. L., Coulombre A. J. 1963. Lens development: fiber elongation and lens orientation. Science 142, 1489–1490 10.1126/science.142.3598.1489 (doi:10.1126/science.142.3598.1489) [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y. 1976. Growth of lens and ocular environment: role of neural retina in the growth of mouse lens as revealed by an implantation experiment. Dev. Growth Differ. 18, 273–278 10.1111/j.1440-169X.1976.00273.x (doi:10.1111/j.1440-169X.1976.00273.x) [DOI] [PubMed] [Google Scholar]
- 4.Lovicu F. J., McAvoy J. W. 2005. Growth factor regulation of lens development. Dev. Biol. 280, 1–14 10.1016/j.ydbio.2005.01.020 (doi:10.1016/j.ydbio.2005.01.020) [DOI] [PubMed] [Google Scholar]
- 5.West-Mays J. A., Pino G., Lovicu F. J. 2009. Development and use of the lens epithelial explant system to study lens differentiation and cataractogenesis. Prog. Retin. Eye Res. 29, 135–143 10.1016/j.preteyeres.2009.12.001 (doi:10.1016/j.preteyeres.2009.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain C. G., McAvoy J. W. 1987. Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr. Eye Res. 6, 1165–1169 10.3109/02713688709034890 (doi:10.3109/02713688709034890) [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain C. G., McAvoy J. W. 1989. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF). Growth Factors 1, 125–134 10.3109/08977198909029122 (doi:10.3109/08977198909029122) [DOI] [PubMed] [Google Scholar]
- 8.McAvoy J. W., Chamberlain C. G. 1989. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development 107, 221–228 [DOI] [PubMed] [Google Scholar]
- 9.Lovicu F. J., McAvoy J. W. 1989. Structural analysis of lens epithelial explants induced to differentiate into fibres by fibroblast growth factor (FGF). Exp. Eye Res. 49, 479–494 10.1016/0014-4835(89)90056-0 (doi:10.1016/0014-4835(89)90056-0) [DOI] [PubMed] [Google Scholar]
- 10.Schulz M. W., Chamberlain C. G., de Iongh R. U., McAvoy J. W. 1993. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development 118, 117–126 [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain C. G., McAvoy J. W. 1997. Fiber differentiation and polarity in the mammalian lens: a key role for FGF. Prog. Retin. Eye Res. 16, 443–478 10.1016/S1350-9462(96)00034-1 (doi:10.1016/S1350-9462(96)00034-1) [DOI] [Google Scholar]
- 12.Lovicu F. J., Chamberlain C. G., McAvoy J. W. 1995. Differential effects of aqueous and vitreous on fiber differentiation and extracellular matrix accumulation in lens epithelial explants. Invest. Ophthalmol. Vis. Sci. 36, 1459–1469 [PubMed] [Google Scholar]
- 13.Lovicu F. J., Overbeek P. A. 1998. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development 125, 3365–3377 [DOI] [PubMed] [Google Scholar]
- 14.Robinson M. L., Overbeek P. A., Verran D. J., Grizzle W. E., Stockard C. R., Friesel R., Maciag T., Thompson J. A. 1995. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development 121, 505–514 [DOI] [PubMed] [Google Scholar]
- 15.de Iongh R. U., Lovicu F. J., Chamberlain C. G., McAvoy J. W. 1997. Differential expression of fibroblast growth factor receptors during rat lens morphogenesis and growth. Invest. Ophthalmol. Vis. Sci. 38, 1688–1699 [PubMed] [Google Scholar]
- 16.de Iongh R. U., Lovicu F. J., Hanneken A., Baird A., McAvoy J. W. 1996. FGF receptor-1 (flg) expression is correlated with fibre differentiation during rat lens morphogenesis and growth. Dev. Dyn. 206, 412–426 (doi:10.1002/(SICI)1097-0177(199608)206:4<412::AID-AJA7>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 17.Robinson M. L. 2006. An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 17, 726–740 10.1016/j.semcdb.2006.10.002 (doi:10.1016/j.semcdb.2006.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H., et al. 2008. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276–288 10.1016/j.ydbio.2008.03.028 (doi:10.1016/j.ydbio.2008.03.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis T. S., Shapiro P. S., Ahn N. G. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74, 49–139 10.1016/S0065-230X(08)60765-4 (doi:10.1016/S0065-230X(08)60765-4) [DOI] [PubMed] [Google Scholar]
- 20.Schaeffer H. J., Weber M. J. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell Biol. 19, 2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovicu F. J., McAvoy J. W. 2001. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development 128, 5075–5084 [DOI] [PubMed] [Google Scholar]
- 22.Le A. C., Musil L. S. 2001. FGF signaling in chick lens development. Dev. Biol. 233, 394–411 10.1006/dbio.2001.0194 (doi:10.1006/dbio.2001.0194) [DOI] [PubMed] [Google Scholar]
- 23.Wang Q., Stump R., McAvoy J. W., Lovicu F. J. 2009. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp. Eye Res. 88, 293–306 10.1016/j.exer.2008.08.023 (doi:10.1016/j.exer.2008.08.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Y., Carbe C., Powers A., Feng G. S., Zhang X. 2010. Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development. Development 137, 1085–1093 10.1242/dev.042820 (doi:10.1242/dev.042820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyengar L., Wang Q., Rasko J. E., McAvoy J. W., Lovicu F. J. 2007. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation 75, 662–668 10.1111/j.1432-0436.2007.00167.x (doi:10.1111/j.1432-0436.2007.00167.x) [DOI] [PubMed] [Google Scholar]
- 26.Iyengar L., Patkunanathan B., Lynch O. T., McAvoy J. W., Rasko J. E., Lovicu F. J. 2006. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp. Eye Res. 83, 667–678 10.1016/j.exer.2006.03.008 (doi:10.1016/j.exer.2006.03.008) [DOI] [PubMed] [Google Scholar]
- 27.Wang Q., McAvoy J. W., Lovicu F. J. 2010. Growth factor signaling in vitreous humour-induced lens fiber differentiation. Invest. Ophthalmol. Vis. Sci. 51, 3599–3610 10.1167/iovs.09-4797 (doi:10.1167/iovs.09-4797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor M. D., McAvoy J. W. 2007. In vitro generation of functional lens-like structures with relevance to age-related nuclear cataract. Invest. Ophthalmol. Vis. Sci. 48, 1245–1252 10.1167/iovs.06-0949 (doi:10.1167/iovs.06-0949) [DOI] [PubMed] [Google Scholar]
- 29.Klok E., Lubsen N. H., Chamberlain C. G., McAvoy J. W. 1998. Induction and maintenance of differentiation of rat lens epithelium by FGF-2, insulin and IGF-1. Exp. Eye Res. 67, 425–431 10.1006/exer.1998.0534 (doi:10.1006/exer.1998.0534) [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Chamberlain C. G., McAvoy J. W. 1996. IGF enhancement of FGF-induced fibre differentiation and DNA synthesis in lens explants. Exp. Eye Res. 63, 621–629 10.1006/exer.1996.0156 (doi:10.1006/exer.1996.0156) [DOI] [PubMed] [Google Scholar]
- 31.Richardson N. A., Chamberlain C. G., McAvoy J. W. 1993. IGF-1 enhancement of FGF-induced lens fiber differentiation in rats of different ages. Invest. Ophthalmol. Vis. Sci. 34, 3303–3312 [PubMed] [Google Scholar]
- 32.Beebe D. C., Silver M. H., Belcher K. S., van Wyk J. J., Svoboda M. E., Zelenka P. S. 1987. Lentropin, a protein that controls lens fiber formation, is related functionally and immunologically to the insulin-like growth factors. Proc. Natl Acad. Sci. USA 84, 2327–2330 10.1073/pnas.84.8.2327 (doi:10.1073/pnas.84.8.2327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ireland M. E., Mrock L. K. 2004. Expression and activation of the epidermal growth factor receptor in differentiating cells of the developing and post-hatching chicken lens. Exp. Eye Res. 79, 305–312 10.1016/j.exer.2004.05.012 (doi:10.1016/j.exer.2004.05.012) [DOI] [PubMed] [Google Scholar]
- 34.Chen F., Mrock L. K., Ireland M. E. 2001. A role for endogenous TGFalpha and associated signaling pathways in the differentiation of lens fiber cells. J. Cell Physiol. 186, 288–297 (doi:10.1002/1097-4652(200002)186:2<288::AID-JCP1031>3.0.CO;2-H) [DOI] [PubMed] [Google Scholar]
- 35.Belecky-Adams T. L., Adler R., Beebe D. C. 2002. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development 129, 3795–3802 [DOI] [PubMed] [Google Scholar]
- 36.Faber S. C., Robinson M. L., Makarenkova H. P., Lang R. A. 2002. Bmp signaling is required for development of primary lens fiber cells. Development 129, 3727–3737 [DOI] [PubMed] [Google Scholar]
- 37.de Iongh R. U., Lovicu F. J., Overbeek P. A., Schneider M. D., Joya J., Hardeman E. D., McAvoy J. W. 2001. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development 128, 3995–4010 [DOI] [PubMed] [Google Scholar]
- 38.Boswell B. A., Lein P. J., Musil L. S. 2008. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol. Biol. Cell. 19, 2631–2641 10.1091/mbc.E08-02-0124 (doi:10.1091/mbc.E08-02-0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boswell B. A., Overbeek P. A., Musil L. S. 2008. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev. Biol. 324, 202–212 10.1016/j.ydbio.2008.09.003 (doi:10.1016/j.ydbio.2008.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyu J., Joo C. K. 2004. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development 131, 1813–1824 10.1242/dev.01060 (doi:10.1242/dev.01060) [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Stump R. J., Lovicu F. J., McAvoy J. W. 2004. Expression of Frizzleds and secreted frizzled-related proteins (sFRPs) during mammalian lens development. Int. J. Dev. Biol. 48, 867–877 10.1387/ijdb.041882yc (doi:10.1387/ijdb.041882yc) [DOI] [PubMed] [Google Scholar]
- 42.Chen Y., Stump R. J., Lovicu F. J., Shimono A., McAvoy J. W. 2008. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev. Biol. 324, 161–176 10.1016/j.ydbio.2008.09.002 (doi:10.1016/j.ydbio.2008.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cain S., Martinez G., Kokkinos M. I., Turner K., Richardson R. J., Abud H. E., Huelsken J., Robinson M., de Iongh R. 2008. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev. Biol. 321, 420–433 10.1016/j.ydbio.2008.07.002 (doi:10.1016/j.ydbio.2008.07.002) [DOI] [PubMed] [Google Scholar]
- 44.Martinez G., Wijesinghe M., Turner K., Abud H. E., Taketo M. M., Noda T., Robinson M. L., de Iongh R. U. 2009. Conditional mutations of beta-catenin and APC reveal roles for canonical Wnt signaling in lens differentiation. Invest. Ophthalmol. Vis. Sci. 50, 4794–4806 10.1167/iovs.09-3567 (doi:10.1167/iovs.09-3567) [DOI] [PubMed] [Google Scholar]
- 45.Trousse F., Esteve P., Bovolenta P. 2001. Bmp4 mediates apoptotic cell death in the developing chick eye. J. Neurosci. 21, 1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler R., Belecky-Adams T. L. 2002. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development 129, 3161–3171 [DOI] [PubMed] [Google Scholar]
- 47.de Iongh R. U., Chen Y., Kokkinos M. I., McAvoy J. W. 2004. BMP and activin receptor expression in lens development. Mol. Vis. 10, 566–576 [PubMed] [Google Scholar]
- 48.Hung F. C., Zhao S., Chen Q., Overbeek P. A. 2002. Retinal ablation and altered lens differentiation induced by ocular overexpression of BMP7. Vision Res. 42, 427–438 10.1016/S0042-6989(01)00242-5 (doi:10.1016/S0042-6989(01)00242-5) [DOI] [PubMed] [Google Scholar]
- 49.Robinson M. L., Ohtaka-Maruyama C., Chan C. C., Jamieson S., Dickson C., Overbeek P. A., Chepelinsky A. 1998. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev. Biol. 198, 13–31 10.1016/S0012-1606(98)80026-2 (doi:10.1016/S0012-1606(98)80026-2) [DOI] [PubMed] [Google Scholar]
- 50.Sanford L. P., Ormsby I., Gittenberger de Groot A. C., Sariola H., Friedman R., Boivin G. P., Cardell E. L., Doetschman T. 1997. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124, 2659–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proetzel G., Pawlowski S. A., Wiles M. V., Yin M., Boivin G. P., Howles P. N., Ding J., Ferguson M. W., Doetschman T. 1995. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat. Genet. 11, 409–414 10.1038/ng1295-409 (doi:10.1038/ng1295-409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shull M. M., et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359, 693–699 10.1038/359693a0 (doi:10.1038/359693a0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vassalli A., Matzuk M. M., Gardner H. A., Lee K. F., Jaenisch R. 1994. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 8, 414–427 10.1101/gad.8.4.414 (doi:10.1101/gad.8.4.414) [DOI] [PubMed] [Google Scholar]
- 54.Matzuk M. M., Kumar T. R., Vassalli A., Bickenbach J. R., Roop D. R., Jaenisch R., Bradley A. 1995. Functional analysis of activins during mammalian development. Nature 374, 354–356 10.1038/374354a0 (doi:10.1038/374354a0) [DOI] [PubMed] [Google Scholar]
- 55.Beebe D., et al. 2004. Contributions by members of the TGFbeta superfamily to lens development. Int. J. Dev. Biol. 48, 845–856 10.1387/ijdb.041869db (doi:10.1387/ijdb.041869db) [DOI] [PubMed] [Google Scholar]
- 56.Kokkinos M. I., Brown H. J., de Iongh R. U. 2007. Focal adhesion kinase (FAK) expression and activation during lens development. Mol. Vis. 13, 418–430 [PMC free article] [PubMed] [Google Scholar]
- 57.Graham H., Peng C. 2006. Activin receptor-like kinases: structure, function and clinical implications. Endocr. Metab. Immune Disord. Drug Targets 6, 45–58 [DOI] [PubMed] [Google Scholar]
- 58.Logan C. Y., Nusse R. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 10.1146/annurev.cellbio.20.010403.113126 (doi:10.1146/annurev.cellbio.20.010403.113126) [DOI] [PubMed] [Google Scholar]
- 59.Nusse R., Fuerer C., Ching W., Harnish K., Logan C., Zeng A., Berge D. T., Kalani Y. 2008. Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol. 73, 59–66 10.1101/sqb.2008.73.035 (doi:10.1101/sqb.2008.73.035) [DOI] [PubMed] [Google Scholar]
- 60.MacDonald B. T., Tamai K., He X. 2009. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 10.1016/j.devcel.2009.06.016 (doi:10.1016/j.devcel.2009.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Amerongen R., Nusse R. 2009. Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 10.1242/dev.033910 (doi:10.1242/dev.033910) [DOI] [PubMed] [Google Scholar]
- 62.Karner C., Wharton K. A., Jr, Carroll T. J. 2006. Planar cell polarity and vertebrate organogenesis. Semin. Cell Dev. Biol. 17, 194–203 10.1016/j.semcdb.2006.05.003 (doi:10.1016/j.semcdb.2006.05.003) [DOI] [PubMed] [Google Scholar]
- 63.Jones C., Chen P. 2007. Planar cell polarity signaling in vertebrates. Bioessays 29, 120–132 10.1002/bies.20526 (doi:10.1002/bies.20526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cong F., Schweizer L., Varmus H. 2004. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131, 5103–5115 10.1242/dev.01318 (doi:10.1242/dev.01318) [DOI] [PubMed] [Google Scholar]
- 65.Liu H., Mohamed O., Dufort D., Wallace V. A. 2003. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev. Dyn. 227, 323–334 10.1002/dvdy.10315 (doi:10.1002/dvdy.10315) [DOI] [PubMed] [Google Scholar]
- 66.Wallingford J. B., Habas R. 2005. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132, 4421–4436 10.1242/dev.02068 (doi:10.1242/dev.02068) [DOI] [PubMed] [Google Scholar]
- 67.Liu H., Thurig S., Mohamed O., Dufort D., Wallace V. A. 2006. Mapping canonical Wnt signaling in the developing and adult retina. Invest. Ophthalmol. Vis. Sci. 47, 5088–5097 10.1167/iovs.06-0403 (doi:10.1167/iovs.06-0403) [DOI] [PubMed] [Google Scholar]
- 68.Liu H., et al. 2007. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev. Biol. 308, 54–67 10.1016/j.ydbio.2007.04.052 (doi:10.1016/j.ydbio.2007.04.052) [DOI] [PubMed] [Google Scholar]
- 69.Fuhrmann S. 2008. Wnt signaling in eye organogenesis. Organogenesis 4, 60–67 10.4161/org.4.2.5850 (doi:10.4161/org.4.2.5850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuhrmann S., Riesenberg A. N., Mathiesen A. M., Brown E. C., Vetter M. L., Brown N. L. 2009. Characterization of a transient TCF/LEF-responsive progenitor population in the embryonic mouse retina. Invest. Ophthalmol. Vis. Sci. 50, 432–440 10.1167/iovs.08-2270 (doi:10.1167/iovs.08-2270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westenskow P., Piccolo S., Fuhrmann S. 2009. Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development 136, 2505–2510 10.1242/dev.032136 (doi:10.1242/dev.032136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ang S. J., Stump R. J., Lovicu F. J., McAvoy J. W. 2004. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Exp. Patterns 4, 289–295 10.1016/j.modgep.2003.11.002 (doi:10.1016/j.modgep.2003.11.002) [DOI] [PubMed] [Google Scholar]
- 73.Stump R. J., Lovicu F. J., Ang S. L., Pandey S. K., McAvoy J. W. 2006. Lithium stabilizes the polarized lens epithelial phenotype and inhibits proliferation, migration, and epithelial mesenchymal transition. J. Pathol. 210, 249–257 10.1002/path.2049 (doi:10.1002/path.2049) [DOI] [PubMed] [Google Scholar]
- 74.Bekman E., Henrique D. 2002. Embryonic expression of three mouse genes with homology to the Drosophila melanogaster prickle gene. Mech. Dev. 119(Suppl. 1), S77–S81 10.1016/S0925-4773(03)00095-9 (doi:10.1016/S0925-4773(03)00095-9) [DOI] [PubMed] [Google Scholar]
- 75.Tissir F., Goffinet A. M. 2006. Expression of planar cell polarity genes during development of the mouse CNS. Eur. J. Neurosci. 23, 597–607 10.1111/j.1460-9568.2006.04596.x (doi:10.1111/j.1460-9568.2006.04596.x) [DOI] [PubMed] [Google Scholar]
- 76.Chen Y., Stump R. J., Lovicu F. J., McAvoy J. W. 2006. A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation? Semin. Cell Dev. Biol. 17, 712–725 10.1016/j.semcdb.2006.11.005 (doi:10.1016/j.semcdb.2006.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao V., Wawrousek E., Tamm E. R., Zigler S., Jr 2002. Rho GTPase inactivation impairs lens growth and integrity. Lab. Invest. 82, 231–239 [DOI] [PubMed] [Google Scholar]
- 78.Maddala R., Reneker L. W., Pendurthi B., Rao P. V. 2008. Rho GDP dissociation inhibitor-mediated disruption of Rho GTPase activity impairs lens fiber cell migration, elongation and survival. Dev. Biol. 315, 217–231 10.1016/j.ydbio.2007.12.039 (doi:10.1016/j.ydbio.2007.12.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maddala R., Deng P. F., Costello J. M., Wawrousek E. F., Zigler J. S., Rao V. P. 2004. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab. Invest. 84, 679–692 10.1038/labinvest.3700105 (doi:10.1038/labinvest.3700105) [DOI] [PubMed] [Google Scholar]
- 80.Maddala R. L., Reddy V. N., Rao P. V. 2001. Lovastatin-induced cytoskeletal reorganization in lens epithelial cells: role of Rho GTPases. Invest. Ophthalmol. Vis. Sci. 42, 2610–2615 [PubMed] [Google Scholar]
- 81.Lawrence P. A., Struhl G., Casal J. 2007. Planar cell polarity: one or two pathways? Nat. Rev. Genet. 8, 555–563 10.1038/nrg2125 (doi:10.1038/nrg2125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y., Nathans J. 2007. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134, 647–658 10.1242/dev.02772 (doi:10.1242/dev.02772) [DOI] [PubMed] [Google Scholar]
- 83.Axelrod J. D. 2008. Basal bodies, kinocilia and planar cell polarity. Nat. Genet. 40, 10–11 10.1038/ng0108-10 (doi:10.1038/ng0108-10) [DOI] [PubMed] [Google Scholar]
- 84.Sugiyama Y., Stump R. J., Nguyen A., Wen L., Chen Y., Wang Y., Murdoch J. N., Lovicu F. J., McAvoy J. W. 2010. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev. Biol. 338, 193–201 10.1016/j.ydbio.2009.11.033 (doi:10.1016/j.ydbio.2009.11.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seifert J. R., Mlodzik M. 2007. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126–138 10.1038/nrg2042 (doi:10.1038/nrg2042) [DOI] [PubMed] [Google Scholar]
- 86.Strutt D. 2008. The planar polarity pathway. Curr. Biol. 18, R898–R902 10.1016/j.cub.2008.07.055 (doi:10.1016/j.cub.2008.07.055) [DOI] [PubMed] [Google Scholar]
- 87.Montcouquiol M., et al. 2006. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26, 5265–5275 10.1523/JNEUROSCI.4680-05.2006 (doi:10.1523/JNEUROSCI.4680-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Guo N., Nathans J. 2006. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 26, 2147–2156 10.1523/JNEUROSCI.4698-05.2005 (doi:10.1523/JNEUROSCI.4698-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le T. T., Conley K. W., Brown N. L. 2009. Jagged 1 is necessary for normal mouse lens formation. Dev. Biol. 328, 118–126 10.1016/j.ydbio.2009.01.015 (doi:10.1016/j.ydbio.2009.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saravanamuthu S. S., Gao C. Y., Zelenka P. S. 2009. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev. Biol. 332, 166–176 10.1016/j.ydbio.2009.05.566 (doi:10.1016/j.ydbio.2009.05.566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jia J., Lin M., Zhang L., York J. P., Zhang P. 2007. The Notch signaling pathway controls the size of the ocular lens by directly suppressing p57Kip2 expression. Mol. Cell. Biol. 27, 7236–7247 10.1128/MCB.00780-07 (doi:10.1128/MCB.00780-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rowan S., Conley K. W., Le T. T., Donner A. L., Maas R. L., Brown N. L. 2008. Notch signaling regulates growth and differentiation in the mammalian lens. Dev. Biol. 321, 111–122 10.1016/j.ydbio.2008.06.002 (doi:10.1016/j.ydbio.2008.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shiels A., Bennett T. M., Knopf H. L., Maraini G., Li A., Jiao X., Hejtmancik J. F. 2008. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol. Vis. 14, 2042–2055 [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang T., et al. 2009. Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum. Mutat. 30, E603–E611 10.1002/humu.20995 (doi:10.1002/humu.20995) [DOI] [PubMed] [Google Scholar]
- 95.Jun G., et al. 2009. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS Genet. 5, e1000584. 10.1371/journal.pgen.1000584 (doi:10.1371/journal.pgen.1000584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cooper M. A., Son A. I., Komlos D., Sun Y., Kleiman N. J., Zhou R. 2008. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc. Natl Acad. Sci. USA 105, 16 620–16 625 10.1073/pnas.0808987105 (doi:10.1073/pnas.0808987105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eldred J. A., Dawes L. J., Wormstone I. M. 2011. The lens as a model for fibrotic disease. Phil. Trans. R. Soc. B 366, 1301–1319 10.1098/rstb.2010.0341 (doi:10.1098/rstb.2010.0341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Faber S. C., Dimanlig P., Makarenkova H. P., Shirke S., Ko K., Lang R. A. 2001. Fgf receptor signaling plays a role in lens induction. Development 128, 4425–4438 [DOI] [PubMed] [Google Scholar]
- 99.Ashery-Padan R., Marquardt T., Zhou X., Gruss P. 2000. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14, 2701–2711 10.1101/gad.184000 (doi:10.1101/gad.184000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajagopal R., et al. 2009. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev. Biol. 335, 305–316 10.1016/j.ydbio.2009.08.027 (doi:10.1016/j.ydbio.2009.08.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajagopal R., Dattilo L. K., Kaartinen V., Deng C. X., Umans L., Zwijsen A., Roberts A. B., Bottinger E. P., Beebe D. C. 2008. Functions of the type 1 BMP receptor Acvr1 (Alk2) in lens development: cell proliferation, terminal differentiation, and survival. Invest. Ophthalmol. Vis. Sci. 49, 4953–4960 10.1167/iovs.08-2217 (doi:10.1167/iovs.08-2217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brisken C., Heineman A., Chavarria T., Elenbaas B., Tan J., Dey S. K., McMahon J. A., McMahon A. P., Weinberg R. A. 2000. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 14, 650–654 [PMC free article] [PubMed] [Google Scholar]
- 103.Itaranta P., Lin Y., Perasaari J., Roel G., Destree O., Vainio S. 2002. Wnt-6 is expressed in the ureter bud and induces kidney tubule development in vitro. Genesis 32, 259–268 10.1002/gene.10079 (doi:10.1002/gene.10079) [DOI] [PubMed] [Google Scholar]
- 104.Miller C., Sassoon D. A. 1998. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125, 3201–3211 [DOI] [PubMed] [Google Scholar]
- 105.Pinto D., Clevers H. 2005. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp. Cell Res. 306, 357–363 10.1016/j.yexcr.2005.02.022 (doi:10.1016/j.yexcr.2005.02.022) [DOI] [PubMed] [Google Scholar]
- 106.Jasoni C., Hendrickson A., Roelink H. 1999. Analysis of chicken Wnt-13 expression demonstrates coincidence with cell division in the developing eye and is consistent with a role in induction. Dev. Dyn. 215, 215–224 (doi:10.1002/(SICI)1097-0177(199907)215:3<215::AID-AJA4>3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 107.Stark M. R., Biggs J. J., Schoenwolf G. C., Rao M. S. 2000. Characterization of avian frizzled genes in cranial placode development. Mech. Dev. 93, 195–200 10.1016/S0925-4773(00)00263-X (doi:10.1016/S0925-4773(00)00263-X) [DOI] [PubMed] [Google Scholar]
- 108.Leimeister C., Bach A., Gessler M. 1998. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech. Dev. 75, 29–42 10.1016/S0925-4773(98)00072-0 (doi:10.1016/S0925-4773(98)00072-0) [DOI] [PubMed] [Google Scholar]
- 109.Stump R. J., et al. 2003. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev. Biol. 259, 48–61 10.1016/S0012-1606(03)00179-9 (doi:10.1016/S0012-1606(03)00179-9) [DOI] [PubMed] [Google Scholar]
- 110.de Iongh R. U., Abud H. E., Hime G. R. 2006. WNT/Frizzled signaling in eye development and disease. Front. Biosci. 11, 2442–2464 10.2741/1982 (doi:10.2741/1982) [DOI] [PubMed] [Google Scholar]
- 111.Gunhaga L. 2011. The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Phil. Trans. R. Soc. B 366, 1193–1203 10.1098/rstb.2010.0175 (doi:10.1098/rstb.2010.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith A. N., Miller L. A., Song N., Taketo M. M., Lang R. A. 2005. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev. Biol. 285, 477–489 10.1016/j.ydbio.2005.07.019 (doi:10.1016/j.ydbio.2005.07.019) [DOI] [PubMed] [Google Scholar]
- 113.Kreslova J., Machon O., Ruzickova J., Lachova J., Wawrousek E. F., Kemler R., Krauss S., Piatigorsky J., Kozmik Z. 2007. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis 45, 157–168 10.1002/dvg.20277 (doi:10.1002/dvg.20277) [DOI] [PubMed] [Google Scholar]
- 114.Rasmussen J. T., Deardorff M. A., Tan C., Rao M. S., Klein P. S., Vetter M. L. 2001. Regulation of eye development by frizzled signaling in Xenopus. Proc. Natl Acad. Sci. USA 98, 3861–3866 10.1073/pnas.071586298 (doi:10.1073/pnas.071586298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maurus D., Heligon C., Burger-Schwarzler A., Brandli A. W., Kuhl M. 2005. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. EMBO J. 24, 1181–1191 10.1038/sj.emboj.7600603 (doi:10.1038/sj.emboj.7600603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lovicu F. J., McAvoy J. W. 1999. Spatial and temporal expression of p57(KIP2) during murine lens development. Mech. Dev. 86, 165–169 10.1016/S0925-4773(99)00106-9 (doi:10.1016/S0925-4773(99)00106-9) [DOI] [PubMed] [Google Scholar]
- 117.Kim J. I., Li T., Ho I. C., Grusby M. J., Glimcher L. H. 1999. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc. Natl Acad. Sci. USA 96, 3781–3785 10.1073/pnas.96.7.3781 (doi:10.1073/pnas.96.7.3781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang-Su S. T., et al. 2003. Proteome analysis of lens epithelia, fibers, and the HLE B-3 cell line. Invest. Ophthalmol. Vis. Sci. 44, 4829–4836 10.1167/iovs.03-0556 (doi:10.1167/iovs.03-0556) [DOI] [PubMed] [Google Scholar]
- 119.Taliana L., Evans M. D., Ang S., McAvoy J. W. 2006. Vitronectin is present in epithelial cells of the intact lens and promotes epithelial mesenchymal transition in lens epithelial explants. Mol. Vis. 12, 1233–1242 [PubMed] [Google Scholar]
- 120.Lovicu F. J., McAvoy J. W. 2008. Epithelial explants and their application to study development processes in the lens. In Animal models in eye research (ed. Tsonis P. A.), pp. 134–147 San Diego, CA: Academic Press [Google Scholar]
- 121.Wederell E. D., de Iongh R. U. 2006. Extracellular matrix and integrin signaling in lens development and cataract. Semin. Cell Dev. Biol. 17, 759–776 10.1016/j.semcdb.2006.10.006 (doi:10.1016/j.semcdb.2006.10.006) [DOI] [PubMed] [Google Scholar]
- 122.De Arcangelis A., Mark M., Kreidberg J., Sorokin L., Georges-Labouesse E. 1999. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development 126, 3957–3968 [DOI] [PubMed] [Google Scholar]
- 123.de Iongh R. U., Teo Z. L., Dedhar S., Robinson M. L. 2008. The effect of conditional null mutation of integrin-linked kinase (Ilk) on lens development. ARVO Meeting Abstracts, Fort Lauderdale, FL, April 27–May 1, 2008. Invest. Ophthalmol. Vis. Sci 49, 5406 [Google Scholar]
- 124.Simirskii V. N., Wang Y., Duncan M. K. 2007. Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev. Biol. 306, 658–668 10.1016/j.ydbio.2007.04.004 (doi:10.1016/j.ydbio.2007.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Teo J., McQueen-Miscamble L., Turner K., Martinez G., Reneker L., Dedhar S., Robinson M., de Iongh R. 2010. Integrin linked kinase (ILK) is required for lens epithelial cell survival and proliferation. In XIX Biennal Meeting of Int. Soc. for Eye Research, Montreal, Canada, 18–23 July 2010, abstract 509 (http://www.kenes.com/iser2010/cd/Index.htm). [Google Scholar]
- 126.McAvoy J. W., Chamberlain C. G. 1990. Growth factors in the eye. Prog. Growth Factor Res. 2, 29–43 10.1016/0955-2235(90)90008-8 (doi:10.1016/0955-2235(90)90008-8) [DOI] [PubMed] [Google Scholar]
- 127.Kok A., Lovicu F. J., Chamberlain C. G., McAvoy J. W. 2002. Influence of platelet-derived growth factor on lens epithelial cell proliferation and differentiation. Growth Factors 20, 27–34 10.1080/08977190290022202 (doi:10.1080/08977190290022202) [DOI] [PubMed] [Google Scholar]
- 128.Ray S., Gao C., Wyatt K., Fariss R. N., Bundek A., Zelenka P., Wistow G. 2005. Platelet-derived growth factor D, tissue-specific expression in the eye, and a key role in control of lens epithelial cell proliferation. J. Biol. Chem. 280, 8494–8502 10.1074/jbc.M413570200 (doi:10.1074/jbc.M413570200) [DOI] [PubMed] [Google Scholar]
- 129.Hyatt G. A., Beebe D. C. 1993. Regulation of lens cell growth and polarity by an embryo-specific growth factor and by inhibitors of lens cell proliferation and differentiation. Development 117, 701–709 [DOI] [PubMed] [Google Scholar]
- 130.Choi J., Park S. Y., Joo C. K. 2004. Hepatocyte growth factor induces proliferation of lens epithelial cells through activation of ERK1/2 and JNK/SAPK. Invest. Ophthalmol. Vis. Sci. 45, 2696–2704 10.1167/iovs.03-1371 (doi:10.1167/iovs.03-1371) [DOI] [PubMed] [Google Scholar]
- 131.Wormstone I. M., Tamiya S., Marcantonio J. M., Reddan J. R. 2000. Hepatocyte growth factor function and c-Met expression in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 41, 4216–4222 [PubMed] [Google Scholar]
- 132.Wunderlich K., Knorr M. 1994. Effect of platelet-derived growth factor PDGF on replication of cultivated bovine lens epithelial cells. Ophthalmology 91, 98–102 [PubMed] [Google Scholar]
- 133.Reddan J. R., Wilson-Dziedzic D. 1983. Insulin growth factor and epidermal growth factor trigger mitosis in lenses cultured in a serum-free medium. Invest. Ophthalmol. Vis. Sci. 24, 409–416 [PubMed] [Google Scholar]
- 134.Iyengar L., Patkunanathan B., McAvoy J. W., Lovicu F. J. 2009. Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors 27, 50–62 10.1080/08977190802610916 (doi:10.1080/08977190802610916) [DOI] [PubMed] [Google Scholar]
- 135.Boros J., Newitt P., Wang Q., McAvoy J. W., Lovicu F. J. 2006. Sef and Sprouty expression in the developing ocular lens: implications for regulating lens cell proliferation and differentiation. Semin. Cell Dev. Biol. 17, 741–752 10.1016/j.semcdb.2006.10.007 (doi:10.1016/j.semcdb.2006.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lovicu F. J., Boros J., McAvoy J. W. 2009. Spreds: sprouty-related proteins antagonistic for multiple cell signalling pathways are expressed in the lens and eye. ARVO Meeting Abstracts, Fort Lauderdale, FL, May 3–7, 2009. Invest. Ophthalmol. Vis. Sci. 50, 4353 [Google Scholar]
- 137.Lin W., Jing N., Basson M. A., Dierich A. E., Licht L., Ang S.-L. 2005. Synergistic activity of Sef and Sprouty proteins in regulating the expression of Gbx2 in the mid-hindbrain region. Genesis 41, 110–115 10.1002/gene.20103 (doi:10.1002/gene.20103) [DOI] [PubMed] [Google Scholar]
- 138.Newitt P., Boros J., Madakashira B., Robinson M. L., Reneker L. W., McAvoy J. W., Lovicu F. J. 2010. Sef is a negative regulator of fiber cell differentiation in the ocular lens. Differentiation 80, 53–67 10.1016/j.diff.2010.05.005 (doi:10.1016/j.diff.2010.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ron D., Fuchs Y., Chorev D. S. 2008. Know thy Sef: a novel class of feedback antagonists of receptor tyrosine kinase signaling. Int. J. Biochem. Cell Biol. 40, 2040–2052 10.1016/j.biocel.2008.03.013 (doi:10.1016/j.biocel.2008.03.013) [DOI] [PubMed] [Google Scholar]