Abstract
Fibrosis affects multiple organs and is associated with hyperproliferation, cell transdifferentiation, matrix modification and contraction. It is therefore essential to discover the key drivers of fibrotic events, which in turn will facilitate the development of appropriate therapeutic strategies. The lens is an elegant experimental model to study the processes that give rise to fibrosis. The molecular and cellular organization of the lens is well defined and consequently modifications associated with fibrosis can be clearly assessed. Moreover, the avascular and non-innervated properties of the lens allow effective in vitro studies to be employed that complement in vivo systems and relate to clinical data. Using the lens as a model for fibrosis has direct relevance to millions affected by lens disorders, but also serves as a valuable experimental tool to understand fibrosis per se.
Keywords: fibrosis, lens, model, matrix, myofibroblast, contraction
1. Introduction
Fibrosis is defined as ‘a pathological condition in which tissue structure is disrupted by production of excessive extracellular matrix (ECM)’ [1,2]. Fibrosis is a chronic multiple organ disorder with some cases having a worse survival rate than cancer [3], and it has been suggested that fibrosing conditions are responsible for almost half of all known deaths [4]. In association with enhanced matrix production, typical features of tissue fibrosis include hyperproliferation, transdifferentiation of both epithelial cells and fibroblasts to myofibroblasts and matrix contraction. The generation of myofibroblasts has long been assumed to be pivotal for the initiation of fibrotic tissue formation and establishment of a pro-contractile apparatus, which leads to matrix contraction. Recently, work carried out on lung and lens cells has begun to question the established dogma and suggest that rather than be profibrogenic, myofibroblasts may in fact play a protective role to regulate the degree of fibrotic response [5–7]. Elucidating the role of myofibroblasts and the signalling mechanisms that drive specific fibrotic events is essential in the quest to develop effective strategies to treat fibrotic disorders. To achieve this, appropriate models need to be employed and the value of the lens should not be underestimated as a model of fibrosis.
2. The lens: a model for fibrosis
The lens is an exquisite biological tool to investigate a host of mechanisms involved in tissue fibrosis. To appreciate the benefits the lens offers it is important to understand the organization and features of the normal lens (figure 1a) [8]. The adult lens is isolated from other tissues and has no innervations or vascular system, receiving all required nutrients from the aqueous and vitreous humours that bathe it. The lens can be separated into distinct cellular groups. Cells within the central anterior epithelium have been present since lens vesicle formation during embryogenesis. Moreover, both cell death and division is negligible in this population. However, the cells in the peripheral epithelium are capable of migration, cell division and differentiation into lens fibre cells that form the bulk of the lens. In association with these cellular compartments markers can be used to define cell phenotype. For example, the forkhead transcription factor FOXE3 is expressed in the lens epithelium [9], along with PAX6, the eye master gene [10], whereas β-crystallin can serve as an early differentiation marker, while γ-crystallin and major intrinsic protein (MIP) are markers of a mature fibre cell [11]. In the normal lens order is maintained, but circumstances can arise that lead to disruption of lens integrity and provoke a fibrotic change. The ability to monitor changes within a well-defined system is critical to understand the nature of both the induction of a wound healing response and fibrotic changes. Models available to study fibrosis such as cell lines [12], transgenic models [13,14] and tissue culture [15–17] are well characterized in the lens. Post-mortem analysis and clinical data also greatly aid the understanding of fibrosis in the lens [17–19]; this information may not strictly inform us of the causal factors, but importantly the data do serve as a blueprint of changes that are seen in patients. This fundamental information of clinical disorders should direct researchers to ensure whichever experimental system is employed has relevance to changes seen in patients. If the model adopted is appropriate then mechanisms giving rise to that change can be determined and advance our knowledge. Fibrotic disorders of the lens affect millions of people [20,21], and for the purpose of this review the two best characterized conditions, anterior subcapsular cataract (ASC) and posterior capsule opacification (PCO), will be discussed.
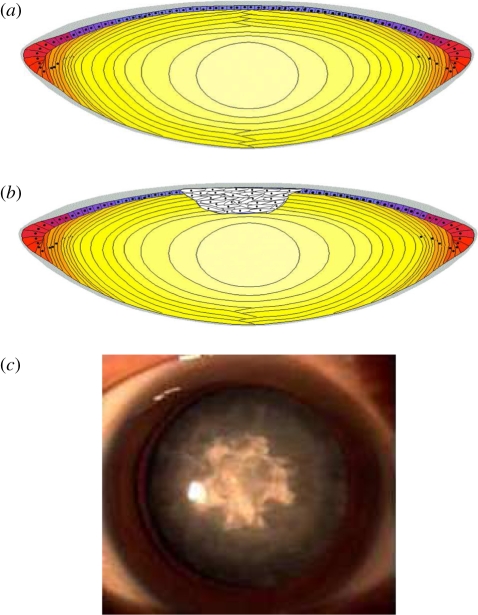
Figure 1.
(a) A schematic illustrating the cellular organization of the human lens. The site of anterior subcapsular cataract (ASC) is depicted in (b); ASC is associated with modification to the epithelium that is clinically seen as a fibrotic plaque (c; image kindly supplied by Dr Hong Zhang, Harbin Medical University, China). Grey area, capsule; purple area, epithelial cells; red area, germinative cells; orange area, elongating fibres; dark yellow area, cortical fibres and light yellow area, nucleus.
(a). Anterior subcapsular cataract
ASC is characterized by dense light scattering fibrotic regions underneath the anterior capsule (figure 1). ASC is the least prevalent form of cataract in the UK with a prevalence of 2–3% [20,22], although ASC is more prevalent in eastern societies such as Japan and Singapore [20,23]. The occurrence of ASC is associated with several conditions that involve ocular trauma, such as impact injury, inflammation or irritation of the eye, such as atopic dermatitis [24–26].
In recent years both analysis of human anterior subcapsular material [23,25] and animal model studies [15,27] have been conducted to determine the molecular and cellular mechanisms that underpin ASC formation. Marcantonio et al. [25] performed a cytochemical analysis of four ex vivo human ASCs. In this study folding and thickening of the anterior lens capsules was observed, with cells in these regions exhibiting a typical myofibroblast morphology, i.e. spindle-shaped, elongated and exhibiting positive staining for alpha smooth muscle actin (αSMA). A previous investigation by Lee & Joo [23] revealed that transdifferentiation of lens epithelial cells in ASC causes the production of large amounts of ECM proteins such as collagen I, III and fibronectin, which are not normally present in the lens capsule. The excessive production and deposition of ECM is a general characteristic of fibrosis [1]. Work initially using isolated whole rat lens cultures demonstrated that the fibrotic changes described above could be mimicked following transforming growth factor β (TGFβ) exposure (figure 2) [15]. Consequently, as with many fibrotic disorders, TGFβ has become the major area of study.
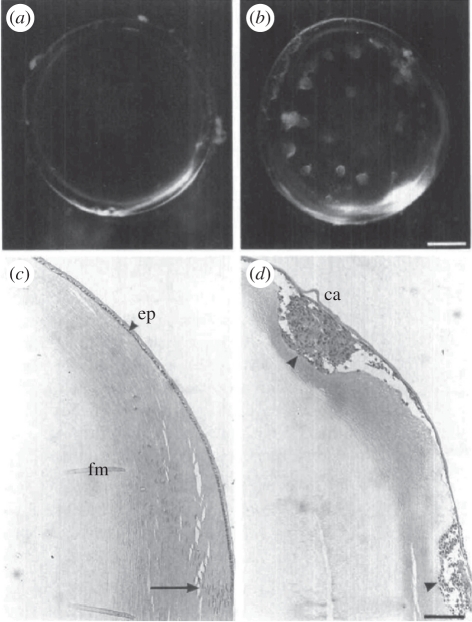
Figure 2.
The effect of TGFβ on lens transparency and morphology. Whole lenses were cultured for 5 days (a,c) without TGFβ or (b,d) with TGFβ2. (a,b) Lenses were photographed through the posterior pole. (c,d) Sagittal sections were stained with haematoxylin and eosin; the equator of the lens is at the bottom edge of the micrograph in each case. In the presence of TGFβ, lenses developed anterior opacities (b) that corresponded with cellular plaques (d, arrowhead) underlying the lens capsule (ca). (a) Without TGFβ lenses remained clear and (c) retained normal histology with a monolayer of epithelial cells (ep) covered by the lens capsule overlying the fibre mass (fm). At the lens equator (arrow), cells began to elongate and to differentiate into fibres. Scale bars, (a,b) 400 µm; (c,d) 250 µm. Previously published in Hales et al. [28] with permission from the copyright holder, the Association for Research in Vision and Ophthalmology (ARVO).
(b). Posterior capsule opacification
PCO is the most common fibrotic condition in the lens that develops following cataract surgery [21]. At present, the only means of treating cataract is by surgical intervention, and this initially restores high visual quality. Unfortunately, PCO develops in a significant proportion of patients to such an extent that a secondary loss of vision occurs (figure 3).
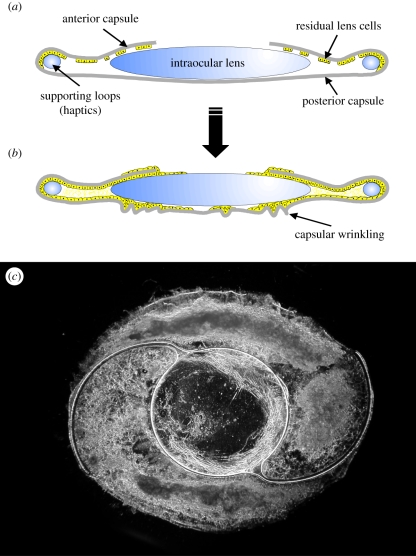
Figure 3.
Schematics of (a) the post-surgical capsular bag and (b) the extensive growth and modification that gives rise to posterior capsule opacification following cataract surgery. (c) A dark-field micrograph of a capsular bag removed from a donor eye that had undergone cataract surgery prior to death that exhibits light scattering regions beneath an intraocular lens. First published in Wormstone [29] with permission from Experimental Eye Research (Elsevier).
A modern cataract operation generates a capsular bag, which comprises a proportion of the anterior and the entire posterior capsule [21]. The bag remains in situ and partitions the aqueous and vitreous humours, and in the majority of cases, houses an intraocular lens. The production of a capsular bag following surgery permits a free passage of light along the visual axis through the transparent intraocular lens and thin acellular posterior capsule. However, on the remaining anterior capsule, lens epithelial cells stubbornly reside despite enduring the rigours of surgical trauma. This resilient group of cells then begin to re-colonize the denuded regions of the anterior capsule, encroach onto the intraocular lens surface, occupy regions of the outer anterior capsule and most importantly begin to colonize the previously cell-free posterior capsule. Cells continue to divide and cover the posterior capsule and can ultimately encroach on the visual axis. A thin cover of cells is insufficient to affect the light path, but subsequent changes to the matrix and cell organization can give rise to light scatter.
PCO is a classical fibrotic disease exhibiting hyperproliferation in response to injury, myofibroblast formation, matrix deposition and contraction [21]. A large complement of methods is available to study PCO; cell lines provide an abundant and reliable source of material to investigate the relationship between signalling pathways and functional effects including cell growth, transdifferentiation, matrix deposition and contraction [5,12,30,31]. Tissue culture models have also served the research field well, and include lens explants [16] and in vitro capsular bag models [17,32–34]. The latter employ a sham cataract operation to produce a capsular bag. These systems have the same cellular organization as in vivo and cells grow on their natural matrix. Moreover, lens cells within these tissue culture systems can be maintained in serum-free medium (without supplements) for extended periods of culture [34]. The culture conditions can therefore be carefully controlled and thus interpretation of the data is easier than in more complex systems. In addition to these in vitro methods, in vivo animal models have been developed [35–37] and it is probable that transgenic animals will be studied further to determine PCO progression.
A number of features are common to both ASC and PCO, and not surprisingly TGFβ is again strongly implicated [21]. Histological analysis of post-mortem specimens demonstrates increased matrix deposition, cell transdifferentiation and matrix contraction; moreover, activated TGFβ signalling molecules, Smads, are present. Moreover, application of TGFβ to in vitro capsular bags, lens epithelial explants or cell lines induces myofibroblast expression, increased matrix production and matrix contraction, thus mimicking the clinically observed features. Consequently, TGFβ again serves as a major focus of research in PCO formation; however a number of other mechanisms are also likely to govern fibrosis and it is important to establish a full picture in our assessment of fibrotic tissue formation.
3. Regulation of fibrosis
A number of cellular and biological processes contribute to fibrotic conditions and it is important to consider the mechanisms underpinning their involvement; these include how inflammation can drive the fibrotic process and the factors that lead to increased matrix production/deposition and modification that ultimately define fibrotic conditions in the lens and throughout the body.
4. Inflammation
Inflammation has long been regarded as ‘setting the stage’ for fibrosis development. Inflammatory response components and angiogenic factors are frequently present in numerous fibrotic disorders; however the essential mechanistic controls involved are poorly understood. Tissue damage or injury including surgery results in inflammation with the aim of repairing and protecting the surrounding tissues. Damaged tissues release chemicals that attract white blood cells such as the lymphocyte T helper cells that help coordinate the ensuing immune response by releasing cytokines such as interleukins. These secreted proteins activate resident macrophages that further enhance the production of cytokines, chemokines and other inflammatory mediators as well as recruiting monocytes [38], with the intention of returning injured tissue back to normal status.
The concentration of several interleukin isoforms along with monocyte chemoattractant protein-1 (MCP-1) and interferon gamma (IFN-γ) have been shown to increase in the aqueous humour of patients experiencing idiopathic anterior uveitus (inflammation of the iris and ciliary structures of the uvea) [39].
A number of people exhibit chronic inflammation of the eye (uveitis) which results in elevated levels of cytokines in the ocular fluids. Uveitis is relatively common and is the fifth leading cause of blindness in Europe [40]. Interestingly, 40 per cent of uveitis patients develop cataracts including a high level of anterior subcapsular opacities [24,41]. Moreover, sustained inflammatory responses are likely to exacerbate the rate of PCO progression. Consequently, gaining an understanding of the defined role inflammation plays in fibrosis is vital.
The lymphocyte T-helper cells upregulated during inflammatory episodes have two variations. The Th1-type produce cytokines and chemokines, such as interleukins (IL)-2, IL-12, IFN-γ and tumour necrosis factor (TNF)-α, which in general facilitate tissue restoration [42]. IFN-γ can decrease cell proliferation along with reducing collagen synthesis and deposition and attenuates expression of pro-fibrotic cytokines [43]. Th1-type cytokines and chemokines also stimulate resident M1 macrophages that subsequently yield reactive oxygen species (ROS) and IL-1β [42]. IL-1β facilitates cells to synthesize matrix metalloproteinases (MMPs), and prostaglandin E2 that prevents proliferation and collagen production. Furthermore, M1 macrophages generate TNF-α, an effective inhibitor of type 1 collagen [44].
In contrast, Th2-type lymphocytes increase expression of factors that encourage fibroblast expansion and deposition of matrix, and thus augment fibrosis. For example, Th2-type cells induce IL-4, a stimulant of proliferation and inducer of both types I and III collagen and fibronectin [45]. IL-4 additionally heightens the production of TGFβ and connective tissue growth factor [46] and decreases IFN-γ. Secretion of IL-5 from Th2-type cells stimulate B cell growth and activates eosinophils which likewise produce the pro-fibrotic ligands TGFβ and PDGF and hence enhance fibroblast proliferation. Eosinophils are also reported to accumulate in the lesions of various fibrotic disorders [47]. Like IL-4 the Th2 specific IL-13 stimulates TGFβ formation and can directly activate fibroblasts [48]. Th2-type cells also initiate M2 macrophages to release PDGF and IL-10 (a pro-inflammatory interleukin that decreases IFN-γ and TNF-α). Class M2 macrophages also secrete TGFβ and inhibitors to MMPs, impairing routine ECM remodelling [49].
The correlation of inflammation with fibrosis has been targeted for innovative therapies directed at ocular fibrotic disorders, such as the treatment of PCO with anti-inflammatory drugs [50]. However, this approach has demonstrated little benefit in preventing fibrosis; this could be due to anti-inflammatory therapies affecting both advantageous Th1-type cells and the potentially damaging Th2 cells, in addition to diminishing prostaglandin E2 responses which permits increases to collagen synthesis [42]. Importantly, Laurell & Zetterstrom [50] found that anti-inflammatory treatment of patients undergoing cataract removal surgery resulted in a higher number of patients developing fibrotic PCO 4 years post-surgery than in subjects treated with a placebo. Moreover, Symonds et al. [51] demonstrated that the steroid dexamethasone, which is routinely administered to patients undergoing cataract surgery, improves cell survival and increase collagen type I synthesis in a rat lens explant model of PCO. This study therefore suggests that lens cells are susceptible to the actions of anti-inflammatory molecules. Chandler et al. [52] tested the direct actions of cyclooxygenase 2 (COX-2) through application of rofecoxib or celecoxib to canine capsular bags; both COX-2 inhibitors suppressed PCO.
It therefore appears that when considering methods to manage inflammation at the site of injury, maintaining homeostasis of specific subsets of lymphocytes must be tightly controlled; an imbalance can have detrimental effects on ECM production (Th2) and loss (Th1). Moreover the actions of anti-inflammatory drugs on cell behaviour, within the affected tissue, and the ability of these cells to locally synthesize and respond to inflammatory cytokines should also be considered.
A number of cytokines become elevated at the site of injury. These can drive hyperproliferation or mediate matrix deposition, transdifferentiation or matrix contraction. Growth factors such as fibroblast growth factor (FGF), epidermal growth factor and platelet-derived growth factor (PDGF) can promote growth [12,31,53], but the growth factor most commonly associated with the fibrotic changes is TGFβ [1]. During inflammation of the eye, TGFβ is believed to suppress the actions of white blood cells and thus control the degree of inflammatory response [54]. While this role is of benefit to the eye, active TGFβ becomes available to responsive cells and in such cases can drive fibrosis in these tissues. Understanding the role of TGFβ in fibrosis is therefore critical if we are to advance our knowledge and apply this to future strategies.
5. TGFβ: the orchestrator of fibrosis
The cytokine TGFβ is widely implicated as being a central mediator of the fibrotic response [1]; consequently, there is a great deal of interest regarding the role of TGFβ in fibrotic disorders of the lens, namely ASC and PCO.
TGFβ is present in the aqueous humour of the eye and exists largely in a latent, inactive form [55,56]. Under normal conditions TGFβ activity is tightly regulated by proteins in the ocular humours such as α2-macroglobulin, which have a high affinity for free active TGFβ [57], however following trauma to the lens, e.g. by surgical injury, active levels of TGFβ can be elevated. Following trauma, a wound healing response is initiated that causes an induction of TGFβ activators such as plasmin proteases MMP-2 and -9 [58] and thrombospondin-1 [59], which cleave latent TGFβ precursor via degradation of pro-segments. Moreover, increased levels of ROS have also been reported to promote TGFβ activity [60,61]. The induction of TGFβ following cutaneous injury is a common repair response; however, the sustained activation of TGFβ during this process has implicated this protein as being a predominant initiator of the fibrotic response in vivo. In human post-burn hypertrophic scars [62] and in dermal fibrotic lesions of scleroderma patients, expression of TGFβ isoforms and TGFβ receptors are elevated [63]. Similarly, with respect to the lens, capsular bag tissue from donor eyes that had undergone cataract surgery has identified all three TGFβ isoforms and receptors [64] and elevated levels of active TGFβ2 [17]. TGFβ2 is the major isoform in the eye, however TGFβ1 and 3 can be activated following surgical injury, such as cataract surgery, where breaching of the blood aqueous barrier initiates an inflammatory response [55].
Much evidence confirms that following trauma to the lens, active levels of TGFβ can induce the cellular responses key to fibrosis, namely: epithelial to mesenchymal transdifferentiation; increased ECM production and deposition and matrix contraction [1,21,65]. One of the major effects of TGFβ on lens epithelial cells is to promote transdifferentiation to a myofibroblast phenotoype. Analysis of human ASC tissue has revealed elevated levels of the myofibroblast markers αSMA and fibronectin [23]. Moreover, TGFβ has been shown to induce ASC in a rat lens culture model (figure 3) [15].
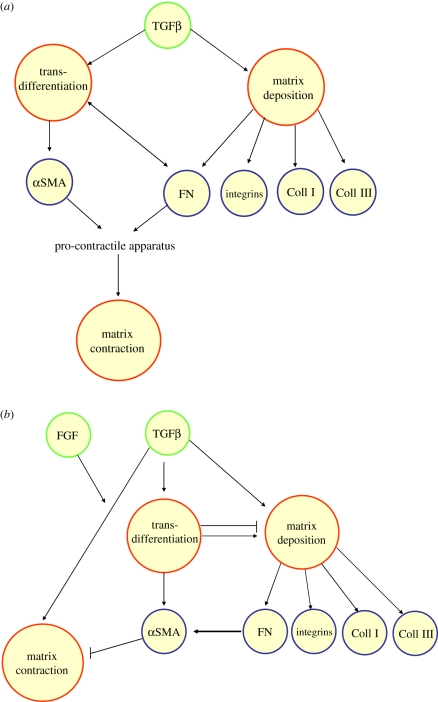
With respect to understanding the role of TGFβ in PCO, the post-mortem analysis of a capsular bag received from a donor 1 month following cataract surgery revealed increased levels of TGFβ-induced fibrotic markers including the transdifferentiation marker αSMA and matrix contraction/wrinkling of the posterior capsule (figure 4) [17]. These changes were replicated by application of TGFβ to in vitro human capsular bags (figure 5). TGFβ-induced transdifferentiation has long been assumed to be critical for inducing the synthesis and contraction of the ECM [66]. Contraction of the ECM of the lens capsule causes the formation of wrinkles which induce light scatter and with increasing severity can lead to visual loss. It is therefore of great importance to understand the cellular mechanisms that regulate TGFβ-induced matrix contraction in fibrotic conditions such as PCO in order to provide potential targets for inhibition by pharmaceutical agents. To permit thorough investigations of the signalling pathways driving fibrotic events, human lens cell lines have been employed. Using this biological tool, specific assays have been developed to assess matrix production, transdifferentiation and matrix contraction (figures 6 and 7) [5,12,30]. Inhibition studies could then be employed to tease out the pathways mediating TGFβ-induced fibrosis. Using this approach, Dawes et al. [5] tested the relationship between transdifferentiation of human lens epithelial cells and matrix contraction. In contrast to conventional wisdom, it was revealed that αSMA expression and fibronectin/fibronectin receptors are not critical to TGFβ-induced matrix contraction (figure 8). The aforementioned study raises provocative questions that concern the importance of TGFβ-mediated transdifferentiation in regulating matrix contraction in non-lenticular fibrotic pathologies (figure 9). In the case of lens fibrosis it cannot be assumed that transdifferentiation is a redundant process in regulating fibrotic events as myofibroblasts are commonly regarded as key mediators of increased ECM production and deposition, a key characteristic of ASC and PCO [21,65] and a common feature of fibrotic pathologies [1]. However, work in lung cells suggests a reduction in αSMA expression can promote matrix deposition. It would therefore appear that the relationship between matrix production deposition and myofibroblast involvement needs further investigation in a number of tissues to determine whether a consensus pattern is present or not.
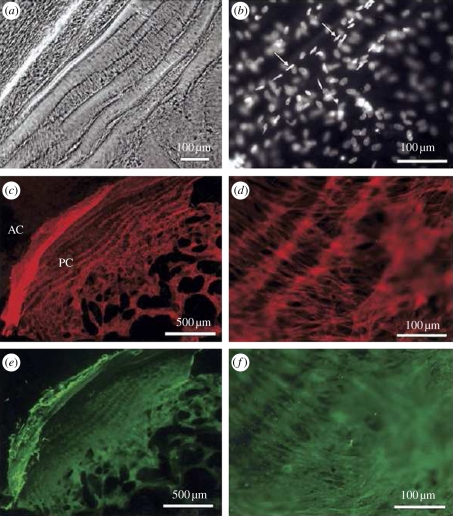
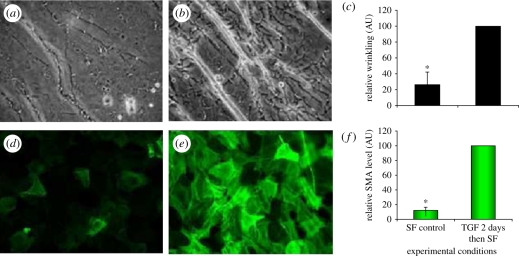
Figure 4.
Examination of a capsular bag removed from a donor eye that had undergone cataract surgery 32 days before the time of death. (a) A phase-contrast micrograph shows wrinkling of the posterior capsule and the associated cellular morphology. (b) Fluorescence micrograph illustrating several layers of nuclei, some of which exhibit a spindle-shape (arrows) and are oriented along capsular wrinkles. (c) Fluorescence micrograph showing F-actin distribution in cells growing across the posterior capsule (PC) and also those growing on the outer surface of the anterior capsule (AC). (d) A higher-magnification fluorescent micrograph demonstrating the F-actin organization of cells residing on the posterior capsule in association with matrix contraction. (e) Fluorescence micrograph showing αSMA distribution in cells growing across the posterior capsule and also those growing on the outer surface of the anterior capsule. (f) A higher-magnification fluorescence micrograph demonstrating the αSMA organization of cells residing on the posterior capsule in association with matrix contraction. Previously published in Wormstone et al. [17] with permission from the copyright holder, the Association for Research in Vision and Ophthalmology (ARVO).
Figure 5.
Replication of clinical features of lens fibrosis in a human in vitro tissue culture model for posterior capsule opacification. (a,b) Phase-contrast and (d,e) fluorescent micrographs demonstrate enhance matrix contraction and αSMA expression in response to TGFβ (10 ng ml−1) exposure (b,e). These images can be converted to a binary form that permits quantification (c,f), which greatly aids comparative assessment. Data were previously published by Wormstone et al. [33] with permission from Experimental Eye Research (Elsevier).
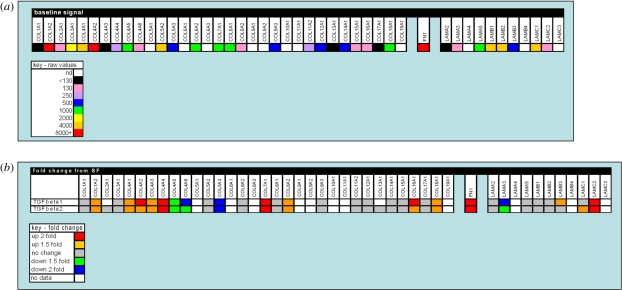
Figure 6.
A gene expression profile of selected matrix components in the human lens cell line FHL 124. (a) The baseline signal of matrix component gene expression detected in non-stimulated serum-free controls using oligonucleotide microarrays. (b) Changes detected in gene expression of matrix components following 24 h culture in TGFβ (10 ng ml−1) conditions using oligonucleotide microarrays. The data are presented in a colorimetric form to indicate level detected (a) and fold change (b). A key for each is provided. Data previously published by Dawes et al. [30] with permission from Molecular Vision.
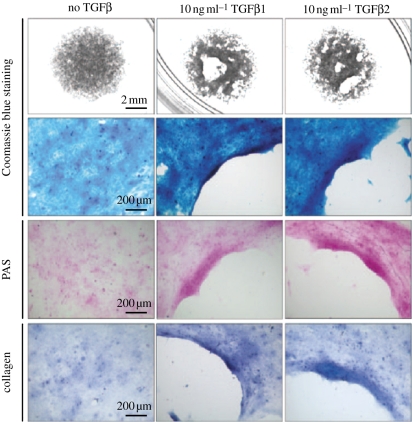
Figure 7.
Validation of an in vitro method, the patch assay, to assess matrix contraction by human lens epithelial cells. The images clearly show the appearance of cell-free regions within the patch area after 3 days of exposure to 10 ng ml−1 TGFβ1 or -β2, which was determined by Coomassie blue staining. Moreover, these cell-free regions did not exhibit positive PAS staining or collagen, thus indicating matrix movement in association with cells. Data previously published by Dawes et al. [5] with permission from the copyright holder, the Association for Research in Vision and Ophthalmology (ARVO).
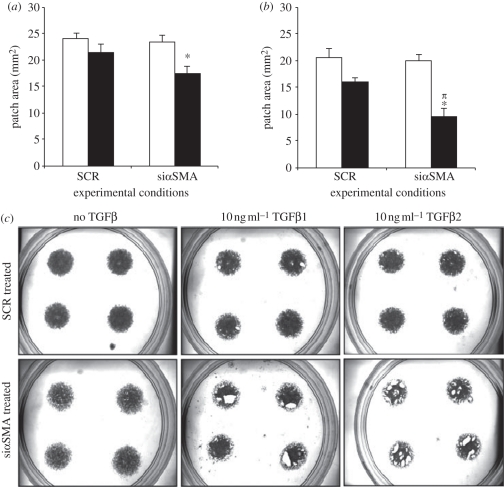
Figure 8.
αSMA is not critical for TGFβ-induced matrix contraction: 24 h patch assay analysis. FHL 124 cells were seeded to form patches, then transfected with siRNA targeted to αSMA (thus reducing αSMA expression) or a scrambled (SCR) negative control and maintained in EMEM supplemented with 2%FCS. Patches were measured after 24 h of culture in control conditions or exposed to 10 ng ml−1 (a) TGFβ1 or (b) TGFβ2. Data represent the mean ± SEM (n = 4). *Significant difference between treated and untreated siαSMA (p ≤ 0.05, ANOVA with the Tukey test); πsignificant difference between siαSMA + TGFβ treated groups and siSCR + TGFβ-treated groups (p ≤ 0.05, ANOVA with the Tukey test). (c) Representative images of dishes for each experimental group ((a) open bar, −TGFβ1; filled black bar, +TGFβ1, (b) open bar, −TGFβ2; filled black bar, +TGFβ2). Data previously published by Dawes et al. [5] with permission from the copyright holder, the Association for Research in Vision and Ophthalmology (ARVO).
Figure 9.
Putative pathways relating TGFβ to fibrotic events, (a) based on conventional dogma and (b) incorporating novel findings that challenge this view. Coll, collagen; FN, fibronectin.
To understand the development of fibrotic pathologies it is of fundamental importance to ascertain the mechanisms by which TGFβ signals mediate the cellular events of transdifferentiation, matrix contraction and ECM synthesis. A body of evidence has been obtained in recent years concerning TGFβ signal transduction pathways (for comprehensive reviews on TGFβ signalling see references [67,68]). The major intracellular signalling system identified for TGFβ is through translocation of Smad proteins. TGFβ initiates its response through a complex of high-affinity cell surface receptors consisting of two type I and two type II transmembrane serine/threonine kinase receptors [69]. TGFβ ligand binding links the constitutively active type II receptor kinases to the dormant type I receptor kinases, allowing the type II receptor to phosphorylate the cytoplasmic domain of the type I. The activated type I receptor recruits and phosphorylates receptor regulated Smads (R-Smads) at their C-terminus enabling them to complex with the common-mediator (Co) Smad Smad4. The R-Smads Smad2 and Smad3 are commonly associated with mediating TGFβ signals [68], although recently TGFβ-induced phosphorylation of R-Smad1/5, associated with bone morphogenetic protein, has been demonstrated in non-ocular epithelial cells [70]. The R-Smad/Smad4 heteromeric complex is free to translocate from the cell cytoplasm to the nucleus and is competent to associate with transcriptional co-activators and co-repressors to direct specific transcriptional responses [68]. R-Smad nuclear translocation has been reported to occur independently of Smad4 binding in both lenticular and non-lenticular cell types [6,71,72]. TGFβ can terminate the induction of its own target genes by recruiting Smad7, which negatively regulates signalling strength and duration, through formation of a negative-feedback loop [73].
A body of evidence has been obtained regarding the involvement of the classical TGFβ/Smad signalling pathway in the pathogenesis of fibrotic disorders of the lens [6,13,14,19]. Work by Saika et al. [19] has shown Smad3 and Smad4 to be present in cell nuclei of post-operative human lenses and in injury-induced epithelial to mesenchymal transition (EMT) murine lenses [74], therefore implicating activation by TGFβ following trauma. The activation of Smad3/4 by endogenous TGFβ was confirmed in injury-induced fibrosis of murine lenses where the application of TGFβ neutralizing antibodies prior to lens injury inhibited the translocation of Smad3/4 to lens cell nuclei [74]. Smad3 rather than Smad2 dependent signalling is widely implicated in fibrotic conditions throughout the body [75]; for example, elevated levels of activated Smad3 have been detected in models of bleomycin-induced fibrosis, hepatic stellate cell (HSC) activation and the leading edge of scleroderma lesions [76–78]. Therefore, in more recent years Smad3 knockout transgenic models have been employed to elucidate the importance of TGFβ/Smad3-dependent pathway in regulating the cellular changes that lead to fibrosis of the lens [13,14] and throughout the body [76]. A key focus of research in the context of lens fibrosis has been the role of Smad3 signalling in the process of EMT. In particular, Saika et al. [14] observed that both TGFβ2-induced and injury-induced EMT and αSMA expression were inhibited in the lens epithelium of Smad3 knockout mice. Furthermore, Banh et al. [13] observed αSMA expression in a TGFβ1/Smad3 knockout mouse, although the level of αSMA expression was reduced compared to wild-type lenses. Loss of Smad3 in mice also reduces the expression of type I collagen following trauma to the lens [14]. The reduction in TGFβ-induced fibrosis, detected by EMT and collagen I synthesis, in Smad3 knockout mice is not exclusive to the lens as these models show reduced scarring of the skin following irradiation [75], protection from renal tubulointerstitial fibrosis [79] and are resistant to bleomycin-induced pulmonary fibrosis [80]. Concurrent with the aforementioned studies, Dawes et al. [6] detected a significant reduction in expression of TGFβ-induced fibrotic markers αSMA and fibronectin in a Smad4 knockdown human lens epithelial cell line, therefore suggesting TGFβ Smad3/Smad4 signalling may regulate transdifferentiation of human lens epithelial cells. Most interestingly, this study found TGFβ-induced matrix contraction and Smad7 expression to be independent of Smad4 expression, suggesting that TGFβ/Smad independent pathways may also regulate lens fibrosis.
Increasing evidence suggests that TGFβ can signal independently of Smad function [67]. For instance, TGFβ type I receptor can phosphorylate both serine and tyrosine residues in the SHCA adaptor, recruiting adaptor protein GRB2 and the Ras guanine exchange factor (GEF) son of sevenless (SOS) to activate Ras-Raf-MEK-ERK mitogen-activated protein (MAP) kinase in mammalian cells [81]. The tyrosine kinase Src can phosphorylate the cytoplasmic domain of the TGFβRII leading to GRB2 and SHC recruitment enabling the activation of the p38 MAP kinase pathway [82]. Moreover the JNK MAP kinase and Rho kinase signalling pathways can be activated by TGFβ [83]. In the context of lens fibrosis, Smad-independent signalling pathways ERK and p38 MAP kinase are activated by TGFβ in human lens epithelial cells [6]. The notion that TGFβ-induced matrix contraction by human lens epithelial cells is mediated by Smad-independent signalling [6] is supported by investigations in non-ocular systems where Rho/Rho kinase [84], ERK [85] and p38 MAPK [86] promote matrix contraction. Moreover, the ERK signalling pathways can promote matrix contraction by activation of myosin light chain kinase, a key enzymatic regulator of contractile force [85]. Therefore, with respect to lens fibrosis, the role of Smad-independent signalling pathways in TGFβ-induced matrix contraction needs further investigation to underpin the regulatory TGFβ mechanism controlling this detrimental fibrotic characteristic.
Growing evidence also suggests that TGFβ Smad-independent pathways can mediate fibrotic events throughout the body. The production of the ECM protein fibronectin is dependent on TGFβ–p38 MAP kinase signalling in human proximal tubular epithelial cells [87] and TGFβ–JNK MAP kinase signalling in a human fibrosarcoma cell line [88]. Moreover, TGFβ Smad-independent MEK/ERK MAP kinase pathway has been implicated in the pathogenesis of scleroderma [89] and Rho kinase inhibitors have been shown to diminish fibrosis, detected by reduced presence of transdifferentiation markers and ECM proteins, in experimental models of vascular fibrosis and renal damage [90–92]. Most interestingly, there is evidence of cross talk between the Smad and MAP kinase pathways in experimental models of fibrosis. For example, TGFβ-induced angiotensin receptor expression in pulmonary fibrosis [93] and αSMA expression in renal proximal tubular cells [87] involve Smad-dependent and -independent MAP kinase signalling pathways. The rationale for cross talk between TGFβ signalling pathways is based on the MAP kinase cascade modifying Smad activity by targeting phosphorylation sites in the linker regions of Smad3 and Smad4 [94].
The Wnt signalling pathway has also been implicated in fibrotic disorders and is reported to play a key role in TGFβ-induced transdifferentiation [95–98]. The canonical Wnt signalling pathway regulates β-catenin activity. Wnt ligand binding to receptors (Frizzled) and co-receptor low-density lipoprotein receptor-related protein (LRP) leads to the phosphorylation of LRP6, by glycogen synthase kinase-3β (GSK-3β) and casein kinase γ, in its cytoplasmic region. This leads to the recruitment of cytosolic proteins, dishevelled and axin [96]. As a result of the association of GSK-3β with Frizzled receptors and LRP, β-catenin phosphorylation is reduced. In this form, β-catenin is no longer targeted for degradation and consequently accumulates in the cytoplasm before translocating to the nucleus where it associates with DNA binding factors Terhorst T cell factor (TCF) and lymphocyte enhancer factor (LEF) to regulate gene expression [96]. TGFβ is believed to influence this pathway through Smad-dependent and Smad-independent mechanisms. Smad-mediated signalling can activate integrin like kinase, which leads to GSK and Akt phosphorylation; this results in β-catenin nuclear translocation [98]. Smad-independent Akt phosphorylation can also suppress GSK activity and promote β-catenin translocation [98]. In both cases the result is to promote transdifferentiation. A report using whole rat lens cultures exposed to TGFβ2 and transgenic mice over expressing TGFβ1 demonstrated increased level of Wnt ligands and Frizzled receptors compared with controls; moreover, this was associated with increased accumulation of β-catenin in the nucleus of cataract forming cells and expression of αSMA/plaque formation [99].
It is therefore of great importance for future research in lens fibrosis to focus on disseminating the importance of both Smad-independent and Smad-dependent pathways and the interactions between them in the cellular events that give rise to fibrosis. The knowledge gained will aid the development of therapeutic drugs to prevent ASC and PCO formation.
6. Extracellular matrix production and deposition
The ECM, once believed to be a static structure, acting as a scaffold to maintain tissue integrity, regulates many aspects of cell function including growth, proliferation and differentiation. Modifications of the ECM by increased synthesis and deposition of ECM proteins is fundamental to the pathogenesis of fibrotic pathologies [1]. Lens epithelial cells and lens fibres are associated with a basement membrane termed the lens capsule, which is predominantly composed of collagen IV, along with laminin, heparin sulphate proteoglycans and tenascin [100]. These ECM components maintain the structural integrity of the lens capsule, enabling lens cell attachment and migration. During the pathogenesis of ASC and PCO, lens cells aberrantly synthesize and deposit new ECM components that contribute to the formation of fibrotic plaques and light scattering regions; the deposited components include fibronectin, vitronectin and collagen types I and III [101]. These ECM proteins are commonly associated with fibrosis and wound healing in many systems throughout the body. For example in patients with scleroderma, an abnormal accumulation of collagens I and III is the most prominent pathological manifestation of the disease in the skin [102]. The excessive accumulation of ECM constituents on the basement membrane of the lens capsule can influence cell behaviour and in particular EMT and cell migration, thus promoting fibrotic pathologies.
The ECM constituents vitronectin and fibronectin have been investigated in PCO-related events [103]. It has also been reported that cells from explant cultures adhere and migrate on vitronectin and fibronectin matrices, which was associated with increased αSMA staining and a more elongated/fibroblast-like cell appearance [103]. Most interestingly, the ED-A domain of fibronectin has been proposed to be crucial for the induction of αSMA expression [104]; application of an inhibiting RGD peptide to corneal fibroblasts, preventing the interaction of fibronectin and its integrin receptor, suppressed αSMA expression. Therefore, fibronectin expression may be of importance to the transdifferentiation events that occur during lens fibrosis. Furthermore, investigations by Saika et al. [105] identified the expression of the proteoglycan lumican in post-mortem capsular bag specimens. Additional investigations employing lumican knockout mice revealed that following trauma by a needle puncture injury of the anterior lens capsule, there was a delay in αSMA expression and appearance of transdifferentiated cells [105]. These studies are consistent with the notion that modifications in ECM constituents influencing lens cell behaviour enable the development of fibrotic pathologies. Similar modifications to the basement membrane and subsequent changes to cell behaviour are observed during the development of liver fibrosis [106]. HSCs are affected by ECM content; in the normal liver quiescent HSCs are surrounded by a basement membrane-like matrix [107]. In response to injury the composition of the ECM changes towards fibrillar collagen which promotes HSCs to transdifferentiate.
Secreted protein acidic and rich in cysteine (SPARC) may play a role in wound healing, tumour progression and cell proliferation by influencing the expression of ECM proteins [108]. Modifications of SPARC expression have particular relevance to lens fibrosis. SPARC-null mice have been shown to develop cataracts by 3-4 months of age due to disruptions in lens cell growth [109]. Investigations comparing lens cells derived from wild-type mice and SPARC knockout mice show SPARC expression to decrease laminin deposition [110]. A follow-on study also tested the effects of TGFβ in lens cells from wild-type and SPARC null mice [111]. Interestingly, TGFβ-induced expression of both fibronectin and αSMA was evident in both groups, but the greatest level observed was in the SPARC null group, suggesting that SPARC can suppress to some degree TGFβ-induced transdifferentiation. In addition, dexamethasone-increased SPARC expression of lens cells was shown to correlate with a reduced level of fibronectin and collagen type IV, therefore suggesting SPARC may modulate the lens ECM to suppress transdifferentiation events; thus, maintaining high levels of SPARC expression following cataract surgery may be of benefit in the prevention of PCO. However, with respect to other fibrotic pathologies increasing SPARC expression may be counter-productive as collagen accumulation is shown to be decreased in SPARC null mice with bleomycin-induced pulmonary fibrosis and SPARC expression is induced following lung injury, implicating SPARC in the development of pulmonary fibrosis [112].
Matrix components play an important role in the function of growth factors. FGF and hepatocyte growth factor (HGF), for example, require heparin binding to facilitate interaction with their corresponding receptor; therefore, distribution of heparin sulphate proteoglycans in association with the lens capsule matrix could play a critical role in FGF and HGF mediated events [113–115]. Growth factors such as FGF can exacerbate the effects of TGFβ [116], which is also known to bind to several matrix components including decorin and collagen type IV [117]. As the level of many growth factors are elevated following surgery, the accumulation of these proteins in association with the capsule is likely to increase accordingly [33,118]. Slow release resulting from proteolytic cleavage of this reservoir of potential modulators of cell function is therefore likely to influence fibrotic progression for significant periods.
7. Proteolytic regulation of the extracellular matrix
Proteases are a group of endogenous enzymes that through the process of proteolysis hydrolyse peptide bonds of proteins. Matrix metalloproteinases (MMPs), calpains, cathepsins the ubiquitin–proteosome pathway and caspases are just a small number of the different types of proteases that can contribute to development and disease [119]. For the purpose of this current review we will concentrate on the role of MMPs, which are commonly implicated in many fibrotic disorders.
The MMPs belong to the larger family of proteases known as the metzincin superfamily. There are 26 MMPs including six membrane tethered (MT)-MMPs in addition to four natural MMP inhibitors, the tissue inhibitors of MMPs (TIMPs). The MMP and TIMP family members can act on multiple targets and perform a variety of functions which maintain and remodel tissue architecture [120].
Elevated levels of MMPs are often associated with pathological disorders despite the fundamental functions MMPs maintain in normal biological processes such as embryogenesis, the creation of space in order for cells to migrate, and regulation of intracellular connection and signalling cascades [121]. However, the involvement of MMPs in disease cannot be ignored and their direct correlation in conditions such as arthritis, cancer, nephritis and importantly fibrosis has been significantly documented. The involvement of MMPs in fibrosis seems counterintuitive as the primary role of MMPs is to breakdown matrix and fibrosis is essentially an accrual of copious ECM. However alternative functions for MMPs include the ability to release growth factors and cytokines such as FGF-2 and TGFβ from supporting matrix and activation of macrophages that have direct roles in fibrosis development [58,122,123].
Numerous fibrotic diseases have MMP involvement. Models for ischaemic injury in normal rat kidney cells have shown that MT1-MMP causes E-cadherin cleavage and a decrease in N-cadherin level; this results in a loss of epithelial cells in the proximal tubules [124]. Ischaemic attack is the principal cause of acute renal failure, leaving only the basement membrane for filtration yielding filtrate return and tubular obstruction. MT1-MMP (MMP-14) is a MT-MMP that is involved in the activation of pro-form MMP-2; increased MMP-2 expression is crucial in the development of chronic kidney disease. In a transgenic mouse model, over-expression of MMP-2 in renal proximal tubular cells transformed the basement membrane instigating tubular EMT with resultant tubular atrophy, fibrosis and renal failure [125]. This study indicated that MMP-2 alone without wounding was enough to induce glomerulosclerosis, interstitial fibrosis, tubular atrophy and renal failure.
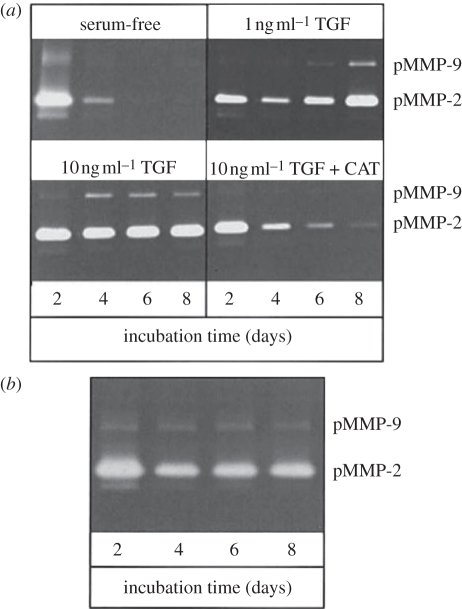
MMPs have also been implicated in complications of the lens. Studies by Dwivedi et al. [126] demonstrated that both TGFβ and MMPs play important roles in the formation of ASC plaques. Whole rat lenses cultured in the presence of TGFβ2 alone developed numerous distinct opacities on the anterior surface of the lens that contained αSMA expression and multilayering of cells [126]. Co-treatment of lenses with TGFβ2 and the broad-spectrum MMP inhibitor GM6001 prevented the formation of TGFβ2-mediated opacities, abrogated the expression of αSMA and inhibited cellular multilayering [126]. Furthermore, analysis of conditioned media from the whole lens cultures showed that pro-form and active MMP-2 was initiated by the presence of TGFβ2; the pro-form of MMP-9 was also elevated along with fragmented E-cadherin. These changes were attenuated by MMP inhibitors [126]. As observed in rat ASC in vitro models, TGFβ2 also augments the level of both MMP-2 and -9 in the media of in vitro human capsular bag cultures (figure 10) [17]; importantly, both MMP-2 and -9 are able to degrade collagen type IV [127], the main constituent of the lens capsule. Neutralization of TGFβ2 with CAT-152 (a human monoclonal anti-TGFβ2 antibody) prevented these TGFβ2-induced events [17]. Interestingly, bathing media obtained from culture of ex vivo capsular bags (donor lenses from patients who have previously had cataract operations) in serum-free media also revealed expression of MMP-2 and -9, indicating that ex vivo capsular bags secrete MMPs in a manner similar to TGFβ2 -treated cultures (figure 10) [17].
Figure 10.
Detection of MMP-2 and -9 in culture media of (a) in vitro capsular bags and (b) cultured ex vivo specimens, by gelatin zymography. Analysis of in vitro capsular bags was performed on the media of three cultures. Data are representative of the pattern observed in all cases in each group. In the case of cultured ex vivo specimens, the medium from one additional culture was analysed and showed a profile similar to the presented data. Data previously published by Wormstone et al. [17] with permission from the copyright holder, the Association for Research in Vision and Ophthalmology (ARVO).
Furthermore, Wong et al. [128] found that released MMP-2 and -9 following sham cataract operations on human lenses was inhibited by application of 100 µM Ilomostat (a broad-specrum MMP inhibitor). Ilomostat significantly reduced cell migration and capsular bag wrinkling. Additionally, radiolabel binding analysis demonstrates that the lens capsule is a store for TGFβ2 [33] along with FGF [123], and insulin-like growth factor [122,129]. As MMPs can function to release growth factors from the ECM it is reasonable to suggest that sustained levels can increase the concentration of cytokines available to lens cells over extended periods. This could therefore act as a link between short-term inflammation and progressive lens fibrosis.
8. Extracellular matrix/cell communication—integrins
The interaction of the ECM with the cell environment is mediated by a group of distinct cell surface receptors termed integrins which are composed of α and β subunits. The interaction of specific integrins with their respective ECM ligands enables cells to adhere to and migrate across the ECM [130]. Moreover, integrins function as cell signalling centres, transducing signals to and from the microenvironment [130]; therefore, any altered regulation of integrin expression or function can promote the development of fibrotic disease.
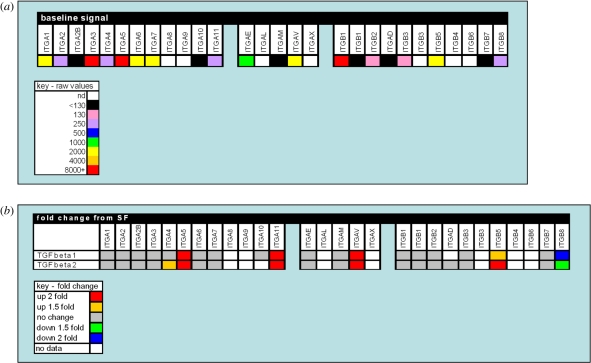
With respect to the lens, Dawes et al. [30] performed a microarray analysis of the human lens epithelial cell line FHL 124 to determine those integrins that are expressed in the human lens and identify those of potential importance to the formation of fibrotic lens pathologies (figure 11). This study revealed that the integrin β1, β2, β3, β5 and β6 subunits are expressed by human lens epithelial cells, with the integrin β1 subunit being the most abundantly expressed. β1 integrin is the most diverse integrin subunit as it can associate with 12 different α integrin chains [131], thus is the most widely expressed integrin in numerous cell types throughout the body [131]. With respect to the alpha sub-units expressed by human lens epithelial cells, α1, α2, α3, α4, α5, α6, α7, α10, α11, αE, αM and αV have been detected [30]. Treatment of human lens epithelial cells with the profibrotic cytokine TGFβ notably increased the expression of α5, α11, αV and β5 integrin subunits.
Figure 11.
A gene expression profile of integrins in FHL 124 cells. (a) The baseline signal of integrin gene expression detected in non-stimulated serum-free controls using oligonucleotide microarrays. (b) Changes detected in gene expression of integrins following 24 hr culture in TGFβ (10 ng ml−1) conditions using oligonucleotide microarrays. The data are presented in a colorimetric form to indicate (a) level detected and (b) fold change. Data previously published by Dawes et al. [30] with permission from Molecular Vision.
Of particular interest to ASC and PCO development is the expression of α5β1 integrin, which along with its matrix ligand fibronectin is significantly upregulated by TGFβ in both the human capsular bag and lens epithelial cell line [5,30]. The interaction between α5β1 integrin and fibronectin can regulate the expression of αSMA and transdifferentiation of corneal fibroblast cells to myofibroblasts [132]. Moreover, it has been proposed that α5β1 integrin and fibronectin form a putative contractile apparatus with αSMA [132,133]. A recent study has investigated the putative role of fibronectin/fibronectin receptor interaction in relation to TGFβ-induced matrix contraction by human lens epithelial cells [5]; application of an RGDS peptide to block the RGD binding site of α5 integrin revealed that the fibronectin/fibronectin receptor interaction was not required to promote matrix contraction by TGFβ [5]. In addition, Marcantonio et al. [134] demonstrated that α5β1 integrin expression was altered in FHL 124 cells following TGFβ exposure, such that a diffuse pattern across the cell was observed, with membrane bound α5β1 integrin having no association with actin filaments. The aforementioned investigations therefore suggest that in the human lens the distribution of α5β1 integrin in response to TGFβ could provide multiple sites of attachment to the underlying matrix to negatively regulate matrix contraction. Abnormal expression of α5β1 integrin plays an important role in the pathogenesis of fibrosis throughout the body. An upregulation in α5β1 integrin expression has been detected in pulmonary tissue sections from patients with idiopathic pulmonary fibrosis [135] and in interstitial fibroblasts in progressive renal fibrosis [136]. In non-ocular systems the importance of the role of integrins in mediating matrix fibrotic events has been widely investigated. With respect to the regulation of matrix contraction in the pathogenesis of fibrosis, it has been suggested that contraction of collagen gels by renal fibroblasts is dependent on β1 integrin [137]; moreover, α1 integrin deficient mice develop severe glomerulosclerosis [138]. The regulation of matrix contraction by the collagen receptors α1β1 integrin along with α2β1 integrin in fibrotic disease remains an intriguing possibility and an interesting area for future investigation with respect to ASC and PCO.
The integrin αVβ5 is of importance in the formation of fibrotic pathologies due to its role in enabling the mechanotransduction of signals from extracellular microenvironments that influence cell behaviour [139]. With respect to fibrosis, αVβ5 integrin is of importance in the transdifferentiation of cells to myofibroblasts [140]. Moreover, αVβ5 integrin mediates the release of TGFβ from lung myofibroblasts undergoing contraction [141]. The expression of αVβ5 integrin is upregulated in scleroderma fibroblasts [142] and interstitial renal fibroblasts [136]. With respect to the lens, TGFβ treatment of lens epithelial cells promotes αVβ5 expression [30] and is a potent inducer of transdifferentiation of lens epithelial cells to myofibroblasts [5,17]. These investigations have led to speculation over the bidirectional role of αVβ5 integrin in the formation of lens fibrosis [143], which could occur through (i) mediating transdifferentiation induced by increasing levels of active TGFβ that result from trauma to the lens or (ii) mediating signals from myofibroblasts back to the ECM that activate matrix-associated TGFβ. Injury by mechanical trauma can modulate integrin expression, which has particular relevance to fibrotic conditions that occur following surgery such as PCO. An interesting study was carried out by Sponer et al. [144] using the human capsular bag system which demonstrated that αVβ6 integrin is increased in capsular bags cultured in protein-free medium compared with cultured intact lenses. Similarly, in non-ocular fibrotic diseases αVβ6 integrin is increased following acute biliary injury [145]. αVβ6 integrin activates TGFβ through its association with an RGD peptide in the latency associated peptide [146]; the importance of αVβ6 integrin to TGFβ activation in fibrotic disease was confirmed in β6 null mice which fail to develop a fibrotic response following biliary injury [145]. Therefore, integrin αVβ6 and αVβ5 may have essential roles in sustaining TGFβ activation, thus enabling the initiation of fibrotic events that follow localized tissue injury to both the lens and throughout the rest of the body.
Weaver et al. [147] have shown that integrin linked kinase (ILK), a serine threonine kinase that binds to the cytoplasmic tail of β1, 2 and 3 integrin subunits is present in both mouse and human lens cells. ILK is a multidomain focal adhesion protein that is an important regulator of both ECM adhesion and signal transduction. Studies suggest ILK to be an important mediator of EMT [11,147,148]; for instance, ILK expression of cultured mouse and human lens cells correlates with increased levels of transdifferentiation markers fibronectin and αSMA. Moreover, transfection of lens epithelial cells with ILK-expressing constructs induces a morphological change that resembles EMT [11]. Similarly, a role for ILK in mediating EMT in renal fibrosis has been proposed [148]. Interestingly, ILK has been reported to co-localize with α5β1 integrin, which was enhanced by the presence of fibronectin [147]. Therefore, the role of ILK in regulating EMT via interaction with fibronectin–α5β1 integrin remains an interesting area of study with respect to the pathogenesis of fibrosis.
9. Summary
Fibrotic disorders of the lens affect millions. The unique biological properties of the lens allow it to serve as an exquisite biological tool to investigate the processes that underpin fibrosis. Employing the lens as a study system, we can observe hyperproliferation following injury, matrix production/deposition, matrix contraction and transdifferentiation to myofibroblasts. Using a variety of strategies employing cell lines, transgenic animals and tissue culture models, a number of pathways driving lens fibrosis are starting to emerge. The lens is therefore an excellent experimental model system to investigate tissue fibrosis per se.
Acknowledgements
The authors would like to thank all members of the Norwich Eye Research Group, both past and present, who have contributed greatly to its reputation and success; in particular we would like to thank the late Prof. George Duncan for being an inspiring mentor and friend. Moreover, we must thank our colleagues at the Norfolk and Norwich University Hospital for their continued collaborations and the wonderful work carried out by Pamela Keeley and her team at the NNUH eye bank.
Footnotes
One contribution of 10 to a Theme Issue ‘The ocular lens: a classic model for development, physiology and disease’.
References
- 1.Leask A., Abraham D. J. 2004. TGF-beta signaling and the fibrotic response. FASEB J. 18, 816–827 10.1096/fj.03-1273rev (doi:10.1096/fj.03-1273rev) [DOI] [PubMed] [Google Scholar]
- 2.Radisky D. C., Przybylo J. A. 2008. Matrix metalloproteinase-induced fibrosis and malignancy in breast and lung. Proc. Am. Thorac. Soc. 5, 316–322 10.1513/pats.200711-166DR (doi:10.1513/pats.200711-166DR) [DOI] [PubMed] [Google Scholar]
- 3.Laurent G. J., McAnulty R. J., Hill M., Chambers R. 2008. Escape from the matrix: multiple mechanisms for fibroblast activation in pulmonary fibrosis. Proc. Am. Thorac. Soc. 5, 311–315 10.1513/pats.200710-159DR (doi:10.1513/pats.200710-159DR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAnulty R. J. 2007. Fibroblasts and myofibroblasts: their source, function and role in disease. Int. J. Biochem. Cell Biol. 39, 666–671 10.1016/j.biocel.2006.11.005 (doi:10.1016/j.biocel.2006.11.005) [DOI] [PubMed] [Google Scholar]
- 5.Dawes L. J., Eldred J. A., Anderson I. K., Sleeman M., Reddan J. R., Duncan G., Wormstone I. M. 2008. TGF beta-induced contraction is not promoted by fibronectin–fibronectin receptor interaction, or alpha SMA expression. Invest. Ophthalmol. Vis. Sci. 49, 650–661 10.1167/iovs.07-0586 (doi:10.1167/iovs.07-0586) [DOI] [PubMed] [Google Scholar]
- 6.Dawes L. J., Sleeman M. A., Anderson I. K., Reddan J. R., Wormstone I. M. 2009. TGFbeta/Smad4-dependent and -independent regulation of human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 50, 5318–5327 10.1167/iovs.08-3223 (doi:10.1167/iovs.08-3223) [DOI] [PubMed] [Google Scholar]
- 7.Liu T., Warburton R. R., Guevara O. E., Hill N. S., Fanburg B. L., Gaestel M., Kayyali U. S. 2007. Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 37, 507–517 10.1165/rcmb.2007-0077OC (doi:10.1165/rcmb.2007-0077OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan G. 2001. Physiology of the lens. In Duane's clinical ophthalmology (ed. Tasman W.), pp. 1–20 Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- 9.Blixt A., Mahlapuu M., Aitola M., Pelto-Huikko M., Enerback S., Carlsson P. 2000. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 14, 245–254 10.1101/gad.14.2.245 (doi:10.1101/gad.14.2.245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashery-Padan R., Gruss P. 2001. Pax6 lights-up the way for eye development. Curr. Opin. Cell Biol. 13, 706–714 10.1016/S0955-0674(00)00274-X (doi:10.1016/S0955-0674(00)00274-X) [DOI] [PubMed] [Google Scholar]
- 11.de Iongh R. U., Wederell E., Lovicu F. J., McAvoy J. W. 2005. Transforming growth factor-beta-induced epithelial–mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs 179, 43–55 10.1159/000084508 (doi:10.1159/000084508) [DOI] [PubMed] [Google Scholar]
- 12.Wormstone I. M., Tamiya S., Eldred J. A., Lazaridis K., Chantry A., Reddan J. R., Anderson I., Duncan G. 2004. Characterisation of TGF-beta2 signalling and function in a human lens cell line. Exp. Eye Res. 78, 705–714 10.1016/j.exer.2003.08.006 (doi:10.1016/j.exer.2003.08.006) [DOI] [PubMed] [Google Scholar]
- 13.Banh A., Deschamps P. A., Gauldie J., Overbeek P. A., Sivak J. G., West-Mays J. A. 2006. Lens-specific expression of TGF-beta induces anterior subcapsular cataract formation in the absence of Smad3. Invest. Ophthalmol. Vis. Sci. 47, 3450–3460 10.1167/iovs.05-1208 (doi:10.1167/iovs.05-1208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saika S., et al. 2004. Smad3 signaling is required for epithelial–mesenchymal transition of lens epithelium after injury. Am. J. Pathol. 164, 651–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hales A. M., Schulz M. W., Chamberlain C. G., McAvoy J. W. 1994. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr. Eye Res. 13, 885–890 10.3109/02713689409015091 (doi:10.3109/02713689409015091) [DOI] [PubMed] [Google Scholar]
- 16.West-Mays J. A., Pino G., Lovicu F. J. Development and use of the lens epithelial explant system to study lens differentiation and cataractogenesis. Progr. Retin. Eye Res. 29, 135–143 10.1016/j.preteyeres.2009.12.001 (doi:10.1016/j.preteyeres.2009.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wormstone I. M., Tamiya S., Anderson I., Duncan G. 2002. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest. Ophthalmol. Vis. Sci. 43, 2301–2308 [PubMed] [Google Scholar]
- 18.Marcantonio J. M., Rakic J. M., Vrensen G. F., Duncan G. 2000. Lens cell populations studied in human donor capsular bags with implanted intraocular lenses. Invest. Ophthalmol. Vis. Sci. 41, 1130–1141 [PubMed] [Google Scholar]
- 19.Saika S., Miyamoto T., Ishida I., Shirai K., Ohnishi Y., Ooshima A., McAvoy J. W. 2002. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br. J. Ophthalmol. 86, 1428–1433 10.1136/bjo.86.12.1428 (doi:10.1136/bjo.86.12.1428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deane J. S., Hall A. B., Thompson J. R., Rosenthal A. R. 1997. Prevalence of lenticular abnormalities in a population-based study: Oxford clinical cataract grading in the Melton eye study. Ophthal. Epidemiol. 4, 195–206 10.3109/09286589709059193 (doi:10.3109/09286589709059193) [DOI] [PubMed] [Google Scholar]
- 21.Wormstone I. M., Wang L., Liu C. S. 2009. Posterior capsule opacification. Exp. Eye Res. 88, 257–269 10.1016/j.exer.2008.10.016 (doi:10.1016/j.exer.2008.10.016) [DOI] [PubMed] [Google Scholar]
- 22.Frost N. A., Sparrow J. M., Moore L. 2002. Associations of human crystalline lens retrodots and waterclefts with visual impairment: an observational study. Invest. Ophthalmol. Vis. Sci. 43, 2105–2109 [PubMed] [Google Scholar]
- 23.Lee E. H., Joo C. K. 1999. Role of transforming growth factor-beta in transdifferentiation and fibrosis of lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 40, 2025–2032 [PubMed] [Google Scholar]
- 24.Fisher R. F. 1981. The lens in uveitis. Trans. Ophthalmol. Soc. UK 101, 317–320 [PubMed] [Google Scholar]
- 25.Marcantonio J. M., Syam P. P., Liu C. S., Duncan G. 2003. Epithelial transdifferentiation and cataract in the human lens. Exp. Eye Res. 77, 339–346 10.1016/S0014-4835(03)00125-8 (doi:10.1016/S0014-4835(03)00125-8) [DOI] [PubMed] [Google Scholar]
- 26.Sasaki K., Kojima M., Nakaizumi H., Kitagawa K., Yamada Y., Ishizaki H. 1998. Early lens changes seen in patients with atopic dermatitis applying image analysis processing of Scheimpflug and specular microscopic images. Ophthalmologica 212, 88–94 10.1159/000027285 (doi:10.1159/000027285) [DOI] [PubMed] [Google Scholar]
- 27.Lovicu F. J., Schulz M. W., Hales A. M., Vincent L. N., Overbeek P. A., Chamberlain C. G., McAvoy J. W. 2002. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br. J. Ophthalmol. 86, 220–226 10.1136/bjo.86.2.220 (doi:10.1136/bjo.86.2.220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hales A. M., Chamberlain C. G., McAvoy J. W. 1995. Cataract induction in lenses cultured with transforming growth factor-beta. Invest. Ophthalmol. Vis. Sci. 36, 1709–1713 [PubMed] [Google Scholar]
- 29.Wormstone I. M. 2002. Posterior capsule opacification: a cell biological perspective. Exp. Eye Res. 74, 337–347 10.1006/exer.2001.1153 (doi:10.1006/exer.2001.1153) [DOI] [PubMed] [Google Scholar]
- 30.Dawes L. J., Elliott R. M., Reddan J. R., Wormstone Y. M., Wormstone I. M. 2007. Oligonucleotide microarray analysis of human lens epithelial cells: TGFbeta regulated gene expression. Mol. Vis. 13, 1181–1197 [PubMed] [Google Scholar]
- 31.Wormstone I. M., Tamiya S., Marcantonio J. M., Reddan J. R. 2000. Hepatocyte growth factor function and c-Met expression in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 41, 4216–4222 [PubMed] [Google Scholar]
- 32.Liu C. S., Wormstone I. M., Duncan G., Marcantonio J. M., Webb S. F., Davies P. D. 1996. A study of human lens cell growth in vitro. A model for posterior capsule opacification. Invest. Ophthalmol. Vis. Sci. 37, 906–914 [PubMed] [Google Scholar]
- 33.Wormstone I. M., Anderson I. K., Eldred J. A., Dawes L. J., Duncan G. 2006. Short-term exposure to transforming growth factor beta induces long-term fibrotic responses. Exp. Eye Res. 83, 1238–1245 10.1016/j.exer.2006.06.013 (doi:10.1016/j.exer.2006.06.013) [DOI] [PubMed] [Google Scholar]
- 34.Wormstone I. M., Liu C. S., Rakic J. M., Marcantonio J. M., Vrensen G. F., Duncan G. 1997. Human lens epithelial cell proliferation in a protein-free medium. Invest. Ophthalmol. Vis. Sci. 38, 396–404 [PubMed] [Google Scholar]
- 35.Behar-Cohen F. F., David T., D'Hermies F., Pouliquen Y. M., Buechler Y., Nova M. P., Houston L. L., Courtoisy Y. 1995. In vivo inhibition of lens regrowth by fibroblast growth factor 2-saporin. Invest. Ophthalmol. Vis. Sci. 36, 2434–2448 [PubMed] [Google Scholar]
- 36.Lois N., Dawson R., McKinnon A. D., Forrester J. V. 2003. A new model of posterior capsule opacification in rodents. Invest. Ophthalmol. Vis. Sci. 44, 3450–3457 10.1167/iovs.02-1293 (doi:10.1167/iovs.02-1293) [DOI] [PubMed] [Google Scholar]
- 37.Lois N., Taylor J., McKinnon A. D., Forrester J. V. 2005. Posterior capsule opacification in mice. Arch. Ophthalmol. 123, 71–77 10.1001/archopht.123.1.71 (doi:10.1001/archopht.123.1.71) [DOI] [PubMed] [Google Scholar]
- 38.Martinez F. O., Gordon S., Locati M., Mantovani A. 2006. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 39.Curnow S. J., et al. 2005. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest. Ophthalmol. Vis. Sci. 46, 4251–4259 10.1167/iovs.05-0444 (doi:10.1167/iovs.05-0444) [DOI] [PubMed] [Google Scholar]
- 40.van Laar J. A., van Hagen P. M. 2006. Cytokines in uveitis. Clin. Med. Res. 4, 248–249 10.3121/cmr.4.4.248 (doi:10.3121/cmr.4.4.248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zigler J. S., Jr, Gery I., Kessler D., Kinoshita J. H. 1983. Macrophage mediated damage to rat lenses in culture: a possible model for uveitis-associated cataract. Invest. Ophthalmol. Vis. Sci. 24, 651–654 [PubMed] [Google Scholar]
- 42.Keane M. P. 2008. The role of chemokines and cytokines in lung fibrosis. Eur. Respir. Rev. 17, 136–151 10.1183/09059180.00010908 (doi:10.1183/09059180.00010908) [DOI] [Google Scholar]
- 43.Bajwa E. K., Ayas N. T., Schulzer M., Mak E., Ryu J. H., Malhotra A. 2005. Interferon-gamma1b therapy in idiopathic pulmonary fibrosis: a metaanalysis. Chest 128, 203–206 10.1378/chest.128.1.203 (doi:10.1378/chest.128.1.203) [DOI] [PubMed] [Google Scholar]
- 44.Ghosh A. K. 2002. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp. Biol. Med. (Maywood) 227, 301–314 [DOI] [PubMed] [Google Scholar]
- 45.Postlethwaite A. E., Holness M. A., Katai H., Raghow R. 1992. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J. Clin. Invest. 90, 1479–1485 10.1172/JCI116015 (doi:10.1172/JCI116015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varga J., Abraham D. 2007. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J. Clin. Invest. 117, 557–567 10.1172/JCI31139 (doi:10.1172/JCI31139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka H., Komai M., Nagao K., Ishizaki M., Kajiwara D., Takatsu K., Delespesse G., Nagai H. 2004. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am. J. Respir. Cell Mol. Biol. 31, 62–68 10.1165/rcmb.2003-0305OC (doi:10.1165/rcmb.2003-0305OC) [DOI] [PubMed] [Google Scholar]
- 48.Lee C. G., et al. 2001. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J. Exp. Med. 194, 809–821 10.1084/jem.194.6.809 (doi:10.1084/jem.194.6.809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strieter R. M. 2008. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc. Am. Thorac. Soc. 5, 305–310 10.1513/pats.200710-160DR (doi:10.1513/pats.200710-160DR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laurell C. G., Zetterstrom C. 2002. Effects of dexamethasone, diclofenac, or placebo on the inflammatory response after cataract surgery. Br. J. Ophthalmol. 86, 1380–1384 10.1136/bjo.86.12.1380 (doi:10.1136/bjo.86.12.1380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Symonds J. G., Lovicu F. J., Chamberlain C. G. 2006. Differing effects of dexamethasone and diclofenac on posterior capsule opacification-like changes in a rat lens explant model. Exp. Eye Res. 83, 771–782 10.1016/j.exer.2006.03.017 (doi:10.1016/j.exer.2006.03.017) [DOI] [PubMed] [Google Scholar]
- 52.Chandler H. L., Barden C. A., Lu P., Kusewitt D. F., Colitz C. M. 2007. Prevention of posterior capsular opacification through cyclooxygenase-2 inhibition. Mol. Vis. 13, 677–691 [PMC free article] [PubMed] [Google Scholar]
- 53.Wormstone I. M., Del Rio-Tsonis K., McMahon G., Tamiya S., Davies P. D., Marcantonio J. M., Duncan G. 2001. FGF: an autocrine regulator of human lens cell growth independent of added stimuli. Invest. Ophthalmol. Vis. Sci. 42, 1305–1311 [PubMed] [Google Scholar]
- 54.Streilein J. W., Ohta K., Mo J. S., Taylor A. W. 2002. Ocular immune privilege and the impact of intraocular inflammation. DNA Cell Biol. 21, 453–459 10.1089/10445490260099746 (doi:10.1089/10445490260099746) [DOI] [PubMed] [Google Scholar]
- 55.Ohta K., Yamagami S., Taylor A. W., Streilein J. W. 2000. IL-6 antagonizes TGF-beta and abolishes immune privilege in eyes with endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 41, 2591–2599 [PubMed] [Google Scholar]
- 56.Schlotzer-Schrehardt U., Zenkel M., Kuchle M., Sakai L. Y., Naumann G. O. 2001. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp. Eye Res. 73, 765–780 10.1006/exer.2001.1084 (doi:10.1006/exer.2001.1084) [DOI] [PubMed] [Google Scholar]
- 57.Schulz M. W., Chamberlain C. G., McAvoy J. W. 1996. Inhibition of transforming growth factor-beta-induced cataractous changes in lens explants by ocular media and alpha 2-macroglobulin. Invest. Ophthalmol. Vis. Sci. 37, 1509–1519 [PubMed] [Google Scholar]
- 58.Yu Q., Stamenkovic I. 2000. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163–176 [PMC free article] [PubMed] [Google Scholar]
- 59.Frazier W. A. 1991. Thrombospondins. Curr. Opin. Cell Biol. 3, 792–799 10.1016/0955-0674(91)90052-Z (doi:10.1016/0955-0674(91)90052-Z) [DOI] [PubMed] [Google Scholar]
- 60.Chamberlain C. G., Mansfield K. J., Cerra A. 2009. Glutathione and catalase suppress TGFbeta-induced cataract-related changes in cultured rat lenses and lens epithelial explants. Mol. Vis. 15, 895–905 [PMC free article] [PubMed] [Google Scholar]
- 61.Fatma N., Kubo E., Sharma P., Beier D. R., Singh D. P. 2005. Impaired homeostasis and phenotypic abnormalities in Prdx6-/-mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ. 12, 734–750 10.1038/sj.cdd.4401597 (doi:10.1038/sj.cdd.4401597) [DOI] [PubMed] [Google Scholar]
- 62.Schmid P., Itin P., Cherry G., Bi C., Cox D. A. 1998. Enhanced expression of transforming growth factor-beta type I and type II receptors in wound granulation tissue and hypertrophic scar. Am. J. Pathol. 152, 485–493 [PMC free article] [PubMed] [Google Scholar]
- 63.Querfeld C., Eckes B., Huerkamp C., Krieg T., Sollberg S. 1999. Expression of TGF-beta 1, -beta 2 and -beta 3 in localized and systemic scleroderma. J. Dermatol. Sci. 21, 13–22 10.1016/S0923-1811(99)00008-0 (doi:10.1016/S0923-1811(99)00008-0) [DOI] [PubMed] [Google Scholar]
- 64.Saika S., Miyamoto T., Kawashima Y., Okada Y., Yamanaka O., Ohnishi Y., Ooshima A. 2000. Immunolocalization of TGF-beta1, -beta2, and -beta3, and TGF-beta receptors in human lens capsules with lens implants. Graefes Arch. Clin. Exp. Ophthalmol. 238, 283–293 10.1007/s004170050354 (doi:10.1007/s004170050354) [DOI] [PubMed] [Google Scholar]
- 65.Saika S., Yamanaka O., Okada Y., Tanaka S., Miyamoto T., Sumioka T., Kitano A., Shirai K., Ikeda K. 2009. TGF beta in fibroproliferative diseases in the eye. Front. Biosci. (Schol. Ed) 1, 376–390 [DOI] [PubMed] [Google Scholar]
- 66.Grinnell F. 1994. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 124, 401–404 10.1083/jcb.124.4.401 (doi:10.1083/jcb.124.4.401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Derynck R., Zhang Y. E. 2003. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577–584 10.1038/nature02006 (doi:10.1038/nature02006) [DOI] [PubMed] [Google Scholar]
- 68.Massague J. 2000. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 1, 169–178 [DOI] [PubMed] [Google Scholar]
- 69.Massague J. 1998. TGF-beta signal transduction. Annu. Rev. Biochem. 67, 753–791 10.1146/annurev.biochem.67.1.753 (doi:10.1146/annurev.biochem.67.1.753) [DOI] [PubMed] [Google Scholar]
- 70.Daly A. C., Randall R. A., Hill C. S. 2008. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol. Cell Biol. 28, 6889–6902 10.1128/MCB.01192-08 (doi:10.1128/MCB.01192-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu G. C., Dunn N. R., Anderson D. C., Oxburgh L., Robertson E. J. 2004. Differential requirements for Smad4 in TGFbeta-dependent patterning of the early mouse embryo. Development 131, 3501–3512 10.1242/dev.01248 (doi:10.1242/dev.01248) [DOI] [PubMed] [Google Scholar]
- 72.Wisotzkey R. G., Mehra A., Sutherland D. J., Dobens L. L., Liu X., Dohrmann C., Attisano L., Raftery L. A. 1998. Medea is a Drosophila Smad4 homolog that is differentially required to potentiate DPP responses. Development 125, 1433–1445 [DOI] [PubMed] [Google Scholar]
- 73.Itoh S., ten Dijke P. 2007. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr. Opin. Cell Biol. 19, 176–184 10.1016/j.ceb.2007.02.015 (doi:10.1016/j.ceb.2007.02.015) [DOI] [PubMed] [Google Scholar]
- 74.Saika S., Okada Y., Miyamoto T., Ohnishi Y., Ooshima A., McAvoy J. W. 2001. Smad translocation and growth suppression in lens epithelial cells by endogenous TGFbeta2 during wound repair. Exp. Eye Res. 72, 679–686 10.1006/exer.2001.1002 (doi:10.1006/exer.2001.1002) [DOI] [PubMed] [Google Scholar]
- 75.Flanders K. C., et al. 2003. Interference with transforming growth factor-beta/Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am. J. Pathol. 163, 2247–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu C., Gaca M. D., Swenson E. S., Vellucci V. F., Reiss M., Wells R. G. 2003. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J. Biol. Chem. 278, 11721–11728 10.1074/jbc.M207728200 (doi:10.1074/jbc.M207728200) [DOI] [PubMed] [Google Scholar]
- 77.Mori Y., Chen S. J., Varga J. 2003. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 48, 1964–1978 10.1002/art.11157 (doi:10.1002/art.11157) [DOI] [PubMed] [Google Scholar]
- 78.Takagawa S., Lakos G., Mori Y., Yamamoto T., Nishioka K., Varga J. 2003. Sustained activation of fibroblast transforming growth factor-beta/Smad signaling in a murine model of scleroderma. J. Invest. Dermatol. 121, 41–50 10.1046/j.1523-1747.2003.12308.x (doi:10.1046/j.1523-1747.2003.12308.x) [DOI] [PubMed] [Google Scholar]
- 79.Sato M., Muragaki Y., Saika S., Roberts A. B., Ooshima A. 2003. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 112, 1486–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao J., Shi W., Wang Y. L., Chen H., Bringas P., Jr, Datto M. B., Frederick J. P., Wang X. F., Warburton D. 2002. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L585–L593 [DOI] [PubMed] [Google Scholar]
- 81.Lee M. K., Pardoux C., Hall M. C., Lee P. S., Warburton D., Qing J., Smith S. M., Derynck R. 2007. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 26, 3957–3967 10.1038/sj.emboj.7601818 (doi:10.1038/sj.emboj.7601818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galliher A. J., Schiemann W. P. 2007. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 67, 3752–3758 10.1158/0008-5472.CAN-06-3851 (doi:10.1158/0008-5472.CAN-06-3851) [DOI] [PubMed] [Google Scholar]
- 83.Moustakas A., Heldin C. H. 2009. The regulation of TGFbeta signal transduction. Development 136, 3699–3714 10.1242/dev.030338 (doi:10.1242/dev.030338) [DOI] [PubMed] [Google Scholar]
- 84.Parizi M., Howard E. W., Tomasek J. J. 2000. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp. Cell Res. 254, 210–220 10.1006/excr.1999.4754 (doi:10.1006/excr.1999.4754) [DOI] [PubMed] [Google Scholar]
- 85.Klemke R. L., Cai S., Giannini A. L., Gallagher P. J., de Lanerolle P., Cheresh D. A. 1997. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137, 481–492 10.1083/jcb.137.2.481 (doi:10.1083/jcb.137.2.481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borbiev T., Birukova A., Liu F., Nurmukhambetova S., Gerthoffer W. T., Garcia J. G., Verin A. D. 2004. p38 MAP kinase-dependent regulation of endothelial cell permeability. Am. J. Physiol. Lung Cell Mol. Physiol. 287, L911–L918 10.1152/ajplung.00372.2003 (doi:10.1152/ajplung.00372.2003) [DOI] [PubMed] [Google Scholar]
- 87.Niculescu-Duvaz I., Phanish M. K., Colville-Nash P., Dockrell M. E. 2007. The TGFbeta1-induced fibronectin in human renal proximal tubular epithelial cells is p38 MAP kinase dependent and Smad independent. Nephron Exp. Nephrol. 105, e108–e116 10.1159/000100492 (doi:10.1159/000100492) [DOI] [PubMed] [Google Scholar]
- 88.Hocevar B. A., Brown T. L., Howe P. H. 1999. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 18, 1345–1356 10.1093/emboj/18.5.1345 (doi:10.1093/emboj/18.5.1345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhattacharyya S., et al. 2008. Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma. Am. J. Pathol. 173, 1085–1099 10.2353/ajpath.2008.080382 (doi:10.2353/ajpath.2008.080382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kataoka C., et al. 2002. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 39, 245–250 10.1161/hy0202.103271 (doi:10.1161/hy0202.103271) [DOI] [PubMed] [Google Scholar]
- 91.Nishikimi T., Akimoto K., Wang X., Mori Y., Tadokoro K., Ishikawa Y., Shimokawa H., Ono H., Matsuoka H. 2004. Fasudil, a Rho-kinase inhibitor, attenuates glomerulosclerosis in Dahl salt-sensitive rats. J. Hypertens. 22, 1787–1796 10.1097/00004872-200409000-00024 (doi:10.1097/00004872-200409000-00024) [DOI] [PubMed] [Google Scholar]
- 92.Ruperez M., Sanchez-Lopez E., Blanco-Colio L. M., Esteban V., Rodriguez-Vita J., Plaza J. J., Egido J., Ruiz-Ortega M. 2005. The Rho-kinase pathway regulates angiotensin II-induced renal damage. Kidney Int. Suppl. 68, S39–S45 10.1111/j.1523-1755.2005.09908.x (doi:10.1111/j.1523-1755.2005.09908.x) [DOI] [PubMed] [Google Scholar]
- 93.Martin M. M., Buckenberger J. A., Jiang J., Malana G. E., Knoell D. L., Feldman D. S., Elton T. S. 2007. TGF-beta1 stimulates human AT1 receptor expression in lung fibroblasts by cross talk between the Smad, p38 MAPK, JNK, and PI3K signaling pathways. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L790–L799 10.1152/ajplung.00099.2007 (doi:10.1152/ajplung.00099.2007) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Mulder K. M. 2000. Role of ras and mapks in TGFbeta signaling. Cytokine Growth Factor Rev. 11, 23–35 10.1016/S1359-6101(99)00026-X (doi:10.1016/S1359-6101(99)00026-X) [DOI] [PubMed] [Google Scholar]
- 95.Crosby L. M., Waters C. M. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 298, L715–L731 10.1152/ajplung.00361.2009 (doi:10.1152/ajplung.00361.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Konigshoff M., Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am. J. Respir. Cell Mol. Biol. 42, 21–31 10.1165/rcmb.2008-0485TR (doi:10.1165/rcmb.2008-0485TR) [DOI] [PubMed] [Google Scholar]
- 97.Liu Y. New insights into epithelial–mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 21, 212–222 10.1681/ASN.2008121226 (doi:10.1681/ASN.2008121226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willis B. C., Borok Z. 2007. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L525–L534 10.1152/ajplung.00163.2007 (doi:10.1152/ajplung.00163.2007) [DOI] [PubMed] [Google Scholar]
- 99.Chong C. C., Stump R. J., Lovicu F. J., McAvoy J. W. 2009. TGFbeta promotes Wnt expression during cataract development. Exp. Eye Res. 88, 307–313 10.1016/j.exer.2008.07.018 (doi:10.1016/j.exer.2008.07.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cammarata P. R., Cantu-Crouch D., Oakford L., Morrill A. 1986. Macromolecular organization of bovine lens capsule. Tissue Cell. 18, 83–97 10.1016/0040-8166(86)90009-1 (doi:10.1016/0040-8166(86)90009-1) [DOI] [PubMed] [Google Scholar]
- 101.Saika S., Miyamoto T., Ishida I., Barbour W. K., Ohnishi Y., Ooshima A. 2004. Accumulation of thrombospondin-1 in post-operative capsular fibrosis and its down-regulation in lens cells during lens fiber formation. Exp. Eye Res. 79, 147–156 10.1016/j.exer.2004.03.003 (doi:10.1016/j.exer.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 102.Krieg T., Takehara K. 2009. Skin disease: a cardinal feature of systemic sclerosis. Rheumatology (Oxford) 48(Suppl. 3), iii14–8 10.1093/rheumatology/kep108 (doi:10.1093/rheumatology/kep108) [DOI] [PubMed] [Google Scholar]
- 103.Taliana L., Evans M. D., Ang S., McAvoy J. W. 2006. Vitronectin is present in epithelial cells of the intact lens and promotes epithelial mesenchymal transition in lens epithelial explants. Mol. Vis. 12, 1233–1242 [PubMed] [Google Scholar]
- 104.Serini G., Bochaton-Piallat M. L., Ropraz P., Geinoz A., Borsi L., Zardi L., Gabbiani G. 1998. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J. Cell Biol. 142, 873–881 10.1083/jcb.142.3.873 (doi:10.1083/jcb.142.3.873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saika S., et al. 2003. Response of lens epithelial cells to injury: role of lumican in epithelial–mesenchymal transition. Invest. Ophthalmol. Vis. Sci. 44, 2094–2102 10.1167/iovs.02-1059 (doi:10.1167/iovs.02-1059) [DOI] [PubMed] [Google Scholar]
- 106.Schuppan D., Ruehl M., Somasundaram R., Hahn E. G. 2001. Matrix as a modulator of hepatic fibrogenesis. Semin. Liver Dis. 21, 351–372 10.1055/s-2001-17556 (doi:10.1055/s-2001-17556) [DOI] [PubMed] [Google Scholar]
- 107.Iredale J. P. 2007. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 117, 539–548 10.1172/JCI30542 (doi:10.1172/JCI30542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan Q., Sage E. H. 1999. SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. 47, 1495–1506 [DOI] [PubMed] [Google Scholar]
- 109.Bassuk J. A., Birkebak T., Rothmier J. D., Clark J. M., Bradshaw A., Muchowski P. J., Howe C. C., Clark J. I., Sage E. H. 1999. Disruption of the Sparc locus in mice alters the differentiation of lenticular epithelial cells and leads to cataract formation. Exp. Eye Res. 68, 321–331 10.1006/exer.1998.0608 (doi:10.1006/exer.1998.0608) [DOI] [PubMed] [Google Scholar]
- 110.Weaver M. S., Sage E. H., Yan Q. 2006. Absence of SPARC in lens epithelial cells results in altered adhesion and extracellular matrix production in vitro. J. Cell Biochem. 97, 423–432 10.1002/jcb.20654 (doi:10.1002/jcb.20654) [DOI] [PubMed] [Google Scholar]
- 111.Gotoh N., Perdue N. R., Matsushima H., Sage E. H., Yan Q., Clark J. I. 2007. An in vitro model of posterior capsular opacity: SPARC and TGF-beta2 minimize epithelial-to-mesenchymal transition in lens epithelium. Invest. Ophthalmol. Vis. Sci. 48, 4679–4687 10.1167/iovs.07-0091 (doi:10.1167/iovs.07-0091) [DOI] [PubMed] [Google Scholar]
- 112.Strandjord T. P., Madtes D. K., Weiss D. J., Sage E. H. 1999. Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. Am. J. Physiol. 277, L628–L635 [DOI] [PubMed] [Google Scholar]
- 113.Aviezer D., Hecht D., Safran M., Eisinger M., David G., Yayon A. 1994. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell 79, 1005–1013 10.1016/0092-8674(94)90031-0 (doi:10.1016/0092-8674(94)90031-0) [DOI] [PubMed] [Google Scholar]
- 114.Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. 1991. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64, 841–848 10.1016/0092-8674(91)90512-W (doi:10.1016/0092-8674(91)90512-W) [DOI] [PubMed] [Google Scholar]
- 115.Zarnegar R., Michalopoulos G. K. 1995. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J. Cell Biol. 129, 1177–1180 10.1083/jcb.129.5.1177 (doi:10.1083/jcb.129.5.1177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cerra A., Mansfield K. J., Chamberlain C. G. 2003. Exacerbation of TGF-beta-induced cataract by FGF-2 in cultured rat lenses. Mol. Vis. 9, 689–700 [PubMed] [Google Scholar]
- 117.Paralkar V. M., Vukicevic S., Reddi A. H. 1991. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev. Biol. 143, 303–308 10.1016/0012-1606(91)90081-D (doi:10.1016/0012-1606(91)90081-D) [DOI] [PubMed] [Google Scholar]
- 118.Ishida I., Saika S., Okada Y., Ohnishi Y. 2005. Growth factor deposition in anterior subcapsular cataract. J. Cataract Refract. Surg. 31, 1219–1225 10.1016/j.jcrs.2004.11.039 (doi:10.1016/j.jcrs.2004.11.039) [DOI] [PubMed] [Google Scholar]
- 119.Wride M. A., Geatrell J., Guggenheim J. A. 2006. Proteases in eye development and disease. Birth Defects Res. C Embryo Today 78, 90–105 10.1002/bdrc.20063 (doi:10.1002/bdrc.20063) [DOI] [PubMed] [Google Scholar]
- 120.Sivak J. M., Fini M. E. 2002. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Progr. Retin. Eye Res. 21, 1–14 10.1016/S1350-9462(01)00015-5 (doi:10.1016/S1350-9462(01)00015-5) [DOI] [PubMed] [Google Scholar]
- 121.Visse R., Nagase H. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839 10.1161/01.RES.0000070112.80711.3D (doi:10.1161/01.RES.0000070112.80711.3D) [DOI] [PubMed] [Google Scholar]
- 122.Imai K., Hiramatsu A., Fukushima D., Pierschbacher M. D., Okada Y. 1997. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem. J. 322, 809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tholozan F. M., Gribbon C., Li Z., Goldberg M. W., Prescott A. R., McKie N., Quinlan R. A. 2007. FGF-2 release from the lens capsule by MMP-2 maintains lens epithelial cell viability. Mol. Biol. Cell 18, 4222–4231 10.1091/mbc.E06-05-0416 (doi:10.1091/mbc.E06-05-0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Covington M. D., Burghardt R. C., Parrish A. R. 2006. Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14). Am. J. Physiol. Renal Physiol. 290, F43–F51 10.1152/ajprenal.00179.2005 (doi:10.1152/ajprenal.00179.2005) [DOI] [PubMed] [Google Scholar]
- 125.Cheng S., Pollock A. S., Mahimkar R., Olson J. L., Lovett D. H. 2006. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J. 20, 1898–1900 10.1096/fj.06-5898fje (doi:10.1096/fj.06-5898fje) [DOI] [PubMed] [Google Scholar]
- 126.Dwivedi D. J., Pino G., Banh A., Nathu Z., Howchin D., Margetts P., Sivak J. G., West-Mays J. A. 2006. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am. J. Pathol. 168, 69–79 10.2353/ajpath.2006.041089 (doi:10.2353/ajpath.2006.041089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. 2003. Regulation of matrix metalloproteinases: an overview. Mol. Cell Biochem. 253, 269–285 10.1023/A:1026028303196 (doi:10.1023/A:1026028303196) [DOI] [PubMed] [Google Scholar]
- 128.Wong T. T., Daniels J. T., Crowston J. G., Khaw P. T. 2004. MMP inhibition prevents human lens epithelial cell migration and contraction of the lens capsule. Br. J. Ophthalmol. 88, 868–872 10.1136/bjo.2003.034629 (doi:10.1136/bjo.2003.034629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fowlkes J. L., Enghild J. J., Suzuki K., Nagase H. 1994. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J. Biol. Chem. 269, 25742–25746 [PubMed] [Google Scholar]
- 130.Schwartz M. A. 2001. Integrin signaling revisited. Trends Cell Biol. 11, 466–470 10.1016/S0962-8924(01)02152-3 (doi:10.1016/S0962-8924(01)02152-3) [DOI] [PubMed] [Google Scholar]
- 131.Elner S. G., Elner V. M. 1996. The integrin superfamily and the eye. Invest. Ophthalmol. Vis. Sci. 37, 696–701 [PubMed] [Google Scholar]
- 132.Jester J. V., Huang J., Barry-Lane P. A., Kao W. W., Petroll W. M., Cavanagh H. D. 1999. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest. Ophthalmol. Vis. Sci. 40, 1959–1967 [PubMed] [Google Scholar]
- 133.Jester J. V., Petroll W. M., Barry P. A., Cavanagh H. D. 1995. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest. Ophthalmol. Vis. Sci. 36, 809–819 [PubMed] [Google Scholar]
- 134.Marcantonio J. M., Reddan J. R. 2004. TGFbeta2 influences alpha5-beta1 integrin distribution in human lens cells. Exp. Eye Res. 79, 437–442 10.1016/j.exer.2004.06.014 (doi:10.1016/j.exer.2004.06.014) [DOI] [PubMed] [Google Scholar]
- 135.Fukuda Y., Basset F., Ferrans V. J., Yamanaka N. 1995. Significance of early intra-alveolar fibrotic lesions and integrin expression in lung biopsy specimens from patients with idiopathic pulmonary fibrosis. Hum. Pathol. 26, 53–61 10.1016/0046-8177(95)90114-0 (doi:10.1016/0046-8177(95)90114-0) [DOI] [PubMed] [Google Scholar]
- 136.Norman J. T., Fine L. G. 1999. Progressive renal disease: fibroblasts, extracellular matrix, and integrins. Exp. Nephrol. 7, 167–177 10.1159/000020597 (doi:10.1159/000020597) [DOI] [PubMed] [Google Scholar]
- 137.Kelynack K. J., Hewitson T. D., Nicholls K. M., Darby I. A., Becker G. J. 2000. Human renal fibroblast contraction of collagen I lattices is an integrin-mediated process. Nephrol. Dial. Transplant. 15, 1766–1772 10.1093/ndt/15.11.1766 (doi:10.1093/ndt/15.11.1766) [DOI] [PubMed] [Google Scholar]
- 138.Chen X., Moeckel G., Morrow J. D., Cosgrove D., Harris R. C., Fogo A. B., Zent R., Pozzi A. 2004. Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am. J. Pathol. 165, 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kass L., Erler J. T., Dembo M., Weaver V. M. 2007. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int. J. Biochem. Cell Biol. 39, 1987–1994 10.1016/j.biocel.2007.06.025 (doi:10.1016/j.biocel.2007.06.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lygoe K. A., Norman J. T., Marshall J. F., Lewis M. P. 2004. AlphaV integrins play an important role in myofibroblast differentiation. Wound Repair Regen. 12, 461–470 10.1111/j.1067-1927.2004.12402.x (doi:10.1111/j.1067-1927.2004.12402.x) [DOI] [PubMed] [Google Scholar]
- 141.Wipff P. J., Rifkin D. B., Meister J. J., Hinz B. 2007. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 179, 1311–1323 10.1083/jcb.200704042 (doi:10.1083/jcb.200704042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Asano Y., Ihn H., Yamane K., Kubo M., Tamaki K. 2004. Increased expression levels of integrin alphavbeta5 on scleroderma fibroblasts. Am. J. Pathol. 164, 1275–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walker J., Menko A. S. 2009. Integrins in lens development and disease. Exp. Eye Res. 88, 216–225 10.1016/j.exer.2008.06.020 (doi:10.1016/j.exer.2008.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sponer U., Pieh S., Soleiman A., Skorpik C. 2005. Upregulation of alphavbeta6 integrin, a potent TGF-beta1 activator, and posterior capsule opacification. J. Cataract Refract. Surg. 31, 595–606 10.1016/j.jcrs.2004.05.058 (doi:10.1016/j.jcrs.2004.05.058) [DOI] [PubMed] [Google Scholar]
- 145.Wang B., Dolinski B. M., Kikuchi N., Leone D. R., Peters M. G., Weinreb P. H., Violette S. M., Bissell D. M. 2007. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology 46, 1404–1412 10.1002/hep.21849 (doi:10.1002/hep.21849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sheppard D. 2004. Roles of alphav integrins in vascular biology and pulmonary pathology. Curr. Opin. Cell Biol. 16, 552–557 10.1016/j.ceb.2004.06.017 (doi:10.1016/j.ceb.2004.06.017) [DOI] [PubMed] [Google Scholar]
- 147.Weaver M. S., Toida N., Sage E. H. 2007. Expression of integrin-linked kinase in the murine lens is consistent with its role in epithelial–mesenchymal transition of lens epithelial cells in vitro. Mol. Vis. 13, 707–718 [PMC free article] [PubMed] [Google Scholar]
- 148.Li Y., Yang J., Dai C., Wu C., Liu Y. 2003. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J. Clin. Invest. 112, 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]