Abstract
Hydroxyapatite (HA) coatings on titanium (Ti) substrates have attracted much attention owing to the combination of good mechanical properties of Ti and superior biocompatibility of HA. Incorporating silver (Ag) into HA coatings is an effective method to impart the coatings with antibacterial properties. However, the uniform distribution of Ag is still a challenge and Ag particles in the coatings are easy to agglomerate, which in turn affects the applications of the coatings. In this study, we employed pulsed electrochemical deposition to co-deposit HA and Ag simultaneously, which realized the uniform distribution of Ag particles in the coatings. This method was based on the use of a well-designed electrolyte containing Ag ions, calcium ions and l-cysteine, in which cysteine acted as the coordination agent to stabilize Ag ions. The antibacterial and cell culture tests were used to evaluate the antibacterial properties and biocompatibility of HA/Ag composite coatings, respectively. The results indicated the as-prepared coatings had good antibacterial properties and biocompatibility. However, an appropriate silver content should be chosen to balance the biocompatibility and antibacterial properties. Heat treatments promoted the adhesive strength and enhanced the biocompatibility without sacrificing the antibacterial properties of the HA/Ag coatings. In summary, this study provided an alternative method to prepare bioactive surfaces with bactericidal ability for biomedical devices.
Keywords: hydroxyapatite, silver nanoparticle, electrochemical deposition, antibacterial properties, biocompatibility
1. Introduction
Biomedical devices and implants have been widely used to save lives and to improve the quality of life of human beings over the past decades. However, the problems of bacterial infections related to biomedical devices and implants persist despite considerable research and development efforts [1–3]. A possible solution that could prevent bacterial infections is to modify the implant surfaces with antibacterial coatings while maintaining good biocompatibility [4]. Titanium and titanium alloys have excellent mechanical strength, good corrosion resistance and biocompatibility and have been widely used for producing implants and biomedical devices, such as hip joint prostheses, knee joint prostheses and dental implants [5]. Titanium implants with bioactive calcium phosphate (Ca-P) coatings, especially hydroxyapatite (HA) coatings, are commonly used in clinics because they are able to generate good osteointegration with bone tissue for Ti implants [6]. However, Ca-P coatings may also raise affinity to bacteria together with their osteoconductivity. It was reported that Ca-P-coated devices were at a higher risk for peri-implantitis than non-coated devices [1,7]. The bacterial infections of Ti implants are usually difficult to treat and may lead to necessary medical antibiotic therapies, and even implant removal. It is imperative to develop coatings that provide excellent antibacterial activity and osteointegration simultaneously [8].
One strategy is to load antibiotic agents into HA coatings on Ti implants. Radin et al. [9] loaded vancomycin into the Ca-P coatings by immersion of Ca-P-coated discs in vancomycin-containing simulated physiological solution. Campbell et al. [10] employed a surface-induced mineralization technique to produce chlorhexidine-incorporated HA coatings on external fixation pins. Alt et al. [11] applied gentamicin coatings both on pure HA and HA-Arg-Gly-Asp coatings using an ink-jet technology and found that antibiotic-contained HA coatings significantly reduced the infection rates in an in vivo model. Stigter et al. [12,13] used a biomimetic co-precipitation technology to incorporate various antibiotics into HA coatings on Ti substrates, including cephalothin, carbenicillin, amoxicillin, cefamandol, tobramycin, gentamicin and vancomycin. Although the antibiotic-HA coatings exhibit significant improvement in infection prophylaxis compared with conventional HA coatings, it remains a challenge to produce antibiotic-laden coatings with a relatively long antibiotic delivery time at an effective concentration. The long-term antibacterial efficacy of antibiotic-HA coatings needs to be further studied [1].
Incorporating silver into Ca-P coatings is an alternative method to impart Ca-P coatings with antibacterial properties. The use of silver has recently become one of the convenient methods to confer antibacterial properties on biomaterials and medical devices [14]. Silver has long been known to have strong inhibitory and bactericidal effects as well as a broad spectrum of antimicrobial activities [15–17]. It also exhibits high thermal stability and low toxicity towards mammalian cells [18,19]. There are several methods to introduce Ag into Ca-P coatings, such as plasma spraying [20,21], ion beam-assisted deposition [22], magnetron sputtering [23], micro-arc oxidation [24] and sol–gel technology [25,26]. The first three methods are line-of-sight technologies and cannot be applied to the medical devices with a complex shape; the last two methods have the problem of controlling the distribution of Ag in the coatings. Electrochemical deposition (ED) is one of the promising methods to prepare Ag-loaded Ca-P coatings. ED becomes a popular coating method owing to its ease of processing control, variability of the coating composition and suitability for complex implant geometries [27,28]. Liu et al. [29,30] produced HA/Ag composite coatings using ED by adding silver powder in the electrolyte. They also tried to deposit Ca-P coatings on Ti surfaces and subsequently electroplate silver on the surfaces of Ca-P coatings in nicotinic acid bath [29,30]. However, the uniform distribution of Ag in coatings is still a challenge since Ag particles in the coatings are ready to agglomerate, which undermines applications of the coatings.
Recently, we demonstrated the possibility of co-depositing HA and Ag simultaneously by pulsed electrochemical deposition (PED), which generated a uniform distribution of Ag particles in the coating [31]. This method was based on the use of a well-designed electrolyte containing Ag ions, calcium ions and l-cysteine (Cys, HSCH2CH(NH2)COOH), in which Cys worked as the coordination agent to stabilize Ag ions. This paper presents our study on investigating the effects of doped Ag on the antibacterial properties/biocompatibility of the coatings and the effects of heat treatments on the coating properties. The mechanism of the PED method is also discussed.
2. Material and methods
2.1. Pre-treatment of Ti specimens
Commercial pure titanium (CP Ti, Baoji Special Iron and Steel Co. Ltd, China) was cut to make plates with the dimension of 10 × 10 × 1 mm. Before ED, the specimens were etched in a mixed acid (volume ratio of H2SO4 : HCl : H2O = 1 : 1 : 1) at 60°C for 2 h to remove natural oxide layers and increase surface roughness. These specimens were washed in distilled water by ultrasonic cleaning for 15 min. Then they went through alkali treatments to increase hydrophilicity, which was performed by immersing specimens in 100 ml of 5.0 mol l−1 NaOH aqueous solution at 60°C for 24 h [32]. Finally, the specimens were washed with distilled water and dried in air.
2.2. Preparation of HA/Ag composite coatings
The HA/Ag composite coatings were prepared by PED, which was conducted in the cell that had three electrodes: a Ti plate as the working electrode, a platinum plate as the counter electrode and a saturated calomel electrode as the reference electrode. The PED was conducted with a pulse width of 100 s at different potentials: −1.3, −2.0, −3.5 and −4.0 V. The cell was kept in a water bath at a constant temperature (40°C). The electrolyte was an aqueous solution of 5 mmol l−1 Ca(NO3)2 and 3 mmol l−1 NH4H2PO4. HA/Ag composite coatings with different Ag contents were prepared by adding AgNO3 and cysteine into the electrolyte. In the present study, electrolytes with the Ag+ concentration of 0, 0.5 and 1 mmol l−1 were prepared and the ratio of Ag+/cysteine in the electrolyte was kept as 2 : 1. Hereafter, these coatings are referred to as HA, 0.5 HA/Ag and 1 HA/Ag, respectively. The pH value of electrolytes was adjusted to 4.0 using ammonia and nitric acid. After deposition, the Ti plates with coatings were rinsed with deionized water and dried in air at 60°C for 3 h. Finally, the specimens went through heat treatments at one of designated temperatures (700°C, 800°C and 900°C) for 2 h in a furnace at a ramp speed of 5°C min−1 and then cooled in the furnace.
2.3. Characterization
Scanning electron microscopy (SEM; Quanta 200, FEI, The Netherlands; JSM 6390, JEOL, Japan) with an X-ray energy dispersive spectrometer (EDS) was used to examine the morphology and composition of the HA/Ag composite coatings. A thin layer of gold (approx. 100 Å) was coated on the specimens to increase conductivity by a sputter coater (K575X, Emitech, UK). During EDS characterization, the accelerated voltage was 15 kV and the working distance was 17 mm. The detected energies ranged from 0 to 20 keV and the dwell time was 3–4 min. A thin-film X-ray diffractometer (TF-XRD; X' pert pro-MPD, PANalytical, The Netherlands) was used to identify the crystalline phases of the coatings. The TF-XRD measurements were performed using a Cu-Kα (wavelength = 1.54056 Å) X-ray source with a scanning rate of 0.066° s−1 and a scanning range of 20–80°.
2.4. Adhesive strength testing
The tensile adhesive strength of the coatings was evaluated using a universal mechanical testing machine (Model 5567, Instron, USA). One side of the sample was fixed on the stage of the testing machine and the other side was glued to a cylindrical stub that was connected to the crosshead through a grip. The glue was cured for 48 h at room temperature before the tensile testing. A tensile load at the crosshead speed of 1 mm min−1 was applied to the coatings until failure occurred. The adhesive strength was calculated as the load at failure divided by the coated area bonded to the stub. The reported adhesive strength of the coatings was the average value of five specimens.
2.5. Evaluation of antibacterial activity
The spread plate method was used to qualitatively analyse the antibacterial activity of HA/Ag coatings. Two types of bacteria, Escherichia coli and Staphylococcus albus, were used in antibacterial experiments. E. coli is Gram-negative, whereas S. albus is Gram-positive. Firstly, a bacterial suspension with the concentration of 1 × 108 cells ml−1 E. coli or S. albus was prepared by mixing bacteria in LB culture medium and phosphate buffer solution (PBS). Then, Ti plates with HA/Ag coatings were placed in flat-bottom test tubes with 10 ml of bacterial suspension. Each tube was shaken at 200 r.p.m., at 36°C, for 12 h. At the same time, 15 ml of the molten yeast extract tryptose agar was poured in a Petri culture dish and left undisturbed for the agar to solidify. Then, 100 µl of the shaken bacterial suspension was inoculated on agar and incubated at 36°C for 12 h in an incubator. The colony formation of bacteria was examined and photographs were taken. The pure HA coating without Ag was tested in the same way as a control.
The film-adhering method was performed to quantitatively analyse the antibacterial activity of the HA/Ag coating. HA/Ag coatings with different Ag contents and pure HA coatings were selected as the experimental groups and the contrastive group, respectively. First, these specimens were put in a culture dish that contained solidified nutritional agar. Subsequently, each of the above mentioned bacterial strain was adjusted to a concentration of 1 × 106 cells ml−1 with PBS. Bacterial suspension of two bacterial strains (50 µl) was added onto Ti plates. Then the plates were incubated at 36°C for 12 h in an incubator. After that, bacteria on the plates were transferred into 10 ml of sterilized PBS and stirred for 5 min. Fifty microlitres of the diluted bacterial suspension was seeded onto a culture dish with yeast extract tryptose agar and incubated for 12 h. Finally, the bacteria in each group were counted through colony-forming units (CFUs) and bactericidal ratios were calculated according to equation (2.1). The tests were performed in triplicate.
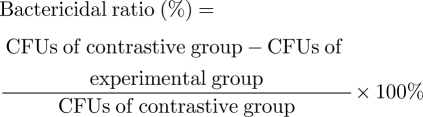 |
2.1 |
2.6. Evaluation of biocompatibility
The biocompatibility of HA/Ag coatings was evaluated by culturing osteoblasts on them. Osteoblasts (SD rat) were cultured in α-MEM (Thermo Fisher Scientific, USA) culture medium with 15 per cent (v/v) foetal bovine serum (FBS, Yuan Heng Sheng Ma, China) and incubated at 37°C in a humidified atmosphere consisting of 95 per cent air and 5 per cent CO2. Cells were seeded onto HA/Ag samples and cultured in a 24-well plate. During cell culture, 3.0 × 104 viable cells were added to each substratum (1 ml each well) and the medium was refreshed every 2 days. After incubation for 1, 3 or 7 days, the samples were rinsed with PBS, transferred to a buffer solution of 2.5 per cent glutaraldehyde (pH 7.4) for 2 h to fix the cells, then dehydrated in a graded ethanol series, followed by vacuum drying for 12 h and sputter-coating with gold prior to SEM analysis.
The proliferation of cells was evaluated by the Alamar Blue assay. The assay is based on detection of the metabolic activity of the cells, which chemically reduces the Alamar Blue and causes it to change colour from blue to red. The assay was performed after culture periods of 3 and 7 days. At each interval, the culture medium in the 24-well plate was replaced by 300 µl of Medium 199 (M199) without phenol red, with 10 per cent (v/v) serum and 10 per cent (v/v) Alamar Blue (BioSource, CA, USA). The cells were incubated under standard cell culture conditions for 4 h. Then the optical absorbance of the medium was read at 600 and 570 nm against a medium blank with Alamar Blue in an ELISA Reader (MQX200, BioTEK, USA). The value of (OD570 nm,cells – OD600 nm,cells) − (OD570 nm,blank – OD600 nm,blank) was recorded and presented as the cell activity. We tested three samples in each case and repeated the assay two times.
3. Results
3.1. Formation of Ag-containing coatings
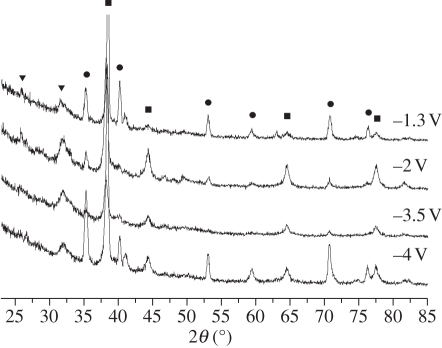
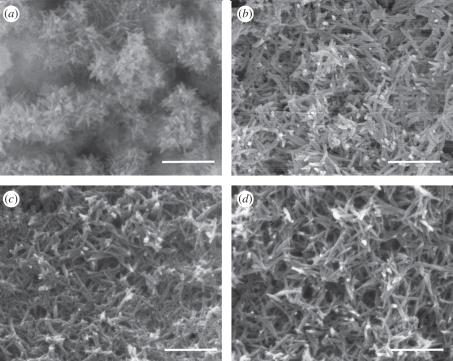
XRD analysis demonstrated that the coatings prepared under different potentials were composed of HA and Ag (figure 1). The diffraction peaks of Ag were distinct, which indicated that Ag was well crystallized under this condition. The morphology of the HA/Ag coatings was revealed by SEM micrographs (figure 2). When the potential was −1.3 V, the coating was mainly composed of needle-like crystals and Ag could not be observed on the surface directly. When the potential was −2.0 V, the coatings had porous network structures that were formed by needle-like crystals. White spot-like Ag nanoparticles were sparsely dispersed in the coatings, as proved by EDS point detection (electronic supplementary material, figure S1). When the potentials were −3.5 and −4.0 V, the Ag nanoparticles were more distinct. The thickness of the coatings was 30 µm, as revealed by the cross-sectional SEM micrograph of the coatings (electronic supplementary material, figure S2). EDS line profile analysis was conducted in order to investigate Ag distribution in the film. The SEM electron beam was scanned along a line across the cross-section of the coatings. The results proved that Ag was uniformly distributed in the film. The tensile adhesive strengths of coatings were 8.4 ± 0.4, 9.7 ± 0.6, 6.5 ± 0.8 and 5.3 ± 0.5 MPa when at −1.3, −2.0, −3.5 and −4 V, respectively. The potential of −2.0 V was the optimum potential for ED in terms of good adhesion between the substrates and coatings.
Figure 1.
XRD patterns of the 1 HA/Ag coatings prepared under different pulsed potentials. Filled squares, Ag; filled circles, Ti; filled inverted triangles, HA.
Figure 2.
SEM micrographs of 1 HA/Ag coatings under different pulsed potentials. (a) −1.3 V; (b) −2.0 V; (c) −3.5 V; (d) −4.0 V. Scale bar, 1 µm.
3.2. Silver contents
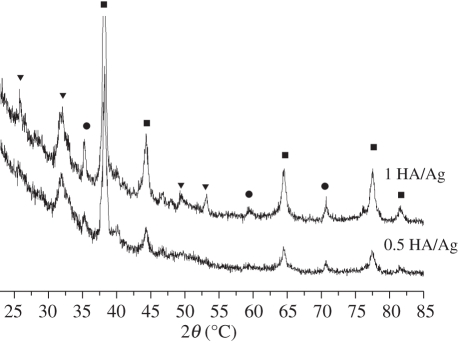
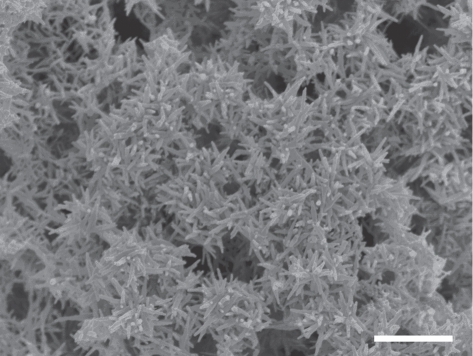
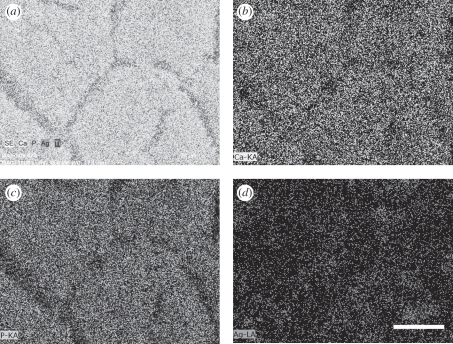
The amount of silver in the coatings is one of the important factors that affect the coating properties. A high silver content leads to poor biocompatibility while a low silver content results in poor antibacterial properties. The appropriate silver content should be chosen to balance the biocompatibility and antibacterial properties of the coatings. We prepared 0.5 HA/Ag and 1 HA/Ag coatings and compared their antibacterial properties and biocompatibility with those of pure HA coatings. XRD analysis indicated that the phases of 0.5 HA/Ag and 1 HA/Ag were the same (figure 3). SEM observation revealed that Ag nanoparticles in 0.5 HA/Ag were less distinct than those in 1 HA/Ag (figure 4). EDS analysis indicated that the Ag contents in the 0.5 HA/Ag and 1 HA/Ag coatings were 6.7 and 14.8 wt%, respectively. EDS elemental mapping demonstrated the uniform distribution of Ag in the coatings (figure 5).
Figure 3.
XRD patterns of (HA/Ag) coatings with different silver contents. The pulse potential was −2.0 V and the deposition time was 2 h. Filled squares, Ag; filled circles, Ti; filled inverted triangles, HA.
Figure 4.
SEM micrographs of 0.5 HA/Ag coatings. The pulse potential was −2.0 V and the deposition time was 2 h. Scale bar, 1 µm.
Figure 5.
The element distribution from EDS elemental mapping: (a) The integrated elemental mapping with secondary electron image; (b) Ca; (c) P; (d) Ag. Scale bar, 30 µm.
Antibacterial properties of HA/Ag coatings were highly dependent on the silver content in the coatings. Antibacterial tests indicated that the 0.5 HA/Ag and 1 HA/Ag coatings exhibited distinct antibacterial effects on two types of bacteria at a high bacterial concentration. Electronic supplementary material, figure S3 shows the photographs of the spread plate method. For pure HA, a large number of colonies of two kinds of bacteria were found. Many colonies stacked together to form a bacterial lawn, and a layer of white sticky substance on the surface of the lawn was observed with the naked eye, which demonstrated that the metabolism of bacteria was exuberant. This indicated that pure HA coatings without any Ag did not possess antibacterial properties. For 0.5 HA/Ag, the number of bacterial colonies decreased remarkably compared with that of pure HA. Spots of the colony were sparsely dispersed on the culture medium substrate. For 1 HA/Ag, there was no colony formation, which indicated the complete inhibition of both bacteria. The bactericidal ability of HA/Ag coatings with different silver contents was also demonstrated by the film-adhering method. As shown in table 1, more than 90 per cent of E. coli and S. albus was killed after 12 h of incubation. Note that the bacterial concentration in the experiments was much higher than that encountered by biomaterials at surgical sites. Thus, the bactericidal ability of the as-prepared coatings could satisfy the requirement of clinic applications.
Table 1.
CFUs of antibacterial tests and bactericidal ratios of the coatings (n = 3).
| specimen | E. coli | bactericidal ratio (%) | S. albus | bactericidal ratio (%) |
|---|---|---|---|---|
| HA | 366.0 ± 16.5 | — | 338.7 ± 17.0 | — |
| 0.5 HA/Ag | 9.7 ± 2.5 | 97.4 | 12.3 ± 4.2 | 96.4 |
| 0.5 HA/Ag (heat-treated) | 18.0 ± 3.6 | 95.1 | 22.7 ± 4.5 | 93.3 |
| 1 HA/Ag | 0 | 100 | 1.3 ± 0.6 | 99.6 |
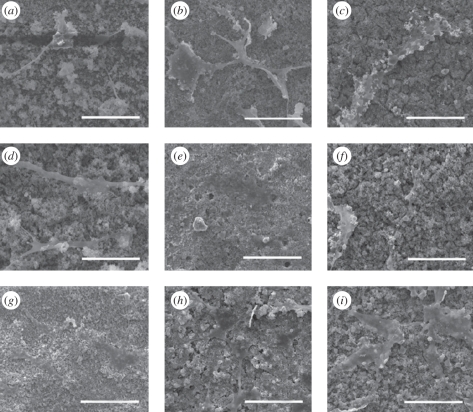
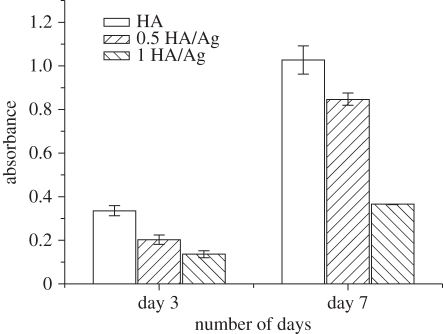
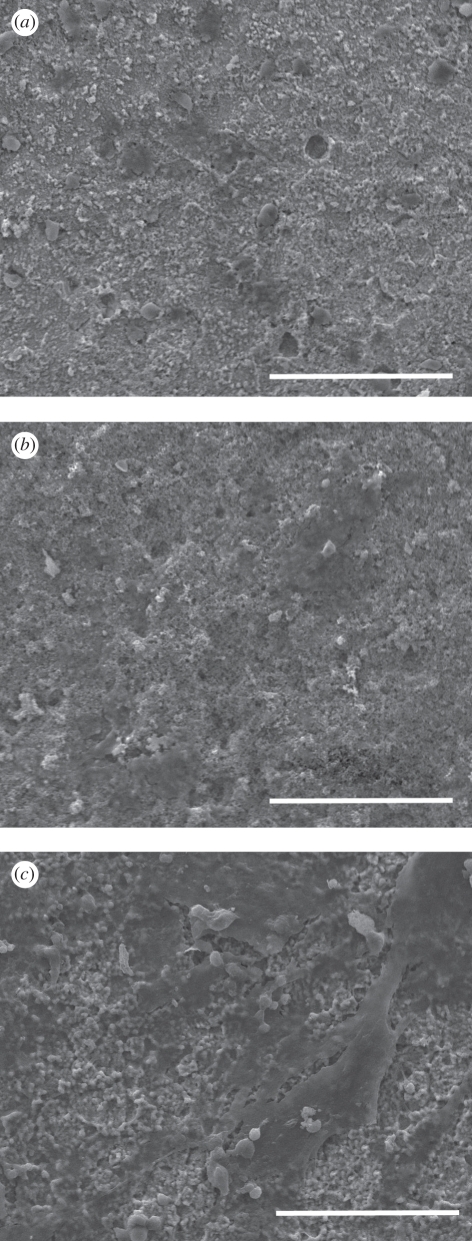
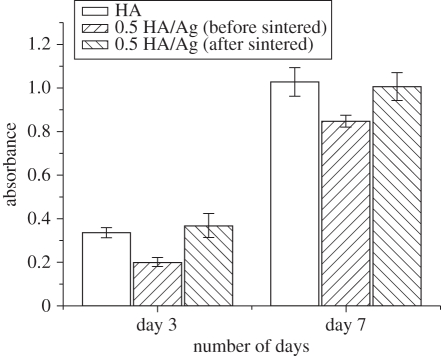
Culturing of osteoblasts on the coatings demonstrated that HA/Ag coatings had good biocompatibility. The SEM micrographs of osteoblasts on the coatings with different silver contents are shown in figure 6. Cells started to adhere on the coatings after 1 day of culture and spread well after 7 days of culture, which revealed that the coatings were able to support adhesion and spreading of osteoblasts. The Alamar Blue assay indicated that cells on pure HA coatings had the highest proliferation rate, followed by 0.5 HA/Ag and 1 HA/Ag after 3 days of culture (figure 7). After 7 days of culture, the inhibitory effect of 1 HA/Ag on cell proliferation became significant. Chen et al. [20] found that the silver-containing coatings with 5 wt% silver had no cytotoxicity, which was consistent with our results. In summary, HA/Ag coatings with low amounts of silver were more favourable for cell growth. The 0.5 HA/Ag coatings could be considered as the optimum that compromised cytocompatibility and antibacterial activity.
Figure 6.
SEM micrographs of osteoblasts cultured on HA/Ag coatings with different silver contents. (a) pure HA, 1 day; (b) 0.5 HA/Ag, 1 day; (c) 1 HA/Ag, 1 day; (d) pure HA, 3 days; (e) 0.5 HA/Ag, 3 days; (f) 1 HA/Ag, 3 days; (g) pure HA, 7 days; (h) 0.5 HA/Ag, 7 days; (i) 1 HA/Ag, 7 days. Scale bar, 100 µm.
Figure 7.
Osteoblast proliferation on HA/Ag coatings with different silver contents evaluated by the Alamar Blue assay.
3.3. Heat treatments
SEM observation revealed that the coatings changed morphology after heat treatments and the coatings with heat treatments were much denser than those without heat treatments. The needle-like crystals showed a tendency to merge together after heat treatments at 700°C (electronic supplementary material, figure S4). Large crystals were observed in the coatings when the temperature was raised to 800°C. The coatings had pores at 900°C, which was probably due to the decomposition of coatings.
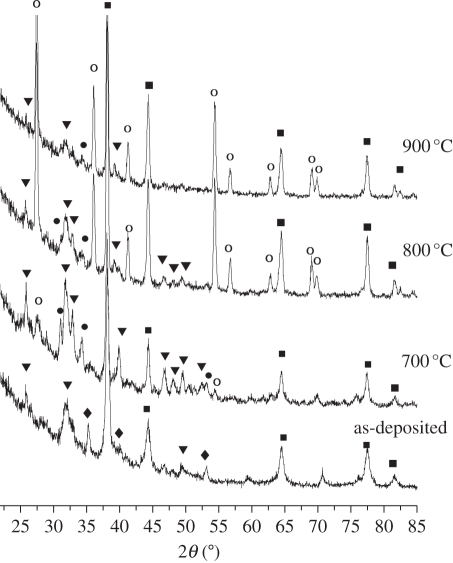
XRD analysis indicated phase transformation in the film after heat treatments (figure 8). The composite coatings sintered at 700°C exhibited typical HA diffraction peaks (211) (112) (300) in the range of 31.7–33°, which indicated that heat treatments improved the crystallinity of coatings. Compared with those without heat treatments, the spectra of the heat-treated coatings exhibited the weak peaks of β-tricalcium phosphate (β-TCP). As the temperature was raised to 800°C and 900°C, the peaks of β-TCP became stronger and sharper and the intensity of HA diffraction peaks became weaker. Diffraction peaks of CaO also appeared in the spectra when the coatings were sintered at 800°C and 900°C, which revealed that substantial HA phase had already transformed into β-TCP and CaO. Combining SEM and XRD analysis, we can conclude that the phase transition temperature of HA/Ag composite coatings is around 700°C. The tensile adhesive strength of coatings increased to 14.1 ± 0.9 MPa after being heat-treated at 700°C, which indicated that heat treatment at the proper temperature was beneficial to the bonding between the coatings and substrates.
Figure 8.
XRD patterns of 0.5 HA/Ag coatings with heat treatments at different temperatures. The coatings were prepared under the pulsed potential of −2.0 V for 2 h. Filled squares, Ag; filled circles, β-TCP; filled inverted triangles, HA; open circles, Rutile; filled diamonds, Ti.
The effect of heat treatments on the antibacterial effects of the coatings was evaluated by the comparison of the antibacterial effects of the heat-treated and untreated 0.5 HA/Ag as well as pure HA coatings. As indicated in table 1, the heat-treated coatings exhibited nearly the same bactericidal ability as the coatings without heat treatments, which suggested that heat treatments were not detrimental to the antibacterial properties of the coatings.
The effect of heat treatments on the biocompatibility of HA/Ag coatings was investigated by the comparison of cell behaviour of the heat-treated and untreated 0.5 HA/Ag as well as pure HA coatings. Figure 9 shows the morphology of osteoblasts on the heat-treated composite coatings after a different period of culture. The cells adhered and spread well on the surface of heat-treated 0.5 HA/Ag coatings, and there was no significant difference between the cells on heat-treated (figure 9) and untreated coatings (figure 6). Figure 10 shows the results of the Alamar Blue assay of osteoblasts on pure HA coatings, 0.5 HA/Ag with and without heat treatments. The cell proliferation rate of heat-treated HA/Ag coatings was nearly equal to that of pure HA coatings, and was higher than that of untreated HA/Ag coatings, which implied that the cytocompatibility of HA/Ag coatings was improved after heat treatments.
Figure 9.
SEM micrographs of osteoblasts cultured on the surfaces of 0.5 HA/Ag after heat treatment at 700°C: (a) 1 day; (b) 3 days; (c) 7 days. Scale bar, 100 µm.
Figure 10.
The Alamar Blue assay of osteoblast proliferation on pure HA coatings, 0.5 HA/Ag coatings (untreated and heat-treated at 700°C).
4. Discussion
In recent years, there has been a tendency to develop composite coatings on Ti surfaces that are biocompatible, bioactive and antibacterial. HA has been proved to possess good biocompatibility as well as bioactive activity, can form a direct bonding with bone and has been used in orthopaedics and dentistry for nearly 20 years [33,34]. Proteins, amino acids and other organic substances are absorbed by HA easily, which in turn favours the adsorption and colonization of bacteria on HA [35]. Silver-loaded HA has shown antibacterial effects, and also good osteoconduction [35–38]. HA/Ag composite coatings are of special interest for biomaterial surface modification in order to achieve the dual aims of bacterial inhibition and enhancement of osteoblast functions.
The uniform distribution of nano-sized Ag in the coatings is one of the critical factors determining the successful application of HA/Ag coatings. We employed the PED method to co-deposit HA and Ag simultaneously, which realized the uniform distribution of Ag. The key to success is to carefully adjust the composition of the electrolyte and the processing parameters to control the deposition rate of HA and Ag. The deposition rates of two phases in the composite coatings should match each other in order to obtain a uniform distribution. To solve this problem, cysteine is added into the electrolyte to work as the coordination agent to stabilize Ag ions. For HA deposition, OH− ions are produced by the electrolysis of H2O on the Ti cathode when a potential is applied (equation (4.1)),
| 4.1 |
Thus, the pH value of the electrolyte in the close vicinity of the cathode increases. Ca2+ and H2PO4− ions in the electrolyte diffuse from bulk solution to the surface of Ti substrates. Then OH− ions react with Ca2+ and H2PO4− to form HA crystals, and deposit on the Ti substrates according to the following reactions [27,28,39,40]:
| 4.2 |
| 4.3 |
| 4.4 |
At the same time of HA formation, the reduction of silver ions (Ag+) in aqueous solution yields Ag:
| 4.5 |
Thus, HA and Ag co-deposit on the surface of Ti substrates and form HA/Ag composite coatings.
Cysteine is selected as the coordination agent, which plays an important role in the formation of nano-sized Ag [41]. Otherwise, only large particles of Ag instead of nanoparticles can be obtained. Cysteine is an inexpensive, simple and environmentally friendly thiol-containing amino acid, and has a strong affinity to metal ions to form metal–ligand complexes, which have been used to prepare inorganic nanocrystals [42,43]. The key role of cysteine is schematically shown in figure 11. Cysteine exists in solution in a partly ionized form (zwitterion) and contains electron-donor groups, -NH2, -COOH and –SH, which enable it to coordinate with Ag+ and form high-affinitive metal–ligand clusters. In the presence of excessive silver ions, there is a coordination and dissociation equilibrium in solution [43,44]:
| 4.6 |
Figure 11.
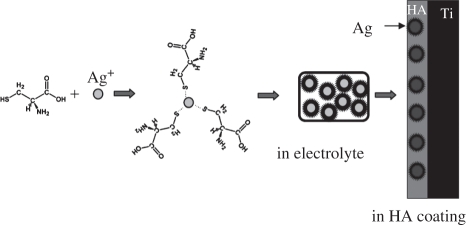
Schematic of the role of cysteine in the PED process. In the electrolyte, cysteine coordinates with Ag+ and forms metal–ligand clusters. After deposition, cysteine combines with Ag and forms Cys-capped Ag nanoparticles in HA coatings. The electrostatic repulsive force of functional groups in cysteine prevents the aggregation of Ag nanoparticles in the film.
Before the reduction of Ag+ ions, the concentration of free Ag+ ions in the electrolyte is controlled by the above reaction; consequently, the reduction of Ag+ on the surface of the Ti cathode is maintained at a mild speed. After reduction, cysteine combines with Ag and forms Cys-capped Ag nanoparticles [41]. The electrostatic repulsive force of functional groups in cysteine prevents the aggregation of Ag nanoparticles, and therefore, a uniform distribution of Ag particles in the coatings is obtained [45].
Heat treatment has two effects on HA/Ag composite coatings. First, it enhances the biocompatibility of the coatings. The Alamar Blue assay indicates that the coatings are more favourable for cell proliferation after heat treatments (figure 10). One possible reason is the phase change of the coatings after heat treatments. XRD analysis reveals that β-TCP appears in the coatings after heat treatments (figure 8), which is due to the decomposition of HA according to the following equation:
| 4.7 |
Pure HA is stable until 1300°C, whereas it decomposes at 700°C with the existence of Ag nanoparticles. Our results are consistent with Rameshbabu's reports and Ag probably plays a role as a catalyst and lowers the thermal stability of HA [35]. β-TCP is a bioresorbable ceramic, and it degrades faster than HA in a physiological environment. The degraded products of TCP are calcium and phosphate ions, which will affect the initial stage behaviour of cells [33].
The second effect of heat treatments is the increase in the adhesive strength of the coatings. Generally speaking, the adhesive strength of Ca-P coatings formed by ED is rather low. In our previous study, tensile tests show that the adhesive strength of pure octacalcium phosphate (OCP) coatings formed by ED is only 1.3 ± 0.3 MPa, while OCP/chitosan composite coatings have the adhesion strength of 7.1 ± 1.9 MPa [46]. In this study, the adhesive strength of HA/Ag coatings is 9.7 ± 0.6 MPa before heat treatments and is raised to 14.1 ± 0.9 MPa after heat treatments, which suggests that silver is beneficial for the improvement of the adhesion between the coatings and Ti substrates. It has been reported that silver can reinforce sintered HA by taking advantage of the ductility of silver, according to the crack-bridging mechanism operated by the elasto-plastic stretching of unbroken silver ligaments along the crack wake [47]. Based on experimental results and the literature, it can be speculated that a portion of silver melts during heat treatments and works as an agglutinant in the film, just as chitosan in OCP/chitosan composite coatings. In summary, heat treatments promote the adhesive strength and enhance the biocompatibility without sacrificing antibacterial properties of the coatings.
5. Conclusions
PED is an effective method for preparing HA/Ag composite coatings with a uniform distribution of silver in them. The preferable conditions of PED are: pulsed voltage, −2 V; Ag+ concentration, 0.5 mmol−1; the ratio of Ag/cysteine, 2 : 1. The HA/Ag coatings prepared by PED have good biocompatibility and antibacterial properties. The tensile adhesive strength of HA/Ag coatings can be enhanced by heat treatments, which improve cytocompatibility without compromising the bactericidal ability of HA/Ag coatings.
Acknowledgements
This project was financially supported by the NSFC (30700172, 31070851), NSFC/RGC Joint Research Funding (N_HKUST601/08, 30831160509), National Key Project of Scientific and Technical Supporting Programmes Fund from MSTC (2006BAI16B01), JSPS Postdoctoral Fellowship for Foreign Researchers and Grants-in-Aid for Scientific Research.
References
- 1.Norowski P. A., Bumgardner J. D. 2009. Biomaterial and antibiotic strategies for peri-implantitis. J. Biomed. Mater. Res. 88B, 530–543 10.1002/jbm.b.31152 (doi:10.1002/jbm.b.31152) [DOI] [PubMed] [Google Scholar]
- 2.Vasilev K., Cook J., Griesser H. J. 2009. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 6, 553–567 10.1586/erd.09.36 (doi:10.1586/erd.09.36) [DOI] [PubMed] [Google Scholar]
- 3.Zhao L. Z., Chu P. K., Zhang Y. M., Wu Z. F. 2009. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. 91B, 470–480 10.1002/jbm.b.31463 (doi:10.1002/jbm.b.31463) [DOI] [PubMed] [Google Scholar]
- 4.Harris L. G., Mead L., Muller-Oberlander E., Richards R. G. 2006. Bacteria and cell cytocompatibility studies on coated medical grade titanium surfaces. J. Biomed. Mater. Res. 78A, 50–58 10.1002/jbm.a.30611 (doi:10.1002/jbm.a.30611) [DOI] [PubMed] [Google Scholar]
- 5.Liu X. Y., Chub P. K., Ding C. 2004. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 47, 49–121 10.1016/j.mser.2004.11.001 (doi:10.1016/j.mser.2004.11.001) [DOI] [Google Scholar]
- 6.Paital S. R., Dahotre N. B. 2009. Calcium phosphate coatings for bio-implant applications: materials, performance factors, and methodologies. Mater. Sci. Eng. R 66, 1–70 10.1016/j.mser.2009.05.001 (doi:10.1016/j.mser.2009.05.001) [DOI] [Google Scholar]
- 7.Oosterbos C. J. M., Vogely H. C., Nijhof M. W., Fleer A., Verbout A. J., Tonino A. J., Dhert W. J. A. 2002. Osseointegration of hydroxyapatite-coated and noncoated Ti6Al4V implants in the presence of local infection: a comparative histomorphometrical study in rabbits. J. Biomed. Mater. Res. 60A, 339–347 10.1002/jbm.1288 (doi:10.1002/jbm.1288) [DOI] [PubMed] [Google Scholar]
- 8.Shi Z. L., Chua P. H., Neoh K. G., Kang E. T., Wang W. 2008. Bioactive titanium implant surfaces with bacterial inhibition and osteoblast function enhancement properties. Int. J. Artif. Organs 31, 777–785 [DOI] [PubMed] [Google Scholar]
- 9.Radin S., Campbellt J. T., Ducheyne P., Cuckler J. M. 1997. Calcium phosphate ceramic coatings as carriers of vancomvcin. Biomaterials 18, 777–782 10.1016/S0142-9612(96)00190-1 (doi:10.1016/S0142-9612(96)00190-1) [DOI] [PubMed] [Google Scholar]
- 10.Campbell A. A., Song L., Li X. S., Nelson B. J., Bottoni C., Brooks D. E., DeJong E. S. 2000. Development, characterization, and anti-microbial efficacy of hydroxyapatite-chlorhexidine coatings produced by surface-induced mineralization. J. Biomed. Mater. Res. 53B, 400–407 [DOI] [PubMed] [Google Scholar]
- 11.Alt V., et al. 2006. The effects of combined gentamicin–hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 27, 4627–4634 10.1016/j.biomaterials.2006.04.035 (doi:10.1016/j.biomaterials.2006.04.035) [DOI] [PubMed] [Google Scholar]
- 12.Stigter M., Bezemer J., de Groot K., Layrolle P. 2004. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 99, 127–137 10.1016/j.jconrel.2004.06.011 (doi:10.1016/j.jconrel.2004.06.011) [DOI] [PubMed] [Google Scholar]
- 13.Stigter M., de Groot K., Layrolle P. 2002. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials 23, 4143–4153 10.1016/S0142-9612(02)00157-6 (doi:10.1016/S0142-9612(02)00157-6) [DOI] [PubMed] [Google Scholar]
- 14.Nair L. S., Laurencin C. T. 2007. Silver nanoparticles: synthesis and therapeutic applications. J. Biomed. Nanotechnol. 3, 301–316 10.1166/jbn.2007.041 (doi:10.1166/jbn.2007.041) [DOI] [Google Scholar]
- 15.Betts A. J., Dowling D. P., McConnell M. L., Pope C. 2005. The influence of platinum on the performance of silver-platinum anti-bacterial coatings. Mater. Design 26, 217–222 10.1016/j.matdes.2004.02.006 (doi:10.1016/j.matdes.2004.02.006) [DOI] [Google Scholar]
- 16.Kawashita M., Tsuneyama S., Miyaji F., Kokubo T., Kozuka H., Yamamoto K. 2000. Antibacterial silver-containing silica glass prepared by sol–gel method. Biomaterials 21, 393–398 10.1016/S0142-9612(99)00201-X (doi:10.1016/S0142-9612(99)00201-X) [DOI] [PubMed] [Google Scholar]
- 17.Zhao Q., Liu Y., Wang C. 2005. Development and evaluation of electroless Ag-PTFE composite coatings with anti-microbial and anti-corrosion properties. Appl. Surf. Sci. 252, 1620–1627 10.1016/j.apsusc.2005.02.098 (doi:10.1016/j.apsusc.2005.02.098) [DOI] [Google Scholar]
- 18.Williams R. L., Doherty P. J., Vince D. G., Grashoff G. J., Df W. 1989. The biocompatibility of silver. Crit. Rev. Biocompat. 5, 221–223 [Google Scholar]
- 19.Zhao G. S. S. J. 1998. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals 11, 27–32 10.1023/A:1009253223055 (doi:10.1023/A:1009253223055) [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Zheng X., Xie Y., Ding C., Ruan H., Fan C. 2008. Anti-bacterial and cytotoxic properties of plasma sprayed silver-containing HA coatings. J. Mater. Sci. Mater. Med. 19, 3603–3609 10.1007/s10856-008-3529-8 (doi:10.1007/s10856-008-3529-8) [DOI] [PubMed] [Google Scholar]
- 21.Ruan H. J., Fan C. Y., Zheng X. B., Zhang Y., Chen Y. 2009. In vitro antibacterial and osteogenic properties of plasma sprayed silver-containing hydroxyapatite coating. Chin. Sci. Bull. 54, 4438–4445 10.1007/s11434-009-0175-6 (doi:10.1007/s11434-009-0175-6) [DOI] [Google Scholar]
- 22.Bai X., More K., Rouleau C. M., Rabiei A. 2010. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 6, 2264–2273 10.1016/j.actbio.2009.12.002 (doi:10.1016/j.actbio.2009.12.002) [DOI] [PubMed] [Google Scholar]
- 23.Chen W., Liu Y., Courtney H. S., Bettenga M., Agrawal C. M., Bumgardner J. D., Ong J. L. 2006. In vitro antibacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 27, 5512–5517 10.1016/j.biomaterials.2006.07.003 (doi:10.1016/j.biomaterials.2006.07.003) [DOI] [PubMed] [Google Scholar]
- 24.Song W. H., Ryu H. S., Hong S. H. 2009. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coating formed by micro-arc oxidation. J. Biomed. Mater. Res. 88A, 246–254 10.1002/jbm.a.31877 (doi:10.1002/jbm.a.31877) [DOI] [PubMed] [Google Scholar]
- 25.Chen W., Oh S., Ong A. P., Oh N., Liu Y., Courtney H. S., Appleford M., Ong J. L. 2007. Antibacterial and osteogenic properties hydroxyapatite coatings produced using of silver-containing a sol gel process. J. Biomed. Mater. Res. 82A, 899–906 10.1002/jbm.a.31197 (doi:10.1002/jbm.a.31197) [DOI] [PubMed] [Google Scholar]
- 26.Chung R. J., Hsieh M. F., Huang C. W., Perng L. H., Wen H. W., Chin T. S. 2006. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol–gel coatings. J. Biomed. Mater. Res. 76B, 169–178 10.1002/jbm.b.30365 (doi:10.1002/jbm.b.30365) [DOI] [PubMed] [Google Scholar]
- 27.Lu X., Zhao Z. F., Leng Y. 2005. Calcium phosphate crystal growth under controlled atmosphere in electrochemical deposition. J. Crystal Growth 284, 506–516 10.1016/j.jcrysgro.2005.07.032 (doi:10.1016/j.jcrysgro.2005.07.032) [DOI] [Google Scholar]
- 28.Lu X., Zhao Z., Leng Y. 2005. Calcium phosphate crystal growth under controlled atmosphere in electrochemical deposition. J. Crystal Growth 284, 506–516 10.1016/j.jcrysgro.2005.07.032 (doi:10.1016/j.jcrysgro.2005.07.032) [DOI] [Google Scholar]
- 29.Liu R. F., Xiao X. F., Xu D. X. 2003. Composite electrodepositing HA/Ag bioceramic coatings. J. Chin. Ceram. Soc. 31, 616–619 [Google Scholar]
- 30.Liu R. F., Xiao X. F., Zhen L. Q., Huang J. M. 2003. HA/Ag composite coatings prepared by two-step electrodeposition. Chin. J. Appl. Chem. 20, 547–551 [Google Scholar]
- 31.Zhang B. L., Wang Y. B., Lu X., Zhou X. L., Qu S. X., Feng B., Weng J. In press Accepted HA/Ag composite coatings prepared by pulse electrochemical deposition on titanium surfaces. Rare Metal Mater. Eng. [Google Scholar]
- 32.Kim H. M., Kokubo T., Fujibayashi S., Nishiguchi S., Nakamura T. 2000. Bioactive macroporous titanium surface layer on titanium substrate. J. Biomed. Mater. Res. 52A, 553–557 (doi:10.1002/1097-4636(20001205)52:3<553::AID-JBM14>3.0.CO;2-X) [DOI] [PubMed] [Google Scholar]
- 33.LeGeros R. Z. 2002. Properties of osteoconductive biomaterials: calcium phosphates. Clin. Orthop. Relat. Res. 395, 81–98 10.1097/00003086-200202000-00009 (doi:10.1097/00003086-200202000-00009) [DOI] [PubMed] [Google Scholar]
- 34.LeGeros R. Z., LeGeros J. P. 2003. Calcium phosphate bioceramics: past, present and future. Key Eng. Mat. 240–242, 3–10 10.4028/www.scientific.net/KEM.240-242.3 (doi:10.4028/www.scientific.net/KEM.240-242.3) [DOI] [Google Scholar]
- 35.Rameshbabu N., Kumar T. S. S., Prabhakar T. G., Sastry V. S., Murty K. V. G. K., Rao K. P. 2007. Antibacterial nanosized silver substituted hydroxyapatite: synthesis and characterization. J. Biomed. Mater. Res. 80A, 581–591 10.1002/jbm.a.30958 (doi:10.1002/jbm.a.30958) [DOI] [PubMed] [Google Scholar]
- 36.Chanki T. K., Wang P. E. 1994. Densification and strengthening of silver-reinforced hydroxyapatite- matrix composite prepared by sintering. J. Mater. Sci. Mater. Med. 5, 533–542 10.1007/BF00124886 (doi:10.1007/BF00124886) [DOI] [Google Scholar]
- 37.Yang L., Ning X., Xiao Q., Chen K., Zhou H. 2007. Development and characterization of porous silver-incorporated hydroxyapatite ceramic for separation and elimination of microorganisms. J. Biomed. Mater. Res. 81B, 50–56 10.1002/jbm.b.30635 (doi:10.1002/jbm.b.30635) [DOI] [PubMed] [Google Scholar]
- 38.Zhao K., Feng Q., Chen G. 1999. Antibacterial effects of silver loaded hydroxyapatite. Tsinghua Sci. Techno. 4, 1570–1573 [Google Scholar]
- 39.Jody R., Tom S., Sandra B., Louis L., John M. 1996. Characterization of electrolytically prepared brushite and hydroxyapatite coatings on orthopedic alloys. J. Biomed. Mater. Res. 30A, 287–294 [DOI] [PubMed] [Google Scholar]
- 40.Lu X., Leng Y. 2005. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 26, 1097–1108 10.1016/j.biomaterials.2004.05.034 (doi:10.1016/j.biomaterials.2004.05.034) [DOI] [PubMed] [Google Scholar]
- 41.Liao X. H., Zhu J. J., Zhao X. N. 2000. Synthesis of silver nanoparticles by electrochemical method. Chem. J. Chin. Univ. 21, 1837–1839 [Google Scholar]
- 42.Panigrahi S., Kundu S., Basu S., Praharaj S., Jana S., Pande S., Ghosh S. K., Pal A., Pal T. 2006. Cysteine functionalized copper organosol: synthesis, characterization and catalytic application. Nanotechnology 17, 5461–5468 10.1088/0957-4484/17/21/028 (doi:10.1088/0957-4484/17/21/028) [DOI] [Google Scholar]
- 43.Xiang J., Cao H., Wu Q., Zhang S., Zhang X., Watt A. A. R. 2008. L-cysteine-assisted synthesis and optical properties of Ag2S nanospheres. J. Phys. Chem. C 112, 3580–3584 10.1021/jp710597j (doi:10.1021/jp710597j) [DOI] [Google Scholar]
- 44.Pakhomov P. M., Ovchinnikov M. M., Khizhnyak S. D., Lavrienko M. V., Nierling W., Lechner M. D. 2004. Study of gelation in aqueous solutions of cysteine and silver nitrate. Colloid J. 66, 65–70 10.1023/B:COLL.0000015059.50285.e9 (doi:10.1023/B:COLL.0000015059.50285.e9) [DOI] [Google Scholar]
- 45.Sun L., Wang L., Song Y., Guo C., Sun Y., Peng C., Liu Z., Li Z. 2008. Aggregation-based growth of silver nanowires at room temperature. Appl. Surf. Sci. 254, 2581–2587 10.1016/j.apsusc.2007.09.093 (doi:10.1016/j.apsusc.2007.09.093) [DOI] [Google Scholar]
- 46.Lu X., Leng Y., Zhang Q. 2008. Electrochemical deposition of octacalcium phosphate micro-fiber/chitosan composite coatings on titanium substrates. Surf. Coat. Techno. 202, 3142–3147 10.1016/j.surfcoat.2007.11.024 (doi:10.1016/j.surfcoat.2007.11.024) [DOI] [Google Scholar]
- 47.Asmus S. M. F., Sakakura S., Pezzotti G. 2003. Hydroxyapatite toughened by silver inclusions. J. Compos. Mater. 37, 2117–2129 10.1177/002199803036242 (doi:10.1177/002199803036242) [DOI] [Google Scholar]