Abstract
Spider silks exhibit remarkable properties, surpassing most natural and synthetic materials in both strength and toughness. Orb-web spider dragline silk is the focus of intense research by material scientists attempting to mimic these naturally produced fibres. However, biomechanical research on spider silks is often removed from the context of web ecology and spider foraging behaviour. Similarly, evolutionary and ecological research on spiders rarely considers the significance of silk properties. Here, we highlight the critical need to integrate biomechanical and ecological perspectives on spider silks to generate a better understanding of (i) how silk biomechanics and web architectures interacted to influence spider web evolution along different structural pathways, and (ii) how silks function in an ecological context, which may identify novel silk applications. An integrative, mechanistic approach to understanding silk and web function, as well as the selective pressures driving their evolution, will help uncover the potential impacts of environmental change and species invasions (of both spiders and prey) on spider success. Integrating these fields will also allow us to take advantage of the remarkable properties of spider silks, expanding the range of possible silk applications from single threads to two- and three-dimensional thread networks.
Keywords: biomaterials, biomimetics, dragline silk, ecology, evolution, orb-web spider
1. Introduction
Some animals produce non-living structures external to their bodies that are critical for survival [1], for example the calcified skeletons of corals, the shells of molluscs, the byssal threads of mussels or the webs of spiders. The interactions between the sizes and shapes of these biological structures and their biomechanical properties have significant ecological and evolutionary implications. For instance, growth architectures and mechanical limitations of coral species fundamentally determine their ecologies across gradients of wave exposure [2]. Predation drives the evolution of gastropod shell structures that are more resistant to crushing [3]. Web-building spiders rely on the critical interplay between web structure and the biomechanical properties of their silks to successfully capture prey [4]. However, many biological systems suffer from a general disconnect between investigations focusing on the evolution and ecologies of structures, and those focusing on material properties, despite their clear interdependence in determining performance and, subsequently, an organism's fitness.
The study of the mechanical properties of spider silks sits at the interface between engineering and biology. Spiders produce multiple silk types, from discrete abdominal glands, that exhibit remarkable strength and elasticity surpassing most natural and artificial fibres, and they are unmatched in toughness ([5,6]; figure 1, area under curve). As a result, spider silks, specifically orb-web spider dragline silks (figure 2g), are the focus of intense research aiming to produce biomimetic fibres (see figure 2 for silk and web descriptions). A consequence of this focus is that silk research rarely includes ecological or evolutionary perspectives. Spiders first began spinning silk approximately 400 Ma [7]. There are at least 41 000 described species of spiders spinning silk in every known terrestrial ecosystem except Antarctica. Many of these spiders produce seven to eight distinct types of silks that are used in a variety of webs, ranging from silk-lined burrows in the ground to aerial orb-webs (figure 2a,b) to the seemingly chaotic three-dimensional cobwebs (figure 2a,c). A better understanding of how silks interact with web structures during prey capture, how and why silk properties vary at individual and interspecific levels, and what selective pressures drive the evolution of silk properties will reveal new possibilities for the application of silk analogues. For example, while past research suggested that small spiders producing thin diameter silks should compensate evolutionarily by producing tougher fibres [4], recent research instead reveals that evolutionary increases in orb spider body sizes are accompanied by concerted improvements in many different silk properties and web architectures [8]. This is in stark contrast to synthetic fibres where improvement in one aspect of performance is typically accompanied by reductions in other properties.
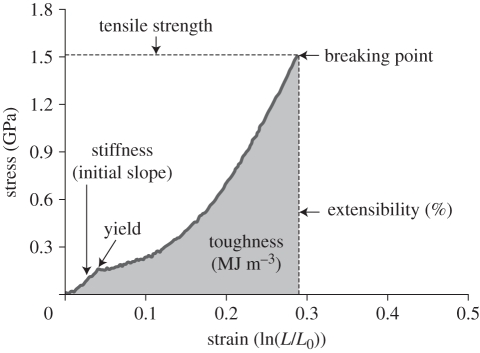
Figure 1.
Representative stress–strain curve of Argiope keyserlingi radial silk showing the key measures of biomechanical properties in spider silks (A. M. T. Harmer, 2010 unpublished data). The biomechanical properties of spider silks (and materials in general) are defined by several key parameters. These include: stress, calculated as force divided by the cross-sectional area of the fibre (engineering stress). For silks, this is usually converted to true stress by multiplying engineering stress by L/L0 (length of stretched fibre/original length; e.g. [79]). This approximates the instantaneous cross-sectional area of the fibre that is important for elastic materials [78]. Strain measures the change in the length of a fibre relative to its original length (engineering strain). It is usually converted to true strain by taking the natural log of L/L0 [146]. Tensile strength is the stress at the breaking point of a material under uniaxial loading [78]. Extensibility describes the stretchiness of a fibre, for example the percentage increase in a fibre's length at breaking when compared with its original length. Stiffness is defined by Young's modulus and is calculated from the slope of the initial elastic region of the stress–strain curve. It is a measure of the ability of a fibre to resist deformation [95]. Yield is the point where a fibre transitions from elastic (and reversible) deformation to plastic deformation. Higher yield values make fibres more resistant to permanent deformation [33]. Toughness is the energy required to break a thread. It is calculated as the area under the stress–strain curve [95]. Hysteresis (not shown on figure) is the proportion of energy lost during a loading–unloading cycle [78]. The energy required to stretch a silk thread is greater than that required to return it to its natural state as some energy is lost as heat.
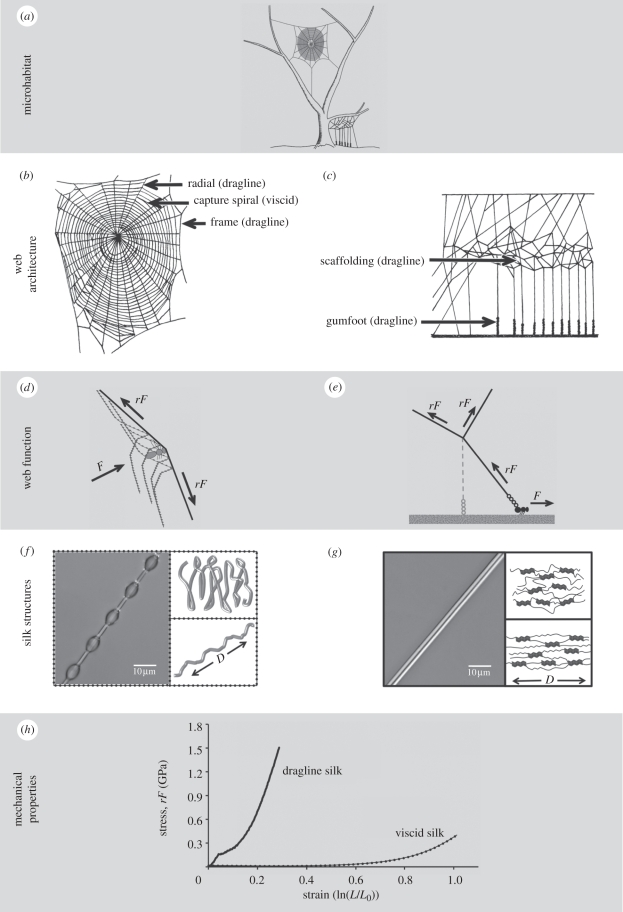
Figure 2.
Hierarchical structure of two common web types, an orb-web and cobweb. (a) Microhabitat in which each web type is generally found. Orb-webs usually span open spaces in and between vegetation. Cobwebs tend to enclose three-dimensional spaces between two substrates. (b) Representative orb-web showing the main structural elements. An orb-web is suspended in space, potentially metres from the nearest vegetation, by three or more anchor threads (dragline silk). The anchor threads attach to frame threads (dragline silk), which form the periphery of the web. The radial threads (dragline silk) attach to the frame and converge on the centre of the web known as the hub [62]. During web construction, the radials are overlaid with a widely spaced, non-viscid temporary spiral. This is then replaced (with the exception of nephilids [18]) by the final, closely spaced, viscid capture spiral, creating a more or less evenly spaced mesh (redrawn and modified from Jocqué & Dippenaar-Schoeman [147]). (c) Representative cobweb showing the main structural elements. Cobwebs are usually built between two substrates and consist of a supporting network of scaffolding threads with the capture (gumfoot) threads spanning the space between the scaffolding and the substrate. Both scaffolding and gumfoot threads are composed of dragline silk, but the gumfoot lines have glue droplets deposited on their lower portions (redrawn and modified from Jocqué & Dippenaar-Schoeman [147]). (d) Distribution of forces during orb-web function. During prey impacts, the relatively strong and stiff radial threads (bold line) probably perform most of the work of dissipating prey energy, acting much like shock absorbers [78]. The much more compliant capture spiral (dotted lines) may contribute to energy dissipation via thread displacement and aerodynamic damping [64]. F = prey force, rF = restoring force of pre-tensioned threads. (e) Distribution of forces during cobweb function. Cobwebs are more likely to encounter ambulatory prey that stumble against a gumfoot thread, which then breaks and restrains or lifts the prey towards the scaffolding and waiting spider. Pre-tensioning of the gumfoot threads and scaffolding helps catapult small prey up into the web (redrawn and modified from Boutry & Blackledge [33]). F = prey force, rF = restoring force of pre-tensioned threads. (f) Orb-web viscid silk (left) and simplified molecular structure of relaxed (top right) and stretched (bottom right) fibre. Note the glue droplets distributed along the viscid silk fibre. Viscid silk is composed largely of β-spirals that act as nanosprings (redrawn from Becker et al. [81]). D = direction of fibre elongation. (g) Dragline silk (left) and simplified molecular structure of relaxed (top right) and stretched (bottom right) fibre. β-sheet crystals are embedded in a semi-amorphous network of β-turns and helices. When a fibre is stretched, there is a transition from β-turns to β-sheets in the amorphous regions (schematic adapted from Keten & Buehler [45]). D = direction of fibre elongation. (h) Representative stress–strain curves for dragline silk and viscid silk in the web of A. keyserlingi (Harmer, unpublished data). The high tensile strength and stiffness of dragline silk [35] allow it to absorb the energy of prey impacts [4]. The extreme compliance of viscid silk, on the other hand, results in lower tensile strength but much greater extensibility (and lower stiffness) than dragline silk [35]. This allows viscid silk to dissipate energy via thread displacement and stretching and prevents insects from ricocheting out of webs [4]. Stress–strain curves for dragline silks typically show an initial phase of high stiffness before the fibre yields. The fibre then shows plastic deformation until rupture. Viscid silks do not show an initial elastic phase, but instead exhibit high extensibility (more than 200–300%) before an exponential increase in stiffness just prior to failure. Such ‘j-shaped’ stress–strain curves are indicative of natural biomaterials that need to be stretchy for performance but also have high safety factors.
Parallel to the field of silk biomechanics, research on behavioural and phylogenetic variation in web structures has advanced greatly over the last two decades [9,10]. However, behavioural and ecological studies on spiders seldom take into account the importance of silk properties, which are critical determinants of prey capture and therefore fundamental to survival. For instance, stored energy in the highly resilient scaffolding silk of black widow cobwebs (Latrodectus hesperus) causes small prey to be catapulted up into the web when they contact a gumfoot thread [11]. This indicates that cobwebs have a very different biomechanical and ecological function to orb-webs, relying on stored energy to catch prey, rather than dissipating prey energy (figure 2d,e). We clearly lack a synthesis of the two seemingly disparate fields of biomechanics and spider biology, despite their potential to mutually inform one another. We know very little about silk biomechanics in the context of spider web architectures. For example, how do web structure and silk properties interact to perform the primary web functions of absorbing prey energy, adhering to prey and transmitting vibrations to spiders? How do spiders vary silk properties within individual webs? Do spiders modify silk properties to suit different structures within webs? How do spiders vary silk properties and web structure in different ecological niches? From an evolutionary perspective, how have silk biomechanics and web architecture interactions influenced the evolution of webs along different structural pathways (e.g. orb-webs, cobwebs and sheet-webs)? We seek here to provide insights into the biomechanics of spider silks in the context of web structural evolution and emphasize the gaps in our knowledge of how these factors interact to produce functional prey capture devices. This paper aims to stimulate new research that integrates overall web structure and silk biomechanics so that we can better understand how webs function in relation to micro-habitat and prey ecology, and subsequently, why such a diversity of web structures has evolved.
Spiders are among the most numerous and diverse terrestrial predators [12]. Many living taxa represent several ancient lineages near the base of the spider phylogeny that often possess unusual silk-producing morphologies and unique web architectures [13]. However, two major spider radiations (the RTA clade and the Orbiculariae) are responsible for more than 75 per cent of extant spider diversity [9]. Both the retrolateral tibial apophysis (RTA) clade (mostly cursorial spiders) and the Orbiculariae (orb-web spiders and their relatives) represent evolutionary shifts away from ancestors that probably built substrate-bound sheet-webs [9]. Within the Orbiculariae, spider diversification is attributed to the evolution of novel silk types, such as the viscid, stretchy capture silks of araneoids [14] or novel web architectures such as three-dimensional cobwebs [15,16]. Among those spiders that rely upon webs for prey capture, orb-web spiders are by far the most studied ([17]; figure 2b). Orb-webs are an ideal model for biomechanical studies for a number of reasons. Recent studies have developed a strong understanding of phylogenetic relationships among orb-web spiders and their relatives (e.g. [9,18]). We also have a strong understanding of the major factors associated with variation in orb-web structure [10,19]. For example, interspecific differences in web structure may reflect adaptations to different types of prey or habitats (e.g. [20]). However, significant gaps remain in our knowledge about the degree to which silk biomechanical properties evolved with the ecological function of webs, potentially constraining or facilitating evolution of different web structures.
In addition to high levels of structural diversity among webs, the biomechanical properties of silks are also highly variable (e.g. [21,22]). This variation may be due to a range of physiological and abiotic mechanisms, such as the rate of spinning [23–25], pH levels in the silk glands [26], humidity [27,28] and temperature [25]. Diet may also influence availability of silk proteins [29,30], which will in turn affect silk properties [31]. Much less understood is the influence of an individual's behaviour on silk properties (e.g. [32–34]). While the properties of spider dragline silk are well described, other silks are largely unexplored. Orb-web spiders produce at least seven different silk types, with highly divergent properties and molecular structures [5,21,35], suggesting there are alternatives to dragline silk as the model for biomimetic silk fibres. Yet, most research on silk biomechanics focuses on only a handful of ecologically similar species from the genera Nephila, Argiope and Araneus [36]. In order to understand how silk and web properties influence web evolution, research is needed on how silk properties vary across different web types. But an understanding of how these properties interact with the structure of the web to absorb energy from prey impacts and to transmit vibrations generated by struggling prey is also critical.
Orb-webs are suspended in space under tension much like human-engineered suspension bridges. To understand how a suspension bridge functions, we need to know the properties of each of the bridge elements (pylons, cables and deck) and how they interact with each of the other elements. In the same way, to understand how a web functions, and the implications of architectural variation, we need to know the properties of the different threads and how they interact with each other as an integrated system (figure 3). While some silks, in particular draglines, are well described, research on how various elements in a web interact has not kept pace. In this paper, we draw together research on silk biomechanics, web structure/function and spider behavioural ecology to emphasize the future directions of this dynamic, interdisciplinary field. This paper is not intended as a comprehensive review of the current knowledge of spider silk biomechanics or biomimetics, which is available elsewhere (e.g. [35,37,38]). Instead, we explore the following areas: (i) the relationship between nanoscale silk structures and silk mechanics, (ii) the evolution of orb-webs and the selective pressures this web structure has placed on the biomechanical properties of silks, (iii) phylogenetic variation in web structure and silk properties, (iv) individual variation in web structure and silk properties, and (v) the future integration of web structure and silk biomechanics research in ecology and evolution.
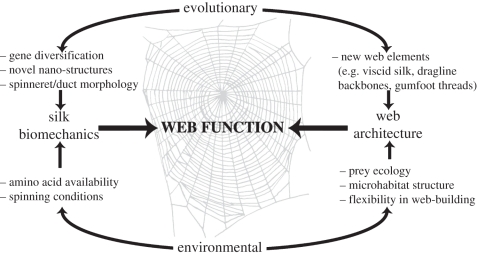
Figure 3.
Evolutionary and environmental influences on spider web function. Silk biomechanics are influenced by both evolutionary effects (such as gene/protein diversification and spider morphology) and by the environmental conditions under which silks are produced. In the same way, overall web architecture can be influenced by genetically determined web-building behaviours and the evolution of novel web elements, as well as by environmental factors (such as prey, microhabitat and flexibility in spider behaviour). Both silk biomechanics and web architecture interact to determine how a web functions to catch prey.
2. Linking nanoscale structures and silk mechanics
Major ampullate (MA) silk is the main constituent of spider draglines (which can also include some minor ampullate silk [37]) and is also used to build various spider web elements, such as the frame and radials in an orb-web. Here, we use dragline silk as a general term for MA silk.
The incredible strength of spider dragline silk is attributable to the formation of β-sheet crystals during fibre extrusion (figure 2g). The β-sheet crystals predominately consist of poly-alanine and poly-(glycine–alanine) repeats [39,40] that are found in two types of fibroin proteins known as MA spidroins 1 and 2 (MaSp1 and MaSp2) [41]. During the spinning process, the liquid-crystalline dope within the abdominal glands is drawn through a tapered duct, causing the silk proteins to elongate, align and hydrogen bonds to form [42]. Despite the relative weakness of hydrogen bonds, silk achieves extraordinary strength through ‘nanoconfinement’ of the β-sheet crystals, where crystal size is constrained, resulting in uniform deformation under loading [43]. Crystal size and arrangement are determined by nanoscale processes during spinning and are influenced to a large degree by reeling speed (and hence shear stress). Faster reeling speeds cause smaller crystals to form that are more uniformly aligned, resulting in increased fibre strength [44]. Decrease in crystal size and greater orientation are associated with faster reeling speeds from about 1 to 10 mm s−1, but then plateau, which corresponds closely with the natural reeling speed of golden orb-web spiders (Nephila) [44]. The strong correlation between reeling speed and fibre strength suggests that spiders may be able to tailor silk properties by adjusting the rate at which they draw silk from the spinnerets. This would seem to be the most economical way for spiders to adjust silk properties, as alternatives require changing amino acid content (dependent on availability), or changing macro-structure such as fibre thickness (requires more material). Despite this, some spiders such as the common house spider (Achaearanea tepidariorum) appear to increase thread thickness in response to greater mechanical challenges from larger prey or their own increased body mass [33].
In addition to its strength, dragline silk is highly elastic when compared with synthetic fibres. Elasticity of dragline silk is determined largely by the semi-amorphous regions interconnecting the β-sheet crystals (figure 2g). These regions include helix-like structures and β-turns that are rich in glycine [45]. When a silk fibre is subjected to tensile loading, the semi-amorphous regions unravel first owing to breaking of hydrogen bonds, which accounts for the high level of extensibility observed in silk [45]. β-sheet crystals also play an important role in elasticity by acting as cross-links between amorphous regions [46] that contribute to elastic recovery after deformation [47]. Higher proline content within the semi-amorphous regions may increase elasticity because it forms kinks in the amino acid chains [47,48]. Closely linked with mechanisms of elasticity is the yield point of dragline silk, where a fibre transitions from elastic (and reversible) deformation to plastic deformation. Yield is again a result of the breaking of hydrogen bonds in the semi-amorphous protein regions [46]. The ratio of β-turns in the semi-amorphous regions determines yield point and allows dragline silk to have an initially high stiffness (which may be important in dealing with high-energy prey impacts), while at the same time not compromising on extensibility [45]. Higher yield values make fibres more resistant to permanent deformation and so may be particularly important in silks and synthetic fibres that must perform repeatedly.
Some nanoscale structures within dragline silk (e.g. β-sheet crystals, β-turns) are found in most spider species. However, variation in biomechanical function can arise owing to diversity in silks at all structural scales. For example, as described above, crystal size and orientation (determined by reeling speed) affect tensile strength. But at micro- and macro-scales, thread diameter and thread architecture within webs also vary extensively. This means that the overall biomechanical function of silks cannot be predicted or controlled by understanding molecular mechanisms alone. Biomechanical function results from complex interactions between structures at all scales. Therefore, understanding the universality and diversity of these structures will allow us to model biomimetic silks for specific applications, as well as target specific features within the hierarchical structure of silks, thereby bettering our understanding of ecological and evolutionary trade-offs.
3. Evolution of orb-webs and associated silk properties
The Orbiculariae, so named for the classic orb-web they build (figure 2b), account for more than 25 per cent of spider diversity (11 880 + species [49]). However, within this diverse group, only about 40 per cent of species actually construct ‘typical’ planar orb-webs [9,50]. The first orb-web spiders probably appeared in the Triassic (more than 200 Ma) [7], and there is strong evidence that by the Late Jurassic to Early Cretaceous (approx. 145 Ma), the major extant orb-web families were already present [7,51–53]. The ancestor of modern orb-web spiders most probably built substrate-bound sheet-webs [9], in which web structure was largely limited by substrate structure and prey limited to mainly ambulatory arthropods. The evolution of the orb-web meant that spiders could build aerial webs suspended in space, freeing them from the limitations in web shape imposed by the substrate [9]. Aerial orb-webs also opened entirely new niches for these spiders by targeting insects in mid-flight. The move to building aerial webs may represent the beginnings of an evolutionary arms race with flying insects [7] that co-radiated alongside the spiders [54]. Several evolutionary innovations in web spinning behaviours were critical for orb-webs to evolve: in particular, a significant increase in the geometric regularity of spider webs and an associated increase in stereotyped web-building behaviours, as well as the suspension of the capture threads on discrete supporting threads [9].
As spiders began to build aerial orb-webs, their silks faced new selective pressures. For example, insects in mid-flight strike webs with greater kinetic energy than experienced by ancestral substrate-bound webs (and derived sheet- and cobwebs) that intercept ambulatory prey, or prey attempting to land ([16,55]). Additionally, webs suspended in space are subjected to much greater wind stress, even more so if anchored to flexible substrates such as grasses. The high degree of geometric regularity of orb-webs may have been an important adaptation for dealing with increased stress from prey and wind by spreading energy out over the web and reducing the likelihood of individual threads breaking [56]. Spiders also responded to greater web stresses by evolving silks with enhanced mechanical properties. Dragline silk evolved early in the evolutionary history of spiders, long before the origin of orb-webs [36]. But, while dragline silk in both orb-web spiders and less-derived (non-orb) spiders is impressively strong and tough, there is a significant increase in material properties associated with the origin of orb-web spiders [57]. Spiders within the Orbiculariae also evolved a new type of capture silk (viscid silk) that is currently considered to play an important role in stopping large, fast-flying prey (discussed below).
4. Phylogenetic variation in webs and silks
4.1. ‘Primitive’ and ‘derived’ orb-webs
Orbicularian spiders are divided into two superfamilies, the Deinopoidea (2 families, 322 species [49]) and the much more diverse Araneoidea (16 families, 11 565 species [49]). The divergence of deinopoids and araneoids is associated with changes in orb-web characteristics, such as the type of capture silk they incorporate into their webs, and, subsequently, the orientation of the planes of webs [9,14]. Most deinopoid webs are horizontal while araneoid webs are usually vertical [58]. Vertical orientation not only helps araneoid orb-webs intercept more prey [59,60] but also to retain them for longer, as escaping prey are more likely to fall into lower parts and become re-entangled [61]. A vertical web may intercept more prey; however, such prey are typically larger, faster flying insects that impact the web with greater energy. This shift to a vertical web orientation was facilitated by a major shift in the type of capture silk used in the web [58].
Capture silk must adhere to intercepted prey and prevent it escaping long enough for the spider to localize, reach and subdue its meal [62], but it may also play a role in absorbing energy as prey are intercepted [63,64]. Deinopoids, like most other web-building non-araneoid spiders, use cribellate capture silk [65]. It is comparatively more expensive than araneoid capture silk, as it requires both a greater volume of material and takes more time to produce [66,67]. Cribellate silk is composed of a pair of axial fibres (produced by the pseudo-flagelliform glands) surrounded by a sheath of microscopic fibrils that are brushed onto the axial fibres from a specialized plate called a cribellum [68]. This silk is dry and achieves its stickiness via hygroscopic and van der Waals forces [69]. Araneoid capture silk, often referred to as viscid or sticky silk, is more economical to produce because of an alternative mechanism of adhesion [70]. As the axial fibres are extruded from the flagelliform glands, an aqueous glue coating is simultaneously deposited from the aggregate glands onto the axial fibres and coalesces into microscopic droplets along the fibre's length (figure 2f; [71]). The glue droplets are composed of about 80 per cent water as well as glycoproteins that adhere to prey and small hygroscopic molecules [37,72]. Viscid silk is stickier per volume when compared with cribellate silk, which increases prey capture potential and ultimately spider fitness [73]. Stickiness is further enhanced by a ‘suspension bridge mechanism’ that recruits adhesion from neighbouring glue droplets, reducing the tendency of capture threads to peel away from prey cuticle [74]. Moreover, the glue droplets act as viscoelastic solids that achieve greater stickiness under fast deformation when insects first impact webs, but also maintain adhesion for long periods under static loading [75]. However, stickiness of viscid silk is ultimately constrained by the tensile strength of its axial fibres. Stickiness increases linearly with tensile strength, but is always less than the force required to break the axial fibres. This safety factor allows threads to repeatedly detach and reattach to prey, rather than breaking, as prey struggle to escape the web [76]. While untested, the high extensibility of viscid silk (up to 1000% [77]) may add an additional safety factor preventing breakage, as a struggling insect is unlikely to extend the silk this far.
Not only do cribellate and viscid silk differ in adhesive mechanisms, but they also react to the energy of prey impacts in different ways [78,79]. Cribellate and viscid threads require similar amounts of energy to break (i.e. are similarly tough; area under curve in figure 1); however, cribellate capture threads are stronger, stiffer and much less extensible than viscid capture threads [63,79]. Although viscid silk is not as strong as cribellate silk, it is better equipped to dissipate prey impact energy via stretching, which may be more important for the higher energy prey typically intercepted by vertical araneoid webs [4,79]. This greater extensibility may also facilitate aerodynamic damping, which is important for energy dissipation in Araneus diadematus webs [64].
Differences in biomechanical properties of important silks, such as MA, minor ampullate and flagelliform silk are due largely to differences in protein structures [39]. While deinopoids and araneoids share capture silk cDNA orthologues unique to the Orbiculariae [80], the mechanical differences between cribellate and viscid silks are likely due in part to differences in protein composition of the axial fibres [63]. Becker et al. [81] showed that the high extensibility of viscid silk is attributable to its high percentages of glycine and proline that are arranged in β-spirals that act as nanosprings (figure 2f). Vollrath & Edmonds [71] suggested viscid silk's extensibility and recovery are largely owing to the aqueous glue coating surrounding the fibres and to microscopic windlasses within each droplet. However, Blackledge et al. [82] demonstrated that these factors alone are not enough to account for the greater extensibility and decreased stiffness of viscid silk. Clearly, more detailed investigation of silk composition across silk types and species is warranted to provide insight into how the properties of synthetic silks may be enhanced.
In reality, the combination of characteristics of araneoid orb-webs—their vertical orientation, greater ability to dissipate prey energy, increased stickiness and greater economy—are all likely to have contributed to the greater success and diversity of this group. Not only do these traits improve prey capture potential, but they also allow spiders to occupy new niches. But, while there is strong selection for viscid silk over less-efficient cribellate silk, subsequent selection to spin webs that minimize or abandon viscid silk may have played an important role in the diversification of derived orbicularian spiders [9].
4.2. Modified orb-webs
Among some orb-web building families (e.g. Araneidae, Nephilidae, Tetragnathidae and Uloboridae), a number of species construct webs highly modified from the typical planar, radially symmetric orb. While most orb-web spiders are considered generalist predators, modified orb-webs are usually adaptations for catching specific prey types [20]. Specialist webs are in most cases greatly reduced in size and complexity when compared with typical orbs, in the most extreme cases consisting of only single lines of silk (e.g. Miagrammopes [83]; Mastophora [84]). The highly elongated ladder-webs of genera such as Scoloderus [85,86], Clitaetra [87,88] and Telaprocera [89,90] are curious exceptions. Whether reduced or extended, modified orb-webs function differently (mechanically) from typical orbs to deal with stresses of prey interception. Particularly for reduced webs, spiders cannot rely on the highly geometric orb-web structure to assist in energy dissipation and must instead depend to a greater extent on the biomechanical properties of individual silk threads [4]. This functional transition may impose selection on the biomechanical properties of silks in reduced orb-webs, for example for greater tensile strength to resist breaking, or greater extensibility to better dissipate force through stretching. While Opell [91] demonstrated increased stickiness of capture threads in reduced webs of the Uloboridae, to date, the tensile properties of silks in reduced webs remain largely uncharacterized. Exceptions are the silks of Cyrtarachne spiders, which specialize upon moths by building orb-webs characterized by very few radial threads and a widely spaced capture spiral. This web structure means that prey encounter single capture threads, which exhibit breaking strengths 7–10 times greater than other araneid viscid silks [92]. Silk of the bolas spider Mastophora hutchinsoni, another moth-specialist that hunts using large glue droplets at the ends of single threads, is similar to typical orb spiders [57], but this was forcibly reeled dragline silk rather than the actual capture silk that forms the bolas thread (although this is probably MA silk also). As the bolas thread must perform all the work of stopping prey, this silk is also likely to exhibit exceptional mechanical properties. More detailed investigation of silks from reduced webs holds promising possibilities for fine-tuning synthetic silk properties as the selective pressures of catching prey with a limited thread network have probably resulted in the evolution of silks with unique and superior mechanical properties.
4.3. Sheet-webs and cobwebs
In addition to modified orb-webs for prey specialization, there is a strong evolutionary trend among derived orbicularian spiders towards smaller body and web size, as well as for webs that enclose three-dimensional spaces [15,50]. Two of the three most diverse families within the Orbiculariae, the Linyphiidae and Theridiidae, no longer build orb-webs at all. Recent evidence that combines both morphological and molecular data indicates that linyphiid sheet-webs and theridiid cobwebs each evolved independently from orb-webs [9]. The huge diversity within these families, along with the frequent reduction of orb-webs within the ‘traditional’ orb-spinning families, suggests that the orb-web has provided a platform for the further rapid evolution of novel web structures [9,50]. While we now have a good understanding of evolutionary patterns of web structure, a large gap remains regarding the selective forces driving the evolution of these different structures. Several hypotheses propose to explain repeated evolution away from the typical planar orb-web in derived orbicularian families. These include competition and adaptation to novel microhabitats [15]. Or, as Craig [4] suggests, the evolution of smaller webs that rely less on orb-web structure to absorb energy and more on the mechanical properties of individual silk threads, may have released spiders from the ‘constraints’ of orb architecture, allowing them to explore new microhabitats. Alternatively, the evolution of three-dimensional webs may be an adaptation to avoid hymenopteran predators by creating a physical barrier between spiders and their predators ([93]; although see [16]).
Sheet-webs and cobwebs generally exhibit reduced geometric regularity when compared with orb-webs, although within species there is still a high degree of structural stereotypy [9,16,94]. The reduced geometric regularity and decreased use of capture silk in cobwebs and sheet-webs make them absorb prey energy very differently from orb-webs (e.g. [11,33]). These webs are also less likely to intercept fast-flying prey as these webs generally occur in more enclosed spaces. Indeed, many theridiid webs specifically target ambulatory prey with their capture threads anchored directly to the substrate ([16,55]). Intuitively, this suggests that the silks in these webs do not need to be as tough because ambulatory prey contact a web with less kinetic energy. However, prey may be more likely to initially encounter single capture strands rather than a network as in an orb-web, and this may select for increased silk strength or extensibility in these webs (e.g. [33]). While the properties of capture threads in three-dimensional derivatives of orb-webs remain largely unquantified, results from L. hesperus webs (Theridiidae) indicate that the sticky gumfoot lines are indeed stronger and tougher than the analogous capture spiral of many orb-webs and have properties similar to orb-web radial threads (and are probably composed of MA silk [95]). Furthermore, three-dimensional webs tend to be more permanent structures when compared with many orb-webs that are often replaced daily [95]. This will perhaps select for silks better equipped to deal with repeated and prolonged stresses, and for silks more resistant to permanent deformation (i.e. higher yield values) [33]. Also, while silks in general are very long-lived materials, theridiid webs do not contain viscid (flagelliform) silk, which shows a much more rapid decline in mechanical properties when compared with dragline silk [96]. Factors driving evolution beyond the orb-web remain largely unexplored and too often neglect the role of silk properties despite silk being central to web function. Only by integrating silk properties and web architecture to understand the biomechanical constraints of different web structures can we gain a complete picture of web diversification.
4.4. Integrating web structure and biomechanical function
The biomechanical properties of some spider silks, particularly the dragline silk of a few selected orb-web species, are now well described. Much of the research on spider silk has focused on describing its properties (e.g. [97,98]) with the ultimate goal of artificially replicating these remarkable, naturally produced fibres. The importance of placing this research in an ecological context is largely neglected.
We now have an improved understanding of patterns of web evolution (e.g. [9]). Yet, how silk biomechanics and the structures of webs interact to function in prey capture is largely unknown, but probably complex. For example, the high tensile strength and hysteresis of radial threads means they not only support the web but also work as shock absorbers during prey impact [78], while the geometric regularity of the web may help to spread prey energy out over more radial threads [56,64]. Also, as radials have high tensile strength and stiffness while capture silks have lower strength but high elasticity (figure 2h), the ratio of radials to capture spiral turns may determine the energy-absorbing capabilities of a web. For example, webs with a low radial to spiral turn ratio can be classed as low-energy absorbing, while webs with a high radial to spiral turn ratio are high-energy absorbing [4].
The next step beyond these descriptions of silk and web properties is to investigate what happens within a web during prey impact, and how work is performed by each of the web elements. How much of the energy-absorbing capabilities of a web are due to properties of individual silk threads and how much to web structure? Although some studies have started to explore this, results to date are limited. For example, Lin et al. [64] used a finite-element model to examine how an orb-web dissipates prey energy, but looked only at a single species (A. diadematus) and hence single web structural type. Craig [4] also used a modelling approach to look at the effects of web structure and silk properties on energy absorption across five orb-web species. She suggested that large, high-energy-absorbing webs rely on the geometry of the web to generate tension in web threads to dissipate the force of prey impacts, while small, low-energy-absorbing webs rely more on the properties of individual threads and thread displacement. However, the range of web sizes in this study only varied from a radius of 4.4 cm to a radius of 10.5 cm [4]. Across orb-web-building species, there is much greater variation in orb-web size and structure than is represented by this study (e.g. Argiope radon webs average over 80 cm in diameter [99]), and so a comprehensive understanding of structural/biomechanical interactions is limited. In Craig's own words, her study ‘ … does not prove or disprove the mechanisms or patterns through which web-weaving spiders have evolved, but it provides a new conceptual framework in which to study the evolution of web spinners’ [4], p. 65.
Ideally, the biomechanical properties of silks from a range of web structures within a closely related group of spiders need to be examined. Using modelling techniques, such as finite-element models, coupled with detailed analyses of web behaviour during prey impact, we can explore how these different structures function in the capture of prey with characteristically different ecologies. Only then can we really understand how the structure of a web interacts with the properties of the silk that make it up, which in turn may provide insights into what influenced the evolution of different web structures; for example, as a function of the types of prey available within different microhabitats. If Craig's [4] proposal holds true that high-energy-absorbing webs rely on web structure to dissipate energy while low-energy-absorbing webs rely on the silks themselves, then we may perhaps expect silk properties to scale nonlinearly with web and spider size. That is, smaller webs should have proportionally superior silk properties when compared with larger, higher energy-absorbing webs, as individual threads must perform more work during prey capture. However, this prediction is not supported by more robust sampling of taxa. Sensenig et al. [8] examined the web structure and silk biomechanics of 22 taxa of orb-web spiders that ranged almost two orders of magnitude in body size. They concluded that small orb-webs could rely primarily on individual threads to dissipate energy, not because they contained superior silk, but rather because the smaller insects that flew into those webs were easier to capture. Sensenig et al. [8] found a strong pattern of evolution between silk and web architecture where the biggest species of spiders repeatedly evolved better performing silks and relatively smaller, higher energy-absorbing webs, possibly to meet the challenges of dissipating the high kinetic energies of their larger prey. As web-building incurs significant material and energetic costs, selection should favour the most efficient web structures that provide the best possible return for a spider's investment [56,100]. Therefore, following from Craig's [4] hypothesis for a shift towards low-energy-absorbing webs, there must be some selective advantage to building webs that rely on the silk properties in localized areas of prey impact rather than on the web structure as a whole. Large spiders with high-energy-absorbing webs may be constrained to a limited range of web structures and hence microhabitats when compared with smaller spiders. Decoupling structure from function in webs would allow spiders to modify their webs to almost any building environment and subsequently facilitate the exploration of new foraging niches [4,50]. However, research on the role of biomechanical function in web diversification must consider whether species with novel web structures are associated with small or large ancestors. Recent progress in spider phylogenies (e.g. [9]) begins to provide a solid foundation for such studies.
A further advantage of being freed from architectural constraints may be that as parts of a web become damaged, the rest of the web remains functional. While recent modelling data suggest that orb-webs are highly resilient to localized damage [101–103], the effects of damage are untested in real webs of varying energy-absorbing capabilities. Even if web damage does not significantly disrupt prey stopping potential, it does reduce capture area. As silk biomechanical properties are fundamental to the foraging ecology of all web-building spiders, adaptations that enhance the biomechanical function or cost effectiveness of webs must have played an important role in shaping silk and web evolution. But further to this, an understanding of variation in web biomechanical function across diverse web types and across structural scales expands the range of synthetic silk applications from single threads, to complex two- and three-dimensional thread networks.
5. Individual variation in webs and silks
5.1. Behavioural flexibility
Individual variation in orb-web structure is well documented and arises for a variety of reasons. Individual spiders adjust web characteristics (e.g. thread spacing, top/bottom asymmetry and area) to suit local conditions [10]. Important factors include the types of prey available [104,105], overall prey availability [106–108], nutritional status [109,110], weather conditions [111,112], spider size [113,114], age and development [115,116], silk supply [117], experience [118–120], the presence of predators and parasites [121–124] and microhabitat structure [90,125,126]. However, individual variation in silk properties, which together with web architecture determine web performance, is poorly understood. Orb-web spiders spin seven to eight different types of silk [127,128] that are produced from discrete glands and vary greatly in tensile properties. Blackledge & Hayashi [21] found 250 per cent variation in performance (across strength, toughness, stiffness and extensibility) among four types of dry silk produced by Argiope argentata, while the viscid capture silk was an order of magnitude stretchier than these dry silks. Similar variation exists in Argiope keyserlingi (Harmer, unpublished data; figure 2h). In addition to variability among silks types, there is substantial inherent variability in silk properties within the same silk types produced by individual spiders [22,129,130]. This may be in part owing to variation in silk fibroin gene sequences and heterogeneous nanoscale morphology of silk fibres [41,131,132].
Several studies investigated how properties of silks produced by the same individual vary with spinning conditions; however, they concentrated largely on dragline silk that was usually forcibly reeled from the spider (e.g. [22,25,97,133,134]). More recent studies look at how individuals vary silk properties in the context of building webs. For example, A. diadematus with artificially increased weight produce radial threads that are thicker, stronger and stiffer, which presumably helps support the heavier spiders [135]. Nephila pilipes build overall stiffer webs when fed large crickets instead of small flies by increasing the diameter of silk fibres and incorporating more radials into the web [34]. It is unclear, however, what these spiders were responding to when altering silk properties; for example nutritional, size or vibratory differences in prey, their own change in weight, or gut distension during feeding [34]. Spiders could perhaps also be attuned to the mechanics of the initial prey impact with the web, when the web is most likely to fail. For instance, in the cobweb spider A. tepidariorum, individuals fed large, fast prey produced thicker silks that were able to withstand greater energy [32]. But again, as body condition and nutritional intake also varied between spiders fed high- and low-energy prey, it is difficult to disentangle behavioural flexibility on the part of the spiders from physiological effects of increased spider weight or nutritional variation between prey [32]. However, by looking at silks from functionally distinct parts of individual cobwebs, this study demonstrated that spiders can actively control silk properties on short time scales. For instance, gumfoot silk and scaffolding silk from individual A. tepidariorum webs show dramatically different mechanical and structural properties despite being produced from the same gland, indicating that spiders can quickly tailor silk properties for specific functions [33].
In addition to prey effects, ambient wind conditions may also influence silk and web properties. Cyclosa mulmeinensis exposed to persistent wind build smaller orb-webs with fewer, but higher performance radial threads, presumably to reduce drag [111]. These spiders clearly alter silk mechanical properties in response to wind conditions, but the effects of changes in silk and web properties on prey capture remain untested. Static forces in spider webs, namely thread pre-tension, help determine web properties such as vibration transmission and overall web stiffness. Some orb-web spiders actively adjust radial pre-tension, which allows them to control the distribution of forces in different radials [136,137], or to adjust the sensitivity of the web to vibrations produced by different-sized prey [138]. While our understanding of how silk material properties relate to static web forces is limited, incorporating static forces into biomechanical function models is critical, because they will interact with dynamic forces generated during prey impacts or by wind.
Understanding how and why individual spiders manipulate silk properties has implications for the enhancement of synthetic silks. Recent research showing that reeling speed affects the size and orientation of β-sheet crystals [44] is an important first step in understanding the mechanisms by which individuals might control silk properties at a molecular level. Despite these initial explorations of how individual spiders might actively tune silk properties to foraging conditions, there are still major gaps in our understanding of the level of control spiders possess over silk properties, as well as the mechanisms involved. Major difficulties lie in teasing apart behavioural flexibility from the effects of (i) variation in the nutritional composition of prey, which may influence silk properties by determining the relative amounts of different amino acids available [29,30], and (ii) the effects of spider weight and condition, which are important as the diameters of silk threads generally scale with spider size, and tensile properties are proportional to thread diameter. A major question that remains unanswered is whether the behavioural flexibility exhibited as variation in web structure is mirrored by variation in silk biomechanical properties and how this affects overall web function.
5.2. Integrating behavioural flexibility and biomechanical function
In variable environments, behavioural flexibility is important for maximizing fitness [139]. With changing climate and ecosystem ecology, spiders (and most other organisms) are exposed to changes in prey type/abundance, microhabitat structure, novel predators and competition from invasive species [140]. The ability of spiders to cope with a changing environment will be determined largely by flexibility in web function, and hence in web architecture and silk production. However, we have very limited understanding of whether, or how, spiders vary the biomechanical function of their webs in response to their physical, chemical and ecological environment. While behavioural flexibility in spiders manifests as variation in the architectures of completed webs, the interactions between web architecture, silk properties and prey ecology ultimately predict web biomechanical function (figure 3). Modifying the structure of a spider web is likely to affect its biomechanical function, and so silk properties may need to be adjusted, either to compensate for or to facilitate such structural shifts. For instance, if an orb-web spider builds a lower energy-absorbing web by modifying the ratio of radials to capture spiral turns, is it capable of increasing the strength of individual threads to alleviate the loss in prey stopping potential? Or, if a spider builds a larger web does it increase thread stiffness and pre-tension to aid transmission of vibrations occurring further from the hub? Alternatively, hungry spiders may build larger webs, spreading their silk more thinly to increase their chances of catching at least something, while well-satiated spiders may become more selective in their prey capture by building smaller webs, concentrating silk in a smaller area, and increasing their chances of stopping large profitable prey.
Further research is needed to explore how silk biomechanics, web architecture and their interactions change with foraging conditions. Tso et al. [34] and Boutry & Blackledge [32] show that spiders adjust silk and web properties according to the type of prey they frequently encounter. The next step is to explore behavioural flexibility in spider web architectures and silk properties and their ultimate effects on web biomechanical function by experimentally manipulating microhabitat and prey characteristics across species with diverse web structures. Understanding flexibility in web function will generate insights into the ability of spiders to cope with variation in microhabitat and prey, as well as the historical selective pressures that have shaped web evolution. For instance, flexibility afforded to spiders by decoupling web architecture and biomechanical function is likely to account for the much greater diversity of derived, non-orb-web species as they were better adapted to cope with novel foraging conditions during their evolutionary history.
6. Outlook: integrating biomechanics, ecology and behaviour
This paper highlights the disconnect between biomechanical research on spider silk and ecological research on spider web function and evolution. However, the time is ripe to make use of recent developments in our understanding of the evolution of webs and new explorations of silk biomechanics from nano- to ecological-scales. Only through the integration of silk biomechanics, web structure and spider biology, can we hope to understand how spider webs function during prey capture and therefore the selective forces shaping silk and web evolution. Through the integrative use of techniques already common in engineering and biomaterials fields we can uncover the key principles driving silk and web evolution. For example, the application of finite-element analyses (e.g. [141]) and other modelling techniques (e.g. [103]) to integrate detailed data on both silk properties and web structure across a diverse range of taxa and web types will highlight constraints in web function that can then be mapped over spider phylogenies. By exploring mechanical trade-offs and constraints in web evolution, we can develop models for assessing spider fitness, and paint theoretical landscapes over which species can adapt to maximize success in relation to environmental conditions and prey ecology. Such models will highlight theoretical fitness optima, illustrating disparities in our understanding of web function and evolution, as well as highlighting gaps in our integrated knowledge of web function that will help inform new research directions.
At the same time, the integrative approach to silk biomechanics and web function described above may open up alternative pathways to biomimetic silks. The protein structures of silks are highly variable across species, as well as between silk types produced by the same spider. For example, both dragline silk and viscid silk are both very high-performance fibres with breaking energies over 150 MJ m−3 (A. diadematus [5]), yet dragline silk is rich in β-sheet crystals [142] while viscid silk is composed largely of β-spirals that act as nanosprings [81,143]. Multi-scale modelling approaches to understand the function of these silks from the nanoscale (molecular) to macro-scale (whole fibres and webs; e.g. [44]) may provide mechanistic insights that can be applied to the production of synthetic fibres [144]. We should look to spiders and the functions of their silks and webs when attempting to reproduce silks and not focus so exclusively on dragline silks and such a limited range of species. For instance, Agnarsson et al. [145] predicted and subsequently discovered dragline silk with a new range of performance properties. In this case, giant orb-web spiders (Caerostris darwini, Araneidae) that suspend their webs across rivers and lakes do so using MA silk that is up to two times tougher than other known silks [145]. Efforts to elucidate the structural basis for these properties are now under way. Furthermore, the covariation between different silk performance parameters (e.g. [129]) may give insight into the refinement of synthetic silks, or, novel webs with novel silks may reveal novel spinning morphologies that make fibre replication easier. The next decade of spider silk research promises to benefit in new ways from this integrative approach. Investigating how silk functions in the context in which it evolved—as part of an interacting web—has the potential to expand the range of possible applications from single threads (one-dimensional) to optimized thread surfaces (two-dimensional) and objects (three-dimensional).
Acknowledgements
A.M.T.H is supported by a Hermon Slade Foundation grant HSF 10/12. T.A.B. is supported by National Science Foundation award IOS-0745379. J.S.M. is supported by Australian Research Council award DP-0987892. M.E.H. is supported by the Department of Biological Sciences, Macquarie University. We thank three anonymous reviewers for valuable feedback on the manuscript.
References
- 1.Hansell M. H. 2005. Animal architecture. 1st edn. New York, NY: Oxford University Press [Google Scholar]
- 2.Madin J. S., Connolly S. R. 2006. Ecological consequences of major hydrodynamic disturbances on coral reefs. Nature 444, 477–480 10.1038/nature05328 (doi:10.1038/nature05328) [DOI] [PubMed] [Google Scholar]
- 3.Palmer A. R. 1979. Fish predation and evolution of gastropod shell sculpture: experimental and geographic evidence. Evolution 33, 697–713 10.2307/2407792 (doi:10.2307/2407792) [DOI] [PubMed] [Google Scholar]
- 4.Craig C. L. 1987. The ecological and evolutionary interdependence between web architecture and web silk spun by orb web weaving spiders. Biol. J. Linn. Soc. 30, 135–162 10.1111/j.1095-8312.1987.tb00294.x (doi:10.1111/j.1095-8312.1987.tb00294.x) [DOI] [Google Scholar]
- 5.Gosline J. M., Guerette P. A., Ortlepp C. S., Savage K. N. 1999. The mechanical design of spider silks: from fibroin sequence to mechanical function. J. Exp. Biol. 202, 3295–3303 [DOI] [PubMed] [Google Scholar]
- 6.Swanson B. O., Anderson S. P., DiGiovine C., Ross R. N., Dorsey J. P. 2009. The evolution of complex biomaterial performance: the case of spider silk. Integr. Comp. Biol. 49, 21–31 10.1093/icb/icp013 (doi:10.1093/icb/icp013) [DOI] [PubMed] [Google Scholar]
- 7.Vollrath F., Selden P. 2007. The role of behavior in the evolution of spiders, silks, and webs. Annu. Rev. Ecol. Evol. S. 38, 819–846 10.1146/annurev.ecolsys.37.091305.110221 (doi:10.1146/annurev.ecolsys.37.091305.110221) [DOI] [Google Scholar]
- 8.Sensenig A., Agnarsson I., Blackledge T. A. 2010. Behavioural and biomaterial coevolution in spider orb webs. J. Evol. Biol. 23, 1839–1856 10.1111/j.1420-9101.2010.02048.x (doi:10.1111/j.1420-9101.2010.02048.x) [DOI] [PubMed] [Google Scholar]
- 9.Blackledge T. A., Scharff N., Coddington J. A., Szüts T., Wenzel J. W., Hayashi C. Y., Agnarsson I. 2009. Reconstructing web evolution and spider diversification in the molecular era. Proc. Natl Acad. Sci. USA 106, 5229–5234 10.1073/pnas.0901377106 (doi:10.1073/pnas.0901377106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiling A. M., Herberstein M. E. 2000. Interpretations of orb-web variability: a review of past and current ideas. Ekol. Bratislava 19, 97–106 [Google Scholar]
- 11.Argintean S., Chen J., Kim M., Moore A. M. F. 2006. Resilient silk captures prey in black widow cobwebs. Appl. Phys. A Mater. 82, 235–241 10.1007/s00339-005-3430-y (doi:10.1007/s00339-005-3430-y) [DOI] [Google Scholar]
- 12.Wise D. H. 1993. Spiders in ecological webs, 1st edn. Cambridge,UK: Cambridge University Press [Google Scholar]
- 13.Coddington J. A., Levi H. W. 1991. Systematics and evolution of spiders (Araneae). Ann. Rev. Ecol. Syst. 22, 565–592 10.1146/annurev.es.22.110191.003025 (doi:10.1146/annurev.es.22.110191.003025) [DOI] [Google Scholar]
- 14.Bond J. E., Opell B. D. 1998. Testing adaptive radiation and key innovation hypotheses in spiders. Evolution 52, 403–414 10.2307/2411077 (doi:10.2307/2411077) [DOI] [PubMed] [Google Scholar]
- 15.Craig C. L. 1987. The significance of spider size to the diversification of spider-web architectures and spider reproductive modes. Am. Nat. 129, 47–68 10.1086/284622 (doi:10.1086/284622) [DOI] [Google Scholar]
- 16.Eberhard W. G., Agnarsson I., Levi H. W. 2008. Web forms and the phylogeny of theridiid spiders (Araneae: Theridiidae): chaos from order. Syst. Biodivers. 6, 415–475 10.1017/S1477200008002855 (doi:10.1017/S1477200008002855) [DOI] [Google Scholar]
- 17.Eberhard W. G. 1990. Function and phylogeny of spider webs. Annu. Rev. Ecol. Syst. 21, 341–372 10.1146/annurev.es.21.110190.002013 (doi:10.1146/annurev.es.21.110190.002013) [DOI] [Google Scholar]
- 18.Kuntner M., Coddington J. A., Hormiga G. 2008. Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): testing morphological and ethological homologies. Cladistics 24, 147–217 10.1111/j.1096-0031.2007.00176.x (doi:10.1111/j.1096-0031.2007.00176.x) [DOI] [Google Scholar]
- 19.Zschokke S. 1997. Factors influencing the size of the orb web. In Proc. 16th European Colloquim of Arachnology, pp. 329–334 Siedlce, Poland [Google Scholar]
- 20.Stowe M. K. 1986. Prey specialization in the Araneidae. In Spiders: Webs, behavior and evolution (ed. Shear W. A.), pp. 101–103 Stanford, CA: Stanford University Press [Google Scholar]
- 21.Blackledge T. A., Hayashi C. Y. 2006. Silken toolkits: biomechanics of silk fibers spun by the orb web spider Argiope argentata (Fabricius 1775). J. Exp. Biol. 209, 2452–2461 10.1242/jeb.02275 (doi:10.1242/jeb.02275) [DOI] [PubMed] [Google Scholar]
- 22.Madsen B., Shao Z. Z., Vollrath F. 1999. Variability in the mechanical properties of spider silks on three levels: interspecific, intraspecific and intraindividual. Int. J. Biol. Macromol. 24, 301–306 10.1016/S0141-8130(98)00094-4 (doi:10.1016/S0141-8130(98)00094-4) [DOI] [PubMed] [Google Scholar]
- 23.Pan Z. J., Li C. P., Xu Q. 2004. Active control on molecular conformations and tensile properties of spider silk. J. Appl. Polym. Sci. 92, 901–905 10.1002/app.20055 (doi:10.1002/app.20055) [DOI] [Google Scholar]
- 24.Pan Z. J., Liu M. 2009. Effects of drawing speed and water on microstructure and mechanical properties of artificially spun spider dragline silk. Fiber. Polym. 10, 285–289 10.1007/s12221-009-0285-4 (doi:10.1007/s12221-009-0285-4) [DOI] [Google Scholar]
- 25.Vollrath F., Madsen B., Shao Z. Z. 2001. The effect of spinning conditions on the mechanics of a spider's dragline silk. Proc R. Soc. Lond. B 268, 2339–2346 10.1098/rspb.2001.1590 (doi:10.1098/rspb.2001.1590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dicko C., Vollrath F., Kenney J. M. 2004. Spider silk protein refolding is controlled by changing pH. Biomacromolecules 5, 704–710 10.1021/bm034307c (doi:10.1021/bm034307c) [DOI] [PubMed] [Google Scholar]
- 27.Blackledge T. A., Boutry C., Wong S. C., Baji A., Dhinojwala A., Sahni V., Agnarsson I. 2009. How super is supercontraction? Persistent versus cyclic responses to humidity in spider dragline silk. J. Exp. Biol. 212, 1981–1989 10.1242/jeb.028944 (doi:10.1242/jeb.028944) [DOI] [PubMed] [Google Scholar]
- 28.Vehoff T., Glisovic A., Scholimeyer H., Zippelius A., Salditt T. 2007. Mechanical properties of spider dragline silk: humidity, hysteresis, and relaxation. Biophys. J. 93, 4425–4432 10.1529/biophysj.106.099309 (doi:10.1529/biophysj.106.099309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig C. L., Riekel C., Herberstein M. E., Weber R. S., Kaplan D., Pierce N. E. 2000. Evidence for diet effects on the composition of silk proteins produced by spiders. Mol. Biol. Evol. 17, 1904–1913 [DOI] [PubMed] [Google Scholar]
- 30.Tso I. M., Wu H. C., Hwang I. R. 2005. Giant wood spider Nephila pilipes alters silk protein in response to prey variation. J. Exp. Biol. 208, 1053–1061 10.1242/jeb.01437 (doi:10.1242/jeb.01437) [DOI] [PubMed] [Google Scholar]
- 31.Hu X., Vasanthavada K., Köhler K., McNary S., Moore A. M. F., Vierra C. A. 2006. Molecular mechanisms of spider silk. Cell. Mol. Life Sci. 63, 1986–1999 10.1007/s00018-006-6090-y (doi:10.1007/s00018-006-6090-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutry C., Blackledge T. A. 2008. The common house spider alters the material and mechanical properties of cobweb silk in response to different prey. J. Exp. Zool. Part A 309A, 542–552 10.1002/jez.487 (doi:10.1002/jez.487) [DOI] [PubMed] [Google Scholar]
- 33.Boutry C., Blackledge T. A. 2009. Biomechanical variation of silk links spinning plasticity to spider web function. Zoology 112, 1–10 10.1016/j.zool.2009.03.003 (doi:10.1016/j.zool.2009.03.003) [DOI] [PubMed] [Google Scholar]
- 34.Tso I. M., Chiang S. Y., Blackledge T. A. 2007. Does the giant wood spider Nephila pilipes respond to prey variation by altering web or silk properties? Ethology 113, 324–333 10.1111/j.1439-0310.2007.01318.x (doi:10.1111/j.1439-0310.2007.01318.x) [DOI] [Google Scholar]
- 35.Vollrath F. 2000. Strength and structure of spiders' silks. Rev. Mol. Biotech. 74, 67–83 10.1016/S1389-0352(00)00006-4 (doi:10.1016/S1389-0352(00)00006-4) [DOI] [PubMed] [Google Scholar]
- 36.Swanson B. O., Blackledge T. A., Beltrán J., Hayashi C. Y. 2006. Variation in the material properties of spider dragline silk across species. Appl. Phys. A Mater. 82, 213–218 [Google Scholar]
- 37.Gosline J. M., DeMont M. E., Denny M. W. 1986. The structure and properties of spider silk. Endeavour 10, 37–43 10.1016/0160-9327(86)90049-9 (doi:10.1016/0160-9327(86)90049-9) [DOI] [Google Scholar]
- 38.Heim M., Keerl D., Scheibel T. 2009. Spider silk: from soluble protein to extraordinary fiber. Angew. Chem. Int. Ed. 48, 3584–3596 10.1002/anie.200803341 (doi:10.1002/anie.200803341) [DOI] [PubMed] [Google Scholar]
- 39.Hayashi C. Y., Shipley N. H., Lewis R. V. 1999. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int. J. Biol. Macromol. 24, 271–275 10.1016/S0141-8130(98)00089-0 (doi:10.1016/S0141-8130(98)00089-0) [DOI] [PubMed] [Google Scholar]
- 40.Simmons A. H., Michal C. A., Jelinski L. W. 1996. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science 271, 84–87 10.1126/science.271.5245.84 (doi:10.1126/science.271.5245.84) [DOI] [PubMed] [Google Scholar]
- 41.Gatesy J., Hayashi C. Y., Motriuk D., Woods J., Lewis R. V. 2001. Extreme diversity, conservation, and convergence of spider silk fibroin sequences. Science 291, 2603–2605 10.1126/science.1057561 (doi:10.1126/science.1057561) [DOI] [PubMed] [Google Scholar]
- 42.Vollrath F., Knight D. P. 2001. Liquid crystalline spinning of spider silk. Nature 410, 541–548 10.1038/35069000 (doi:10.1038/35069000) [DOI] [PubMed] [Google Scholar]
- 43.Keten S., Xu Z., Ihle B., Buehler M. J. 2010. Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nat. Mater. 9, 359–367 10.1038/nmat2704 (doi:10.1038/nmat2704) [DOI] [PubMed] [Google Scholar]
- 44.Du N., Liu X. Y., Narayanan J., Li L., Lim M. L. M., Li D. 2006. Design of superior spider silk: from nanostructure to mechanical properties. Biophys. J. 91, 4528–4535 10.1529/biophysj.106.089144 (doi:10.1529/biophysj.106.089144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keten S., Buehler M. J. 2010. Nanostructure and molecular mechanics of spider dragline silk protein assemblies. J. R. Soc. Interface 7, 1709–1721 10.1098/rsif.2010.0149 (doi:10.1098/rsif.2010.0149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Termonia Y. 1994. Molecular modelling of spider silk elasticity. Macromolecules 27, 7378–7381 10.1021/ma00103a018 (doi:10.1021/ma00103a018) [DOI] [Google Scholar]
- 47.Liu Y., Shao Z., Vollrath F. 2008. Elasticity of spider silks. Biomacromolecules 9, 1782–1786 10.1021/bm7014174 (doi:10.1021/bm7014174) [DOI] [PubMed] [Google Scholar]
- 48.Savage K. N., Gosline J. M. 2008. The role of proline in the elastic mechanism of hydrated spider silks. J. Exp. Biol. 211, 1948–1957 10.1242/jeb.014225 (doi:10.1242/jeb.014225) [DOI] [PubMed] [Google Scholar]
- 49.Platnick N. I. 2010. The world spider catalog, version 11.0. New York, NY: American Museum of Natural History; See http://research.amnh.org/entomology/spiders/catalog/index.html [Google Scholar]
- 50.Griswold C. E., Coddington J. A., Hormiga G., Scharff N. 1998. Phylogeny of the orb-web building spiders (Araneae, Orbiculariae: Deinopoidea, Araneoidea). Zool. J. Linn. Soc. 123, 1–99 10.1111/j.1096-3642.1998.tb01290.x (doi:10.1111/j.1096-3642.1998.tb01290.x) [DOI] [Google Scholar]
- 51.Penney D., Ortuño V. M. 2006. Oldest true orb-weaving spider (Araneae: Araneidae). Biol. Lett. 2, 447–450 10.1098/rsbl.2006.0506 (doi:10.1098/rsbl.2006.0506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selden P. 1989. Orb-web weaving spiders in the early Cretaceous. Nature 340, 711–713 10.1038/340711a0 (doi:10.1038/340711a0) [DOI] [Google Scholar]
- 53.Zschokke S. 2003. Spider-web silk from the early Cretaceous. Nature 424, 636–637 10.1038/424636a (doi:10.1038/424636a) [DOI] [PubMed] [Google Scholar]
- 54.Penney D. 2004. Does the fossil record of spiders track that of their principal prey, the insects? Trans. R. Soc. Edin. Earth 94, 275–281 10.1017/s0263593300000675 (doi:10.1017/s0263593300000675) [DOI] [Google Scholar]
- 55.Hodar J. A., Sanchez-Piñero F. 2002. Feeding habits of the blackwidow spider Latrodectus lilianae (Araneae: Theridiidae) in an arid zone of south-east Spain. J. Zool. 257, 101–109 10.1017/S0952836902000699 (doi:10.1017/S0952836902000699) [DOI] [Google Scholar]
- 56.Denny M. 1976. The physical properties of spider's silk and their role in the design of orb-webs. J. Exp. Biol. 65, 483–506 [Google Scholar]
- 57.Swanson B. O., Blackledge T. A., Summers A. P., Hayashi C. Y. 2006. Spider dragline silk: correlated and mosaic evolution in high-performance biological materials. Evolution 60, 2539–2551 [PubMed] [Google Scholar]
- 58.Opell B. D. 1997. A comparison of capture thread and architectural features of deinopoid and araneoid orb-webs. J. Arachnol. 25, 295–306 [Google Scholar]
- 59.Chacón P., Eberhard W. G. 1980. Factors affecting numbers and kinds of prey caught in artificial spider webs, with considerations of how orb webs trap prey. B. Brit. Arachnol. Soc. 5, 29–38 [Google Scholar]
- 60.Opell B. D., Bond J. E., Warner D. A. 2006. The effects of capture spiral composition and orb-web orientation on prey interception. Zoology 109, 339–345 10.1016/j.zool.2006.04.002 (doi:10.1016/j.zool.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 61.Eberhard W. G. 1989. Effects of orb web orientation and spider size on prey retention. B. Brit. Arachnol. Soc. 8, 45–48 [Google Scholar]
- 62.Zschokke S. 2002. Form and function of the orb-web. In European Arachnology 2000 (eds Toft S., Scharff N.), pp. 99–106 Aarhus, Denmark: Aarhus University Press [Google Scholar]
- 63.Blackledge T. A., Hayashi C. Y. 2006. Unraveling the mechanical properties of composite silk threads spun by cribellate orb-weaving spiders. J. Exp. Biol. 209, 3131–3140 10.1242/jeb.02327 (doi:10.1242/jeb.02327) [DOI] [PubMed] [Google Scholar]
- 64.Lin L. H., Edmonds D. T., Vollrath F. 1995. Structural engineering of an orb-spider's web. Nature 373, 146–148 10.1038/373146a0 (doi:10.1038/373146a0) [DOI] [Google Scholar]
- 65.Opell B. D. 1998. Economics of spider orb-webs: the benefits of producing adhesive capture thread and of recycling silk. Funct. Ecol. 12, 613–624 10.1046/j.1365-2435.1998.00222.x (doi:10.1046/j.1365-2435.1998.00222.x) [DOI] [Google Scholar]
- 66.Lubin Y. D. 1986. Web building and prey capture in the Uloboridae. In Spiders: webs, behavior and evolution (ed. Shear W. A.), pp. 132–171 Stanford, CA: Stanford University Press [Google Scholar]
- 67.Zschokke S., Vollrath F. 1995. Unfreezing the behaviour of two orb spiders. Physiol. Behav. 58, 1167–1173 10.1016/0031-9384(95)02062-4 (doi:10.1016/0031-9384(95)02062-4) [DOI] [PubMed] [Google Scholar]
- 68.Opell B. D. 2002. How spider anatomy and thread configuration shape the stickiness of cribellar prey capture threads. J. Arachnol. 30, 10–19 10.1636/0161-8202(2002)030[0010:HSAATC]2.0.CO;2 (doi:10.1636/0161-8202(2002)030[0010:HSAATC]2.0.CO;2) [DOI] [Google Scholar]
- 69.Hawthorn A. C., Opell B. D. 2003. van der Waals and hygroscopic forces of adhesion generated by spider capture threads. J. Exp. Biol. 206, 3905–3911 10.1242/jeb.00618 (doi:10.1242/jeb.00618) [DOI] [PubMed] [Google Scholar]
- 70.Opell B. D. 1997. The material cost and stickiness of capture threads and the evolution of orb-weaving spiders. Biol. J. Linn. Soc. 62, 443–458 10.1111/j.1095-8312.1997.tb01635.x (doi:10.1111/j.1095-8312.1997.tb01635.x) [DOI] [Google Scholar]
- 71.Vollrath F., Edmonds D. T. 1989. Modulation of the mechanical properties of spider silk by coating with water. Nature 340, 305–307 10.1038/340305a0 (doi:10.1038/340305a0) [DOI] [Google Scholar]
- 72.Vollrath F., Tillinghast E. K. 1991. Glycoprotein glue beneath a spider web's aqueous coat. Naturwissenschaften 78, 557–559 10.1007/BF01134447 (doi:10.1007/BF01134447) [DOI] [Google Scholar]
- 73.Opell B. D. 1999. Redesigning spider webs: stickiness, capture area and the evolution of modern orb-webs. Evol. Ecol. Res. 1, 503–516 [Google Scholar]
- 74.Opell B. D., Hendricks M. L. 2007. Adhesive recruitment by the viscous capture threads of araneoid orb-weaving spiders. J. Exp. Biol. 210, 553–560 10.1242/jeb.02682 (doi:10.1242/jeb.02682) [DOI] [PubMed] [Google Scholar]
- 75.Sahni V., Blackledge T. A., Dhinojwala A. 2010. Viscoelastic solids explain spider web stickiness. Nat. Commun. 1, 19. 10.1038/ncomms1019 (doi:10.1038/ncomms1019) [DOI] [PubMed] [Google Scholar]
- 76.Agnarsson I., Blackledge T. A. 2009. Can a spider web be too sticky? Tensile mechanics constrains the evolution of capture spiral stickiness in orb-weaving spiders. J. Zool. 278, 134–140 10.1111/j.1469-7998.2009.00558.x (doi:10.1111/j.1469-7998.2009.00558.x) [DOI] [Google Scholar]
- 77.Opell B. D., Bond J. E. 2000. Capture thread extensibility of orb-weaving spiders: testing punctuated and associative explanations of character evolution. Biol. J. Linn. Soc. 70, 107–120 10.1111/j.1095-8312.2000.tb00203.x (doi:10.1111/j.1095-8312.2000.tb00203.x) [DOI] [Google Scholar]
- 78.Köhler T., Vollrath F. 1995. Thread biomechanics in the two orb-weaving spiders Araneus diadematus (Araneae, Araneidae) and Uloborus walckenaerius (Araneae, Uloboridae). J. Exp. Zool. 271, 1–17 10.1002/jez.1402710102 (doi:10.1002/jez.1402710102) [DOI] [Google Scholar]
- 79.Opell B. D., Bond J. E. 2001. Changes in the mechanical properties of capture threads and the evolution of modern orb-weaving spiders. Evol. Ecol. Res. 3, 567–581 [Google Scholar]
- 80.Garb J. E., DiMauro T., Vo V., Hayashi C. Y. 2006. Silk genes support the single origin of orb webs. Science 312, 1762–1762 10.1126/science.1127946 (doi:10.1126/science.1127946) [DOI] [PubMed] [Google Scholar]
- 81.Becker N., Oroudjev E., Mutz S., Cleveland J. P., Hansma P. K., Hayashi C. Y., Makarov D. E., Hansma H. G. 2003. Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2, 278–283 10.1038/nmat858 (doi:10.1038/nmat858) [DOI] [PubMed] [Google Scholar]
- 82.Blackledge T. A., Summers A. P., Hayashi C. Y. 2005. Gumfooted lines in black widow cobwebs and the mechanical properties of spider capture silk. Zoology 108, 41–46 10.1016/j.zool.2004.11.001 (doi:10.1016/j.zool.2004.11.001) [DOI] [PubMed] [Google Scholar]
- 83.Lubin Y. D., Eberhard W. G., Montgomery G. G. 1978. Webs of Miagrammopes (Araneae: Uloboridae) in the neotropics. Psyche 85, 1–23 10.1155/1978/72579 (doi:10.1155/1978/72579) [DOI] [Google Scholar]
- 84.Yeargan K. V. 1994. Biology of bolas spiders. Annu. Rev. Entomol. 39, 81–99 10.1146/annurev.en.39.010194.000501 (doi:10.1146/annurev.en.39.010194.000501) [DOI] [Google Scholar]
- 85.Eberhard W. G. 1975. The ‘inverted ladder' orb web of Scoloderus sp. and the intermediate orb of Eustala (?) sp. Araneae: Araneidae. J. Nat. Hist. 9, 93–106 10.1080/00222937500770071 (doi:10.1080/00222937500770071) [DOI] [Google Scholar]
- 86.Stowe M. K. 1978. Observations of two nocturnal orbweavers that build specialized webs: Scoloderus cordatus and Wixia ectypa (Araneae: Araneidae). J. Arachnol. 6, 141–146 [Google Scholar]
- 87.Kuntner M., Haddad C. R., Aljancic G., Blejec A. 2008. Ecology and web allometry of Clitaetra irenae, an arboricolous African orb-weaving spider (Araneae, Araneoidea, Nephilidae). J. Arachnol. 36, 583–594 10.1636/T07-54.1 (doi:10.1636/T07-54.1) [DOI] [Google Scholar]
- 88.Kuntner M., Agnarsson I. 2009. Phylogeny accurately predicts behaviour in Indian Ocean Clitaetra spiders (Araneae: Nephilidae). Invert. Syst. 23, 193–204 10.1071/IS09002 (doi:10.1071/IS09002) [DOI] [Google Scholar]
- 89.Harmer A. M. T. 2009. Elongated orb-webs of Australian ladder-web spiders (Araneidae: Telaprocera) and the significance of orb-web elongation. J. Ethol. 27, 453–460 10.1007/s10164-008-0142-8 (doi:10.1007/s10164-008-0142-8) [DOI] [Google Scholar]
- 90.Harmer A. M. T., Herberstein M. E. 2009. Taking it to extremes: what drives extreme web elongation in Australian ladder web spiders (Araneidae: Telaprocera maudae)? Anim. Behav. 78, 499–504 10.1016/j.anbehav.2009.05.023 (doi:10.1016/j.anbehav.2009.05.023) [DOI] [Google Scholar]
- 91.Opell B. D. 1994. Increased stickiness of prey capture threads accompanying web reduction in the spider family Uloboridae. Funct. Ecol. 8, 85–90 10.2307/2390115 (doi:10.2307/2390115) [DOI] [Google Scholar]
- 92.Cartan C. K., Miyashita T. 2000. Extraordinary web and silk properties of Cyrtarachne (Araneae, Araneidae): a possible link between orb-webs and bolas. Biol. J. Linn. Soc. 71, 219–235 10.1111/j.1095-8312.2000.tb01255.x (doi:10.1111/j.1095-8312.2000.tb01255.x) [DOI] [Google Scholar]
- 93.Blackledge T. A., Coddington J. A., Gillespie R. G. 2003. Are three-dimensional spider webs defensive adaptations? Ecol. Lett. 6, 13–18 10.1046/j.1461-0248.2003.00384.x (doi:10.1046/j.1461-0248.2003.00384.x) [DOI] [Google Scholar]
- 94.Benjamin S. P., Zschokke S. 2003. Webs of theridiid spiders: construction, structure and evolution. Biol. J. Linn. Soc. 78, 293–305 10.1046/j.1095-8312.2003.00110.x (doi:10.1046/j.1095-8312.2003.00110.x) [DOI] [Google Scholar]
- 95.Blackledge T. A., Swindeman J. E., Hayashi C. Y. 2005. Quasistatic and continuous dynamic characterization of the mechanical properties of silk from the cobweb of the black widow spider Latrodectus hesperus. J. Exp. Biol. 208, 1937–1949 10.1242/jeb.01597 (doi:10.1242/jeb.01597) [DOI] [PubMed] [Google Scholar]
- 96.Agnarsson I., Boutry C., Blackledge T. A. 2008. Spider silk aging: initial improvement in a high performance material followed by slow degradation. J. Exp. Zool. Part A 309A, 494–504 10.1002/jez.480 (doi:10.1002/jez.480) [DOI] [PubMed] [Google Scholar]
- 97.Guinea G. V., Elices M., Real J. I., Gutierrez S., Pérez-Rigueiro J. 2005. Reproducibility of the tensile properties of spider (Argiope trifasciata) silk obtained by forced silking. J. Exp. Zool. Part A 303A, 37–44 10.1002/jez.a.111 (doi:10.1002/jez.a.111) [DOI] [PubMed] [Google Scholar]
- 98.Pérez-Rigueiro J., Elices M., Guinea G. V. 2003. Controlled supercontraction tailors the tensile behaviour of spider silk. Polymer 44, 3733–3736 10.1016/S0032-3861(03)00245-3 (doi:10.1016/S0032-3861(03)00245-3) [DOI] [Google Scholar]
- 99.Rao D., Webster M., Heiling A. M., Bruce M. J., Herberstein M. E. 2009. The aggregating behaviour of Argiope radon, with special reference to web decorations. J. Ethol. 27, 35–42 10.1007/s10164-007-0080-x (doi:10.1007/s10164-007-0080-x) [DOI] [Google Scholar]
- 100.Peakall D. B., Witt P. N. 1976. The energy budget of an orb web-building spider. Comp. Biochem. Phys. A 54, 187–190 10.1016/S0300-9629(76)80094-1 (doi:10.1016/S0300-9629(76)80094-1) [DOI] [PubMed] [Google Scholar]
- 101.Alam M. S., Jenkins C. H. 2005. Damage tolerance in naturally compliant structures. Int. J. Damage Mech. 14, 365–384 10.1177/1056789505054313 (doi:10.1177/1056789505054313) [DOI] [Google Scholar]
- 102.Alam M. S., Wahab M. A., Jenkins C. H. 2007. Mechanics in naturally compliant structures. Mech. Mater. 39, 145–160 10.1016/j.mechmat.2006.04.005 (doi:10.1016/j.mechmat.2006.04.005) [DOI] [Google Scholar]
- 103.Aoyanagi Y., Okumura K. 2010. Simple model for the mechanics of spider webs. Phys. Rev. Lett. 104, 038102. 10.1103/PhysRevLett.104.038102 (doi:10.1103/PhysRevLett.104.038102) [DOI] [PubMed] [Google Scholar]
- 104.Sandoval C. P. 1994. Plasticity in web design in the spider Parawixia bistriata: a response to variable prey type. Funct. Ecol. 8, 701–707 10.2307/2390229 (doi:10.2307/2390229) [DOI] [Google Scholar]
- 105.Schneider J. M., Vollrath F. 1998. The effect of prey type on the geometry of the capture web of Araneus diadematus. Naturwissenschaften 85, 391–394 10.1007/s001140050521 (doi:10.1007/s001140050521) [DOI] [Google Scholar]
- 106.Herberstein M. E., Craig C. L., Elgar M. A. 2000. Foraging strategies and feeding regimes: web and decoration investment in Argiope keyserlingi Karsch (Araneae: Araneidae). Evol. Ecol. Res. 2, 69–80 [Google Scholar]
- 107.Higgins L. E., Buskirk R. E. 1992. A trap-building predator exhibits different tactics for different aspects of foraging behaviour. Anim. Behav. 44, 485–489 10.1016/0003-3472(92)90058-H (doi:10.1016/0003-3472(92)90058-H) [DOI] [Google Scholar]
- 108.Sherman P. M. 1994. The orb-web: an energetic and behavioural estimator of a spider's dynamic foraging and reproductive strategies. Anim. Behav. 48, 19–34 10.1006/anbe.1994.1208 (doi:10.1006/anbe.1994.1208) [DOI] [Google Scholar]
- 109.Crews S. C., Opell B. D. 2006. The features of capture threads and orb-webs produced by unfed Cyclosa turbinata (Araneae: Araneidae). J. Arachnol. 34, 427–434 10.1636/S04-106.1 (doi:10.1636/S04-106.1) [DOI] [Google Scholar]
- 110.Mayntz D., Toft S., Vollrath F. 2009. Nutrient balance affects foraging behaviour of a trap-building predator. Biol. Lett. 5, 735–738 10.1098/rsbl.2009.0431 (doi:10.1098/rsbl.2009.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liao C. P., Chi K. J., Tso I. M. 2009. The effects of wind on trap structural and material properties of a sit-and-wait predator. Behav. Ecol. 20, 1194–1203 10.1093/beheco/arp119 (doi:10.1093/beheco/arp119) [DOI] [Google Scholar]
- 112.Vollrath F., Downes M., Krackow S. 1997. Design variability in web geometry of an orb-weaving spider. Physiol. Behav. 62, 735–743 10.1016/S0031-9384(97)00186-8 (doi:10.1016/S0031-9384(97)00186-8) [DOI] [PubMed] [Google Scholar]
- 113.Heiling A. M., Herberstein M. E. 1998. The web of Nuctenea sclopetaria (Araneae, Araneidae): relationship between body size and web design. J. Arachnol. 26, 91–96 [Google Scholar]
- 114.Herberstein M. E., Heiling A. M. 1999. Asymmetry in spider orb webs: a result of physical constraints? Anim. Behav. 58, 1241–1246 10.1006/anbe.1999.1255 (doi:10.1006/anbe.1999.1255) [DOI] [PubMed] [Google Scholar]
- 115.Hesselberg T. 2010. Ontogenetic changes in web design in two orb-web spiders. Ethology 116, 535–545 10.1111/j.1439-0310.2010.01760.x (doi:10.1111/j.1439-0310.2010.01760.x) [DOI] [Google Scholar]
- 116.Kuntner M., Kralj-Fiser S., Gregoric M. 2010. Ladder webs in orb-web spiders: ontogenetic and evolutionary patterns in Nephilidae. Biol. J. Linn. Soc. 99, 849–866 10.1111/j.1095-8312.2010.01414.x (doi:10.1111/j.1095-8312.2010.01414.x) [DOI] [Google Scholar]
- 117.Eberhard W. G. 1988. Behavioral flexibility in orb web construction: effects of supplies in different silk glands and spider size and weight. J. Arachnol. 16, 295–302 [Google Scholar]
- 118.Heiling A. M., Herberstein M. E. 1999. The role of experience in web-building spiders (Araneidae). Anim. Cogn. 2, 171–177 10.1007/s100710050037 (doi:10.1007/s100710050037) [DOI] [Google Scholar]
- 119.Nakata K., Ushimaru A. 1999. Feeding experience effects web relocation and investment in web threads in an orb-web spider, Cyclosa argenteoalba. Anim. Behav. 57, 1251–1255 10.1006/anbe.1999.1105 (doi:10.1006/anbe.1999.1105) [DOI] [PubMed] [Google Scholar]
- 120.Nakata K., Ushimaru A. 2004. Difference in web construction behavior at newly occupied web sites between two Cyclosa species. Ethology 110, 397–411 10.1111/j.1439-0310.2004.00983.x (doi:10.1111/j.1439-0310.2004.00983.x) [DOI] [Google Scholar]
- 121.Eberhard W. G. 2000. Spider manipulation by a wasp larva. Nature 406, 255–256 10.1038/35018636 (doi:10.1038/35018636) [DOI] [PubMed] [Google Scholar]
- 122.Eberhard W. G. 2010. Recovery of spiders from the effects of parasitic wasps: implications for fine-tuned mechanisms of manipulation. Anim. Behav. 79, 375–383 10.1016/j.anbehav.2009.10.033 (doi:10.1016/j.anbehav.2009.10.033) [DOI] [Google Scholar]
- 123.Higgins L. E. 1992. Developmental changes in barrier web structure under different levels of predation risk in Nephila clavipes (Araneae: Tetragnathidae). J. Insect Behav. 5, 635–655 10.1007/BF01048010 (doi:10.1007/BF01048010) [DOI] [Google Scholar]
- 124.Nakata K. 2008. Spiders use airborne cues to respond to flying insect predators by building orb-webs with fewer silk threads and larger silk decorations. Ethology 114, 686–692 10.1111/j.1439-0310.2008.01506.x (doi:10.1111/j.1439-0310.2008.01506.x) [DOI] [Google Scholar]
- 125.Blamires S. J., Thompson M. B., Hochuli D. F. 2007. Habitat selection and web plasticity by the orb spider Argiope keyserlingi (Argiopidae): do they compromise foraging success for predator avoidance? Austral Ecol. 32, 551–563 10.1111/j.1442-9993.2007.01727.x (doi:10.1111/j.1442-9993.2007.01727.x) [DOI] [Google Scholar]
- 126.Krink T., Vollrath F. 2000. Optimal area use in orb webs of the spider Araneus diadematus. Naturwissenschaften 87, 90–93 10.1007/s001140050017 (doi:10.1007/s001140050017) [DOI] [PubMed] [Google Scholar]
- 127.Foelix R. F. 1996. Biology of Spiders, 2nd edn. New York, NY: Oxford University Press [Google Scholar]
- 128.Vollrath F. 2006. Spider silk: thousands of nano-filaments and dollops of sticky glue. Curr. Biol. 16, 925–927 10.1007/BF00223966 (doi:10.1007/BF00223966) [DOI] [PubMed] [Google Scholar]
- 129.Garrido M. A., Elices M., Viney C., Pérez-Rigueiro J. 2002. The variability and interdependence of spider drag line tensile properties. Polymer 43, 4495–4502 10.1016/S0032-3861(02)00254-9 (doi:10.1016/S0032-3861(02)00254-9) [DOI] [Google Scholar]
- 130.Pérez-Rigueiro J., Elices M., Llorca J., Viney C. 2001. Tensile properties of Argiope trifasciata drag line silk obtained from the spider's web. J. Appl. Polym. Sci. 82, 2245–2251 10.1002/app.2072 (doi:10.1002/app.2072) [DOI] [Google Scholar]
- 131.Higgins L. E., White S., Nuñez-Farfán J., Vargas J. 2007. Patterns of variation among distinct alleles of the Flag silk gene from Nephila clavipes. Int. J. Biol. Macromol. 40, 201–216 10.1016/j.ijbiomac.2006.07.007 (doi:10.1016/j.ijbiomac.2006.07.007) [DOI] [PubMed] [Google Scholar]
- 132.Shao Z., Hu X. W., Frische S., Vollrath F. 1999. Heterogeneous morphology of Nephila edulis spider silk and its significance for mechanical properties. Polymer 40, 4709–4711 10.1016/S0032-3861(99)00072-5 (doi:10.1016/S0032-3861(99)00072-5) [DOI] [Google Scholar]
- 133.Garrido M. A., Elices M., Viney C., Pérez-Rigueiro J. 2002. Active control of spider silk strength: comparison of drag line spun on vertical and horizontal surfaces. Polymer 43, 1537–1540 10.1016/S0032-3861(01)00713-3 (doi:10.1016/S0032-3861(01)00713-3) [DOI] [Google Scholar]
- 134.Zax D. B., Armanios D. E., Horak S., Malowniak C., Yang Z. 2004. Variation of mechanical properties with amino acid content in the silk of Nephila clavipes. Biomacromolecules 5, 732–738 10.1021/bm034309x (doi:10.1021/bm034309x) [DOI] [PubMed] [Google Scholar]
- 135.Vollrath F., Köhler T. 1996. Mechanics of silk produced by loaded spiders. Proc. R. Soc. Lond. B 263, 387–391 10.1098/rspb.1996.0059 (doi:10.1098/rspb.1996.0059) [DOI] [Google Scholar]
- 136.Wirth E., Barth F. G. 1992. Forces in the spider orb web. J. Comp. Physiol. A 171, 359–371 10.1016/j.cub.2006.09.050 (doi:10.1016/j.cub.2006.09.050) [DOI] [Google Scholar]
- 137.Zschokke S. 2000. Radius construction and structure in the orb-web of Zilla diodia (Araneidae). J. Comp. Physiol. A 186, 999–1006 10.1007/s003590000155 (doi:10.1007/s003590000155) [DOI] [PubMed] [Google Scholar]
- 138.Watanabe T. 2000. Web tuning of an orb-web spider, Octonoba sybotides, regulates prey-catching behaviour. Proc. R. Soc. Lond. B 267, 565–569 10.1098/rspb.2000.1038 (doi:10.1098/rspb.2000.1038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hazlett B. A. 1987. Behavioural plasticity as an adaptation to a variable environment. In Behavioral adaptation to intertidal life (eds Chelazzi G., Vannini M.), pp. 317–332 New York, NY: Plenum Press [Google Scholar]
- 140.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 141.Ko F. K., Jovicic J. 2004. Modeling of mechanical properties and structural design of spider web. Biomacromolecules 5, 780–785 10.1021/bm0345099 (doi:10.1021/bm0345099) [DOI] [PubMed] [Google Scholar]
- 142.Simmons A. H., Ray E., Jelinski L. W. 1994. Solid-state 13C NMR of Nephila clavipes dragline silk establishes structure and identity of crystalline regions. Macromolecules 27, 5235–5237 10.1021/ma00096a060 (doi:10.1021/ma00096a060) [DOI] [Google Scholar]
- 143.Hayashi C. Y., Lewis R. V. 1998. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 275, 773–784 10.1006/jmbi.1997.1478 (doi:10.1006/jmbi.1997.1478) [DOI] [PubMed] [Google Scholar]
- 144.Buehler M. J., Yung Y. C. 2009. Deformation and failure of protein materials in physiologically extreme conditions and disease. Nat. Mater. 8, 175–188 10.1038/nmat2387 (doi:10.1038/nmat2387) [DOI] [PubMed] [Google Scholar]
- 145.Agnarsson I., Kuntner M., Blackledge T. A. 2010. Bioprospecting finds the toughest biological material: extraordinary silk from a giant riverine orb spider. PLoS ONE 5, e11234. 10.1038/nmat2387 (doi:10.1371/journal.pone.0011234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guinea G. V., Pérez-Rigueiro J., Plaza G. R., Elices M. 2006. Volume constancy during stretching of spider silk. Biomacromolecules 7, 2173–2177 10.1021/bm060138v (doi:10.1021/bm060138v) [DOI] [PubMed] [Google Scholar]
- 147.Jocqué R., Dippenaar-Schoeman A. S. 2007. Spider families of the world, 2nd edn. Tervuren, Belgium: Royal Museum for Central Africa [Google Scholar]