Abstract
The study focuses on the synthesis of a novel polymeric scaffold having good porosity and mechanical characteristics synthesized by using natural polymers and their optimization for application in cartilage tissue engineering. The scaffolds were synthesized via cryogelation technology using an optimized ratio of the polymer solutions (chitosan, agarose and gelatin) and cross-linker followed by the incubation at sub-zero temperature (−12°C). Microstructure examination of the chitosan–agarose–gelatine (CAG) cryogels was done using scanning electron microscopy (SEM) and fluorescent microscopy. Mechanical analysis, such as the unconfined compression test, demonstrated that cryogels with varying chitosan concentrations, i.e. 0.5–1% have a high compression modulus. In addition, fatigue tests revealed that scaffolds are suitable for bioreactor studies where gels are subjected to continuous cyclic strain. In order to confirm the stability, cryogels were subjected to high frequency (5 Hz) with 30 per cent compression of their original length up to 1 × 105 cycles, gels did not show any significant changes in their mass and dimensions during the experiment. These cryogels have exhibited degradation capacity under aseptic conditions. CAG cryogels showed good cell adhesion of primary goat chondrocytes examined by SEM. Cytotoxicity of the material was checked by MTT assay and results confirmed the biocompatibility of the material. In vivo biocompatibility of the scaffolds was checked by the implantation of the scaffolds in laboratory animals. These results suggest the potential of CAG cryogels as a good three-dimensional scaffold for cartilage tissue engineering.
Keywords: chitosan–agarose–gelatin cryogels, cartilage tissue engineering, mechanical compression, three-dimensional scaffolds, biocompatibility
1. Introduction
Tissue engineering is an emerging multi-disciplinary field to repair or replace the damaged tissue or organs, it merges the aspects of two important fields, i.e. engineering and biology. Many rapid achievements in this field have risen in part from significant advances in cell, molecular biology and biomaterials [1]. The goal of tissue engineering is to surpass the limitations of conventional treatments like organ transplantation and biomaterial implantation [2]. While the application of tissue engineering is important in the repair of tissues like skin, liver and bone, cartilage degeneracy is a major concern in tissue engineering owing to its limited self-repair ability. Cartilage is predominately avascular, aneural and alymphatic tissue composed of just 2–5% chondrocytes embedded within a dense extracellular matrix (ECM). Current methods used for the repair of damaged cartilage include drilling, abrasion arthroplasty, microfractures, osteochondral autograft or allograft and autologous chondrocytes transplantation [3]. There are some disadvantages associated with these methods such as lack of cell retention where cell suspensions are directly transplanted at the cartilage defect site in case of autologous chondrocyte transplantation [4] or issues of donor site morbidity and uncertainty with the acceptance of the graft in case of osteochondral allograft or autograft [5]. The alternative approach for the repair of articular defects is tissue engineering using porous three-dimensional scaffolds to facilitate cellular attachment along with mechanical support. The scaffolds that are used as an implant for cartilage tissue engineering have certain prerequisites like biodegradability, which should match with the formation of neo-cartilage [6], biocompatibility, should attach to the defect site, have good porosity, should regulate cell expression and must have a good mechanical strength [7]. Out of these properties, good mechanical stability is the most important characteristic because dynamic combined compression–shear stimulation has reported to increase the collagen and proteoglycan synthesis over the static cultures [8]. So the material chosen for the construction of scaffold for cartilage tissue engineering should possess good mechanical and tensile properties, while it is also important that the selected material should provide a native environment for the cells. To date, wide varieties of synthetic and natural materials have been investigated as scaffolds for cartilage tissue engineering [9]. But synthetic materials possess a surface chemistry that does not promote cell adhesion or material components may create a local environment in the scaffold that may inhibit the biological activity of the cells [10]. With scaffolds made from natural materials, there is a problem of reproducibility, but they provide biological cues that enhance cell activity for adhesion and proliferation thereby enhancing the ECM production, making these polymers more appropriate for cartilage tissue engineering.
However, many natural polymers have shown good potential for tissue engineering applications. Out of the series of natural polymers few have the unique properties required for cartilage tissue engineering, like agarose which is a polysaccharide consisting alternate repeating units of 1, 3-linked β-D-galactopyranose and 1, 4-linked 3, 6-anhydro-α-L-galactopyranose. Agarose hydrogels have been used for seeding chondrocytes and when subjected to dynamic deformational loading have been demonstrated to enhance the matrix elaboration [11]. Chondrocytes seeded in agarose gels at high density have been reported to synthesize and assemble a mechanically functional cartilage like ECM [12] along with well-documented ability to promote and maintain the chondrocytes phenotype [13]. The property of agarose, i.e. good mechanical strength and capacity to retain chondrocytes phenotype makes it suitable for the construction of scaffolds for cartilage tissue engineering. On the other hand, chitosan is a natural polysaccharide composed of glucosamine and N-acetyl-glucosamine, obtained by the deacetylation of chitin. Structurally chitosan is an analogue of glycosaminoglycans (GAGs), which is a component of cartilage ECM. These biomaterials have not only been reported to support chondrogenic activities but have also been demonstrated to support the expression of cartilage ECM proteins by human chondrocytes [14]. Chitosan solution injected into knee articular cavity of rats has been reported to increase the proliferation of chondrocytes in knee articular cartilage suggesting that chitosan could be beneficial for the repair of articular cartilage lesions. The biocompatibility of chitosan was proved by chitosan-based biomaterial ingestion or injection in the human body without any allergic or inflammatory reaction [15]. Chitosan augmented with chondroitin sulphate in the form of hydrogels has been reported to maintain many morphological and functional features of chondrocytes in the native cartilage [16]. Gelatin is basically a denatured collagen and presumably retains informational signals owing to the presence of Arg–Gly–Asp (RGD)-like sequences, which enhances cell attachment and proliferation. A resorbable gelatin sponge derived from porcine skin has been shown to support chondrocyte proliferation and matrix synthesis [17].
A polymer or combination of polymers can be fabricated into a porous scaffold through various techniques, such as solvent casting, particulate leaching, gas foaming, fibre meshes, phase separation, melt moulding, emulsion free drying, solution casting, freeze-drying, etc. But these scaffold fabrication techniques have certain limitations like they are incapable of controlling pore size and pore distribution is uneven [2]. Considering an example of particulate leaching technology for scaffold fabrication it has the limitation that it mainly produces thin membranes (2–3 mm) owing to difficulty in ensuring the complete removal of the embedded particles [18]. This technology is also unable to generate large and interconnected porous network in the fabricated matrices. We have recently shown new possibilities of cryogel technology for the construction of interconnected macroporous three-dimensional scaffolds for tissue engineering applications [19,20]. Cryogels have also been used for other biotechnological applications [21]. Cryogelation technology has an advantage over other scaffold fabrication technologies as scaffolds can be fabricated in different formats like disc, sheets and monoliths with varying dimensions. Cryogelation generates the matrices those are interconnected and possess larger pore sizes. Cryogels are gel matrices formed at moderately frozen condition, where most of the solvent gets frozen while part of the solvent is left unfrozen (unfrozen liquid microphase), where monomeric or polymeric precursors concentrate and undergo chemical reactions. This chemical reaction in the liquid microphase leads to gel formation that is converted into porous a scaffold on thawing the frozen part (act as porogens; [20]). These gels facilitate the unhindered diffusion of solutes and nutrients owing to the presence of interconnected macropores [22]. Cryogels possess continuous interconnected pores up to 200 µm that provide a surface for the proliferation of chondrocytes making these suitable matrices for cartilage tissue engineering [23]. Cryogels made from polyvinyl alcohol (PVA) have been used as an artificial cartilage layer for the replacement of arthritic cartilage in shoulder joints [24].
In our previous work [19], we have constructed the scaffolds from chitosan and gelatin for application in tissue engineering. In extension to our previous studies [19,25], the aim of the present study is to synthesize a novel polymeric scaffold using natural polymers like chitosan, agarose and gelatin because they have shown some advantages for cartilage tissue engineering as discussed earlier. The synthesized scaffold was further optimized and characterized physically, chemically as well as mechanically to check their capacity to withstand deformation loading either static or dynamic during cell culture bioreactor studies. The cell compatibility analysis was done with primary goat chondrocytes and further cell adhesion and proliferation were examined. In vivo studies with Wistar rats showed that these blends are biocompatible and are biodegradable without eliciting any adverse effects on the host tissue.
2. Material and methods
2.1. Materials
Agarose (low EEO, gelling temperature approx. 38–40°C) was supplied from Sisco Research Laboratories (Mumbai, India). Low viscosity chitosan (LVC; viscosity: ≤200 mPa.s and MW: 150 000) was purchased from Fluka (Buchs, Switzerland). Gelatin (from cold water fish skin; MW: ∼ 60 000), Dulbecco's modified Eagle's medium (DMEM), 3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyl tetrazolium bromide (MTT, 98%) reagent, trypsin, collagenase type I and penicillin–streptomycin antibiotic were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Glutaraldehyde solution (25%) was obtained from S.D. Fine Chemicals (Mumbai, India). Foetal bovine serum (FBS) was purchased from Hyclone (UT, USA). All the other reagents used were of analytical grade and were used without further purification.
For in vivo studies, five to six-week-old male Wistar rats (200–250 gm) were brought form Central Drug Research Institute (CDRI) Lucknow, India.
2.2. Preparation of supermacroporous chitosan–agarose–gelatin blend
Chitosan–agarose–gelatin (CAG) cryogels were prepared by the cryogelation process. Gelatin was used as a substrate and was cross-linked with chitosan using well-known cross-linker i.e. glutaraldehyde for peptide bond formation, while agarose has the property of self-gelation at low temperature. CAG cryogels were prepared in two different polymer ratios by changing the chitosan polymer concentration in the reaction mixture. LVC (final concentration 0.5 and 1.0%, w/v) was dissolved in 4.5 ml of 1 per cent aqueous acetic acid solution (pH 2.4) with the help of mechanical stirring at room temperature. After the complete dissolution of chitosan, gelatin (100 mg; final concentration 1%, w/v) was added in the same tube containing chitosan solution and stirring continued. The final concentration of chitosan–gelatin in the polymer reaction mixture was dependent upon the chitosan concentration used, i.e. 1.5 per cent or 2 per cent. In the meantime, agarose (300 mg; final concentration 3%, w/v) was dissolved in 5 ml of de-ionized water by boiling in 50 ml plastic tube till the solution become clear indicating that agarose has dissolved properly. Agarose solution was cooled at room temperature till the temperature dropped to 50–55°C. At this point, heterogeneous chitosan–gelatin solution was added to the aqueous solution of agarose and thoroughly mixed by vortexing, followed by the addition of 0.5 ml glutaraldehyde solution (0.2%, v/v) from the stock solution (25%, v/v). The solution was immediately poured into 5 ml plastic syringes and incubated at −12°C for 16 h in liquid cooling bath (cryostat). After that, the gels were thawed in de-ionized water at room temperature and removed from the plastic syringe moulds. Gels were washed in de-ionized water overnight at low speed on a shaker to washout the unreacted aldehyde groups of cross-linker. CAG monoliths were dried at room temperature for studying their physical and chemical behaviour and examination of biocompatibility.
2.3. Chondrocytes isolation and in vitro cultivation
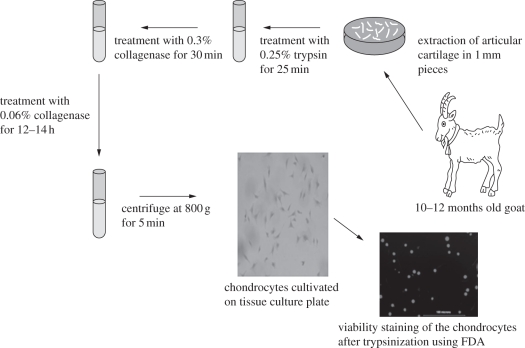
Goat chondrocytes were isolated from the articular cartilage of a 10–12 month old goat. Goat knee was obtained from the local abattoir house within 2 h of slaughter. As shown in figure 1, the cartilage was sliced and minced into 1–2 mm cube pieces. These small pieces were washed with 0.1 M phosphate-buffered saline (PBS; pH 7.4) containing a 2 per cent antibiotic mixture of penicillin and streptomycin. Tissue fragments were incubated with 0.05 per cent trypsin solution prepared in plain DMEM (without FBS) with moderate agitation for 25 min under sterile conditions at room temperature. Cartilage fragments were then digested with 0.3 per cent collagenase type I prepared in plain DMEM (without FBS) for 30 min with moderate agitation in sterile condition at room temperature. Partially digested fragments were then transferred to 60 mm Petri plate inside the laminar flow followed by the addition of 0.06 per cent freshly prepared collagenase type I. Tissues were then incubated for approximately 14–16 h at 37°C in the incubator with 5 per cent CO2. The overnight digested cell containing medium was centrifuged at 800 g for 5 min, and the resulting pellet was re-suspended in DMEM medium supplemented with FBS. Isolated chondrocytes were suspended in DMEM supplemented with 10 per cent FBS and 1 per cent penicillin–streptomycin and cultivated into 75 mm tissue culture flask (Nunc, Germany) followed by incubation at 37°C in presence of 5 per cent CO2.
Figure 1.
Schematic diagram showing the procedure for isolation of chondrocytes from goat knee joint and FDA-stained cells (viable) at the time of seeding on the scaffolds.
2.4. Physical characterization of CAG cryogels
2.4.1. Determination of the flow characteristics
The rate of flow of solvent through the cryogels was determined by preparing the cryogel test samples of specific dimension (diameter: 13 mm and thickness: 20 mm). The test monolith was inserted into the plastic syringe that was connected to a peristaltic pump. Maximum flow of the solvent that can pass through the cryogel was examined using water passing at a controlled speed up to the flow at which cryogel does not show any backpressure. The control for this experiment was established by keeping the same set-up except not keeping the cryogel sample in between the flow path [26] .
2.4.2. Swelling/deswelling behaviour and relative swelling ratio
Swelling kinetics is the property of the gels, which explains their solvent uptake capacity, determined according to the conventional gravimetric procedure [27,28]. Cyclic swelling and deswelling were done up to five cycles for the detection of any changes in the behaviour of the cryogels from initial to the final cycle. Initially, the dry weights of cryogel sections (diameter: 13 mm and thickness: 5 mm) were taken followed by incubation in 0.1 M PBS (pH 7.4) for a particular time duration. The wet weight was recorded at specific time intervals till the samples were equilibrated with the solvent. Experimental error was reduced by taking samples of equal dimension in triplicates. The per cent retention of water (Wr) is given by
where Wr is the water retention capacity, Wt the weight at regular time interval, Wg the weight of dry cryogel and We the weight of water in swollen gels at swelling equilibrium at a room temperature.
In the continuity of the experiment, the equilibrated cryogel sections were dried at 60°C in the oven. Completely dried cryogels sections were again subjected to swelling–deswelling and kinetics repeated up to five cycles. The weight–swelling ratio was taken as a parameter to calculate solvent absorption capacity. It was calculated as:
where SR is the swelling ratio, Ws is the weight of swollen gel and Wd is the weight of dry gel.
2.4.3. Microstructure analysis
Morphology of the CAG cryogels was examined by scanning electron microscopy (SEM, FEI Quanta 200) and cryo-SEM. Before analysing by SEM, the cryogel samples were pre-treated with gradually increasing concentrations of alcohol (ethanol: 20–100%) for the duration of 10–15 min in each step. Dehydrated cryogel sections were then vacuum dried overnight and coated with gold using a sputter coater (Vacuum Tech, Bangalore, India). The coated surface of cryogels was scanned by SEM operated at high vacuum at 20 kV. The pore size was determined by the SEM-associated image software. Different areas were selected for the determination of the average pore size of the gels and samples were examined in triplicate to reduce the error. Three-dimensional pore morphology analysis of cryogels was also done using fluorescent microscopy, which was done by embedding the cryogel monoliths in wax and then slicing 5–10 µm thin sections using a microtome. The porosity of the cryogel samples, i.e. the presence of overall pores in the cryogel test sample was determined using the Archimedes' principle [19].
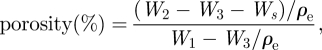 |
where W1 is the specific gravity bottle weight filled with ethanol, W2 the specific gravity bottle weight including ethanol and cryogel sample, W3 the specific gravity bottle weight measured after taking out ethanol-saturated cryogel section from W2, Ws the ethanol-saturated cryogel section weight, and ρe the density of ethanol. Thus, (W1 − W3)/ρe is the total volume of the scaffold including pores and (W2 − W3 − Ws)/ρe the pore volume in the scaffold.
2.4.4. In vitro degradation studies in aseptic mode
To determine the degree of degradation, the initial dry weight of the cryogel samples was recorded and samples were sterilized by treating them with a gradually increasing concentration of ethanol i.e. 20–100% for 10–15 min in each step. Finally, cryogels were incubated in 100 per cent fresh ethanol for 20 min. Ethanol-sterilized CAG cryogel samples were then vacuum dried overnight in a vacuum desiccators under UV light to maintain the aseptic conditions. Completely dried samples were then incubated with 0.1 M sterile PBS in sterile plastic tube. Tightly capped tubes with cryogel samples were incubated for eight weeks. Every week pre-defined samples were removed and washed with de-ionized water to remove the digested polymer. Samples were dried initially at room temperature and then were kept in an oven for the removal of moisture. The samples were in triplicate and average dry weight of the two samples was taken for the calculation of degree of degradation (D.D) (%),
where D.D is the degree of degradation, WI is the average dry weight of samples before incubation (initial weight) and WF is the average dry weight of samples after incubation (final weight). Change in the morphology of degraded cryogels was further analysed by the SEM analysis.
2.4.5. Analysis of permeability
Permeability of the cryogels was determined using Darcy's law, which demonstrates the relationship between liquid flow rates versus pressure in the scaffold. The experiment was performed in two sets, i.e. control and test, in control, water subjected to the constant pressure was passed through the outlet for 1 min and the outflow was collected and mass of liquid was calculated at room temperature. In the test set-up, the flow was collected for 1 min from the outlet by placing the cryogel sample in between the path and mass of liquid was calculated at room temperature. These recorded weights of flow of control and test samples were used for the determination of hydraulic permeability of the scaffold using the following equation:
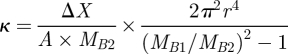 |
where κ is the hydraulic permeability of the porous scaffold, ΔX the thickness of the scaffold, A the flushing area of the porous scaffold, MB1 the mass flow rate of the control, MB2 the mass flow rate of the test and r the internal diameter of the outlet.
Further for the analysis of different functional groups in CAG gels Fourier transform infrared analysis (FT-IR) was done using Perkin Elemer 1000 Paragon spectrometer.
2.5. Mechanical characterization of CAG blend
2.5.1. Determination of compression stability and elasticity
Freshly thawed cryogel monoliths were sliced into uniform parallel discs of specific diameter and thickness, i.e. 13 and 5 mm, respectively. Samples were saturated with 0.1 M PBS (pH 7.4). The mechanical stability of cryogels was investigated by applying uniaxial compression using Zwick/Roell Z010 machine (Germany). Samples were compressed (strain applied) up to 90 per cent of their original length under a load cell of 10 kN at the displacement rate of 1 mm min−1. The compressive modulus of the CAG cryogels was calculated by plotting a graph of stress (kN) versus strain (%).
2.5.2. Cyclic compression analysis (fatigue test)
CAG cryogels were sectioned into slices of 5 mm thickness and 13 mm diameter. These samples were then subjected to varying strain, i.e. 10 per cent, 20 per cent and 30 per cent up to 1 × 105 cycles at a frequency of 2 and 5 Hz using MTS, 810 Material Test System (USA). During the experiment, samples were kept in a specially customized plate having grooves of 20 mm diameter filled with PBS. This laboratory-made set-up helped to maintain the positioning of samples on applying the strain during the experiment. The initial and final wet weight and respective dimensions of cryogel samples that were used for comparing the changes which might have occurred in the samples after applying strain in different set of experiments were recorded.
2.6. In vitro biocompatibility analysis of CAG cryogels
2.6.1. Seeding of primary goat chondrocytes on scaffolds
CAG monoliths were sliced into discs (thickness: 5 mm and diameter: 13 mm) and sterilized by gradually increasing concentration of ethanol. Ethanol-saturated sections were kept in vacuum desiccators for 15–20 h followed by washing with sterile PBS for the removal of traces of ethanol under aseptic conditions. The cryogel sections were also treated with UV light for 15–20 min to ensure their sterility. Sections were then equilibrated with DMEM by placing the sections in a 24-well polystyrene plate (Axygen Scientific, CA, USA) containing 1.5 ml media and allowing it to stand for 3–4 h at 37°C. The primary cell line of chondrocytes isolated from goat was sub-cultured into tissue culture flask for increasing the cell density, which was seeded on DMEM-saturated cryogel sections in its second passage. Chondrocytes cell suspension (500 µl) was gently poured on a cryogel section drop by drop at the cell density of 5 × 105 cells ml−1. The cell culture experiment was run up to 10 days at 37°C with 5 per cent CO2 in a humidified atmosphere, with media change after every 3 days. Before seeding the cells on the cryogel scaffolds, viability of the cells was confirmed by staining the cells with fluorescein diacetate (FDA).
2.6.2. Microscopic analysis of cell-seeded scaffolds
Chondrocytes cultivated on cryogel sections were analysed by SEM on 5th and 10th day of the experiment. Cell-seeded test samples were gently washed with ice cold 0.1 M PBS (pH 7.4) and cells were fixed by treating these sections with 2.5 per cent glutaraldehyde for 5–6 h. After the incubation period, scaffolds were carefully rinsed with PBS and dehydrated by gradually increasing concentration of ethanol followed by drying in vacuum desiccators. The dried samples were then coated with gold and analysed by SEM.
2.6.3. Determination of articular chondrocytes proliferation (MTT assay)
Cell proliferation and scaffold cytotoxicity were analysed by 3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay [29]. DMEM-saturated cryogel sections were seeded with chondrocytes at a seeding density 8 × 105 cells ml−1. In the control, chondrocytes were directly seeded on the surface of the cell culture treated well. Cell-seeded sections were incubated at 37°C in humidified environment containing 5 per cent CO2 and media was changed after every 3 days up to 44 days of cell culture experiment. An MTT assay was performed after every 3 days. The media was removed from the wells (test and control) and followed by the gentle washing with PBS. MTT solution (0.5 ml) of the working concentration of 0.5 mg ml−1 was added in the test well and plate was incubated for 4–5 h at 37°C with 5 per cent CO2. After the incubation, MTT reagent was gently aspirated without disturbing the scaffold followed by the addition of 1.5 ml dimethyl sulphoxide (DMSO) in test wells. The samples were again incubated for 10–15 min at 37°C to dissolve the reduced MTT. The developed colour was measured spectrophotmetrically at 570 nm to calculate the relative viability of chondrocytes on the scaffolds.
2.7. In vivo implantation of cell-seeded cryogel scaffold
In vivo experiments were performed on Wistar rats that were kept in fully ventilated and well-aerated animal facility rooms during the course of experiment and were fed at regular intervals. After the completion of the experiment, animals were euthanized and carcasses were buried. After the characterization of the CAG scaffolds for the physical and biological characterization, the next goal was to check the in vivo biocompatibility of the seeded constructs. CAG cryogel scaffolds were sterilized by treatment with the gradient ethanol concentrations (20%, 40%, 60%, 80% and 100%) followed by the UV sterilization for 20 min. Sterilized scaffolds were then treated with sterile 0.1 M PBS for the removal of remaining ethanol. Treated scaffolds were then incubated in plain DMEM for the equilibration of the scaffolds and proper cell adhesion. Primary goat chondrocytes were isolated by the method as described earlier, and 500 µl cell suspension was seeded at the density of 1 × 106 cells ml−1 on the scaffolds. Scaffolds were incubated under in vitro conditions for 48 h for the cell adherence and ECM synthesis. Seeded scaffolds were then implanted subcutaneously in Wistar rats. All the experiments were done in triplicate with one control. The study was undertaken for a period of six weeks. Wistar rats were anaesthetized using CO2, after which mice were transferred to the sterile hood. An incision was made on the dorsal side with the help of surgical scissors and seeded constructs (cell-seeded scaffolds) were implanted subcutaneously. The incision was stitched using surgical bioresorbable thread to minimize the displacement of the seeded construct. The first group of animals was scarified after two weeks of the implantation, followed by the second and third groups after four and six weeks of study, respectively. Samples were treated separately for the SEM analysis and histological examination. For SEM assay, samples were fixed in paraformaldehyde and dehydrated by treatment with gradient ethanol. Samples were gold coated and analysed for ECM deposition and cell infiltration. For histological examination, samples were fixed in paraformaldehyde for 24 h followed by the treatment with gradient sucrose solution. Samples were then embedded in optical cutting temperature (OCT) compound, which was followed by the sectioning of the specimens. Sections of 5–10 µm thickness were used for the histological studies. Sections were stained with haematoxylin and eosin to investigate cell migration and the effect of the implant on the native tissue.
3. Results and discussion
3.1. Synthesis, optimization and characterization of CAG cryogels
CAG cryogels (4.5% and 5%) were prepared by the process of cryogelation at −12°C. The three-dimensional scaffold was synthesized using a mixture of agarose and chitosan polymers with gelatin as a substrate for cells. The agarose polymer has self-gelation property while chitosan could be cross-linked physically or chemically using different cross-linkers. In the cryogelation process of polymer reaction mixture, agarose self-gelates and chitosan was cross-linked by glutaraldehyde (0.2%) in the presence of gelatin. Glutaraldehyde was used to cross-link the free amino groups present in chitosan and gelatin chains. There is another possibility of self-cross-linking of chitosan and gelatin chains. Eventually it provides stability to gelatin, which is added in the polymer reaction mixture because its presence causes augmentation of cell adhesion and proliferation. In this case, gelatin was also involved in the chitosan cross-linking to increase the elastic strength of scaffold. Scaffolds should have good mechanical strength and elastic nature which are the prerequisites for cartilage tissue engineering. The porosity could enhance the chondrocyte passage, waste transport and nutrient supply efficiently throughout the scaffold. The cross-linker and polymer concentration were optimized in such a manner to synthesize a macroporous scaffold that can withstand the mechanical forces during tissue engineering and also provide native environment to the cells. Individually, the polymers used could not display the desirable properties to be used for cartilage tissue engineering scaffolds. Agarose is an inert polymer, which does not favour cell adhesion effectively but has good mechanical and elastic behaviour, chitosan mimics GAG structure but its gels are brittle, while gelatin is very good for cell cultivation but its gels have little mechanical integrity. So, we have used these polymers to align the properties required for cartilage tissue engineering and overcome their drawbacks by the synthesis of a blend scaffold. Different concentrations of polymers were tried (data not shown). Among the different combinations of varying polymer concentrations, we found good elastic and mechanical stability in 0.5 per cent and 1 per cent chitosan concentration with stable 3 per cent agarose and 1 per cent gelatin concentration in polymer solution mixture (table 1). In other combinations of varying polymer concentrations, gels exhibited the unique property of one of the polymers used in synthesis, which enhanced certain properties in the gel that were not required. In disparity to other concentrations, 4.5 per cent and 5 per cent CAG cryogels were spongy and did not deform on applying external pressure. There are many other parameters affecting the potentiality of a tissue engineering scaffold. Beside the biological factors, i.e. nutrient, cell density, etc, the microstructure of the scaffold itself plays an important role during tissue engineering like cell attachment, cell proliferation ability and tissue vascularization. For instance, scaffolds with high porous architecture were expected to perform better than those that have lower porosity. So, the optimized cryogels (4.5% and 5%) were further characterized by different aspects to check their potentiality for cartilage tissue engineering.
Table 1.
Comparative study of chitosan–agarose–gelatin cryogels.
| parameters | chitosan–agarose (4.5%) | chitosan–agarose (5%) |
|---|---|---|
| chitosan–agarose ratio | 0.5 : 3 | 1 : 3 |
| gelatin (%) | 1 | 1 |
| glutaraldehyde (%) | 0.2 | 0.2 |
| porosity (%) | 85 | 80 |
| permeability (m4 N−1s−1) | 2.2 × 109 | 2.7 × 109 |
| compressive modulus (kPa) | 39 | 44 |
| flow rate (ml min−1) | up to 10 | up to 10 |
| degree of degradation (%) | 13.85 | 13.70 |
3.2. Physical characterization of CAG cryogels
3.2.1. Determination of the flow characteristics
Flow rate is the volume of aqueous solvent travelling through a porous matrix in a unit time and is determined by allowing the liquid to travel through the matrix in unit time without showing any backpressure. This property of the material is conferred by the porous nature of the matrix. In cartilage tissue engineering, generally chondrocyte-seeded scaffolds are subjected to cyclic mechanical loading in a mechanical bioreactor, which increases the secretion of ECM following increase in mechanical properties of neo-tissue. Under this kind of situation, it is important that the scaffold should have a substantial interconnected porous network for the nutrient flow and waste exchange. Interconnected networks can even prevent cell death when cells are seeded at a high density. So, it is important to know the flow passage ability of the scaffold designed for the cartilage tissue engineering. CAG (4.5% and 5%) cryogels did not show backpressure against water up to the flow rate of 10 ml min−1, which indicates that these cryogels have good porous architecture with the interconnected porosity so they offer little resistance to the flow of solvent. The interconnectivity was also examined by SEM and permeability analysis.
3.2.2. Microstructure analysis
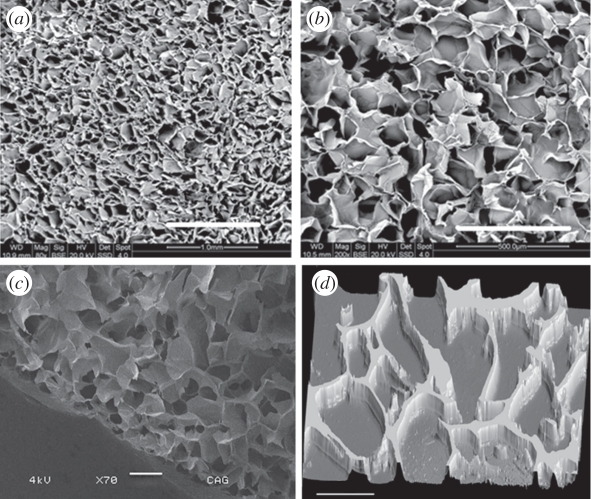
Morphology of chitosan–agarose cryogels was determined by the SEM (figure 2a,b,c). CAG cryogels of 4.5 per cent and 5 per cent showed pore size ranging between 40 and 135 µm, and the average pore diameter was 85–100 µm. The macroporous structure was generated within the scaffold by the nicely arranged interconnected pores formed during cryogelation. The porosity generated in the CAG scaffolds might be appropriate for cartilage tissue engineering because the dimensions of chondrocytes are approximately 10 times lower than the pore diameter present in the scaffold, so that cells can migrate and proliferate without any restriction. Both the concentrations of gels, i.e. 4.5 per cent and 5 per cent exhibited an excellent porosity in the range of 80–85% analysed based on Archimedes' principle. The highly porous network within the scaffold facilitates the good nutrient flow and waste exchange for the healthy growth of chondrocytes. Three-dimensional images (figure 2d) of CAG cryogels as taken with the aid of fluorescence microscopy further support the structure of the cryogels displayed by the SEM.
Figure 2.
Scanning electron microscopy (SEM) images showing microstructure of chitosan–agarose–gelatin CAG gels at (a) 80× and (b) 200× magnification. (c) Cryo-SEM image of CAG cryogels showing porous structure of the gel. (d) Fluorescence microscopic image of CAG cryogel section (thickness: 5 µm) analysed by staining with ethidium bromide. Scale bars, (a) 1 mm; (b) 500 µm; (c) 200 µm; (d) 100 µm.
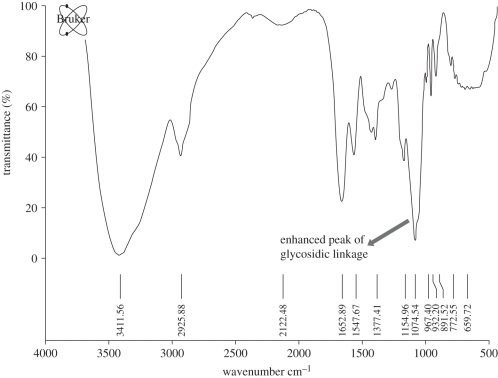
The presence of different polymers in the cryogel blend was confirmed by Fourier transform infrared (FTIR) spectroscopy (figure 3). The peak at 3500–3300 cm−1 confirmed the presence of chitosan owing to the NH group stretching in the polysaccharide, which was prominent in the blend. Agarose was validated owing to the presence of peaks at 930 cm−1 owing to 3,6-anhydrogalactose. Peak at 1077 cm−1 is enhanced in the blend, which is due to the glycosidic linkages in the polymers. Few other peaks are overlapping that can be due to the similar functional groups in the polymers.
Figure 3.
FTIR spectroscopy analysis of the CAG blend showing a prominent peak for glycosidic linkage.
3.2.3. Swelling/de-swelling behaviour and relative swelling ratio
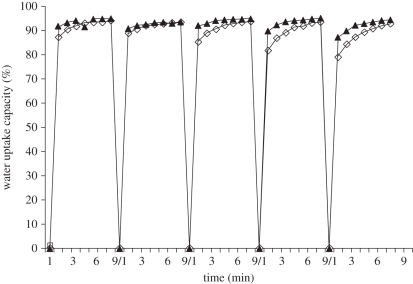
The swelling and de-swelling of the CAG cryogels were determined by the gravimetric method. Cryogels were swelled up to 80 per cent within 1 min and attains equilibrium within 3–4 min (figure 4). Swelling and de-swelling were carried out in a cyclic manner till five cycles with the same cryogel samples. As kinetics obtained from the graph showed almost a constant behaviour from the first cycle to the fifth cycle. Though, we observed slight difference after third cycle in the water uptake rate (figure 4). The difference was not significant and still they reached their equilibrium, so we could infer that these cryogels do not change their behaviour, when repeatedly subjected to swelling and de-swelling during the experiment or during their application in cartilage tissue engineering.
Figure 4.
Cyclic swelling and de-swelling kinetics of CAG cryogels 4.5% (filled triangles), 5% (open squares).
3.2.4. In vitro degradation studies
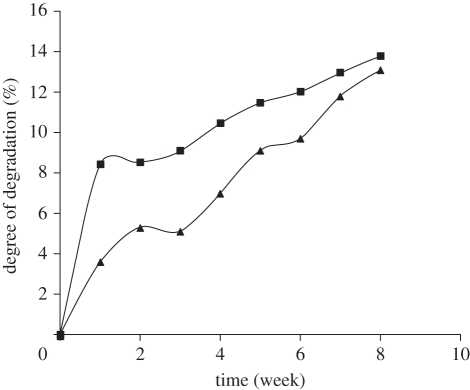
The aim of tissue engineering is the regeneration of tissue, with simultaneous degradation of support matrix used for the cells at a rate which can match the formation of neo-tissue. The implanted cells face the variation in the interface during the surface erosion and new surface exposure that may affect the different cellular process. So, the degradation of CAG cryogels was examined under aseptic conditions to examine the degradation pattern. The degree of degradation was estimated in terms of change of dry weight of the cryogel. Gels were incubated in 0.1 M PBS (pH 7.4) at 37°C for the duration of eight weeks. CAG cryogels of both the concentrations, i.e. 4.5 per cent and 5 per cent were showing approximate by 13 per cent degradation till the end of experiment, i.e. until eight weeks of incubation. The per cent degree of degradation was determined from the values recorded as initial dry weight and final dry weight of cryogels after incubation. The cryogel of 4.5 per cent concentration degraded quickly in the first two weeks but then it aligned with the degradation kinetic of 5 per cent cryogel (figure 5), which might be due to the varying concentrations of chitosan. As previous studies have suggested that gelatin degrades quickly but if it cross-linked with chitosan, the cross-linking decreases the rate of degradation [30]. In the 5 per cent cryogel, the high concentration of chitosan cross-linked more efficiently with the gelatin present in mixture, which may reduce the degradation rate of 5 per cent gel in comparison to 4.5 per cent gel. After two weeks, the imperceptibly cross-linked gelatin almost degrades and then the gel followed the same degradation pattern of 5 per cent cryogel. So besides providing cell adherence and proliferation properties, the presence of gelatin in the CAG cryogels might also be a useful factor by which we can control the rate of degradation. SEM micrographs have revealed the generation of cracks in the walls of the degraded cryogel incubated for eight weeks (figure 6). The rate of degradation is very slow, which may be due to the fact that the degradation process is chemical. Further, the rate of degradation exhibited a significant enhancement under in vivo conditions, owing to presence of different enzymes and factors.
Figure 5.
Degree of degradation of chitosan–agarose cryogels 4.5% (filled squares), 5% (filled triangles), after eight weeks of incubation with 0.1 M PBS under aseptic conditions at 37°C.
Figure 6.
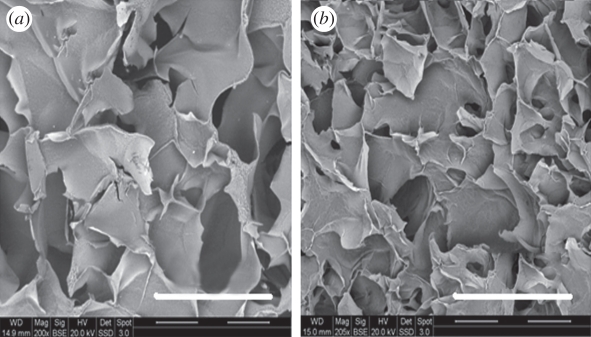
SEM images of cryogel samples after eight week of degradation showing cracks in the pore walls at a magnification of 200×. (a) 4.5% (b) 5%. Scale bars, (a,b) 500 µm.
3.2.5. Permeability analysis
Permeability is expressed as a quantitative measure related to the resistance, to the flow of a fluid through a solid porous material. Where, liquid flow was collected for 1 min from the outlet by placing the cryogel sample in between the path and without the sample. Flow of liquid was collected at room temperature and mass was calculated. The cell growth into a scaffold depends on how well the nutrient containing growth medium can permeate through the porous material during the cultivation of cells on the scaffold. So, the scaffold should have good permeability to increase nutrient flux across the scaffold yielding better cell growth and proliferation. The average hydraulic permeability of CAG cryogels was 2.2 × 109 m4 N−1 s−1, which quantifies the ability of CAG cryogels to transmit fluids efficiently through its interconnected pores. Permeability reflects a combination of several micro-structural parameters of a scaffold including porosity, pore size, interconnectivity, etc. So, permeability can be viewed as a system property of a tissue engineering scaffold, which can be readily measured and related to its biological performance.
3.3. Mechanical characterization of CAG cryogels
3.3.1. Unconfined compression testing
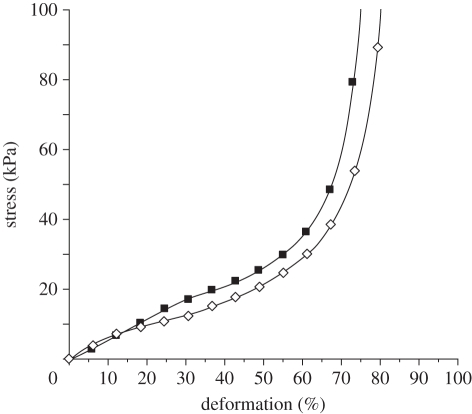
Far from being a passive component, porous scaffolds play a significant role in tissue engineering by preserving tissue volume, providing temporary mechanical function and delivering bio-factors (cells and proteins). The mechanical strength of a scaffold will be changed by changing the porosity. So, a successful scaffold should possess balanced mechanical properties with amiable porosity, providing a sequential transition in which the regenerated tissue assumes function as the scaffold degrades. The dynamic mechanical strength of CAG (4.5% and 5%) cryogels was determined by unconfined compression. The CAG cryogels showed an affable compression modulus, i.e. aprroximately 39 kPa of 4.5 per cent cryogel and approximately 44 kPa of 5 per cent cryogel at 15 per cent deformation, calculated from the graph plotted between stress and strain (figure 7). These gels showed elastic behaviour till around 60–70% compression of the gel length. Thereafter, the uni-axial stress was transferred from the cryogel sample to the instrument fixture, which could be seen from the precipitous increase in stress. Mechanical strength of a designed scaffold is a very important parameter in cartilage tissue engineering providing integrity to chondrocytes to grow without distress and to withstand the mechanical load in case the scaffold has to be implanted in the joint as a tissue replacement. So, this analysis revealed that CAG cryogels can provide substantial mechanical strength, which can be used as a temporary porous architecture for the cultivation of chondrocytes in three-dimensional environments. Different types of scaffolds have been used for cartilage tissue engineering applications. For example, hybrid scaffolds from gelatin–chondroitin–sulphate and hyaluronan have shown potential for cartilage tissue engineering [31], where the authors have fabricated the scaffolds by freeze drying. In comparison in this work, we constructed the scaffolds from natural polymers like chitosan–agarose and gelatin, such scaffolds have a high Young's modulus and do not show any signs of deformation on the application of cyclic compression. Scaffolds were fabricated using cryogelation technology, which generates an interconnected porous network with a pore size in the range of 40–135 µm (as discussed earlier), such large pore sizes can support the growth and proliferation of cells like chondrocytes.
Figure 7.
Unconfined compression stress–strain curve of chitosan–agarose cryogels 4.5% (filled squares) and 5% (open squares).
3.3.2. Unconfined cyclic deformation analysis (fatigue test)
Scaffolds were subjected to the cyclic deformation test to confirm their capacity to bear the applied load. Cryogel sections (diameter: 13 mm and thickness: 5 mm) were compressed to 30 per cent of their original height at frequency up to 5 Hz for 1 × 105 cycles. On applying high strain and frequency during the experiment, the cryogels did not show any remarkable changes in their dimensions and weights. The mechanical stimulation is important during cell culture experiments like bioreactor studies and during the application of the scaffold in cartilage tissue engineering. The continuous cyclic mechanical loading enhances the activity of cells like enhancement in ECM secretion, i.e. synthesis of collagen and GAGs. For continuous cyclic loading, it is important that scaffold should be elastic, so that cyclic compression can be applied for the generation of the mechanical signals that will be delivered to cells followed by their activation. So cryogels made form chitosan, agarose and gelatin can be seeded with chondrocytes and subjected to the mechanical stimulation in a bioreactor for the production of neo-cartilage, which is expected to have good mechanical properties resembling native tissue.
3.4. In vitro biocompatibility analysis of CAG cryogel
3.4.1. Microscopic analysis of articular chondrocytes adhesion in vitro
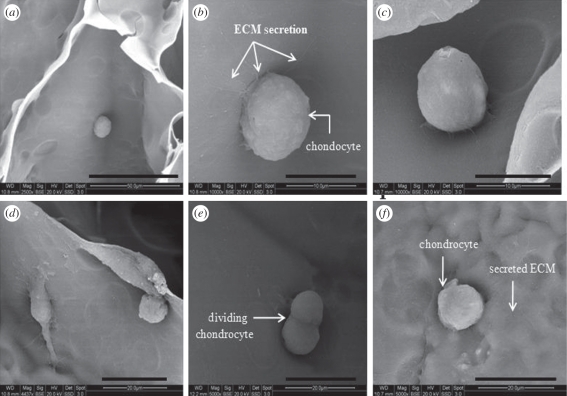
Articular chondrocytes isolated from young goat knee were seeded on sterile CAG cryogels. Chondrocytes were seeded (0.5 ml) on the scaffolds in their second passage at a density of 3.1 × 105 cells ml−1. Viability of the cells was checked by staining the cells with florescent dye, i.e. FDA, which penetrates the cell membrane of the viable cells and is metabolized to fluorescein exhibiting green fluorescence within 515–555 nm. Chondrocytes in large numbers attach to the walls of blend polymeric scaffolds (figure 8) as visualized by SEM. The microscopic observations were done at 24 and 96 h during the cell culture experiment. The growth of chondrocytes was significant, i.e. they produced a high amount of ECM rather than quick division (figure 8e,f). As the previous studies suggested that chondrocytes retain phenotype till the third to fourth passage and after that they lose the phenotype characteristics and behave like fibroblasts, under unfavourable conditions [32]. As shown in figure 8b,c, chondrocytes have retained their native morphology when grown in three-dimensional CAG cryogel scaffolds even after 4 days of culture. Production of the ECM by chondrocytes on the walls of CAG gels indicated that chondrocytes recognized the surface of gels as native. In contrast, the pore size of the blend cryogels was sufficient to facilitate the unhindered passage of cells, nutrient diffusion and waste removal. Both the gels, i.e. 5 per cent and 4.5 per cent CAG cryogels displayed almost the same properties of cell adhesion. This suggests that macroporous CAG cryogels with gelatin substrates provide the conditions that favour cartilage tissue formation by influencing cell adhesion and providing the native conditions that help to maintain chondrocyte phenotype.
Figure 8.
SEM images of (a) chondrocytes adhesion to the surface of the gel after 24 h at the magnification of 2500×, (b,c,d) Chondrocytes synthesizing extracellular matrix (ECM) after 4 days at a magnification of 10 000×, (e) dividing chondrocytes at a magnification of 10 000×, (f) Chondrocytes with secreted ECM. Scale bars, (a) 50 µm; (b,c) 10 µm; (d,e, f) 20 µm.
3.4.2. Determination of chondrocytes proliferation (MTT assay)
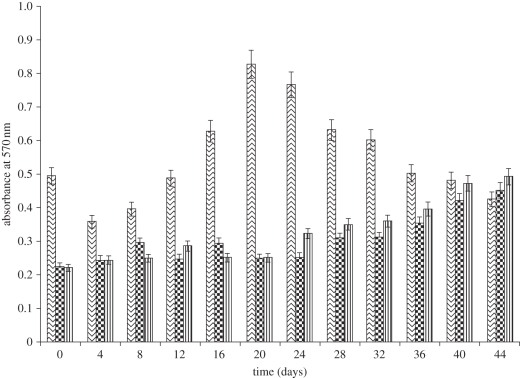
The viability and proliferation of chondrocytes in CAG cryogels were determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT) reagent. This assay involves the conversion of tetrazolium salt MTT by viable proliferating cells to an insoluble product, purple formazan. These formazan crystals can be solubilized in an organic solvent like DMSO and can be quantified by checking the optical density via spectrophotometric analysis. An increase in optical density indicates cell adhesion and proliferation in the scaffolds. The effect of the blend CAG cryogels on the metabolic activity of chondrocytes was assessed in vitro at physiological pH of 7.4 up to 44 days. Both concentrations (4.5% and 5%) of blend CAG cryogels demonstrated increasing cellular metabolic activity with time (figure 9), while the control showed increased cell viability till the 20th day of cell culture but after that the cell viability decreases. It might be because the chondrocytes divide and they reach confluence quickly in a monolayer culture system in comparison to the three-dimensional culture system. After the cell line becomes confluent, owing to the limited surface area for cell proliferation and owing to apoptosis viability in the control experiment decreases. Conversely, the test samples of blend cryogels showed a continuous increase in viability and proliferation of cells till the end of experiment because scaffolds provide a high surface area owing to the three-dimensional morphology with amiable mechanical strength. Eventually, cells are retain round morphology while growing on the scaffold. These results imply good biocompatibility of blend scaffold synthesized from naturally derived polymers.
Figure 9.
Cell viability and proliferation of chondrocytes in CAG cryogels as evaluated by MTT assay. The cell culture experiment was run up to 44 days with chondrocytes seeded in 4.5% (vertical line bars) and 5% (large checker board bars) with positive controls (zig-zag bars). The experiment was done in triplicate with p > 0.5.
3.5. In vivo implantation of cell-seeded cryogel scaffold
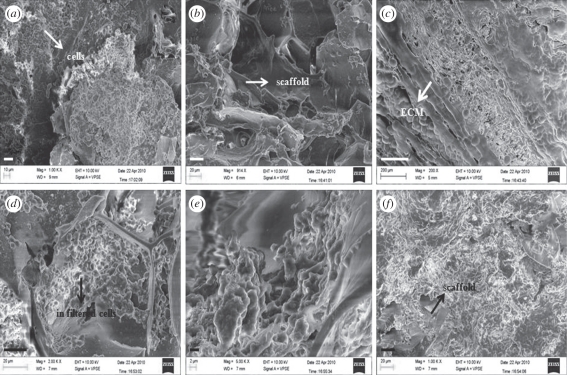
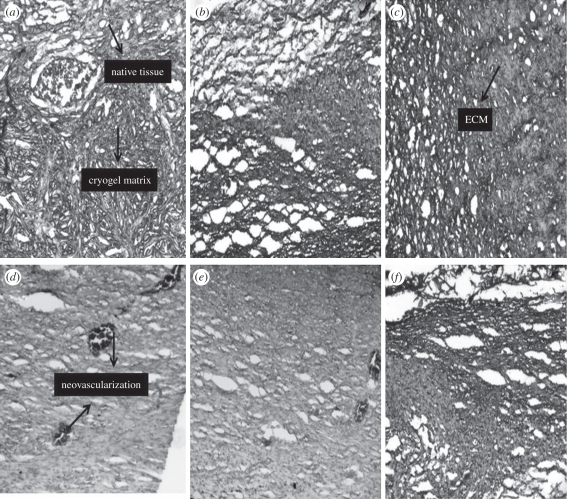
In vivo implantation of the cell-seeded scaffolds was done to check the biocompatibility of the CAG scaffolds together with the in vivo degradability of these scaffolds. Cell-seeded scaffolds were implanted in the animals to check whether the goat chondrocytes elicit any adverse effects in rats which further increases the scope of sources used for the isolation of cells for cartilage tissue engineering. The study was conducted for the duration of six week in triplicate with one control. As obvious from the results after the two weeks of the implantation, the number of cells on the scaffolds has increased (figure 10a,b). The cell population on the scaffold is presumed to be a mixed population as some of cells from the native tissue infiltrated into the scaffold or a few blood cells have adhered to the surface of the scaffold. In histological studies, a clear distinction can be made between the surface of native tissue and the cryogel surface (figure 11a,b). Neo-vascularization is observed at the site of the interface indicating that CAG cryogel matrices are biocompatible and also induce the formation of new blood vessels (figure 11a). In the control (scaffold without cells) neo-vascularization is observed in the gel along with the infiltration of cells from the native tissue (figure 11d,e). After four weeks of the implantation, cell proliferation shows an enhancement with the synthesis of ECM but at this stage the cryogel scaffold is visible, indicating that the scaffolds are not degraded completely after four weeks (figure 10b). Histology shows that the cryogel matrix has merged with the native tissue together with the degradation of the cryogel matrix (figure 11c,f). These results demonstrate that cryogel matrices have a good porous interconnected network that allows the infiltration of the cells from the native tissue or the blood cells. After six weeks of implantation most of the scaffold has degraded and rest of it is surrounded by the ECM in the test (figures 10c and 11c) while in the control (figures 10f and 11f) cells from the native tissue proliferate on the scaffold without the synthesis of ECM. So it can be interpreted from these results that chondrocyte-seeded scaffolds degraded faster than the scaffolds that are not seeded with cells, which may be due to the production of enzymes by the cells along with the other enzymatic factors present in in vivo conditions. So in vivo studies give a better insight into the degradation of the scaffolds and prove that these scaffolds induce neo-vascularization and do not elicit any adverse effects on the native tissue.
Figure 10.
SEM images of (a) implanted scaffolds after two weeks of implantation showing cells attached to the scaffold surface (b) after four weeks of implantation and (c) after six weeks of implantation showing degraded cryogel matrix and deposition of ECM. (d) Control scaffold after two weeks showing the attachment of infiltrated cells on the scaffolds; (e) control scaffold after four weeks; (f) control scaffold after six weeks showing the proliferation of infiltrated cells on the scaffold. Scale bars, (a) 10 µm; (b,d,f) 20 µm; (c) 200 µm; (e) 2 µm.
Figure 11.
Histological examination of the implanted constructs stained by H&E (a) after two weeks showing the integration of scaffolds with the native tissue and exhibiting the process of neo-vascularization (b) after four weeks showing the disintegration of the cryogel matrix (c) after six weeks showing the deposition of the ECM (d) control scaffold (scaffold implanted without chondorcytes) exhibiting the neo-vascularization process and infiltration of the cells from the native tissue (e) control scaffold after four weeks showing neo-vascularization (f) control scaffold after six weeks showing the disintegrated cryogel matrix and its integration with the native tissue.
4. Conclusion and future directions
This study has sufficed in developing a novel polymeric scaffold using natural and biocompatible polymers, which not only provides appropriate physical, chemical and degradation properties but also mechanical and biological properties for effective cell growth following tissue development. As evident from the results, the CAG cryogels showed significant characteristics required for cartilage tissue engineering. These cryogels have supermacroporous architecture with interconnected pores and amiable mechanical stability with biodegradable and biocompatible nature. These findings exhibit the potential of CAG cryogels as a substrate for chondrocytes and its application in cartilage tissue engineering. In vivo experiments have confirmed that these matrices are suitable for the implantation at lesion sites developed during the development of osteoarthritis. Further studies will be conducted by developing an osteoarthritic model and implantation of these scaffolds at lesion sites. The future assessment of these materials for the neo-cartilage development both in vitro and in vivo will be studied, besides making attempts for the pre-clinical testing.
Acknowledgements
All handling of animals was done with the approval of Institutional Authority for Laboratory Animal Care.
The authors would like to acknowledge Department of Biotechnology, Ministry of Science and Technology, Government of India; India–UK (DST-UKIERI) award project and Indo-US Science and Technology Forum.
References
- 1.Butler D. L., Goldstein S. A., Guilak F. 2000. Functional tissue engineering: the role of biomechanics. J. Biomech. Eng. 122, 570–576 10.1115/1.1318906 (doi:10.1115/1.1318906) [DOI] [PubMed] [Google Scholar]
- 2.Sachlos E., Czernuszka J. T. 2003. Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Euro. Cells Mater. 5, 29–40 [DOI] [PubMed] [Google Scholar]
- 3.Capito R. M., Spector M. 2003. Scaffold-based articular cartilage repair. IEEE Eng. Med. Biol. Mag. 22, 42–50 10.1109/MEMB.2003.1256271 (doi:10.1109/MEMB.2003.1256271) [DOI] [PubMed] [Google Scholar]
- 4.Aston J. E., Bentely G. 1986. Repair of articular surfaces by allografts of articular and growth-plate cartilage. J. Bone Joint Surg. 68-B, 29–35 [DOI] [PubMed] [Google Scholar]
- 5.Zelent M. E., Neese D. J. 2005. Osteochondral autograft transfer of the first metatarsal head: a case report. J. foot Ankle Surg. 44, 406–411 10.1053/j.jfas.2005.07.008 (doi:10.1053/j.jfas.2005.07.008) [DOI] [PubMed] [Google Scholar]
- 6.Muller F. A., Muller L., Hoffmann I., Greil P., Wenzel M. M., Staudenmaier R. 2006. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials 27, 3955–3963 10.1016/j.biomaterials.2006.02.031 (doi:10.1016/j.biomaterials.2006.02.031) [DOI] [PubMed] [Google Scholar]
- 7.Coutts R. D., Healey R. M., Ostrander R., Sah R. L., Goomer R., Amiel D. 2001. Matrices for cartilage repair. Clin. Orthop. Relat. Res. 391, S271–S279 10.1097/00003086-200110001-00025 (doi:10.1097/00003086-200110001-00025) [DOI] [PubMed] [Google Scholar]
- 8.Waldman S. D., Couto D. C., Grynpas M. D., Pilliar R. M., & Kandel R. A. 2007. Multi-axial mechanical stimulation of tissue engineered cartilage: review. Euro. Cells Mater. 13, 66–75 [DOI] [PubMed] [Google Scholar]
- 9.Chang C. H., Lin F. H., Lin C. C., Chou C. H., Liu H. C. 2004. Cartilage tissue engineering on the surface of a novel gelatin-calcium-phosphate biphasic scaffold in a double chamber bioreactor. J. Biomed. Mater. Res. B Appl. Biomater. 71, 313–321 10.1002/jbm.b.30090 (doi:10.1002/jbm.b.30090) [DOI] [PubMed] [Google Scholar]
- 10.Grande D. A., Halberstadt C., Naughton G., Schwartz R., Manji R. 1997. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J. Biomed. Mater. Res. Part A 34, 211–220 (doi:10.1002/(SICI)1097-4636(199702)34:2<211::AID-JBM10>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 11.Hauselmann H. J., Fernandes R. J., Mok S. S., Schmid T. M., Block J. A., Aydelotte M. B., Kuettner K. E., Eugene J. M., Thonar E. J. 1994. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J. Cell Sci. 107, 17–27 [DOI] [PubMed] [Google Scholar]
- 12.Buschmann M. D., Gluzband Y. A., Grodzinsky A. J., Kimura J. H., Hunziker E. 1992. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J. Orthop. Res. 10, 745–758 10.1002/jor.1100100602 (doi:10.1002/jor.1100100602) [DOI] [PubMed] [Google Scholar]
- 13.Ng K. W., Wang C. C., Mauck R. L., Kelly T. N., Chahine N. O., Costa K. D., Ateshian G. A., Hung C. T. 2005. A layered agarose approach to fabricate depth-dependent inhomogenity in chondrocyte-seeded constructs. J. Orthop. Res. 23, 134–141 10.1016/j.orthres.2004.05.015 (doi:10.1016/j.orthres.2004.05.015) [DOI] [PubMed] [Google Scholar]
- 14.Suh J. K. F., Matthew H. W. T. 2000. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterial 21, 2589–2598 10.1016/S0142-9612(00)00126-5 (doi:10.1016/S0142-9612(00)00126-5) [DOI] [PubMed] [Google Scholar]
- 15.Pianigiani E., Andreassi A., Taddeucci P., Alessandrini C., Fimiani M., Andreassi L. 1999. A new model for studying differentiation and growth of epidermal cultures on hyaluronan-based carrier. Biomaterials 20, 1689–1694 10.1016/S0142-9612(99)00056-3 (doi:10.1016/S0142-9612(99)00056-3) [DOI] [PubMed] [Google Scholar]
- 16.Sechriest V. F., Miao Y. J., Niyibizi C., Westerhausen-Larson A., Matthew H. W., Evans C. H., Fu F. H., Suh J. K. 2000. GAG-augmented polysaccharide hydrogel: a novel biocompatible and biodegradable material to support chondrogenesis. J. Biomed. Mater. Res. 49, 534–541 (doi:10.1002/(SICI)1097-4636(20000315)49:4<534::AID-JBM12>3.0.CO;2-#) [DOI] [PubMed] [Google Scholar]
- 17.Goodstone N. J., Cartwright A., Ashton B. 2004. Effects of high molecular weight hyaluronan on chondrocytes cultured within a resorbable gelatin sponge. Tissue Eng. 10, 621–631 10.1089/107632704323061979 (doi:10.1089/107632704323061979) [DOI] [PubMed] [Google Scholar]
- 18.Buckley C. T., O'Kelly K. U. 2004. Regular scaffold fabrication techniques for investigations in tissue engineering. In Topics in Bio-mechanical Engineering: Proc. of the 1st Symp. on Biomechanical Engineering (eds Prendergast P. J., McHugh P. E.), Dublin and Galway, Ireland, pp. 147–166. Ann Arbor, MI: TCBE, The University of Michigan. [Google Scholar]
- 19.Kathuria N., Tripathi A., Kar K. K., Kumar A. 2009. Synthesis and characterization of elastic and macroporous chitosan-gelatin cryogels for tissue engineering. Acta Biomater. 5, 406–418 10.1016/j.actbio.2008.07.009 (doi:10.1016/j.actbio.2008.07.009) [DOI] [PubMed] [Google Scholar]
- 20.Tripathi A., Kathuria N., Kumar A. 2009. Elastic and macroporous agarose–gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. J. Biomed. Mater. Res. A 90, 680–694 10.1002/jbm.a.32127 (doi:10.1002/jbm.a.32127) [DOI] [PubMed] [Google Scholar]
- 21.Lozinsky V. I., Galaev I. Y., Plieva F. M., Savina I. N., Jungvid H., Mattiasson B. 2003. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 21, 445–481 10.1016/j.tibtech.2003.08.002 (doi:10.1016/j.tibtech.2003.08.002) [DOI] [PubMed] [Google Scholar]
- 22.Lozinsky V. I., Plieva F. M., Galaev I. Y., Mattiasson B. 2002. The potential of polymeric cryogels in bioseparation. Bioseparation 10, 163–188 10.1023/A:1016386902611 (doi:10.1023/A:1016386902611) [DOI] [PubMed] [Google Scholar]
- 23.Swieszkowski W., Ku D. N., Bersee H. E. N., Kurzydlowski K. J. 2006. An elastic material for cartilage replacement in an arthritic shoulder joint. Biomaterials 27, 1534–1541 10.1016/j.biomaterials.2005.08.032 (doi:10.1016/j.biomaterials.2005.08.032) [DOI] [PubMed] [Google Scholar]
- 24.Adrados B. P., Galaev I. Y., Nilsson K., Mattiasson B. 2001. Size exclusion behavior of hydroxypropylcellulose beads with temperature-dependent porosity. J. Chromatogr. A 930, 73–78 10.1016/S0021-9673(01)01142-6 (doi:10.1016/S0021-9673(01)01142-6) [DOI] [PubMed] [Google Scholar]
- 25.Bhat S., Singh D., Shakesheff K., Kumar A. 2009. An overview on polymeric cryogels scaffolds with a special approach to cartilage tissue engineering. Eur. Cell Mater. 18(Suppl. 2), 46 [Google Scholar]
- 26.Xue W., Champ S., Huglin M. B., Jones T. G. J. 2004. Rapid swelling and deswelling in cryogels of crosslinked poly(N-isopropylacrylamide-co-acrylic). Eur. Polym. J. 40, 703–712 10.1016/j.eurpolymj.2003.10.021 (doi:10.1016/j.eurpolymj.2003.10.021) [DOI] [Google Scholar]
- 27.Srivastava A., Jain E., Kumar A. 2007. The physical characterization of supermacroporous poly(N-isopropylacrylamide) cryogel: mechanical strength and swelling/de-swelling kinetics. Mater. Sci. Eng. A 464, 93–100 10.1016/j.msea.2007.03.057 (doi:10.1016/j.msea.2007.03.057) [DOI] [Google Scholar]
- 28.Li J., Mak A. F. T. 2005. Hydraulic permeability of polyglycolic acid scaffolds as a function of biomaterial degradation. J. Biomater. Appl. 19, 253–266 10.1177/0885328205047219 (doi:10.1177/0885328205047219) [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 10.1016/0022-1759(83)90303-4 (doi:10.1016/0022-1759(83)90303-4) [DOI] [PubMed] [Google Scholar]
- 30.Zhuang H., Zheng J. P., Gao H., Yao K. D. 2007. In vitro biodegradation and biocompatibility of gelatin/montmorillonite–chitosan intercalated nanocomposite. J. Mater. Sci. Mater. Med. 18, 951–957 10.1007/s10856-006-0093-y (doi:10.1007/s10856-006-0093-y) [DOI] [PubMed] [Google Scholar]
- 31.Chang C. H., Liu H. C., Lin C. C., Chou C. H., Lin F. H. 2003. Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 24, 4853–4858 10.1016/S0142-9612(03)00383-1 (doi:10.1016/S0142-9612(03)00383-1) [DOI] [PubMed] [Google Scholar]
- 32.Yamane S., Iwasaki N., Majima T., Funakoshi T., Masuko T., Harada K., Minami A., Monde K., Nishimura S. 2005. Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterilas 26, 611–619 10.1016/j.biomaterials.2004.03.013 (doi:10.1016/j.biomaterials.2004.03.013) [DOI] [PubMed] [Google Scholar]