Abstract
Antibodies to the pre-erythrocytic antigens, circumsporozoite protein (CSP), thrombospondin-related adhesive protein (TRAP) and liver-stage antigen 1, have been measured in field studies of semi-immune adults and shown to correlate with protection from Plasmodium falciparum infection. A mathematical model is formulated to estimate the probability of sporozoite infection as a function of antibody titres to multiple pre-erythrocytic antigens. The variation in antibody titres from field data was used to estimate the relationship between the probability of P. falciparum infection per infectious mosquito bite and antibody titre. Using this relationship, we predict the effect of vaccinations that boost baseline CSP or TRAP antibody titres. Assuming the estimated relationship applies to vaccine-induced antibody titres, then single-component CSP or TRAP antibody-mediated pre-erythrocytic vaccines are likely to provide partial protection from infection, with vaccine efficacy of approximately 50 per cent depending on the magnitude of the vaccine-induced boost to antibody titres. It is possible that the addition of a TRAP component to a CSP-based vaccine such as RTS,S would provide an increase in infection-blocking efficacy of approximately 25 per cent should the problem of immunological interference between antigens be overcome.
Keywords: malaria, vaccine, antibody, sporozoite
1. Introduction
Malaria, caused by the Plasmodium falciparum parasite, continues to pose a major public health problem with approximately one million deaths recorded each year [1], predominantly in young children in Sub-Saharan Africa. An efficacious malaria vaccine would reduce the burden of disease in the world's most vulnerable populations.
When an infected Anopheline mosquito takes a blood meal, it inserts its proboscis into the skin or capillaries just beneath the skin. The mosquito salivates during the initial stages of feeding and a small number of Plasmodium sporozoites are inoculated, enter the bloodstream and make their way to the liver. In the liver, the sporozoite will invade a hepatocyte, shed its cytoskeleton and transform into a trophozoite. The trophozoite then undergoes schizogonic development and differentiates into approximately 20 000 merozoites [2]. Approximately 6.5 days later, hepatic merozoites enter the blood to begin the erythrocytic stage of their life cycle. Humans living in malaria endemic areas have a degree of naturally acquired pre-erythrocytic immunity [3], comprising of an antibody response to sporozoites, a cell-mediated response during liver-stage development and an immune response that clears emerging hepatic merozoites before they begin to replicate. The most promising candidate vaccine, RTS,S/ASO1, currently in Phase III trials, boosts this natural pre-erythrocytic immune response [4,5].
The outcome of an infectious bite is often viewed as a binary event in which the host either does or does not develop blood-stage malaria. However, every bite can inject from 0 to 100+ sporozoites [6,7], with the probability of blood-stage infection increasing for larger doses. Sporozoites that have been deposited in the skin or capillaries will remain at the injection site for up to an hour before trickling into the blood stream and migrating to the liver [8,9]. Sporozoites are susceptible to antibody opsonization from immunoglobulin G (IgG) antibodies, recognizing sporozoite antigens at any stage in this journey [10].
Antibodies to the pre-erythrocytic antigens, circumsporozoite protein (CSP), thrombospondin-related adhesive protein (TRAP) and liver-stage antigen 1 (LSA-1), have been shown to correlate with protection from P. falciparum infection in field studies [11–13]. CSP covers the entire surface of the sporozoite and is found on the plasma membrane of liver-stage parasites [14]. Antibodies to CSP immobilize sporozoites and inhibit parasite invasion of hepatocytes [15]. TRAP is found primarily within the sporozoite's micronemes and on the sporozoite surface [16]. Antibodies to TRAP inhibit sporozoite gliding motility [17] and hepatocyte invasion [18]; however, there is some evidence to suggest that TRAP antibodies do not inhibit sporozoite infectivity in vivo [19]. LSA-1 is expressed soon after the sporozoite invades the hepatocyte in the liver [20]. As LSA-1 is only expressed inside the hepatocyte, which antibodies are unable to access, LSA-1 antibodies are not expected to provide protection from infection although LSA-1 is a likely target of cell-mediated immunity [20]. As pre-erythrocytic antibodies are directed at different aspects of sporozoite biology, they are likely to interact cooperatively in the prevention of infection.
John et al. [11] demonstrated a significant correlation between titres to the pre-erythrocytic antibodies CSP, TRAP and LSA-1 and protection from infection. Individuals with titres to all three antibodies above a cut-off of 2 arbitrary units (AU) were found to have a reduced risk of infection of 0.43 (95% confidence interval (CI) = 0.23–0.80) compared with individuals with low antibody titres. Here, we develop a mathematical model to characterize the relationship between pre-erythrocytic antibody levels and the probability of infection per infectious bite based on these data. This relationship is then used to explore the efficacy of antibody-boosting vaccines based on combinations of the antigens CSP and TRAP.
2. Methods
(a). Data
We analysed data on IgG antibody titres to CSP, TRAP and LSA-1, as well as the blood-stage antigens apical membrane antigen-1 (AMA-1), erythrocyte binding antigen-175 (EBA-175) and merozoite surface protein-1 (MSP-1), and time to re-infection with P. falciparum, from a study of 68 adults conducted in Kanyawegi, Nyanza Province, Kenya, from August to December 2001. Approval for this study was obtained from the Ethical Review Board for the Kenya Medical Research Institute and University Hospitals of Cleveland at Case Western Reserve University. Full details of the trial can be found in John et al. [11]. In brief, the population of Kanyawegi is approximately 3500 and is located in a malaria-endemic region with an approximate entomological inoculation rate (EIR) of 120 infectious bites per person per year [21]. Individuals agreeing to participate were given quinine for 5 days and doxycycline for 7 days to clear parasitaemia, and then followed for malaria infection by microscopic inspection of blood smears obtained weekly for 14 weeks. Plasmodium falciparum infection was confirmed by polymerase chain reaction. Individuals who missed more than two weeks of blood smear testing were included in analysis up to the time of their last blood smear. Blood for laboratory studies to measure antibody titres was obtained by venepuncture prior to anti-malarial treatment. Antibody titres were measured in AU. The IgG antibody titres to CSP, TRAP and LSA-1 were approximately lognormally distributed with mean and standard deviation of 6.83 (5.54) AU, 4.80 (4.31) AU and 8.60 (12.34) AU, respectively.
(b). Probability of infection per infectious bite
Data from experiments by Beier et al. [6,7] suggest that the number of sporozoites injected per bite follows an approximately geometric distribution with arithmetic mean 10 and range 0–100+. For an infectious bite to progress to blood-stage malaria, just one sporozoite must evade the pre-erythrocytic immune response. Denote the probability that a single sporozoite causes blood-stage malaria infection by p1 = r. If sporozoites from an infectious bite act independently, the probability that k sporozoites cause blood-stage malaria is pk = 1 − (1 − r)k. If the distribution of the number of sporozoites per bite follows a geometric distribution with mean n, then the probability that k sporozoites will be injected is
Thus the probability that a bite will cause blood-stage malaria infection, b, is
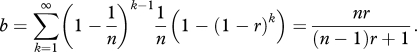 |
(c). Dose–response curves
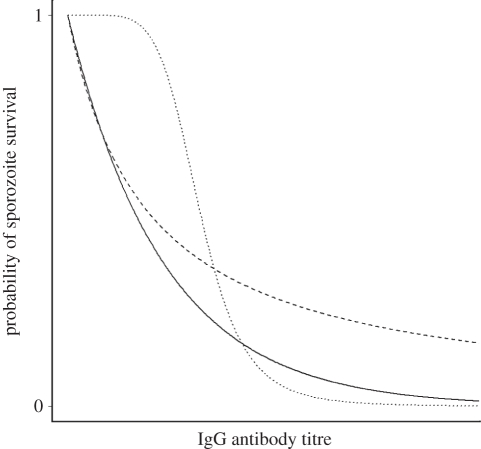
We assumed that IgG antibodies to each of the CSP, TRAP and LSA-1 antigens will induce the opsonization and phagocytosis of invading sporozoites as they make their way from the skin to the liver, and hence antibody titres constitute a correlate of risk [22], with higher titres reducing the probability of infection. The relationship between IgG antibody titre and the probability of a sporozoite evading the associated immune response is modelled using a dose–response curve. These fall into two qualitatively different categories: (i) convex, where increases in dose lead to reduced increases in response; and (ii) threshold, where the antibody response has a significant effect only once a threshold dose has been reached (figure 1). A convex dose–response will result in leaky immunity and a threshold dose–response will give rise to all-or-nothing immunity.
Figure 1.
Example dose–response curves. Dose–response curves for the probability of sporozoite survival as a function of IgG antibody titre. The solid and dashed curves depict a convex dose–response relationship where the antibody is effective even at low levels. The dotted curve depicts a threshold dose–response curve where the antibody only becomes effective once a threshold has been reached. Solid line, exponential; dashed line, convex Hill; dotted line, threshold Hill.
Let xij be the IgG antibody titre to antigen j in individual i. Let rij be the probability that a sporozoite survives the immune response to antigen j. For a Hill function, dose–response in individual i with antibody titre to antigen j of xij, the probability that a sporozoite evades that antibody's immune response is
where βj is the antibody titre that reduces the probability of sporozoite infection by half and αj is a shape parameter. αj ≤ 1 gives a convex dose-response curve and αj > 1 gives a threshold dose–response curve. For an exponential dose–response in individual i, the probability that a sporozoite evades the immune response to antibody j is 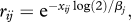 where βj is the antibody titre that reduces the probability of sporozoite infection by half. An exponential function is always convex.
where βj is the antibody titre that reduces the probability of sporozoite infection by half. An exponential function is always convex.
(d). Pre-erythrocytic immune response
For a sporozoite to successfully evade the pre-erythrocytic immune response, it must survive the attack from each of the different antibodies and the cell-mediated immune response. Following the approach of Saul et al. [23,24], we assume each immune response is independent of the others so that ri = r0 ri,CSP ri,TRAP ri,LSA, where r0 is the probability of a sporozoite evading the cell-mediated immune response. An alternative model incorporating interactions was also considered (see the electronic supplementary material). The probability that a bite from an infectious mosquito causes blood-stage infection is therefore
Given a constant EIR ɛ and infection probability bi, the probability that individual i will have developed blood-stage infection by time t will be I(t) = 1 − e−ɛbi(t−tL), where tL = 10 days is the time between sporozoite inoculation and the appearance of detectable blood-stage parasites.
(e). Parameter estimation
The parameters α, β and r0 were estimated by fitting this model to the data on time to re-infection using maximum-likelihood methods. The data used are the antibody titres x = (xCSP, xTRAP, xLSA), and the time to re-infection or final censoring t. For individuals infected in the trial, the likelihood of them developing infection at time ti is ɛbi e−ɛbi(ti−tL) while for those censored at time ti the likelihood of remaining free from infection is e−ɛbi(ti−tL). If individuals (1, …, m) become infected and individuals (m + 1, …, I) are censored, the data likelihood is
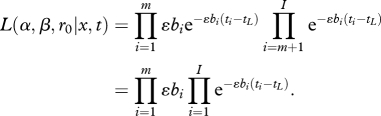 |
Model fits were compared using the Akaike information criterion (AIC). For the model parameters, 95 per cent CI were estimated by calculating the inverse hessian of the log-likelihood surface at the maximum-likelihood estimate. When fitting the model using Hill functions, an exponential function was retained for the LSA-1 dose–response curve as this was better able to model the low level of protection offered by LSA-1 antibodies. In the model described here, the pre-erythrocytic immune response is directed towards sporozoites, with the assumption that individual sporozoites act independently of each other. A model where the immune response was directed towards the infectious bite as a whole was also fitted. This gave an inferior fit to the data and predicted similar results to the sporozoite model, but it was not possible to distinguish between the two assumptions with the available data.
(f). Infection probability of humans
Let xi be the vector of antibody titres from study participant i and assume that each data point xi is sampled from a multivariate lognormal distribution. Then, y= log(x) follows the distribution
where μ = mean(log(xi)) is the mean of the observed log antibody titres, and V is the covariance matrix of the data. The mean infectivity bbase is obtained by integrating over the range of all possible titres to the three antibodies.
where b(y) is the infection probability given log antibody titre y.
(g). Antibody-boosting pre-erythrocytic vaccination
If antibody titres to antigen j in a population are lognormally distributed before vaccination, xj ∼ ln N (μbase, Vjj), then the mean antibody titre is
Assume that vaccination will boost the antibody titres so that they are lognormally distributed about a higher mean, xj ∼ ln N (μvac, Vjj), with μvac > μbase. The mean antibody titre after vaccination is then
The mean vaccine-induced antibody boost is, therefore,
Hence, the log antibody titres of the vaccinated population are normally distributed with a mean of μvac and variance matrix V.
The probability that a vaccinated person will become infected after a bite from an infectious mosquito is
and vaccine efficacy is given by
3. Results
(a). Relationship between antibody titre and probability of infection
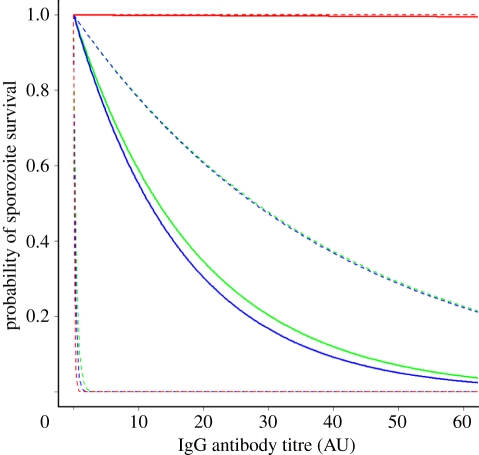
The exponential dose–response curves provided a better and more parsimonious fit than the Hill function dose–response curves (table 1). The fitted dose–response curves for CSP and TRAP indicate that increased antibody titres will provide increased protection against sporozoite infection (figure 2). By contrast, and in accordance with biological expectations, the fitted dose–response curve for LSA-1 indicates no significant role for anti-LSA-1 antibodies in protection from infection.
Table 1.
Maximum-likelihood estimates (MLE) for dose–response curve parameters (α, β) for each of the antibodies CSP, TRAP and LSA-1, and the probability of sporozoite survival in the absence of antibodies (r0). (The exponential dose–response model provides the most parsimonious fit based on the AIC.)
| dose–response curve | MLE parameter estimates (95% CI) |
model fit |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| r0 | βCSP | βTRAP | βLSA | αCSP | αTRAP | αLSA | log(L) | AIC | |
| exponential | 0.011 (0.005–0.017) | 13.07 (0–27.96) | 11.64 (0–27.75) | 9085.91 (0–192 936) | — | — | — | −223.7 | 455.5 |
| Hill function | 0.010 (0.000–0.033) | 20.30 (0–274.3) | 6.51 (0–49.3) | 7777 (0–177 461) | 96.2 (0–8667) | 0.77 (0–3.79) | — | −222.5 | 457.1 |
Figure 2.
Estimated exponential dose–response curves for the relationship between probability of sporozoite survival and IgG antibody titre to each of the antigens CSP, TRAP and LSA-1. Dashed lines represent 95% CI for estimates. Green curve, CSP; blue curve, TRAP; red curve, LSA-1.
In the absence of any antibody response, we estimated the probability of sporozoite survival r0 = 0.011 (95% CI, 0.004–0.017). This captures the failure of a sporozoite to initiate blood-stage infection owing to cell-mediated immunity, chance (e.g. migrating to tissues other than the liver) and the possibility that inoculated sporozoites are immature or damaged [25]. There is expected to be a significant cell-mediated immune response directed at liver-stage parasites as the data were collected from semi-immune adults in a region of stable malaria transmission.
(b). Probability of infection per infectious bite
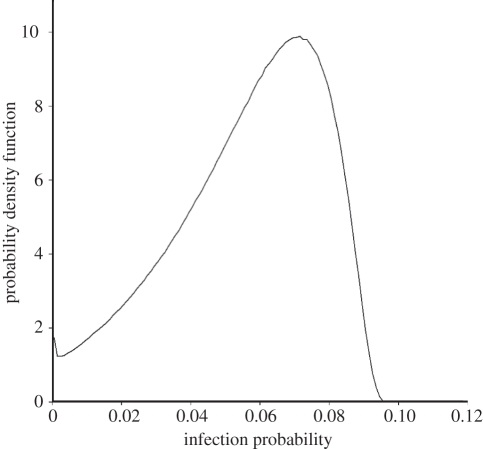
We estimate the mean probability of infection per infectious bite for this population to be b = 0.056, which is in good agreement with the previously reported values of 0.012–0.086 [26] and 0.05–0.13 [27] for semi-immune adults in areas of intense malaria exposure. Figure 3 shows the estimated distribution of infection probability. Note that this variation is only owing to variation in the IgG antibody titres as we assume the cell-mediated immune response is constant. The maximum infection probability is b = 0.09, corresponding to the case of a person with very low IgG antibody titres and an average cell-mediated immune response.
Figure 3.
Estimated distribution of infection probability per infectious bite in the study population. The variation in infection probability represented here is owing only to variation in the CSP, TRAP and LSA-1 IgG antibody titres.
(c). Pre-erythrocytic vaccination
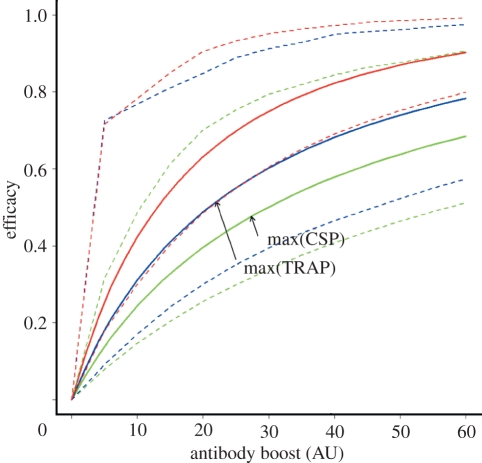
The maximum CSP antibody titre observed in the study by John et al. [11] was 27 AU, four times the mean CSP titre. A vaccine that boosts average CSP antibody titres above the baseline titre by this amount would have a predicted efficacy (VE) of 0.47 (figure 4), assuming that the parameters estimated for the protection associated with naturally acquired antibody levels also apply to vaccine-induced antibodies. The maximum TRAP antibody titre observed in the study was 22 AU, 4.5 times the mean TRAP titre. A vaccine that boosts average TRAP antibody titres by this amount would have a predicted efficacy of 0.52 (figure 4). A multi-component vaccine combining CSP and TRAP is predicted to give an increase in efficacy of approximately 25 per cent over a single-component vaccine, with a predicted VE of 0.75 for a vaccine that gives a boost equal to the maximum CSP and TRAP antibody titres observed in the study (figure 4).
Figure 4.
Predicted pre-erythrocytic vaccine efficacy using fixed boost vaccination. The maximum CSP and TRAP titres observed in the study participants are marked to indicate the vaccine efficacy that could be obtained by giving a boost equal to the maximum observed antibody titre. Dashed lines represent 95% CI. Green curves, CSP; blue curves, TRAP; red curves, CSP+TRAP.
(d). Vaccine efficacy in different transmission settings
People living in a region of intense exposure to malaria will have a high baseline level of IgG antibodies. The benefit of a pre-erythrocytic vaccine that gives an antibody boost to a partially immune individual is likely to depend on the vaccinee's baseline antibody level, as the relative increase in antibody levels owing to boosting by vaccination may be highest in those people with a low baseline antibody level. Therefore, when comparing the measured efficacy of a vaccine given in adults between regions of high and low malaria transmission, higher efficacy may be observed in the region of low transmission.
Using our model, the estimated additional efficacy over and above that already provided by naturally acquired immunity is observed to decrease monotonically as transmission intensity is increased (figure 5). This result highlights the fact that if trials are undertaken in semi-immune adults with high baseline antibody titres, then the measured efficacy in terms of the proportion of infections averted could be lower than the efficacy measured in a population with lower baseline titres.
Figure 5.
Predicted vaccine efficacy as a function of baseline antibody titre. The average baseline antibody titre relative to the study site at Kanyawegi is used as a marker of transmission intensity. Dashed lines represent 95% CI. Green curves, CSP; blue curves, TRAP; red curves, CSP+TRAP.
4. Discussion
Our results demonstrate a clear dose–response relationship between titres of antibodies to two of the pre-erythrocytic antigens, CSP and TRAP, and time to re-infection with P. falciparum. By contrast, we did not observe such a relationship between antibody titres for LSA-1 and time to re-infection. This is biologically plausible given that LSA-1 antigens are only expressed inside hepatocytes in the liver, where they are inaccessible to antibodies; the presence of these functionally redundant LSA-1 antibodies in the blood is probably owing to immune recognition of the remains of eliminated parasites. The apparent correlation between LSA-1 antibody titres and protection from infection observed in field studies [11,12] is therefore probably owing to the correlation between anti-LSA-1 titres and titres of other effective pre-erythrocytic antibodies.
Despite the importance of the infection-blocking immune response, it is unclear whether it is primarily mediated by immune responses directed against sporozoites in the skin and liver, by a blood-stage response that clears emerging hepatic merozoites before replication, or by a blood-stage response that suppresses parasite density below detectable levels. The prevalence and titre of pre-erythrocytic and blood-stage antibodies are likely to be correlated with exposure to malaria, and hence with each other. We tested for such associations in our data by calculating the correlations between the pre-erythrocytic antibodies CSP, TRAP and LSA-1 and the blood-stage antibodies AMA-1, EBA-175 and MSP-1 (see the electronic supplementary material). Anti-CSP titres were not significantly correlated with other antibody titres, suggesting that CSP has a genuine role in protection from infection, although it is possible that some of the immune response is not mediated through the antibodies considered. Conversely, anti-TRAP titres were found to be highly correlated with anti-AMA-1 and anti-LSA-1 titres. Although AMA-1 is usually thought of as a blood-stage antigen, there is evidence that AMA-1 is expressed together with TRAP in the sporozoite micronemes during hepatocyte invasion [28]. Therefore, anti-TRAP-associated infection-blocking activity could be owing to either direct anti-TRAP mediated inhibition of hepatocyte invasion, combined anti-TRAP and anti-AMA-1 inhibition of hepatocyte invasion or simply reflect anti-AMA-1 mediated clearance of blood-stage parasites.
Several studies, including this one, have observed a role for naturally acquired pre-erythrocytic antibodies [12,13] and naturally acquired cell-mediated immune responses [29] in preventing P. falciparum infection, although this association has not been observed in all studies [30]. On the other hand, some studies have concluded that pre-erythrocytic antibodies simply act as markers of exposure [31,32], with protection from infection being mediated by correlated blood-stage immune responses. Moreover, studies showing significant correlation between presence of parasitaemia before treatment and extended time to re-infection [33], or to symptomatic disease [34], suggest that blood-stage antibodies induced by previous infections are able to prevent subsequent infections. However, the observation that vaccine-induced pre-erythrocytic immune responses can prevent sporozoite infection [35,36] provides strong evidence for the hypothesis that naturally acquired immune responses against the sporozoite prevent infection and are not just a marker for exposure.
Our model predicts that both CSP and TRAP-based malaria vaccines should be efficacious vaccine candidates. The most advanced candidate malaria vaccine, RTS,S, is a pre-erythrocytic CSP-based sub-unit vaccine. Phase II trials of RTS,S/AS have demonstrated significant efficacy against time to first infection across all ages [4,37,38]. The principal component of RTS,S is the circumsporozoite antigen that induces high anti-CSP IgG antibody titres [10,36,39] and moderate CSP-specific T-cell responses [36,40,41] in vaccinated humans. Phase II trials have confirmed an association between anti-CSP IgG antibody titre and protection from infection in a challenge model [36,42] and in the field [43]. Although the immunological mechanisms induced by RTS,S are not yet fully understood, it is believed that CSP antibodies play a primary role in protection from infection, with a secondary role for CSP-specific CD4+ T-cell responses [44]. Our results agree with the observation from Phase II trials that RTS,S efficacy depends on the induced CSP antibody titre. However, our model was fitted to antibody levels acquired under conditions of natural exposure and so care must be taken when extrapolating to the significantly higher titres observed after RTS,S vaccination. An additional caveat is that if an antibody is directly protective, we do not know how much of the protection is owing to a direct causal mechanism, which is likely to confer protection in vaccine-induced boosting, and how much is owing to the antibody's role as a marker of exposure and hence associated with other protective mechanisms, which would not confer protection in vaccine-induced boosting.
Our results suggest that a multi-component vaccine expressing both CSP and TRAP antigens could give an increase in efficacy of approximately 25 per cent over a single-component vaccine. A multi-component RTS,S/TRAP/AS02 vaccine has been trialled [45] but proved less efficacious than RTS,S/AS02 alone, possibly owing to immunological interference between the antigens. Despite this, considerable progress is being made in the development of a sub-unit pre-erythrocytic vaccine based on the TRAP sporozoite antigen. ME-TRAP, a heterologous prime-boost vaccine encoding the TRAP antigen and a string of T-cell epitopes from various pre-erythrocytic antigens, offered 10 per cent efficacy against infection in Gambian adults in a Phase II trial [46]. However, protection from P. falciparum infection owing to ME-TRAP is associated with high levels of interferon-γ secreting T-cells [47], rather than IgG antibody titres, which were not explicitly included in our model. One limitation of our current model is the assumption that non-antibody mechanisms of protection such as cell-mediated immunity can be described by a constant factor r0. This assumption will be considered further in future models.
Our model can be used to investigate properties of vaccines other than efficacy. For example, evidence from clinical trials of RTS,S indicates that it is a leaky vaccine offering partial protection to everyone [4,48]. This may be owing to the convex dose–response curve we observed linking anti-CSP IgG antibody titre with protection from malaria infection. This does not, however, exclude the possibility of a threshold existing at the antibody titres induced by RTS,S, which are an order of magnitude higher than naturally occurring titres [38]. The mechanics of sporozoite inoculation may further define vaccine type. When sporozoites are injected into the skin, they remain at the bite site for up to an hour [8,9]. Anti-sporozoite antibodies may opsonize and remove those remaining in the skin but fail to protect against those that enter the bloodstream quickly. Furthermore, the number of sporozoites injected during an infectious mosquito bite is highly variable [6,7], so a vaccine may fail to prevent infection from bites injecting a large number of sporozoites. Therefore, despite the high CSP antibody titres induced by RTS,S, some sporozoites may still invade hepatocytes in the liver and initiate blood-stage infection. A combination vaccine incorporating LSA-1 may provide added protection by reducing the probability that infected hepatocytes survive to produce viable merozoites. Even if a pre-erythrocytic vaccine fails to prevent infection, it can still provide partial protection by reducing the number of exo-erythrocytic forms in the liver [49], thus reducing the number of merozoites emerging from the liver and reducing the severity of blood-stage disease [50]. The increased efficacy against severe as compared with uncomplicated disease in Phase II trials of RTS,S may support this contention [4,5].
RTS,S is currently undergoing extensive testing in Phase III clinical trials, enrolling up to 16 000 children and infants across 11 sites in seven different African countries. Our results predict that lower efficacy may be measured in the trial sites with the highest transmission intensity. This does not mean that the vaccine will be less effective, rather, that in areas of high transmission intensity, the ratio of infections prevented by naturally acquired pre-erythrocytic immunity (or maternally acquired antibodies) to infections prevented by vaccination will be higher than in areas of low transmission intensity. Continued sporozoite boosting in populations with higher exposure may also result in greater long-term vaccine efficacy and a need for fewer doses of vaccine.
Should RTS,S satisfy the necessary criteria to progress from Phase III trials to licensure, it will become the first fully licensed malaria vaccine. After licensure, the route to a second-generation vaccine with improved efficacy could be a multi-stage, multi-component vaccine based on RTS,S encoding a selection of pre-erythrocytic antigens such as TRAP and LSA-1, or blood-stage antigens such as AMA-1 and MSP-1 [51]. Our results suggest that if the problems of immunological interference can be overcome, the addition of a TRAP component to a CSP-based antibody-boosting vaccine could provide significant improvements in efficacy.
Acknowledgements
Approval for this study was obtained from the Ethical Review Board for the Kenya Medical Research Institute and University Hospitals of Cleveland at Case Western Reserve University.
We would like to thank Bob Sinden for helpful discussions. This manuscript has been approved by the Director of the Kenya Medical Research Institute.
References
- 1.Snow R. W., Guerra C. A., Noor A. M., Myint H. Y., Hay S. I. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434, 214–217 10.1038/nature03342 (doi:10.1038/nature03342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meis J., et al. 1986. Fine-structure of the malaria parasite Plasmodium falciparum in human hepatocytes in vitro. Cell Tissue Res. 244, 345–350 [DOI] [PubMed] [Google Scholar]
- 3.Doolan D. L., Dobano C., Baird J. K. 2009. Acquired immunity to malaria. Clin. Microbiol. Rev. 22, 13–36 10.1128/CMR.00025-08 (doi:10.1128/CMR.00025-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso P. L., et al. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364, 1411–1420 10.1016/S0140-6736(04)17223-1 (doi:10.1016/S0140-6736(04)17223-1) [DOI] [PubMed] [Google Scholar]
- 5.Bejon P., et al. 2008. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. New Engl. J. Med. 359, 2521–2532 10.1056/NEJMoa0807381 (doi:10.1056/NEJMoa0807381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beier J. C., Davis J. R., Vaughan J. A., Noden B. H., Beier M. S. 1991. Quantitation of Plasmodium falciparum sporozoites transmitted in vitro by experimentally infected Anopheles gambiae and Anopheles stephensi. Am. J. Trop. Med. Hyg. 44, 564–570 [DOI] [PubMed] [Google Scholar]
- 7.Beier M. S., Davis J. R., Pumpuni C. B., Noden B. H., Beier J. C. 1992. Ingestion of Plasmodium falciparum sporozoites during transmission by Anopheline mosquitoes. Am. J. Trop. Med. Hyg. 47, 195–200 [DOI] [PubMed] [Google Scholar]
- 8.Hamilton Fairley N. 1947. Sidelights on malaria in man obtained by subinoculation experiments. Trans. R. Soc. Trop. Med. Hyg. 40, 621–676 10.1016/0035-9203(47)90025-4 (doi:10.1016/0035-9203(47)90025-4) [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi L. M., Coppi A., Snounou G., Sinnis P. 2007. Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 9, 1215–1222 10.1111/j.1462-5822.2006.00861.x (doi:10.1111/j.1462-5822.2006.00861.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwenk R., et al. 2003. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 25, 17–25 10.1046/j.1365-3024.2003.00495.x (doi:10.1046/j.1365-3024.2003.00495.x) [DOI] [PubMed] [Google Scholar]
- 11.John C. C., Moormann A. M., Pregibon D. C., Sumba P. O., McHugh M. M., Narum D. L., Lanar D. E., Schluchter M. D., Kazura J. W. 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am. J. Trop. Med. Hyg. 73, 222–228 [PubMed] [Google Scholar]
- 12.John C. C., Tande A. J., Moormann A. M., Sumba P. O., Lanar D. E., Min X. M., Kazura J. W. 2008. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J. Infect. Dis. 197, 519–526 10.1086/526787 (doi:10.1086/526787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John C. C., Zickafoose J. S., Sumba P. O., King C. L., Kazura J. W. 2003. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect. Immun. 71, 4320–4325 10.1128/IAI.71.8.4320-4325.2003 (doi:10.1128/IAI.71.8.4320-4325.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nussenzweig R. S., Nussenzweig V. 1989. Antisporozoite vaccine for malaria: experimental basis and current status. Rev. Infect. Dis. 11, S579–S585 [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S., Wery M., Sharma P., Chauhan V. S. 1995. Conserved peptide sequence of the Plasmodium falciparum circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of hep-g2 cells and protect immunized mice against P. berghei sporozoite challenge. Infect. Immun. 63, 4375–4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowan G., Krishna S., Crisanti A., Robson K. 1992. Expression of thrombospondin-related anonymous protein in Plasmodium falciparum sporozoites. Lancet 339, 1412–1413 10.1016/0140-6736(92)91229-2 (doi:10.1016/0140-6736(92)91229-2) [DOI] [PubMed] [Google Scholar]
- 17.Sultan A. A., Thathy V., Frevert U., Robson K. J. H., Crisanti A., Nussenzweig V., Nussenzweig R. S., Menard R. 1997. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90, 511–522 10.1016/S0092-8674(00)80511-5 (doi:10.1016/S0092-8674(00)80511-5) [DOI] [PubMed] [Google Scholar]
- 18.Morahan B. J., Wang L., Coppel R. L. 2009. No TRAP, no invasion. Trends Parasitol. 25, 77–84 10.1016/j.pt.2008.11.004 (doi:10.1016/j.pt.2008.11.004) [DOI] [PubMed] [Google Scholar]
- 19.Gantt S., Persson C., Rose K., Birkett A. J., Abagyan R., Nussenzweig V. 2000. Antibodies against thrombospondin-related anonymous protein do not inhibit Plasmodium sporozoite infectivity in vivo. Infect. Immun. 68, 3667–3673 10.1128/IAI.68.6.3667-3673.2000 (doi:10.1128/IAI.68.6.3667-3673.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtis J. D., Hollingdale M. R., Luty A. J. F., Lanar D. E., Krzych U., Duffy P. E. 2001. Pre-erythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol. 17, 219–223 10.1016/S0169-4758(00)01862-7 (doi:10.1016/S0169-4758(00)01862-7) [DOI] [PubMed] [Google Scholar]
- 21.Beier J. C., et al. 1994. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am. J. Trop. Med. Hyg. 50, 529–536 [DOI] [PubMed] [Google Scholar]
- 22.Qin L., Gilbert P. B., Corey L., McElrath M. J., Self S. G. 2007. A framework for assessing immunological correlates of protection in vaccine trials. J. Infect. Dis. 196, 1304–1312 10.1086/522428 (doi:10.1086/522428) [DOI] [PubMed] [Google Scholar]
- 23.Saul A. 2008. Efficacy model for mosquito stage transmission blocking vaccines for malaria. Parasitology 135, 1497–1506 10.1017/S0031182008000280 (doi:10.1017/S0031182008000280) [DOI] [PubMed] [Google Scholar]
- 24.Saul A., Fay M. P. 2007. Human immunity and the design of multi-component, single target vaccines. PLoS ONE 2, e850. 10.1371/journal.pone.0000850 (doi:10.1371/journal.pone.0000850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinnis P., Zavala F. 2008. The skin stage of malaria infection: biology and relevance to the malaria vaccine effort. Future Microbiol. 3, 275–278 10.2217/17460913.3.3.275 (doi:10.2217/17460913.3.3.275) [DOI] [PubMed] [Google Scholar]
- 26.Nedelman J. 1984. Inoculation and recovery rates in the malaria model of Dietz, Molineaux, and Thomas. Math. Biosci. 69, 209–233 10.1016/0025-5564(84)90086-5 (doi:10.1016/0025-5564(84)90086-5) [DOI] [Google Scholar]
- 27.Krafsur E. S., Armstrong J. C. 1978. Integrated view of entomological and parasitological observations on falciparum malaria in Gambela, western Ethiopian lowlands. Trans. R. Soc. Trop. Med. Hyg. 72, 348–356 10.1016/0035-9203(78)90125-6 (doi:10.1016/0035-9203(78)90125-6) [DOI] [PubMed] [Google Scholar]
- 28.Silvie O., et al. 2004. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J. Biol. Chem. 279, 9490–9496 10.1074/jbc.M311331200 (doi:10.1074/jbc.M311331200) [DOI] [PubMed] [Google Scholar]
- 29.Riley E. M., Allen S. J., Bennett S., Thomas P. J., O'Donnell A., Lindsay S. W., Good M. F., Greenwood B. M. 1990. Recognition of dominant T cell-stimulating epitopes from the circumsporozoite protein of Plasmodium falciparum and relationship to malaria morbidity in Gambian children. Trans. R. Soc. Trop. Med. Hyg. 84, 648–657 10.1016/0035-9203(90)90133-Y (doi:10.1016/0035-9203(90)90133-Y) [DOI] [PubMed] [Google Scholar]
- 30.Perraut R., Marrama L., Diouf B., Fontenille D., Tall A., Sokhna C., Trape J. F., Garraud O., Mercereau-Puijalon O. 2003. Distinct surrogate markers for protection against Plasmodium falciparum infection and clinical malaria identified in a Senegalese community after radical drug cure. J. Infect. Dis. 188, 1940–1950 10.1086/379838 (doi:10.1086/379838) [DOI] [PubMed] [Google Scholar]
- 31.Greenwood B. M. 1990. Immune-responses to sporozoite antigens and their relationship to naturally acquired-immunity to malaria. Bull. World Health Org. 68, 184–190 [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh K., Hayes R. H., Carson D. C., Otoo L., Shenton F., Byass P., Zavala F., Greenwood B. M. 1988. Anti-sporozoite antibodies and immunity to malaria in a rural Gambian population. Trans. R. Soc. Trop. Med. Hyg. 82, 532–537 10.1016/0035-9203(88)90495-6 (doi:10.1016/0035-9203(88)90495-6) [DOI] [PubMed] [Google Scholar]
- 33.Tall A., et al. 2009. Assessment of the relative success of sporozoite inoculations in individuals exposed to moderate seasonal transmission. Malaria J. 8, 161. 10.1186/1475-2875-8-161 (doi:10.1186/1475-2875-8-161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinyanjui S. M., Mwangi T., Bull P. C., Newbold C. I., Marsh K. 2004. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J. Infect. Dis. 190, 1527–1533 10.1086/424675 (doi:10.1086/424675) [DOI] [PubMed] [Google Scholar]
- 35.Hoffman S. L., et al. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185, 1155–1164 10.1086/339409 (doi:10.1086/339409) [DOI] [PubMed] [Google Scholar]
- 36.Kester K. E., et al. & RTS, S Vaccine Evaluation Group 2009. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 200, 337–346 10.1086/600120 (doi:10.1086/600120) [DOI] [PubMed] [Google Scholar]
- 37.Abdulla S., et al. 2008. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. New Engl. J. Med. 359, 2533–2544 10.1056/NEJMoa0807773 (doi:10.1056/NEJMoa0807773) [DOI] [PubMed] [Google Scholar]
- 38.Bojang K. A., et al. 2001. Efficacy of RTS,S/ASO2 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358, 1927–1934 10.1016/S0140-6736(01)06957-4 (doi:10.1016/S0140-6736(01)06957-4) [DOI] [PubMed] [Google Scholar]
- 39.Kester K. E., et al. & RTS, S Vaccine Evaluation Group 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183, 640–647 10.1086/318534 (doi:10.1086/318534) [DOI] [PubMed] [Google Scholar]
- 40.Reece W. H. H., et al. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10, 406–410 10.1038/nm1009 (doi:10.1038/nm1009) [DOI] [PubMed] [Google Scholar]
- 41.Sun P. F., et al. 2003. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4(+) and CD8(+) T cells producing IFN-gamma. J. Immunol. 171, 6961–6967 [DOI] [PubMed] [Google Scholar]
- 42.Olotu A. I., Fegan G., Bejon P. 2010. Further analysis of correlates of protection from a phase 2a trial of the falciparum malaria vaccines RTS, S/AS01B and RTS, S/AS02A in malaria-naive adults. J. Infect. Dis. 201, 970–971 10.1086/651025 (doi:10.1086/651025) [DOI] [PubMed] [Google Scholar]
- 43.Guinovart C., et al. 2009. Insights into long-lasting protection induced by RTS,S/AS02A malaria vaccine: further results from a phase IIb trial in Mozambican children. PLoS ONE 4, article no. e5165. (doi:10.1371/journal.pone.0005165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moorthy V., Ballou W. R. 2009. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malaria J. 8, 312. 10.1186/1475-2875-8-312 (doi:10.1186/1475-2875-8-312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heppner D. G., Cummings J. F., Ockenhouse C. F., Kester K. E., Cohen J., Ballou W. R. 2004. Adjuvanted RTS,S and other protein-based pre-erythrocytic stage malaria vaccines. In New generation vaccines (eds Levine M. M., Kaper J. B., Rappuoli R., Liu M. A., Good M. F.), pp. 851–860 New York, NY: Marcel Dekker [Google Scholar]
- 46.Moorthy V. S., et al. 2004. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 1, 128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webster D. P., et al. 2005. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc. Natl Acad. Sci. USA 102, 4836–4841 10.1073/pnas.0406381102 (doi:10.1073/pnas.0406381102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alonso P. L., et al. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366, 2012–2018 10.1016/S0140-6736(05)67669-6 (doi:10.1016/S0140-6736(05)67669-6) [DOI] [PubMed] [Google Scholar]
- 49.Bejon P., Andrews L., Andersen R. F., Dunachie S., Webster D., Walther M., Gilbert S. C., Peto T., Hill A. V. S. 2005. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J. Infect. Dis. 191, 619–626 10.1086/427243 (doi:10.1086/427243) [DOI] [PubMed] [Google Scholar]
- 50.Richie T. 2006. High road, low road? Choices and challenges on the pathway to a malaria vaccine. Parasitology 133, S113–S144 10.1017/S0031182006001843 (doi:10.1017/S0031182006001843) [DOI] [PubMed] [Google Scholar]
- 51.Heppner D. G., et al. 2005. Towards an RTS,S-based, multi-stage, multi-antigen falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine 23, 2243–2250 [DOI] [PubMed] [Google Scholar]