Abstract
In ant–plant symbioses, plants provide symbiotic ants with food and specialized nesting cavities (called domatia). In many ant–plant symbioses, a fungal patch grows within each domatium. The symbiotic nature of the fungal association has been shown in the ant-plant Leonardoxa africana and its protective mutualist ant Petalomyrmex phylax. To decipher trophic fluxes among the three partners, food enriched in 13C and 15N was given to the ants and tracked in the different parts of the symbiosis up to 660 days later. The plant received a small, but significant, amount of nitrogen from the ants. However, the ants fed more intensively the fungus. The pattern of isotope enrichment in the system indicated an ant behaviour that functions specifically to feed the fungus. After 660 days, the introduced nitrogen was still present in the system and homogeneously distributed among ant, plant and fungal compartments, indicating efficient recycling within the symbiosis. Another experiment showed that the plant surface absorbed nutrients (in the form of simple molecules) whether or not it is coated by fungus. Our study provides arguments for a mutualistic status of the fungal associate and a framework for investigating the previously unsuspected complexity of food webs in ant–plant mutualisms.
Keywords: mutualism, symbiosis, Myrmecophyte, nutrient transfer, stable isotopes, Leonardoxa
1. Introduction
Ants are predominant components of tropical ecosystems [1]. It is thus not surprising that their interactions with other organisms have strong impacts on nutrient fluxes and thus on food webs. Ants of the tribe Attini in America farm symbiotic fungi for food [2]. Some species feed their fungus with fresh leaves, and because colonies can comprise hundreds of thousands of individuals, they represent a strong herbivory pressure [3]; they also process a large amount of organic matter and thus have a noticeable impact on the structure of plant communities [4]. Ants and plants may also interact mutualistically. Ant–plant interactions have a structuring role in tropical arboreal communities through their impact on populations of other arthropods and thereby on food webs [5–7]. The diverse feeding behaviours of tropical arboreal ants predispose them to be protective mutualists of plants: they prey on many arthropod enemies of plants, they are attracted by energy-rich, solid or liquid food rewards offered by plants and they often live in symbiosis with other organisms, such as hemipteran trophobionts or bacteria that help them adapt to the nutritional imbalances that dependence on plant-produced rewards often entails [5,7,8]. In particular, diets of tropical arboreal ants are often characterized by high C : N ratios, reflecting caloric richness relative to paucity of nitrogen and mineral nutrients [5,6].
While provision of food rewards to ants imposes costs to plants, these are often outweighed by the benefits they confer. Thus, opportunistic interactions between ants and plants are often mutualistic, and have repeatedly given rise to specialized symbiotic mutualisms between so-called myrmecophytes (also called ant-plants), which provide symbiotic ants with nesting cavities (specialized hollow structures, called domatia) in addition to food rewards, and specialist ‘plant-ants’ [9]. Plant-ants often protect their hosts against herbivores and pathogens, as well as against competing plants [10–12], and also confer nutritional benefits to their host plants (reviewed in [13]). It was suggested that nutritional benefits were more important than protection in epiphytic myrmecophytes, ants acting as ‘mobile root systems’ in a class of plants with conspicuously low access to mineral nutrients [14]. Consequently, the first studies documenting nutrient flux from ants to plants were conducted on epiphytic myrmecophytes [15–19]. Nevertheless, more recent studies have shown that nutrient transfer from ants may also be important in some ‘protection’ mutualisms involving free-standing myrmecophytes [20–23]. Studies on nutrient transfer from ants to plants have focused on carbon (C) and/or nitrogen (N). However, these are probably not the elements most limiting to non-epiphytic plants in tropical ecosystems [24–26].
A new source of complexity in the trophic structure of ant–myrmecophyte associations is added by the recent findings that many ant–plant symbioses include long-ignored fungal partners [27–29]. These ant–plant–fungi symbioses have been shown to have two benefits. In some, the fungi have a structural role in galleries built by ants around host branches to capture large prey [28,29]. In others, the fungi grow on inner surfaces of domatia and their role remains unknown [27,30]. The role of fungi in trophic fluxes in these tripartite symbioses is not yet clear. The fungi involved in these two types of symbioses belong to the order Chaetothyriales (Ascomycota [27,29]). Although only two plant-ant species are known to build fungal galleries as insect traps, fungi growing in domatia have been observed in many ant–plant symbioses [27,30]. The trophic relationships between plant-ants and their symbiotic fungi may prove particularly important to a better understanding of the nutritional ecology of ant–plant symbioses. As in other ant–microbe mutualisms [8,31], these relationships could help ants adapt to nutritional imbalances.
In order to disentangle the trophic relationships between ants, plants and fungi in ant–plant symbioses, we studied N and C flux from ant to fungi and plant in situ for an understorey myrmecophytic tree, Leonardoxa africana subsp. africana, in which protection conferred by the obligate specific ant associate Petalomyrmex phylax is well documented [32]. The tree provides food (extrafloral nectar) and domatia (hollow internodes) for the ants ([33,34]; electronic supplementary material, figure S1). Moreover, a symbiotic fungus was recently shown to be always present inside domatia occupied by P. phylax, a small patch growing on the inner surface of each inhabited domatium [27]. Each internode of L. a. subsp. africana has a discrete hollow cavity (domatium) with its own entrance hole. Each tree is thus composed of a multitude of domatia that are not interconnected. A single ant colony occupies a single tree, and nearly all domatia are occupied by ants and contain a fungal patch [27]. Ants move freely on the plant's surface from one domatium to another. There is evidence that this fungus is introduced into newly produced domatia and manipulated by the ants. The fungus species involved in this symbiosis, identified by Defossez et al. [27], was not previously known, and other fungal species were found in other ant–plant symbioses. However, wider investigation is required before conclusions can be made about the degree of specificity of the association. Here, we conducted pulse–chase experiments using stable isotopes to address the following questions: (i) is there a nutrient transfer from the ants to their symbiotic fungus? This is an important point to elucidate for testing the mutualistic nature of the association. (ii) Is there a nutrient transfer from the ants to the plant? (iii) Does the presence of the fungus improve the capacity of plant surfaces to incorporate nutrients, and do the plant tissues underlying the fungus present any specialized absorptive structures? Nutrient fluxes are discussed in the context of a possible ant–plant–fungus mutualism.
2. Material and methods
(a). Study species and site
We studied interactions between L. a. africana, P. phylax ants and their fungal associate in secondary rainforest near the village of Nkollo, Cameroon (3°13.278′ N, 10°14.888′ E).
(b). Experiment 1: isotope pulse–chase
On five trees in situ, we carried out isotopic labelling with 13C and 15N in order to study trophic flux from ants to the fungal and plant compartments of the system. On each tree, ants were given 0.5 ml of honey mixed with 40 mg of glucose and 80 mg of glycine, enriched, respectively, in 13C (one atom of carbon marked on each molecule) and 15N (98% of molecules marked). We provided labelled glucose and glycine in a single mix so that the same subsequent samples could be used for both δ13C and δ15N analysis, in order to avoid doubling the sample size. To focus ant foraging activity on the labelled mixture, we prevented access of the ants to the plant's foliar nectaries by applying horticultural glue on tape around the petiole of each leaf before the peak of ant activity (which corresponds to the peak of nectar production, usually between 11.00 and 14.00 [35]). The mixture was then deposited at several sites on the tree's branches. To avoid any potential direct transfer of the stable isotopes from the mixture to the plant through the epidermis, the site of application was wrapped with a piece of L. a. africana leaflet lamina before deposition of the mixture. We used leaflets from the host tree species because these ants are usually reluctant to walk on other surfaces. At the end of the peak of activity, leaflet pieces with remains of mixture and tape with horticultural glue were removed. Thus, the enrichment pulse lasted about three hours. On two additional trees treated the same way (control trees), we prevented access of the ants to the mixture with horticultural glue in order to test for potential contamination of the plant through the rolled leaflet. For each experimental tree, the following compartments were sampled on day − 1, day 1, day 4, day 10 and day 660 (day 0 being the day we provided the labelled mixture): workers, larvae, sexuals (if any), fungal patches, inner surface of domatia not covered by fungus, stems, petioles and leaves (one tree could not be relocated on day 660). Each internode is composed of a short, thin basal portion that is not hollow (which we call stem) and a longer, swollen, hollow portion (which we call domatium). Leonardoxa africana africana has pinnately compound leaves. For the sake of simplicity, hereafter we refer to the leaflet lamina simply as ‘leaf’. In some cases, the amount of material was not enough to perform isotope analysis. This was especially true for fungal patches and ants, which in terms of standing biomass are very small items in this symbiosis. Therefore, to increase the sample size of unlabelled fungi (day − 1), we collected fungi from five unmanipulated trees at the end of the experiment. Isotopic abundance values for these samples were treated as values for day − 1. We collected fungus for analysis by gently scraping it off the surface of the domatium; as a consequence samples could contain a very small amount of plant tissue. However, this is unlikely to have influenced the results because the inner surface of domatia was several times less enriched than the fungal patch (see §3). Control trees were sampled for workers, leaves, petioles and the portion of stems situated immediately under the site of deposition of the labelled mixture on day − 1 and day 1. Samples were dried in the field under silica gel immediately after collecting. Isotopic abundances were measured with an elemental analyser (C, N) connected to an isotopic mass spectrometer (Finnigan Delta S, Finnigan MAT, Bremen, Germany) at the Service Central d'Analyse of the CNRS (SCA, Solaize, France).
(c). Experiment 2: capacity of the domatia surfaces to absorb nutrients
The aim of this experiment was to compare the efficiency of nutrient transfer from bark, inner surface of domatia and fungal patch to the plant tissues (stems, petioles and leaves). Three branches on each of five trees were cut and fitted into a tube filled with water. Domatia were immediately opened and ants removed. For each tree, we immediately applied a drop of the labelled mixture (the same used in the pulse–chase experiment) on a different surface (bark, inner surface of domatium, fungal patch) for each of the three branches. Plant tissues (stems, petioles and leaves) were sampled on day − 1 and day 4 (day 0 being the day when branches were cut and the mixture was applied). Samples were processed as in the pulse–chase experiment. For each branch, and for both δ15N and δ13C, we computed the mean of the values for the three tissues (stems, petiole and leaves). We then computed the difference between day 4 and day − 1 and compared these values with zero and among the types of surface tested.
(d). Morphology of the inner surface of domatia
In order to detect any morphological structure of the inner surface of domatia that could be involved in nutrient transfer, we performed environmental scanning electron microscopy (ESEM) on live domatia with an FEI Quanta FEG200 (FEI, Hillsboro, USA).
(e). Statistical analysis
We used non-parametric tests for independent samples in experiment 1 because missing data precluded tests for paired samples in many cases. We used non-parametric tests for paired samples in experiment 2. All tests were performed with the software R v. 2.10.1 (R Development Core Team).
3. Results
(a). Experiment 1: isotope pulse–chase
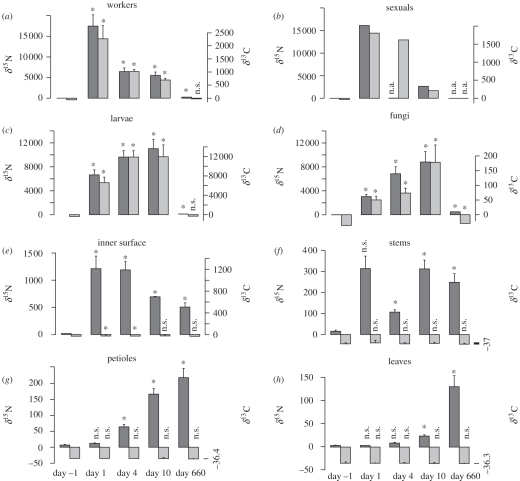
Control trees with labelled mixture inaccessible to the ants showed no enrichment (electronic supplementary material, table S1), so that no direct contamination by the labelled mixture occurred. In experimental trees, enrichments varied significantly according to time for both 15N and 13C for workers (Kruskal–Wallis tests: δ15N : H = 19.8, p = 0.001; δ13C : H = 12.4, p = 0.014), larvae (δ15N : H = 17.3, p = 0.001; δ13C : H = 17.7, p = 0.001), fungi (δ15N : H = 19.3, p = 0.001; δ13C : H = 16.1, p = 0.002) and inner surface of domatia (δ15N : H = 12.8, p = 0.012; δ13C : H = 11.2, p = 0.024), but only for 15N for stems (δ15N : H = 9.3, p = 0.053; δ13C : H = 3.3, p = 0.50), petioles (δ15N : H = 18.8, p = 0.001; δ13C : H = 3.9, p = 0.42) and leaves (δ15N : H = 16.5, p = 0.002; δ13C : H = 0.7, p = 0.96). Sexuals were not tested because of the small sample size for this compartment (see raw data in electronic supplementary material, table S2). The enrichment of workers and sexuals in both δ15N and δ13C was very high as early as day 1, and then gradually decreased (figure 1a,b). Conversely, larvae and fungal patches were enriched more progressively (figure 1c,d). This result suggests a different feeding behaviour of workers towards sexuals and larvae: workers distribute nutrients quickly to sexuals (which may be more efficient at begging for food) and more slowly to larvae. Moreover, larvae do not defaecate nor excrete, and thus accumulated labelled nutrients. The inner surface of domatia (part not covered by a fungal patch) was enriched in 15N from day 1, enrichment then progressively decreasing (figure 1e), but was very little enriched in 13C (significantly enriched at day 1 but barely detectable at the scale of figure 1). Stems, petioles and leaves were enriched progressively, but only in 15N, and displayed the strongest enrichment at day 660 (i.e. almost 2 years after the labelling; figure 1f–h).
Figure 1.
Change in δ13C and δ15N (‰) with time in the different compartments of the ant–plant–fungus symbiosis (experiment 1). The compartment called ‘leaves’ corresponds to the lamina of leaflets. Ants received food enriched in 13C and 15N at day 0. Values are means ± s.e from five experimental trees (sample size is lower in some cases where data are missing; see electronic supplementary material, table S2 for details). Asterisks denote values significantly different from day − 1 (Mann–Whitney tests, p < 0.05). n.a., data not available. n.s., not significant. Dark grey bars, δ15N; light grey bars, δ13C.
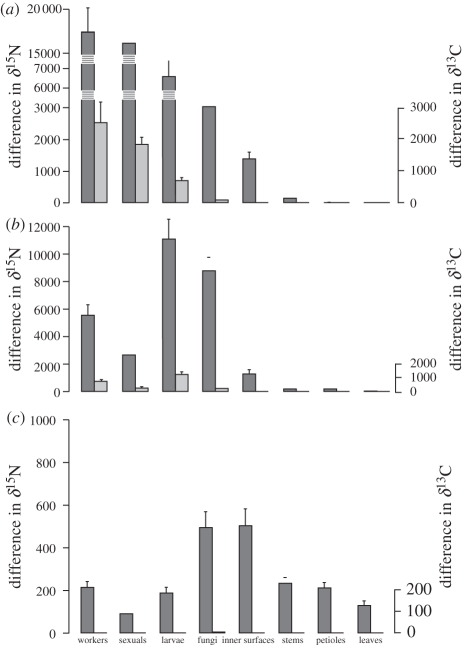
Figure 2 displays the differences of enrichment between day − 1 and each of day 1, day 10 and day 660, allowing comparison between compartments at the same scale. Although precise calculation of uptake and transfer rates would require calculating atom per cent excess, taking into account the dilution of the tracer by solutes present in the honey used in the experiment and in the plant's foliar nectar, it is clear that nutrient flux from ants to fungal patches was much greater for N than for C. Indeed, 1 and 10 days after labelling, the increase in 15N was about seven times greater than the increase in 13C in workers, but about 50 times greater than the increase in 13C in the fungal patches (figure 2). In contrast, the nutrient flux from workers to larvae and sexuals, which also received 15N and 13C directly from the workers, appeared similar for N and C: the relative increase between 15N and 13C was about the same for workers, larvae and sexuals. Enrichment of the inner surface of domatia was also biased in favour of N. Figure 2 shows that ants transferred most of the labelled nutrients to larvae and sexuals. Fungal patches also received a fairly large amount of labelled N. After 10 days, enrichment of the inner surfaces of domatia was six times lower than for the fungal patches. Stems, petioles and leaves received very low amounts of labelled elements, mostly N, but seem to accumulate it because δ15N was highest at day 660. All compartments were still enriched in 15N after 660 days. However, 13C was lost (figures 1 and 2), except in fungi, for which the difference between day 660 and day − 1 was still significant (figure 1d). The distribution of introduced 15N and 13C showed a significant difference among compartments after 1 and 10 days, but not after 660 days (figure 2; Kruskal–Wallis tests: after 1 day, for 15N : H = 26.6, p = 0.001, for 13C : H = 20.8, p = 0.002; after 10 days, for 15N : H = 27.2, p = 0.001, for 13C : H = 20.0, p = 0.002; after 660 days, for 15N : H = 8.9, p = 0.26, for 13C : H = 4.1, p = 0.77).
Figure 2.
Enrichment in 13C and 15N in the different compartments of the ant–plant–fungus symbiosis, 1, 10 and 660 days after the ants were fed with food enriched in these stable isotopes (experiment 1). This figure does not incorporate the data for fungi from the five unmanipulated trees sampled at the end of the experiment (and treated as values for day − 1 in figure 1). Means ± s.e. (a) Enrichment between day − 1 and day 1; (b) enrichment between day − 1 and day 10; (c) enrichment between day − 1 and day 660. Dark grey bars, δ15N; light grey bars, δ13C.
(b). Experiment 2: capacity of fungal and different plant surfaces to absorb nutrients
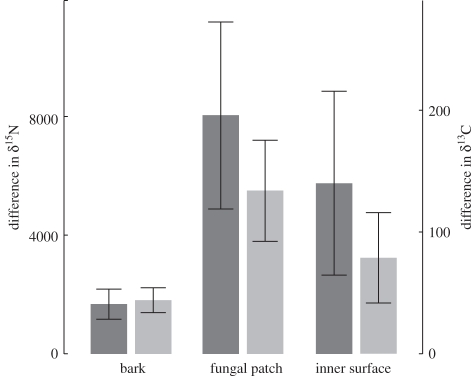
The differences in enrichment of plant parts between day 4 and day − 1 were greater than zero for δ15N and δ13C for the three surfaces that received the labelled mixture (figure 3; Wilcoxon tests: t = 0, p = 0.043 in each case; see raw data in electronic supplementary material, table S3), showing that they were all capable of C and N transfer. Capacity for nutrient transfer did not differ significantly among surfaces tested (Friedman test: χ2 = 2.8, p = 0.25 for δ15N and δ13C).
Figure 3.
Enrichment in 15N and 13C in the plant after deposition of a mixture enriched in the stable isotopes on three different surfaces: bark, fungal patch within domatia, inner surface of domatia (experiment 2). Values are differences between day 4 and day − 1 (day 0 being the day when the mixture was deposited). Means ± s.e. Dark grey bars, δ15N; light grey bars, δ13C.
(c). Morphology of the inner surface of domatia
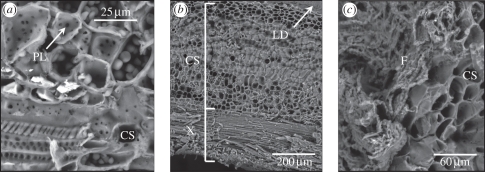
Figure 4 shows that the inner surface of the domatia is formed by a canaliculated, lignified sclerenchyma with numerous plasmodesmata (intercellular pits), underlain by xylem vessels. The same tissue occurred under fungal patches, and no fungal hyphae were seen to penetrate the pits in this tissue, or the tissue itself.
Figure 4.
(a,b) ESEM view of a transversal section of the inner surface of a domatium of L. a. africana reveals a canaliculate sclerenchyma (CS) with numerous plasmodesmata (PL). (c) A transversal section through the fungal patch shows that the fungal hyphae do not penetrate host plant tissue. LD, lumen of domatium; X, xylem vessels; F, fungal hyphae.
4. Discussion
(a). The ants transfer nutrients to their host plant
Our study shows that the ant P. phylax transfers N to its host plant L. a. africana. A significant enrichment in 15N was noticed 4 days after the ants were supplied with enriched food. However, we could not detect any transfer of C. A potential selective absorption of compounds containing N cannot account for the difference between C and N because the absorption experiment (experiment 2) showed that both glucose and glycine can be absorbed by the plant through its domatium surfaces. This difference may be due to the combination of two phenomena. First, the glucose used in the experiments had only one 13C atom out of six atoms of carbon, and we introduced only half as much glucose as glycine into the symbiosis. The effect of enrichment was thus expected to be much stronger for δ15N than for δ13C. Second, workers are expected to need more carbohydrates than amino acids and, thus, should defaecate more N (and thus 15N) than C (and thus 13C). Moreover, C is used by the insect for respiration and thus released as CO2, whereas N is only released in the faeces and excretion products, which are deposited on fungal patches [27]. Therefore, the low transfer observed for N may not be detectable for C. Nutrient transfer from ant cadavers and exuviae (containing both labelled N and C) to the plant did not seem to be greater than that from faeces and excretion because enrichment in 13C was not detectable even 660 days after labelling (at this time, most ants that received the labelling mixture had probably died).
The amount of N transferred to the plant is quite low in comparison with that transferred to the other compartments during the first 10 days of the experiment. However, Leonardoxa, belonging to the tribe Detarieae, is one of the many caesalpinioid legumes that do not fix atmospheric N [36]. A low but regular N transfer may thus be important over the lifespan of the plant. Previous studies of nutrient transfer from ants to host plants have mainly focused on ant–epiphyte symbioses, for which N is likely to be limiting. Our study, along with a few previous ones providing direct evidence of nutrient transfer [20,21], shows that nutrient transfer may also be widespread in free-standing myrmecophytes. Moreover, a recent correlative study conducted in eight forest sites in Venezuela suggested that the uptake of rare nutrients could be the main benefit of bearing domatia [37]. ‘Protection mutualisms’ and ‘nutritional mutualisms’ are thus not exclusive categories. Indeed, other symbioses display the same duality, with nutritional and protection benefits. For example, the mycorrhizal symbiosis, often viewed as a nutritional mutualism, has overlooked roles in plant protection [38]. However, N may not be the best nutrient on which to focus because it is often not a limiting nutrient for free-standing trees in tropical ecosystems. Phosphorus (P) appears more limiting [24–26], and it can be questioned whether it can also be transferred. P has only one stable isotope, which makes field study of transfer from ant to plant much more difficult.
(b). The fungus does not improve the nutrient transfer to the host plant
It has already been suggested that the domatia-inhabiting fungi of the epiphytic myrmecophyte Myrmecodia could be involved in the degradation of ant wastes and could facilitate nutrient incorporation into the plant [16]. However, our study is the first to provide data on the role of the fungal partner in trophic fluxes in ant–plant–fungus associations, giving information on the relationships among the three symbiotic partners. What role the fungus may play in transfer of nutrients to the plant remains unclear. Our results show that inner surfaces of domatia devoid of fungus can transfer simple compounds such as glucose or glycine to other parts of the plant, with no requirement for a fungal intermediary. However, the plant surface may not be able to absorb nutrients from more complex organic molecules, such as the dead ants and pieces of cuticle of unidentified arthropods often found in the fungal patch [27]. It remains to be tested whether the fungal patch could degrade complex molecules into simpler ones that can be incorporated by the plant through the inner surface of domatia.
Observation by ESEM revealed a lignified sclerenchyma on the inner surface of domatia, which probably functions in structural support and protection of domatia and their contents, and for which its unusual canaliculated structure may allow molecule absorption, despite the presence of lignified cell walls. First, the presence of plasmodesmata suggests that the plant needs to maintain the sclerenchymatous cells alive, in spite of their lignified wall; second, the large number of plasmodesmata and their small size may allow molecules to pass through but prevent entry of pathogenic micro-organisms into the cell. However, we could not detect any difference in absorption capacities between different plant surfaces (for example, bark and inner surface of domatia). Thus, the canaliculate sclerenchyma has probably not evolved for the sole function of transfer of simple molecules such as those used in this study (glycine and glucose). The exact role of this tissue remains to be elucidated, but its structure and the presence of xylem within it are compatible with transfer of molecules.
(c). The ants transfer nutrients to their symbiotic fungus
Tracing nutrient transfers with stable isotopes showed that the domatia-inhabiting fungi received N from the ant P. phylax. The fact that the fungus was enriched in stable isotopes as soon as 1 day after ants were given enriched food indicates that ants are manuring the fungus, and that the enrichment is not just a mere consequence of occasional deposition of dead ants and other debris. Defossez et al. [27] showed that the fungus is strictly associated with the presence of the ants, but did not demonstrate reciprocal benefits in the association. Their only evidence that the fungus was valuable to the ants was that when a domatium was highly disturbed by the experimenter, ants removed from the domatium not only their brood but also the fungal patch. Feeding of the fungus, as demonstrated in this study, is an additional strong argument in favour of a mutualistic relationship. The observation that ants also gnaw at the fungus [27] suggests that nutrients may flow in both directions between ant and fungus, opening the possibility that the latter could play a role in recycling of nutrients, particularly N and perhaps mineral nutrients. Whereas N is unlikely to be limiting for the plant [24–26], it is often strongly limiting for tropical arboreal ants [5,6]. Fungi and bacteria possess enzymes capable of converting nitrogenous wastes of insects into nutrients ants could use [39], and this could be an important benefit in this symbiosis, as it is in other ant–microbial symbioses [39]. Moreover, pieces of cuticle (sometimes attributable to ants) were commonly found on the fungal patch, and ants were observed defaecating and depositing refuse on it [27], suggesting a recycling role of the fungus.
The pattern of nutrient transfer from workers to larvae was very different from that of transfer from workers to the fungal patches. While the enrichment in 15N was roughly similar for both larvae and fungal patches, the enrichment in 13C was five times greater for the larvae than for the fungal patches. This result suggests that larvae and fungal patches received nutrients by two different pathways. Larvae, fed through trophallaxis, were provided with the original labelled mixture, perhaps slightly modified by salivary enzymes. In contrast, ants defaecate on the fungal patches [27], which may thus be provided mainly with ant faeces and products of excretion. The high worker metabolism of simple sugars (the C of which is released as CO2) may have resulted in waste products containing a greater proportion of the original labelled nutrients for N than for C.
Comparing enrichment between the fungal patch and the inner surface of parts of domatia devoid of a fungal patch indicates that the ants have a specific feeding behaviour towards the fungal patch. Indeed, after 10 days, six times more N was deposited on the fungal patch than on the inner surface of domatia not covered by a fungal patch. This may be due to preferential defaecation by ants on the fungal patch, as suggested by our previous observation that ant wastes accumulate on the fungal patch [27].
During the first 10 days of the pulse–chase experiment, enrichment increased over time on the fungal patch, whereas it decreased on the inner surface of domatia outside the fungal patch. Moreover, enrichment of plant tissues was low and progressive. The canaliculate sclerenchyma constituting the inner surface of domatia could facilitate absorption and transfer of nutrients to other plant parts. The small quantities of nutrients deposited by the ants on the inner surface could thus have been progressively incorporated into plant tissues, whereas those deposited on the fungal patch would have been incorporated and accumulated by the fungus. It is possible that the fungus could release nutrients to the plant after some delay.
(d). Nitrogen is efficiently recycled within the symbiosis
The most surprising result was that all compartments of the symbiosis were still enriched in 15N almost 2 years (660 days) after the ants were given enriched food. Moreover, all compartments were similarly enriched in 15N. This means that part of the N introduced has been cycling in the system and that there were reciprocal exchanges of N among the three partners. Indeed, the 15N did not accumulate in one or two of the partners at the expense of the other(s), again a strong argument in favour of a mutualistic relationship. Ants that were labelled at the beginning of the experiment were probably dead after 660 days and, thus, the 15N detected in ant compartments 2 years after the pulse–chase experiment was obtained from either the plant or the fungus. The fact that plant-ants feed from their host plant is well known, but the trophic flux from the fungus to the ants remains to be tested experimentally. The fact that ants have been observed gnawing the fungus [27] may constitute a first clue for what appears to be a recycling mechanism within this symbiosis.
(e). Conclusion
Tracking nutrient flux in a three-way symbiosis revealed unexpected interactions and gave a more detailed picture of ant–fungus–myrmecophyte relationships (figure 5). The most striking fact is that ants do feed the fungus. Furthermore, the absence of fungal hyphae in plant tissues suggests that the fungus depends for its growth on these or other substrates supplied to it in the domatia. Even if the function of the fungus still remains unclear, it certainly has a central role in the nutritional ecology of the Leonardoxa–Petalomyrmex symbiosis, perhaps in recycling N or other nutrients, thereby helping ants adapt to nutritional imbalances. In contrast, much less N is transferred from ants to the host plant. This makes sense, because ants would stand to gain little benefit from transferring to the plant a nutrient that is in crucially short supply to ants but unlikely to be limiting to the plant. Quantifying trophic fluxes among all partners could greatly modify our current view of the balance between costs and benefits, with consequences for ideas about the evolution and maintenance of these mutualisms. The stability of such mutualisms is often questioned because cheating could easily evolve. Indeed, horizontal transmission could lead to conflict over reproduction, each partner having no interest in the reproduction of the other. However, in the absence of detailed quantification of costs and benefits, conclusions about evolutionary stability can hardly be made. Our study underlines the fact that even the very nature of the types of benefits provided is not well known, let alone the quantification of flux. Moreover, the roles in trophic fluxes of partners other than plants and ants have been neglected. Despite the widespread presence in ant–plant associations of fungi, they have never been taken into account in studies of trophic fluxes between the ant and the plant. Fungi are not the only neglected actors in these systems. Bacteria [40,41] and domatia inhabitants [42] are also important components of these interaction webs. Our study provides a new framework, demonstrating interactions that should stimulate more detailed analyses of food webs in ant–plant mutualisms.
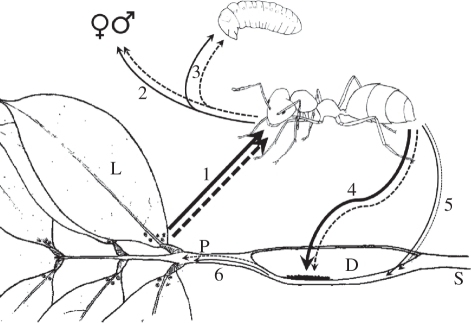
Figure 5.
Schematic diagram combining trophic fluxes between the partners in the ant–plant symbiosis P. phylax–L. a. africana. Arrows represent carbon (dashed arrow) and nitrogen (solid arrow) fluxes. Arrow thickness gives an indication of the relative fluxes. Workers collect foliar nectar (1) [33] and distribute it to sexuals (2) and brood (3) by trophallaxis. Workers also collect small prey items and bird droppings (not figured). Workers transfer nutrients, probably by defaecation, to the fungal patch (4) and to a much lesser extent to the inner surface of the domatium (5). Nutrients are then transferred to the plant (6). Brood and sexuals are normally confined in the domatia but are displayed outside for clarity. Each internode is constituted by a short, thin basal part that is not hollow (stem) and a longer, swollen distal hollow part (domatium). P, petiole of the compound leaf; L, lamina of a leaflet; S, stem; D, domatium.
Acknowledgements
We thank the Ministry of Scientific Research and Innovation of the Republic of Cameroon for permission to carry out this study. We thank Xavier Garde and the IRD in Yaoundé for providing logistic help in the field, and the family of Big John and the traditional chief for their hospitality at NkolloBondé village. We also thank Wolfgang Wanek, Veronika Mayer, Finn Kjellberg and three anonymous referees for their valuable comments on the data and on the manuscript. This study was funded by two grants from the French Agence Nationale de la Recherche: one from the ‘Young scientists’ programme to R.B. (research agreement no. ANR-06-JCJC-0127) and one from the ‘Biodiversity’ programme to D.M. and R.B. (IFORA project).
References
- 1.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, UK: Belknap Press [Google Scholar]
- 2.Mueller U. G., Gerardo N. M., Aanen D. K., Six D. L., Schultz T. R. 2005. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 36, 563–595 10.1146/annurev.ecolsys.36.102003.152626 (doi:10.1146/annurev.ecolsys.36.102003.152626) [DOI] [Google Scholar]
- 3.Herz H., Beyschlag W., Holldobler B. 2007. Herbivory rate of leaf-cutting ants in a tropical moist forest in Panama at the population and ecosystem scales. Biotropica 39, 482–488 10.1111/j.1744-7429.2007.00284.x (doi:10.1111/j.1744-7429.2007.00284.x) [DOI] [Google Scholar]
- 4.Farji-Brener A. G., Illes A. E. 2000. Do leaf-cutting ant nests make ‘bottom-up’ gaps in neotropical rain forests? A critical review of the evidence. Ecol. Lett. 3, 219–227 10.1046/j.1461-0248.2000.00134.x (doi:10.1046/j.1461-0248.2000.00134.x) [DOI] [Google Scholar]
- 5.Davidson D. W. 1997. The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol. J. Linn. Soc. 61, 153–181 10.1111/j.1095-8312.1997.tb01785.x (doi:10.1111/j.1095-8312.1997.tb01785.x) [DOI] [Google Scholar]
- 6.Davidson D. W., Cook S. C., Snelling R. R., Chua T. H. 2003. Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972 10.1126/science.1082074 (doi:10.1126/science.1082074) [DOI] [PubMed] [Google Scholar]
- 7.McKey D., Gaume L., Brouat C., Di Giusto B., Pascal L., Debout G., Dalecky A., Heil M. 2005. The trophic structure of tropical ant-plant–herbivore interactions: community consequences and coevolutionary dynamics. In Biotic interactions in the tropics: their role in the maintenance of species diversity (eds Burslem D., Pinard M., Hartley S.), pp. 386–413 Cambridge, UK: Cambridge University Press [Google Scholar]
- 8.Russell J. A., Moreau C. S., Goldman-Huertas B., Fujiwara M., Lohman D. J., Pierce N. E. 2009. Bacterial gut symbionts are tightly linked with the evolution of herbivory in ants. Proc. Natl. Acad. Sci. USA 106, 21 236–21 241 10.1073/pnas.0907926106 (doi:10.1073/pnas.0907926106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson D. W., McKey D. 1993. The evolutionary ecology of symbiotic ant-plant relationships. J. Hymenopt. Res. 2, 13–83 [Google Scholar]
- 10.Frederickson M. E., Greene M. J., Gordon D. M. 2005. ‘Devil's gardens’ bedevilled by ants. Nature 437, 495–496 10.1038/437495a (doi:10.1038/437495a) [DOI] [PubMed] [Google Scholar]
- 11.Letourneau D. K. 1998. Ants, stem-borers, and fungal pathogens: experimental tests of a fitness advantage in Piper ant-plants. Ecology 79, 593–603 [Google Scholar]
- 12.Rosumek F. B., Silveira F. A. O., Neves F. D., Barbosa N. P. D., Diniz L., Oki Y., Pezzini F., Fernandes G. W., Cornelissen T. 2009. Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia 160, 537–549 10.1007/s00442-009-1309-x (doi:10.1007/s00442-009-1309-x) [DOI] [PubMed] [Google Scholar]
- 13.Rico-Gray V., Oliveira P. S. 2007. The ecology and evolution of ant-plant interactions. Chicago, IL: The University of Chicago Press [Google Scholar]
- 14.Janzen D. H. 1974. Epiphytic myrmecophytes in Sarawak: mutualism through the feeding of plants by ants. Biotropica 6, 237–259 10.2307/2989668 (doi:10.2307/2989668) [DOI] [Google Scholar]
- 15.Gay H. 1993. Animal-fed plants: an investigation into the uptake of ant derived nutrients by the far-eastern epiphytic fern Lecanopteris Reinw. (Polypodiaceae). Biol. J. Linn. Soc. 50, 221–233 10.1111/j.1095-8312.1993.tb00928.x (doi:10.1111/j.1095-8312.1993.tb00928.x) [DOI] [Google Scholar]
- 16.Huxley C. R. 1978. Ant-plants Myrmecodia and Hydnophytum (Rubiaceae), and the relationships between their morphology, ant occupants, physiology and ecology. New Phytol. 80, 231–268 10.1111/j.1469-8137.1978.tb02285.x (doi:10.1111/j.1469-8137.1978.tb02285.x) [DOI] [Google Scholar]
- 17.Rickson F. R. 1979. Absorption of animal tissue breakdown products into a plant stem—the feeding of a plant by ants. Am. J. Bot. 66, 87–90 10.2307/2442629 (doi:10.2307/2442629) [DOI] [Google Scholar]
- 18.Rico-Gray V., Barber J. T., Thien L. B., Ellgaard E. G., Toney J. J. 1989. An unusual animal–plant interaction: feeding of Schomburgkia tibicinis (Orchidaceae) by ants. Am. J. Bot. 76, 603–608 10.2307/2444355 (doi:10.2307/2444355) [DOI] [Google Scholar]
- 19.Treseder K. K., Davidson D. W., Ehleringer J. R. 1995. Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature 375, 137–139 10.1038/375137a0 (doi:10.1038/375137a0) [DOI] [Google Scholar]
- 20.Cabrera M., Jaffe K. 1994. A trophic mutualism between the myrmecophytic melastomataceae Tococa guianensis Aublet and an Azteca ant species. Ecotropicos 7, 1–10 [Google Scholar]
- 21.Fischer R. C., Wanek W., Richter A., Mayer V. 2003. Do ants feed plants? A 15N labelling study of nitrogen fluxes from ants to plants in the mutualism of Pheidole and Piper. J. Ecol. 91, 126–134 10.1046/j.1365-2745.2003.00747.x (doi:10.1046/j.1365-2745.2003.00747.x) [DOI] [Google Scholar]
- 22.Sagers C. L., Ginger S. M., Evans R. D. 2000. Carbon and nitrogen isotopes trace nutrient exchange in an ant-plant mutualism. Oecologia 123, 582–586 10.1007/PL00008863 (doi:10.1007/PL00008863) [DOI] [PubMed] [Google Scholar]
- 23.Solano P. J., Dejean A. 2004. Ant-fed plants: comparison between three geophytic myrmecophytes. Biol. J. Linn. Soc. 83, 433–439 10.1111/j.1095-8312.2004.00381.x (doi:10.1111/j.1095-8312.2004.00381.x) [DOI] [Google Scholar]
- 24.Hedin L. O., Brookshire E. N. J., Menge D. N. L., Barron A. R. 2009. The nitrogen paradox in tropical forest ecosystems. Annu. Rev. Ecol. Evol. Syst. 40, 613–635 10.1146/annurev.ecolsys.37.091305.110246 (doi:10.1146/annurev.ecolsys.37.091305.110246) [DOI] [Google Scholar]
- 25.Huston M. A., Wolverton S. 2009. The global distribution of net primary production: resolving the paradox. Ecol. Monogr. 79, 343–377 10.1890/08-0588.1 (doi:10.1890/08-0588.1) [DOI] [Google Scholar]
- 26.Vitousek P. M. 1984. Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65, 285–298 10.2307/1939481 (doi:10.2307/1939481) [DOI] [Google Scholar]
- 27.Defossez E., Selosse M. A., Dubois M. P., Mondolot L., Faccio A., Djieto-Lordon C., McKey D., Blatrix R. 2009. Ant-plants and fungi: a new threeway symbiosis. New Phytol. 182, 942–949 10.1111/j.1469-8137.2009.02793.x (doi:10.1111/j.1469-8137.2009.02793.x) [DOI] [PubMed] [Google Scholar]
- 28.Dejean A., Solano P. J., Ayroles J., Corbara B., Orivel J. 2005. Arboreal ants build traps to capture prey. Nature 434, 973. 10.1038/434973a (doi:10.1038/434973a) [DOI] [PubMed] [Google Scholar]
- 29.Mayer V. E., Voglmayr H. 2009. Mycelial carton galleries of Azteca brevis (Formicidae) as a multi-species network. Proc. R. Soc. B 276, 3265–3273 10.1098/rspb.2009.0768 (doi:10.1098/rspb.2009.0768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blatrix R., Bouamer S., Morand S., Selosse M. A. 2009. Ant-plant mutualisms should be viewed as symbiotic communities. Plant Signal. Behav. 4, 554–556 10.4161/psb.4.6.8733 (doi:10.4161/psb.4.6.8733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldera E. J., Poulsen M., Suen G., Currie C. R. 2009. Insect symbioses: a case study of past, present, and future fungus-growing ant research. Environ. Entomol. 38, 78–92 10.1603/022.038.0110 (doi:10.1603/022.038.0110) [DOI] [PubMed] [Google Scholar]
- 32.Gaume L., McKey D., Anstett M. C. 1997. Benefits conferred by ‘timid’ ants: active anti-herbivore protection of the rainforest tree Leonardoxa africana by the minute ant Petalomyrmex phylax. Oecologia 112, 209–216 10.1007/s004420050302 (doi:10.1007/s004420050302) [DOI] [PubMed] [Google Scholar]
- 33.McKey D. 1984. Interaction of the ant-plant Leonardoxa africana (Caesalpiniaceae) with its obligate inhabitants in rainforest in Cameroon. Biotropica 16, 81–99 10.2307/2387840 (doi:10.2307/2387840) [DOI] [Google Scholar]
- 34.McKey D. 2000. Leonardoxa africana (Leguminosae: Caesalpinioideae): a complex of mostly allopatric subspecies. Adansonia 22, 71–109 [Google Scholar]
- 35.Gaume L., McKey D. 1999. An ant-plant mutualism and its host-specific parasite: activity rhythms, young leaf patrolling, and effects on herbivores of two specialist plant-ants inhabiting the same myrmecophyte. Oikos 84, 130–144 10.2307/3546873 (doi:10.2307/3546873) [DOI] [Google Scholar]
- 36.Sprent J. I. 2007. Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytol. 174, 11–25 10.1111/j.1469-8137.2007.02015.x (doi:10.1111/j.1469-8137.2007.02015.x) [DOI] [PubMed] [Google Scholar]
- 37.Goitia W., Jaffe K. 2009. Ant-plant associations in different forests in Venezuela. Neotrop. Entomol. 38, 7–31 [DOI] [PubMed] [Google Scholar]
- 38.Newsham K. K., Fitter A. H., Watkinson A. R. 1995. Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol. Evol. 10, 407–411 10.1016/S0169-5347(00)89157-0 (doi:10.1016/S0169-5347(00)89157-0) [DOI] [PubMed] [Google Scholar]
- 39.Douglas A. E. 2009. The microbial dimension in insect nutritional ecology. Funct. Ecol. 23, 38–47 10.1111/j.1365-2435.2008.01442.x (doi:10.1111/j.1365-2435.2008.01442.x) [DOI] [Google Scholar]
- 40.Eilmus S., Heil M. 2009. Bacterial associates of arboreal ants and their putative functions in an obligate ant-plant mutualism. Appl. Environ. Microbiol. 75, 4324–4332 10.1128/AEM.00455-09 (doi:10.1128/AEM.00455-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Borm S., Buschinger A., Boomsma J. J., Billen J. 2002. Tetraponera ants have gut symbionts related to nitrogen-fixing root-nodule bacteria. Proc. R. Soc. Lond. B 269, 2023–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaume L., McKey D., Terrin S. 1998. Ant–plant–homopteran mutualism: how the third partner affects the interaction between a plant-specialist ant and its myrmecophyte host. Proc. R. Soc. Lond. B 265, 569–575 10.1098/rspb.1998.0332 (doi:10.1098/rspb.1998.0332) [DOI] [Google Scholar]