Abstract
Natural abundance is shaped by the abiotic requirements and biotic interactions that shape a species' niche, yet these influences are rarely decoupled. Moreover, most plant mortality occurs during early life stages, making seed recruitment critical in structuring plant populations. We find that natural abundance of two woodland herbs, Hexastylis arifolia and Hepatica nobilis, peaks at intermediate resource levels, a pattern probably formed by concurrent abiotic and biotic interactions. To determine how this abundance patterning reflects intrinsic physiological optima and extrinsic biotic interactions, we translocate adults and seeds to novel locations across experimentally extended abiotic gradients. These experiments indicate that the plant distributions probably reflect biotic interactions as much as physiological requirements, and that adult abundance provides a poor indication of the underlying niche requirements. The positive response exhibited by adult transplants in the wettest conditions is offset by increased fungal attack on buried seeds consistent with peak natural abundance where soil moisture is intermediate. This contraction of niche space is best described by Connell's model—species are limited by physiological tolerances where resources are low and biotic interactions where resources are high.
Keywords: Hexastylis arifolia, Hepatica nobilis, regeneration niche, biotic interactions, species distribution, recruitment
1. Introduction
Species distributions are influenced by physiological requirements for resources (e.g. light and moisture) and interactions with other species (e.g. competition and facilitation). The interplay between biotic and abiotic drivers is recognized in Hutchinson's [1] fundamental and realized niche dichotomy. The ‘fundamental’ niche includes the resource requirements that ensure species persistence, but these are rarely met owing to biotic interactions, such as competition that create a contracted ‘realized niche’. Thus, when species abundance peaks at intermediate resources to form a unimodal curve [2,3], this pattern may represent a self-limiting optimum [4–6] or a contraction owing to negative biotic interactions that overwhelm the species where resources are high [1,7,8]. Conversely, positive biotic interactions can extend the fundamental niche beyond that of the realized niche by enhancing access to limiting resources [9–11]. This requires, however, that resource conditions are optimal for both mutualists, and failed or limited facilitation can result in contracted response patterns similar to those created by competitive interactions [12,13]. Connell [14] suggested that species experience physical stress where resources are low—though this can be ameliorated by facilitation [9,15]—and biological stress where resources are high, but fundamental to realized niche contractions have been tested rarely since Connell's studies [16–18].
Individual life-history stages (e.g. recruitment, survival) contribute unequally to plant population viability [19,20], and each stage may respond differentially to abiotic conditions [21–23] and biotic interactions [24,25]. Most plant mortality occurs during the transition from seed to juvenile [26,27], making recruitment success (the ‘regeneration niche’) a critical phase in structuring the spatial distribution of plant populations [20,21,28]. Warren et al. [13] found that ant-mediated seed dispersal often fails where soil moisture is relatively high in deciduous forests so that seedlings may be absent from habitat where they would otherwise thrive. However, even though wet soils may provide ample resource for plant growth and function, such conditions also promote the growth and spread of pathogenic fungi, a detriment to both seedlings and adults [29–32]. For these reasons, careful study must be made to decouple the physiological and biological limits to a species' niche.
We first surveyed two woodland herbs, Hexastylis arifolia and Hepatica nobilis, in natural understory forest populations. As this revealed a unimodal abundance pattern along both soil moisture and diffuse light gradients, we manipulated the environmental conditions by randomly transplanting adults and seeds into novel locations. We further manipulated the experimental gradients via watering to augment soil moisture and shadecloth to decrease irradiance. The translocations of the study plants removed ant-dispersal, and probably reduced the impact of inter- and intraspecific competition as confounding biotic interactions that probably shape the observed abundance patterns. This permitted us to examine whether the unimodal abundance in the natural populations across resource gradients is consistent with (1i) an intrinsic physiological resource optima or (1ii) biotic interactions. We conducted a second translocation experiment with augmented watering and fungicide to determine whether increased seed mortality in wetter soils is because of (2i) an intrinsic physiological resource optima, ‘waterlogging,’ or (2ii) fungal pathogens, ‘damping off disease’. Our combined observational and experimental approaches permit lines of enquiry not commonly pursued together in ecological research. Specifically, we examine the contraction of the fundamental to realized niche, focusing on both adult and recruitment niche requirements and the role of potential biotic interactions and physiological tolerances at both ends of environmental gradients where natural populations decline.
2. Material and methods
(a). Study species and sites
The study species are small (10–15 cm tall), perennial, understory, evergreen herbs that occur in the temperate deciduous forests of the eastern United States (US). Hexastylis arifolia (Michx.) Small (Aristolochiaceae) is limited to the southeastern US from Florida to Virginia, North Carolina to the Mississippi River. Hepatica nobilis Schreb. var. obtusa (Pursh) Steyerm. (Ranunculaceae) has a greater range, occurring from northern Florida to Nova Scotia, west to Alabama and Missouri and Montana. Both plants depend on ants for propagule dispersal and neither produce clonal offspring [33,34]. This guild of ant-dispersed herbs comprises a great part of plant species richness in southeastern US forests [35–37].
Natural populations were surveyed and transplants collected at Whitehall Forest (WHF) in Clarke County, GA, USA (33°53′ N, 83°21′ W; 150–240 m elevation, 122 cm mean annual precipitation (MAP), 17°C mean annual temperature (MAT)). Hexastylis arifolia is relatively widespread at WHF, while Hp. nobilis occurs in smaller populations. The transplant experiment was replicated at a second site: Coweeta Hydrologic Laboratory (CWT) in Macon County, NC, USA (35°03′ N, 83°25′ W; 750–1025 m elevation, 183 cm MAP, 13°C MAT). Both study species occur at CWT, and our rationale for selecting this location was that it extended the moisture gradient (being a wetter site). The observational and experimental sites at WHF, and the experimental sites at CWT, were located in relatively mature (approx. 80 years) oak-hickory forests without extensive understory shrubs/saplings. A third site was established for a seed germination experiment with water and fungicide treatments in Jackson County, NC, USA (35°17′ N, 83°17′ W, 760 m elevation, 131 cm MAP, 13°C MAT) in forest habitat similar to the other sites. The third site was created for its accessibility to an irrigation source, which the other sites lacked (requiring much more intensive efforts towards remote watering methods).
(b). Observational survey
Natural populations were sampled in 480 m2 grids divided into 4 m2 cells, and abundance was calculated as the number of plants per cell. The grid size and location were designed to assess population-level distributions across understory environmental gradients, which include heterogeneous soil moisture and light. The 4 m2 cells capture the scale at which ant-dispersed plants operate as the propagules are rarely dispersed at greater than 1 m yr−1 [38]. Plant abundance data (n = 2641 Hx. arifolia; n = 1573 Hp. nobilis) were collected at WHF in 2004.
Across the six survey grids, volumetric soil moisture percentage was measured per cell (five averaged points per cell) with a hand-held Hydrosense Soil Water Content Measurement System (Campbell Scientific, Inc., Logan, UT, USA). Per cent photosynthetically active radiation (PAR; wavelength: 400–700 nm) was calculated as the difference between the understory PAR readings at each cell and a fully exposed PAR reference site (diffuse light). The understory per cell measurements were taken with a 0.5 m hand-held AccuPAR ceptometer (Decagon Devices, Inc. Pullman, WA, USA) sequentially in a subset of 16, equally spaced cells within each grid and the open reference measurements were taken with an LI-200 spherical PAR sensor and logged with a LI-1400 datalogger (LiCor, Inc., Lincoln, NE, USA). Measurements were taken on cloudy days between 10.00 and 14.00 h to minimize relative error in diffuse light. For both the study plants, spring and summer (March, June 2004) soil moisture and diffuse light data best-predicted plant abundance, and was used here for analysis. Data collection on these grids for a larger ecological project (see [33,34,39]) indicated that additional environmental drivers, including soil organic matter, pH, total nitrogen, total carbon, phosphorus and carbon : nitrogen, did not provide the robust predictive power of soil moisture and diffuse light (data not shown).
(c). Experimental transplants
For adult transplants, 288 individuals of each species were collected in January 2006 at WHF (proximate to the observational survey populations). Only reproductive-sized individuals [34] were translocated. The plants were extracted with roots intact (maximum rooting depth = 12 cm; approx. digging depth = 20 cm) and transported in closed containers to experimental grids. At each site, we established six 35 m2 experimental grids at similar elevations and slope angles across north- and south-facing slopes. Two individuals of each species were placed at random in 1 m2 cells within these grids, leaving 1 m2 spaces between each cell. This gave 12 cells per grid, with each grid containing 24 individuals of each species. The transplants were placed in 12 cm2 circular holes during the month of collection, which is prior to the new leaf emergence. Overall then, we had 12 experimental grids (six at WHF and six at CWT), containing 144 cells with two transplants of each species, and therefore 576 experimental individuals. Transplants that died within the first 14 days were replaced. To measure recruitment success, seeds were collected from natural populations of Hx. arifolia (June 2006) and Hp. nobilis (March 2006) and placed in clusters of five in a nylon screen mesh held together by a plastic slide-mount. The slide-mounts were placed 2 cm deep so that the seeds rested just below the soil in a subset of eight cells in each experimental grid (n = 12) for a total of 480 seeds per species. However, because the seeds were planted in clusters, there were essentially 96 units and recruitment success was analysed as proportion surviving. The seeds were exposed to the same treatments as adults (see next).
(d). Experimental manipulation of light and water availability
Soil moisture was augmented by delivering water from two 200 l reservoirs via drip irrigation (Dripworks, Inc., Willits, CA, USA). Watered cells were always the lower (slope position) two rows of each grid, so gravitational flow would not contaminate unwatered cells. However, the maximum distance between watered and unwatered rows was 4 m, and all rows shared the same slope angle and position, so that the placement of watered rows, while systematic to the grid design, was not systematic to ambient environmental conditions. Approximately 70 l of water was delivered weekly per plot during May–August 2006. Because empirical data [40] suggest that high light levels inhibit understory evergreen herbs, we attenuated solar irradiation using custom-made, polyvinyl chloride frames (1 × 1 × 0.5 m), which were randomly assigned to half the plots in each grid. Black knitted 60 per cent neutral shadecloth (International Greenhouse Co., Georgetown, IL, USA) was placed over the top and partially down the sides of each frame. We also measured temperature, given that it can vary greatly across slope aspects. Specifically, A HOBO Pendent 64k datalogger (Onset Computer Co., Bourne, MN, USA) was placed at the soil surface in each experimental grid to record hourly surface temperatures (February 2006–February 2007). Four temperature loggers were placed beneath shadecloth treatments to monitor any temperature effects from light treatments.
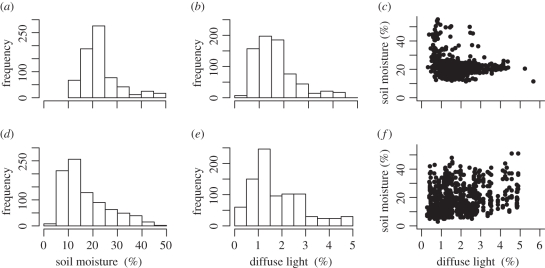
Water augmentation increased soil moisture (mean ± SD; range across grids) from 13.8 ± 5.8% (before watering) to 28.9 ± 8.5% (after watering), and shadecloth treatments reduced closed canopy diffuse light from 2.5 ± 1% (before shadecloth) to 1.0 ± 0.5% (after shadecloth). There was no indication that either treatment impacted conditions in non-target plots. The shade treatment also did not appear to impact mean temperature (22.0 ± 9.5°C shaded; 22.3 ± 7.3°C unshaded) or soil moisture (11.2 ± 4.3% shaded; 12.2 ± 4.7% unshaded). Mean soil moisture was higher in the observational populations (23.8 ± 8.9%) than in the experimental grids (17.6 ± 9.4%), while mean diffuse light was essentially the same between the observational populations (1.7 ± 0.9%) and the experimental grids (1.8 ± 1.1%; figure 1). The use of north- and south-facing grids and experimental manipulations successfully exposed transplants to relatively even gradients of soil moisture and diffuse light (figure 1).
Figure 1.
Frequency distributions of soil moisture and diffuse light values for (a,b) the observational survey and (d,e) experimental plots, along with scatterplots showing the relationship between soil moisture and diffuse light for (c) the observational survey and (f) experimental plots. The variance inflation factors for soil moisture and diffuse light in the observational and experimental models were less than 1.5, indicating they independently predict variance.
Transplants were surveyed the January following placement (i.e. in 2007). Survival was calculated per individual that survived from February 2006. Although augmentation of soil moisture and light attenuation was discontinued in February 2007, we observed across the first year of this experimental work that the fine-scale (slope aspect: north or south-facing) and broad-scale (site: WHF or CWT) locations themselves provided substantial variation in moisture and light availabilities. Hence, in January 2009, we surveyed the transplants a second time and calculated survival from those surviving since January 2007. Soil moisture was sampled within each cell (n = 144) in June and July 2007, January 2008 and April 2009; diffuse light was sampled in each cell (n = 144) in July and January 2007 and 2008.
(e). Disease experiments
Seeds were collected from natural populations of Hx. arifolia (June 2009) and Hp. nobilis (March 2009) and placed in clusters of 10 in a nylon screen mesh held together by a plastic slide-mount. Two 4 × 6 m grids (24.1 m2 plots per grid) were established in mature deciduous forest habitat, and the slide-mounts were placed 2 cm deep so that the seeds rested just below the soil in each plot (n = 48) for a total of 480 seeds per species. Seeds were exposed to two treatments: augmented watering delivered via drip irrigation from a non-chlorinated well and fungicide. Watering increased mean soil moisture in treatment plots from 19 ± 7 to 31 ± 7%. Half the seeds were dusted with 50 per cent Captan (hi-yield captan 50% wp fungicide, Arysta Lifescience North America, LLC, Cary, NC, USA), a chloroalkylthio fungicide, before being placed in slide-mounts. After planting, a 1 : 1000 dilution of Captan was applied to the planted slide mounts. Both water and fungicide treatments were delivered weekly between March–October 2009 and March–May 2010. The treatments were halted during November 2009–April 2010 owing to sub 0°C temperatures. The slide-mounts were retrieved and germinating seeds counted in May 2010. Whereas neither species is known to form a seed bank, we observed seeds that failed to germinate for intact embryos or other signs of viability but consistently found empty seed coats or partially decomposed seeds.
(f). Statistical analysis
The experimental design included several discrete treatments—slope aspect, watering, shading—but the interface between these treatments and the ambient environment resulted in continuous abiotic gradients across sites, grids and plots. The intensive environmental monitoring employed in this research allows the assessment of these combined conditions in creating microhabitat environments. We use these gradients to assess the response pattern of each species—the intricacies of which are lost in discrete analysis—to elucidate fundamental versus realized niche responses.
For the observational survey and transplant experiments, we employed a random-effects mixed model with grid and site as potential random effects, and soil moisture and diffuse light as fixed effects. In the fungal disease experiment, we used the same approach but site was the random effect, and watering and fungicide were fixed effects. Generalized linear models with the Poisson error distribution were used for abundance (observational survey) and the binomial error distribution for survival and recruitment success (both transplant experiments). Generalized linear mixed models (GLMMs) were fitted using the Laplace approximation in the ‘lme4’ package for the ‘R’ statistical program [41]. We evaluated the models based on the inclusion or exclusion of the fixed and random effects, second-order fixed effect terms (to account for unimodal responses) and interaction terms. Model selection was based on the Akaike information criterion (AIC), except when overdispersion was detected, in which case we used quasi-AIC (QAIC; [42]). Similar ‘best’ models (AIC < 2) were averaged to determine the relative AIC weights of the parameters. Collinearity was not high between the predictor variables (variance inflation factors < 1.5), indicating that they independently predict variance. Overdispersion occurred only in the Hx. arifolia abundance data, and this was accounted for by using the quasi-Poisson error distribution and QAIC.
3. Results
(a). Observational survey
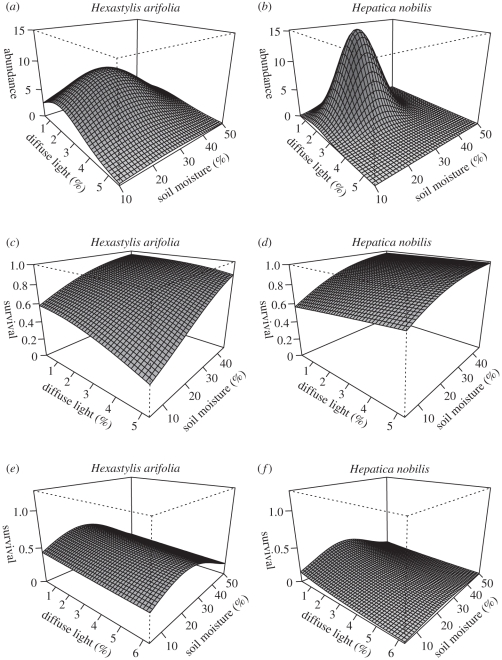
Strong statistical relationships were observed between abiotic variables and the abundance of both plant species at intermediate soil moisture and diffuse light levels (figure 2a,b). For both species, the best-fit abundance models included grid as a random effect and the significant first- and second-order terms for both fixed effects (abundance ∼ grid + soil moisture − soil moisture2 + diffuse light − diffuse light2), indicating unimodal abundance distributions (table 1). Specifically, Hx. arifolia and Hp. nobilis abundance peaked (>75th quartile) in 17–33% soil moisture, while Hx. arifolia abundance peaked at 1.6–3.2% diffuse light (figure 2a), and Hp. nobilis abundance peaked a little lower at 1.4–2.8% diffuse light (figure 2b).
Figure 2.
(a,b) Natural abundance, (c,d) adult transplant survival and (e,f) recruitment success for Hexastylis arifolia and Hepatica nobilis as functions of soil moisture (volumetric (%)) and diffuse light ((%) photosynthetically active radiation penetrating the forest canopy). The response surfaces are parametrized with the fitted coefficients from the regression models that best predicted Hx. arifolia and Hp. nobilis abundance in (a,b) natural populations, (c,d) adults transplanted into experimental plots and (e,f) seeds grown in experimental plots.
Table 1.
Fixed effects from generalized linear mixed models for Hexastylis arifolia and Hepatica nobilis (*p < 0.05; **p < 0.01; ***p < 0.001; †p < 0.1).
| model | fixed effects | estimate | s.e. | z-value |
|---|---|---|---|---|
| observational abundancea | ||||
| Hexastylis arifolia | intercept | 1.11 | 0.30 | 3.79*** |
| soil moisture | 0.21 | 0.05 | 4.59*** | |
| soil moisture2 | −8.17 | 1.11 | −7.37*** | |
| diffuse light | 0.39 | 0.12 | 3.14** | |
| diffuse light2 | −9.31 | 2.29 | −4.06*** | |
| Hepatica nobilis | intercept | 0.72 | 1.08 | 0.67 |
| soil moisture | 1.04 | 0.06 | 16.19*** | |
| soil moisture2 | −20.50 | 1.84 | −11.15*** | |
| diffuse light | 0.29 | 0.06 | 4.52*** | |
| diffuse light2 | −7.56 | 1.79 | −4.22*** | |
| experimental transplant survivalb | ||||
| Hexastylis arifolia | intercept | 1.10 | 0.28 | 3.98*** |
| soil moisture | 0.38 | 0.16 | 2.37* | |
| diffuse light | −0.01 | 0.19 | −0.03 | |
| Diffuse light2 | −5.73 | 2.94 | −1.95† | |
| Hepatica nobilis | intercept | 1.56 | 0.19 | 8.02*** |
| soil moisture | 0.42 | 0.19 | 2.24* | |
| diffuse light | 0.14 | 0.18 | 0.79 | |
| experimental recruitment successc | ||||
| Hexastylis arifolia | intercept | −3.75 | 1.43 | −2.62** |
| soil moisture | 0.11 | 0.05 | 1.99* | |
| soil moisture2 | −12.68 | 6.33 | −2.00* | |
| Hepatica nobilis | intercept | −7.30 | 2.46 | −2.97** |
| soil moisture | 0.26 | 0.12 | 2.17* | |
| soil moisture2 | −32.06 | 14.61 | −2.19* | |
| diffuse light | −0.41 | 0.22 | −1.88† | |
a2007 abundance in natural populations assuming a Poisson error distribution—Hx. arifolia. Quasi-Poisson error distribution, with t-value, owing to overdispersion.
b2007 adult survival in experimental plots assuming a binomial error distribution.
c2007 recruitment success in experimental plots assuming a binomial error distribution.
(b). Experimental adult transplants
The adult transplant survival models for both species exhibited positive, significant responses to increasing soil moisture (figure 2c,d and table 1). The diffuse light responses were weak and either marginally- or non-significant (figure 2c,d and table 1). Specifically, Hx. arifolia transplants responded negatively to increasing diffuse light, particularly at the highest levels, while Hp. nobilis had a non-significant, positive light response (table 1). Both Hx. arifolia and Hp. nobilis transplant survival was highest (>75th quartile) where soil moisture was greater than 30 per cent. Hexastylis arifolia survived best where diffuse light was less than 1.6 per cent (figure 2c), while Hp. nobilis survival was highest where diffuse light was greater than 3.8 per cent (figure 2d). Analysis of 2009 adult transplant survival models indicated no substantive change from 2007 in both the relative importance of soil moisture and the response pattern of plant survival (see electronic supplementary material, appendix A for the full GLMM table). This confirmed that the 2007 results reflected more than transplant shock, and indicated that the ambient gradients invoked the same response patterns as the experimental treatments. For both species, the best-fit models excluded the random site effect.
(c). Experimental seed transplants
Significant statistical relationships were observed between soil moisture and the recruitment success of both plant species, with survival peaking at intermediate soil moisture levels (figure 2e,f and table 1). Specifically, for both species, the best-fit recruitment models included grid and site as random effects and significant first- and second-order terms for soil moisture (recruitment ∼ grid + site + soil moisture − soil moisture2; table 1). The Hp. nobilis recruitment model also included a marginally significantly, negative diffuse light coefficient. Hexastylis arifolia recruitment success peaked (>75th quartile) in 23–33% soil moisture (figure 2e), while Hp. nobilis peaked somewhat lower in 17–26% soil moisture (figure 2f). Hexastylis arifolia recruitment was highest at less than 1.8 per cent diffuse light, while Hp. nobilis was slightly lower at less than 1.6 per cent.
The unimodal pattern observed in the recruitment models did not continue through 2009 seedling survival. In 2009, neither soil moisture nor diffuse light predicted Hx. arifolia seedling survival, and the second-order soil moisture term did not fit Hp. nobilis data (see electronic supplementary material, appendix A). Hexastylis arifolia had greater recruitment success (22.1 ± 17%) than Hp. nobilis (6.5 ± 6%) in 2007, but survival of the established seedlings to 2009 was the same for both species (61 ± 23%).
(d). Disease experiment
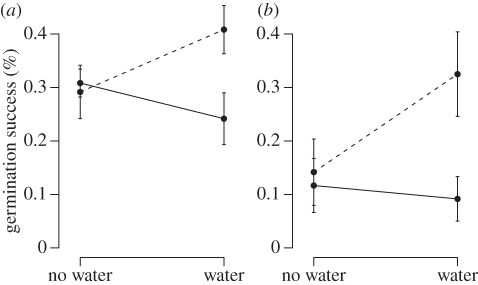
Significant treatment interaction effects indicated that watering only increased germination success when combined with fungicide (electronic supplementary material, appendix B and figure 3). Indeed, germination success for both species was highest in plots treated with both watering and fungicide (Hx. arifolia: 40.8 ± 16%; Hp. nobilis: 16.3 ± 14%) and lowest in those that only received augmented water (Hx. arifolia: 24.2 ± 17%; Hp. nobilis: 4.6 ± 7%).
Figure 3.
Interaction plots of the effects of water augmentation and fungicide treatments on (a) Hexastylis arifolia and (b) Hepatica nobilis germination success. Dashed lines, fungicide; solid line, no fungicide.
4. Discussion
Plant populations are dynamic, and individual life-history stages determine population trajectories and, ultimately, distribution patterns across the landscape [43]. These patterns may reflect intrinsic physiological tolerances, biotic interactions or both. Thus, correlations between species abundance and associated environmental drivers may reflect physiological requirements (e.g. drought tolerance) and/or biotic interactions (e.g. competition) at each ontogenetic stage.
We investigate adult and seed stages in determining the natural abundance patterns of two woodland herbs across soil moisture and diffuse light gradients. We achieve this by comparing the natural distributions to the survival of adult and seed transplants across the same environmental gradients extended via experimental manipulation. In natural populations, the abundance of both Hx. arifolia and Hp. nobilis peaks at intermediate levels of soil moisture and diffuse light, forming a unimodal response pattern (figure 2a,b). In these bell-shaped patterns, the natural abundance of both species declines as soil moisture approaches 20 per cent volume. However, when transplanted as adults, both species survive best at the highest levels of soil moisture—even when it is raised above the characteristic availability (up to 30–50% volume) by watering (figure 2c,d)—indicating no physiological limit in the adult stage. By contrast, recruitment success exhibits a unimodal soil moisture pattern similar to the surveyed, natural populations (figure 2e,f). Specifically, Hx. arifolia recruitment success peaked at intermediate soil moisture (23–33%), as did recruitment success for Hp. nobilis (17–26% moisture). A second experimental test of seed germination also shows lower success with augmented soil moisture; however, the application of fungicide reverses these trends.
The contrast between observed abundances in natural distributions, where the plants are absent from habitats with relatively high soil moisture (figure 2a,b), to survival experiments, in which even augmented soil moisture increases adult survival (figure 2c,d), appears to be a contraction from the fundamental to the realized niche. This suggests that biotic rather than physiological limitations prevent adult plants from occupying the wettest habitats in deciduous forests. Indeed, our fungicide experiment suggests that it is biotic interactions at the recruitment stage that causes the decline in natural herb abundance at higher soil moisture. Recruitment is generally thought to be a critical stage in plant population growth [20,28], yet it is commonly overlooked when coupling plant distributions with environmental variables [28]. Despite the demonstratively deleterious effect of soil fungal pathogens on seed survival [30,31], the impact of fungal attack on buried seeds in natural systems has received limited attention [31]. Notably, plant fungal pathogens are typically prevalent where soil moisture is high [29,31,32]. We find that Hx. arifolia and Hp. nobilis germination success decreases with watering (figure 2) but rebounds significantly with the addition of fungicides (figure 3). Adult evergreen plants typically have relatively high stress resistance [44], and we found no indication of biotic limitation to adult survival along experimental soil moisture gradients. This indicates that fungal pathogens buoyed by moist conditions may shape the abundance distributions of Hx. arifolia and Hp. nobilis in the recruitment stage.
The recruitment stage might also explain declines in natural abundance of both herbs at low soil moisture values. Although differences in seed and adult stages might be expected to be greatest in large stature species such as trees [45,46], Warren [23] showed that Hx. arifolia and Hp. nobilis adults and seedlings responded differently across slope aspects. This may be because perennial herbs are relatively long-lived (potentially centuries for Hp. nobilis [47]), and so the environmental conditions surrounding adults may vary from those experienced by seeds and seedlings. Further, newly emerged seedlings have diminutive roots and so may be far more sensitive to drought than adults, making soil moisture a major determinant of the regeneration niche [48,49]. Consequently, the high mortality during the transition from seed to seedling [26] might be a mechanism that helps explain the low abundance of the natural populations in drier conditions (figure 2). Specifically, we find that translocation does not release seeds from moisture limitations as recruitment success for both species peaked at intermediate soil moisture (figure 2). The most parsimonious explanations from our translocation studies is that natural herb distributions are a product of seedling physiological intolerance of low moisture, and biotic intolerance of fungal pathogens at high moisture.
Our study species are slow-growing, ant-dispersed, shade-tolerant woodland herbs which are, by all measures, competitively inferior in securing understory light [8,50]. Evergreen leaves are relatively advantageous in poor soils [51] but the requirements for maintaining leaves year round—e.g. slow growth, physiological inflexibility, diminutive height—may contain a trade-off with competitive ability at higher resource levels. Neither adult transplants nor recruitment success explains the observed diffuse light response pattern in the natural populations, and competition from co-occurring understory species remains a plausible, and testable, possibility.
Another avenue for further investigation is ant-mediated seed dispersal. Dispersal is an essential component in determining species distributions [43], and mutualisms may structure niche responses so that microhabitat distributions can be shaped as much by positive biotic interactions as negative [9]. Both Hx. arifolia and Hp. nobilis depend on ant-mediated seed dispersal [13,33], but this mutualism breaks down where soil moisture is high [13,33]. Thus, a lack of facilitation also may influence the declines in abundance of the understory evergreen herbs in wetter conditions. Although Warren et al. [13] found no relationship between ant-mediated seed dispersal and understory light, Smallwood [52] demonstrated that shading decreased emigration from seed-dispersing ants. This indicates that ant-mediated dispersal may also influence plant distributions along light gradients.
Moore & Elmendorf [53] suggest that observational studies can detect dispersal- versus propagule-limited distributions because dispersal-limited species will have weak habitat affinities owing to the inability to access the full range of abiotic gradients. Ant-dispersed herbs are strongly dispersal-limited [38], yet Hx. arifolia and Hp. nobilis show strong niche affinities for specific ranges of soil moisture and diffuse light in natural populations in contrast with the predictions of Moore & Elmendorf [53]. The apparent environmental limitations for Hx. arifolia and Hp. nobilis along soil moisture gradients seem driven by drought limitation at the low end and biological interactions at the high end. The niche model proposed by Connell [7] appears to be the best fit for our data. That is, abiotic factors are critical at the harsh end of environmental gradients while biotic interactions become important where the gradient is benign.
Our results are important for evaluating the likelihood that predictive models of plant distribution will yield reliable forecasts. Such models typically are parameterized using adult survey data, yet our data indicate that the species distributions reflect the regeneration more than adult niche of the two understory herbs that we consider. Moreover, species distribution models fail to incorporate biotic interactions, instead assuming that species are in equilibrium with their abiotic resource requirements [54–57]. Our experimental data suggest that biotic interactions—particularly seed fungal pathogens—better explain the plant niche patterning than abiotic responses. Indeed, the impact of resource availability on species interactions, and the impact of those interactions on populations and communities, remains a longstanding theoretical dilemma in ecology [58,59]. If biotic interactions between species remain constant across resource gradients, mapping the variables defining an organism's niche may provide a reasonable approximation of potential distributions. However, the nature and intensity of biotic interactions can vary with resource gradients [13,54,57]. This makes the omission of biotic responses problematic when predicting species distributions based on current patterns. Thus, if soil fungi always attack herbaceous seeds proportional to soil moisture levels, soil moisture can act as a proxy for the niche response. However, geographical variation in fungal species and abiotic interactions—possibilities not addressed within the bounds of this study—may undermine the proportionality. A caveat to such criticisms, however, is that species distributions at geographical scales are theorized to be more influenced by climate than biotic interactions [60,61], and efforts are being made to incorporate biotic interactions into prediction [62].
Our data suggest that the distribution of two ant-dispersed woodland herbs across resource gradients probably depends on both the recruitment niche and biotic interactions. We show that the natural abundance peaks at intermediate levels of diffuse light and soil moisture in mesic deciduous forests, and we demonstrate that this is best explained along the soil moisture gradients by fungal attack on buried seeds. The contraction of niche requirements from experimental survival to natural abundance suggests that the niche model proposed by Connell best describes the species' distributions. That is, physiological tolerances limit the species where resources are low and biotic interactions where resources are high. Within this framework, our data emphasize the need to consider recruitment stages and biotic interactions in delineating a species niche.
Acknowledgements
This research was supported by National Science Foundation grants to H. Ronald Pulliam and the Coweeta LTER Programme. We thank staff and administrators of the Coweeta Hydrological Laboratory and Whitehall Forest for access to the properties and for logistical support, particularly Mike Hunter, Jim Vose and Ted Gragson. The authors thank H. Ronald Pulliam, Lisa Donovan, Ron Hendrick Jr, Marc Van Iersel, Jeff Diez and Jeff Lake for manuscript suggestions.
References
- 1.Hutchinson G. E. 1957. Population studies—animal ecology and demography—concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 22, 415–427 [Google Scholar]
- 2.Austin M. P. 2002. Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecol. Model. 157, 101–118 10.1016/S0304-3800(02)00205-3 (doi:10.1016/S0304-3800(02)00205-3) [DOI] [Google Scholar]
- 3.Guisan A., Lehmann A., Ferrier S., Austin M., Overton J. M. C., Aspinall R., Hastie T. 2006. Making better biogeographical predictions of species' distributions. J. Appl. Ecol. 43, 386–392 10.1111/j.1365-2664.2006.01164.x (doi:10.1111/j.1365-2664.2006.01164.x) [DOI] [Google Scholar]
- 4.Bigelow S. W., Canham C. D. 2002. Community organization of tree species along soil gradients in a north-eastern USA forest. J. Ecol. 90, 188–200 10.1046/j.0022-0477.2001.00655.x (doi:10.1046/j.0022-0477.2001.00655.x) [DOI] [Google Scholar]
- 5.May R. M., MacArthur R. H. 1972. Niche overlap as a function of environmental variability. Proc. Natl Acad. Sci. USA 69, 1109–1113 10.1073/pnas.69.5.1109 (doi:10.1073/pnas.69.5.1109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittaker R. H. 1975. Communities and ecosystems. New York, NY: MacMillan [Google Scholar]
- 7.Connell J. H. 1975. Some mechanisms producing structure in natural communities: a model and evidence from field experiments. In Ecology and evolution of communities (eds Cody M. L., Diamond J. M.), pp. 460–490 Cambridge, MA: Harvard University Press [Google Scholar]
- 8.Keddy P. A. 2001. Competition. Dordrecht, The Netherlands: Kluwer [Google Scholar]
- 9.Bruno J. F., Stachowicz J. J., Bertness M. D. 2003. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125 10.1016/S0169-5347(02)00045-9 (doi:10.1016/S0169-5347(02)00045-9) [DOI] [Google Scholar]
- 10.Choler P., Michalet R., Callaway R. M. 2001. Facilitation and competition on gradients in alpine plant communities. Ecology 82, 3295–3308 10.1890/0012-9658(2001)082[3295:FACOGI]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[3295:FACOGI]2.0.CO;2) [DOI] [Google Scholar]
- 11.Pages J. P., Michalet R. 2006. Contrasted responses of two understorey species to direct and indirect effects of a canopy gap. Plant Ecol. 187, 179–187 10.1007/s11258-005-0976-x (doi:10.1007/s11258-005-0976-x) [DOI] [Google Scholar]
- 12.Bronstein J. L. 1989. A mutualism at the edge of its range. Experientia 45, 622–636 10.1007/BF01975679 (doi:10.1007/BF01975679) [DOI] [Google Scholar]
- 13.Warren R., Giladi I., Bradford M. A. 2010. Ant-mediated seed dispersal does not facilitate niche expansion. J. Ecol. 98, 1178–1185 10.1111/j.1365-2745.2010.01694.x (doi:10.1111/j.1365-2745.2010.01694.x) [DOI] [Google Scholar]
- 14.Connell J. H. 1961. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stallatus. Ecology 42, 710–723 10.2307/1933500 (doi:10.2307/1933500) [DOI] [Google Scholar]
- 15.Bronstein J. L., Wilson W. G., Morris W. E. 2003. Ecological dynamics of mutualist/antagonist communities. Am. Nat. 162, S24–S39 10.1086/378645 (doi:10.1086/378645) [DOI] [PubMed] [Google Scholar]
- 16.Brown J. H. 1995. Macroecology. Chicago, IL: University of Chicago Press [Google Scholar]
- 17.Chase J. M., Leibold M. A. 2003. Ecological niches: linking classical and contemporary approaches. Chicago, IL: University of Chicago Press [Google Scholar]
- 18.Pearman P. B., Guisan A., Broennimann O., Randin C. F. 2008. Niche dynamics in space and time. Trends Ecol. Evol. 23, 149–158 10.1016/j.tree.2007.11.005 (doi:10.1016/j.tree.2007.11.005) [DOI] [PubMed] [Google Scholar]
- 19.Caswell H. 2001. Matrix population models. Construction, analysis and interpretation. Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- 20.Grubb P. J. 1977. Maintenance of species-richness in plant communities: importance of regeneration niche. Biol. Rev. Camb. Phil. Soc. 52, 107–145 10.1111/j.1469-185X.1977.tb01347.x (doi:10.1111/j.1469-185X.1977.tb01347.x) [DOI] [Google Scholar]
- 21.Albrecht M. A., McCarthy B. C. 2009. Seedling establishment shapes the distribution of shade-adapted forest herbs across a topographical moisture gradient. J. Ecol. 97, 1037–1049 10.1111/j.1365-2745.2009.01527.x (doi:10.1111/j.1365-2745.2009.01527.x) [DOI] [Google Scholar]
- 22.Clark J. S., Macklin E., Wood L. 1998. Stages and spatial scales of recruitment limitation in southern Appalachian forests. Ecol. Monogr. 68, 213–235 10.1890/0012-9615(1998)068[0213:SASSOR]2.0.CO;2 (doi:10.1890/0012-9615(1998)068[0213:SASSOR]2.0.CO;2) [DOI] [Google Scholar]
- 23.Warren R. 2010. An experimental test of well-described vegetation patterns across slope aspects using woodland herb transplants and manipulated abiotic drivers. New Phytol. 185, 1038–1049 10.1111/j.1469-8137.2009.03147.x (doi:10.1111/j.1469-8137.2009.03147.x) [DOI] [PubMed] [Google Scholar]
- 24.Boege K., Marquis R. 2005. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol. Evol. 20, 441–448 10.1016/j.tree.2005.05.001 (doi:10.1016/j.tree.2005.05.001) [DOI] [PubMed] [Google Scholar]
- 25.Jarosz A. M., Davelos A. L. 1995. Effects of disease in wild plant-populations and the evolution of pathogen aggressiveness. New Phytol. 129, 371–387 10.1111/j.1469-8137.1995.tb04308.x (doi:10.1111/j.1469-8137.1995.tb04308.x) [DOI] [Google Scholar]
- 26.Fenner M., Kitajima K. 1999. Seed and seedling ecology. In Handbook of functional plant ecology (eds Pugnaire F. I., Valladares F.), pp. 589–611 New York, NY: Marcel-Dekker [Google Scholar]
- 27.Harper J. L. 1977. Population biology of plants. New York, NY: Academic Press [Google Scholar]
- 28.Moore K. A. 2009. Fluctuating patch boundaries in a native annual forb: the roles of niche and dispersal limitation. Ecology 90, 378–387 10.1890/07-1753.1 (doi:10.1890/07-1753.1) [DOI] [PubMed] [Google Scholar]
- 29.Agrios G. N. 2005. Plant pathology. San Diego, CA: Academic Press [Google Scholar]
- 30.Leishman M. R., Masters G. J., Clark I. P., Brown V. K. 2000. Seed bank dynamics and the role of fungal pathogens and climate change. Funct. Ecol. 14, 293–299 10.1046/j.1365-2435.2000.00425.x (doi:10.1046/j.1365-2435.2000.00425.x) [DOI] [Google Scholar]
- 31.Schafer M., Kotanen P. M. 2003. The influence of soil moisture on losses of buried seeds to fungi. Acta Oecol. Int. J. Ecol. 24, 255–263 10.1016/j.actao.2003.09.001 (doi:10.1016/j.actao.2003.09.001) [DOI] [Google Scholar]
- 32.Warren R. J., Mordecai E. 2010. Soil moisture mediated interaction between Polygonatum biflorum and leaf spot disease. Plant Ecol. 209, 1–9 10.1007/s11258-009-9713-1 (doi:10.1007/s11258-009-9713-1) [DOI] [Google Scholar]
- 33.Giladi I. 2004. The role of habitat-specific demography, habitat-specific dispersal, and the evolution of dispersal distances in determining current and future distributions of the ant-dispersed forest herb, Hexastylis arifolia. Athens, GA: University of Georgia; See http://coweeta.uga.edu/coweeta_publications_grad_desc.php [Google Scholar]
- 34.Warren R. J. 2007. Linking understory evergreen herbaceous distributions and niche differentiation using habitat-specific demography and experimental common gardens. Athens, GA: University of Georgia; See http://coweeta.uga.edu/coweeta_publications_grad_desc.php [Google Scholar]
- 35.Beattie A. J., Hughes L. 2002. Ant–plant interactions. In Plant–animal interactions: and evolutionary approach (eds Herrera C. M., Pellmyr O.), pp. 211–235 Oxford, UK: Blackwell Science [Google Scholar]
- 36.Flinn K. M., Vellend M. 2005. Recovery of forest plant communities in post-agricultural landscapes. Front. Ecol. Environ. 3, 243–250 10.1890/1540-9295(2005)003[0243:ROFPCI]2.0.CO;2 (doi:10.1890/1540-9295(2005)003[0243:ROFPCI]2.0.CO;2) [DOI] [Google Scholar]
- 37.Ness J. H., Morin D. F., Giladi I. 2009. Uncommon specialization in a mutualism between a temperate herbaceous plant guild and an ant: are Aphaenogaster ants keystone mutualists? Oikos 12, 1793–1804 10.1111/j.1600-0706.2009.17430.x (doi:10.1111/j.1600-0706.2009.17430.x) [DOI] [Google Scholar]
- 38.Cain M. L., Damman H., Muir A. 1998. Seed dispersal and the Holocene migration of woodland herbs. Ecol. Monogr. 68, 325–347 10.1890/0012-9615(1998)068[0325:SDATHM]2.0.CO;2 (doi:10.1890/0012-9615(1998)068[0325:SDATHM]2.0.CO;2) [DOI] [Google Scholar]
- 39.Diez J. M., Pulliam H. R. 2007. Hierarchical analysis of species distributions across environmental gradients. Ecology 88, 3144–3152 10.1890/07-0047.1 (doi:10.1890/07-0047.1) [DOI] [PubMed] [Google Scholar]
- 40.Warren R. J. 2008. Mechanisms driving understory evergreen herb distributions across slope aspects: as derived from landscape position. Plant Ecol. 198, 297–308 10.1007/s11258-008-9406-1 (doi:10.1007/s11258-008-9406-1) [DOI] [Google Scholar]
- 41.R Development Core Team 2005. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 42.Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H., White J. S. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 43.Pulliam H. R. 2000. On the relationship between niche and distribution. Ecol. Lett. 3, 349–361 10.1046/j.1461-0248.2000.00143.x (doi:10.1046/j.1461-0248.2000.00143.x) [DOI] [Google Scholar]
- 44.Westoby M., Falster D. S., Moles A. T., Vesk P. A., Wright I. J. 2002. Plant ecological strategies: some leading dimensions of variation between species. Ann. Rev. Ecol. Syst. 33, 125–159 10.1146/annurev.ecolsys.33.010802.150452 (doi:10.1146/annurev.ecolsys.33.010802.150452) [DOI] [Google Scholar]
- 45.Comita L. S., Condit R., Hubbell S. P. 2007. Developmental changes in habitat associations of tropical trees. J. Ecol. 95, 482–492 10.1111/j.1365-2745.2007.01229.x (doi:10.1111/j.1365-2745.2007.01229.x) [DOI] [Google Scholar]
- 46.Latham R. E. 1992. Co-occurring tree species change rank in seedling performance with resources varied experimentally. Ecology 73, 2129–2144 10.2307/1941461 (doi:10.2307/1941461) [DOI] [Google Scholar]
- 47.Inghe O., Tamm C. O. 1985. Survival and flowering of perennial herbs. IV. The behavior of Hepatica nobilis and Sanicula europaea on permanent plots during 1943–1981. Oikos 45, 400–420 10.2307/3565576 (doi:10.2307/3565576) [DOI] [Google Scholar]
- 48.Engelbrecht B. M. J., Dalling J. W., Pearson T. R. H., Wolf R. L., Galvez D. A., Koehler T., Tyree M. T., Kursar T. A. 2006. Short dry spells in the wet season increase mortality of tropical pioneer seedlings. Oecologia 148, 258–269 10.1007/s00442-006-0368-5 (doi:10.1007/s00442-006-0368-5) [DOI] [PubMed] [Google Scholar]
- 49.Moles A. T., Westoby M. 2004. What do seedlings die from and what are the implications for evolution of seed size? Oikos 106, 193–199 10.1111/j.0030-1299.2004.13101.x (doi:10.1111/j.0030-1299.2004.13101.x) [DOI] [Google Scholar]
- 50.Neufeld H. S., Young D. R. 2003. Ecophysiology of the herbaceous layer in temperate deciduous forests. In The herbaceous layer in forests of eastern North America (eds Gilliam F., Roberts M.), pp. 38–90 Oxford, UK: Oxford University Press [Google Scholar]
- 51.Givnish T. J. 2002. Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fennica 36, 703–743 [Google Scholar]
- 52.Smallwood J. 1982. The effect of shade and competition on emigration rate in the ant Aphaenogaster-Rudis. Ecology 63, 124–134 10.2307/1937038 (doi:10.2307/1937038) [DOI] [Google Scholar]
- 53.Moore K. A., Elmendorf S. C. 2006. Propagule vs. niche limitation: untangling the mechanisms behind plant species' distributions. Ecol. Lett. 9, 797–804 10.1111/j.1461-0248.2006.00923.x (doi:10.1111/j.1461-0248.2006.00923.x) [DOI] [PubMed] [Google Scholar]
- 54.Davis A. J., Jenkinson L. S., Lawton J. H., Shorrocks B., Wood S. 1998. Making mistakes when predicting shifts in species range in response to global warming. Nature 391, 783–786 10.1038/35842 (doi:10.1038/35842) [DOI] [PubMed] [Google Scholar]
- 55.Guisan A., Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 10.1111/j.1461-0248.2005.00792.x (doi:10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 56.Kearney M. 2006. Habitat, environment and niche: what are we modelling? Oikos 115, 186–191 10.1111/j.2006.0030-1299.14908.x (doi:10.1111/j.2006.0030-1299.14908.x) [DOI] [Google Scholar]
- 57.Pearson R. G., Dawson T. P. 2003. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371 10.1046/j.1466-822X.2003.00042.x (doi:10.1046/j.1466-822X.2003.00042.x) [DOI] [Google Scholar]
- 58.Agrawal A. A., et al. 2007. Filling key gaps in population and community ecology. Front. Ecol. Environ. 5, 145–152 10.1890/1540-9295(2007)5[145:FKGIPA]2.0.CO;2 (doi:10.1890/1540-9295(2007)5[145:FKGIPA]2.0.CO;2) [DOI] [Google Scholar]
- 59.Darwin C. 1859. The origin of species by means of natural selection or the preservation of favoured races in the struggle for life. London: UK: Murray; [PMC free article] [PubMed] [Google Scholar]
- 60.Hirzel A. H., Le Lay G. 2008. Habitat suitability modelling and niche theory. J. Appl. Ecol. 45, 1372–1381 10.1111/j.1365-2664.2008.01524.x (doi:10.1111/j.1365-2664.2008.01524.x) [DOI] [Google Scholar]
- 61.Soberon J. 2007. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123 10.1111/j.1461-0248.2007.01107.x (doi:10.1111/j.1461-0248.2007.01107.x) [DOI] [PubMed] [Google Scholar]
- 62.Araujo M. B., Luoto M. 2007. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743–753 10.1111/j.1466-8238.2007.00359.x (doi:10.1111/j.1466-8238.2007.00359.x) [DOI] [Google Scholar]