Abstract
Many spiders possess myrmecomorphy, and species of the jumping spider genus Myrmarachne exhibit nearly perfect ant mimicry. Most salticids are diurnal predators with unusually high visual acuity that prey on various arthropods, including conspecifics. In this study, we tested whether predation pressure from large jumping spiders is one possible driving force of perfect ant mimicry in jumping spiders. The results showed that small non-ant-mimicking jumping spiders were readily treated as prey by large ones (no matter whether heterospecific or conspecific) and suffered high attack and mortality rates. The size difference between small and large jumping spiders significantly affected the outcomes of predatory interactions between them: the smaller the juvenile jumping spiders, the higher the predation risk from large ones. The attack and mortality rates of ant-mimicking jumping spiders were significantly lower than those of non-ant-mimicking jumping spiders, indicating that a resemblance to ants could provide protection against salticid predation. However, results of multivariate behavioural analyses showed that the responses of large jumping spiders to ants and ant-mimicking salticids differed significantly. Results of this study indicate that predation pressure from large jumping spiders might be one selection force driving the evolution of nearly perfect myrmecomorphy in spiders and other arthropods.
Keywords: Batesian mimicry, Salticidae, myrmecomorphy, Myrmarachne
1. Introduction
Myrmecomorphy (ant mimicry) is a phenomenon in which organisms mimic ants morphologically and/or behaviourally, which is widely seen in various terrestrial arthropod taxa [1]. Ants are one of the most abundant organisms in terrestrial ecosystems and can be found in most habitats. They prey on almost everything, and their social behaviours, chemical defence, powerful mandible and unpleasant sting have made most animals avoid them [2]. Therefore, ants are regarded as a popular model in Batesian mimicry systems. Although all spiders are predators, most of them are small, soft-bodied arthropods without prominent physical or chemical defences. Therefore, many spiders are also the prey of other animals [3]. Many anti-predator devices have evolved in spiders to prevent spiders from being attacked, and Batesian mimicry is one common anti-predator device commonly seen in spiders [4]. In spiders, numerous species mimic other organisms in various ways, and in many of them (around 80%) ants are the models [5,6]. Myrmecomorphy has been described in more than 300 spider species belonging to 13 families and the majority is found among jumping spiders [5,7,8]. Myrmecomorphy is believed to have evolved convergently several times in families such as Salticidae and Clubionidae [9]. The function of ant mimicry in most spiders is considered as a case of Batesian mimicry, which prevents spiders from being attacked by their predators [1,10–16]. In Salticidae, myrmecomorphy is found in 14 genera, and among them Myrmarachne is the best studied genus [5]. Members of Myrmarachne are regarded as perfect ant mimics and are very similar to ants both morphologically and behaviourally.
Ant avoidance of many visual predators is generally regarded as the major driving force of spider myrmecomorphy. Oliveira [14] discussed several possible selection agents including vertebrate and invertebrate predation pressures that might cause the evolution of Batesian ant mimicry in spiders. Diurnal insectivorous vertebrates such as birds, reptiles and amphibians were assumed to be potential selective agents. In addition, Edmunds [10] showed that spider wasps use visual cues to hunt spiders; therefore, spider wasps have long been suggested as one major selection agent driving the evolution of ant mimicry in spiders [3,5,14,15]. However, for several reasons, we propose that predation pressure from spider wasps might not be the major driving force of ant mimicry in jumping spiders. First, although major spider wasp families such as Sphecidae and Pompilidae prey on spiders, most of their prey are web-building spiders rather than jumping spiders. A review by Blackledge et al. [17] reported that web-building spiders constituted about 76 per cent in spider wasps' diet. Secondly, constrained by structural and physical optical properties of compound eyes, the visual resolution and acuity of spider wasps are relatively low [18,19]. Therefore, it is unlikely that ant mimicry in jumping spiders, especially the perfect ant mimicry of Myrmarachne, is driven by organisms with such visual properties.
Jumping spiders are predators of small arthropods, including other spider families and jumping spiders themselves. Several jumping spiders (such as certain species of Portia) have a distinctive preference to prey on jumping spiders [20,21]. Jumping spiders regularly prey on other jumping spiders and about 5–10% of their diet is salticids [22–26]. Therefore, jumping spiders might potentially be the prey of jumping spiders themselves. Most jumping spiders, however, do not prey on ants. Instead, certain ants treat jumping spiders as potential prey. Some ants readily attacked and killed jumping spiders in laboratory conditions [27]. Halaj et al. [28] showed that ants could affect the spider communities (especially wandering spiders) in Douglas fir, and the abundance of jumping spiders was higher in trees without ants. Those studies indicate that certain ants are natural enemies of jumping spiders and resembling ants might gain protection against salticid predators.
In this study, we test the hypothesis that predation of jumping spiders is one potential driving force of ant mimicry in this taxa. Jumping spiders possess the best visual acuity among terrestrial arthropods with similar size [19,29]. Several behavioural studies also demonstrated that jumping spiders are able to use visual cues alone to distinguish different prey types [20,30–32] and to tell ant-mimicking jumping spiders from ants [33,34]. Therefore, this visual ability might have facilitated the evolution of perfect behavioural and morphological ant mimicry in jumping spiders. If jumping spiders readily prey on small jumping spiders but avoid ants of similar size, can ant mimicry of small jumping spiders provide protection from the predation of larger ones? In this study, we tested this hypothesis by asking the following questions. First, do large jumping spiders treat small ones as prey, no matter whether they are hetero- or conspecifics? Second, can ant mimicry of small jumping spiders provide protection from the predation of large ones? Third, does size difference among two interacting jumping spiders affect the predatory outcome? Based on the results of laboratory experiments, we demonstrate that jumping spiders themselves might be one driving force of the evolution of perfect ant mimicry in jumping spiders.
2. Material and methods
(a). Study organisms
In this study, three large jumping spider species Telamonia festiva, Ptocasius strupifer and Myrmarachne magnus were used as predators. These species are commonly seen in Taiwan and are usually sympatric with ant-mimicking jumping spiders and ants. Telamonia festiva and P. strupifer are non-ant-mimicking predators and in the field they do not seem to attack ants (J. N. Huang 2009, personal observations). Myrmarachne magnus are large ant-mimicking jumping spiders that do not prey on ants but seem to stay close to their model ants Polyrhachis dives (J. N. Huang, personal observations). All the predators used were adult or subadult females with body length ranging from 6 to 10 mm (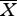 ± s.e. = 10.58 ± 1.07 for T. festiva; 6.92 ± 0.85 for P. strupifer and 8.63 ± 0.89 for M. magnus). In this study, we used the following four types of prey: non-ant-mimicking jumping spiders (further divided into conspecifics and heterospecifics), ant-mimicking jumping spiders, ants and flies (table 1). Conspecific non-ant-mimicking jumping spiders were the juvenile individuals of predator species used. Heterospecific jumping spiders were the juvenile individuals of species other than the predators. The non-ant-mimicking jumping spider prey species used in this study were the juvenile individuals of T. festiva, P. strupifer, Hasarius adansoni, Menemerus fulvus and Evarcha crassipes. Ant-mimicking jumping spider prey used in this study were the juveniles of M. magnus. The ant prey used were the workers of P. dives. House flies Musca sp. were used as fly prey. We collected the spiders by sweep-netting or by hand from the fields in southern Taiwan. All the spiders were reared individually in plastic tubes (diameter 2.7 cm, length 9.5 cm) in the laboratory in Tunghai University, Taiwan. The laboratory was kept at 25 ± °C and 12 L : 12 D light condition. The spiders were fed three to four fruitflies (Drosophila melanogaster) twice per week. In addition, sugar water placed in a micro-centrifuge vial (300 µl) was given occasionally. To ensure that the predators were hungry enough, they were kept without food for 4 days before the tests were conducted. Ants and house flies were collected as needed on the campus of Tunghai University and were fed sugar water during the experimental period.
± s.e. = 10.58 ± 1.07 for T. festiva; 6.92 ± 0.85 for P. strupifer and 8.63 ± 0.89 for M. magnus). In this study, we used the following four types of prey: non-ant-mimicking jumping spiders (further divided into conspecifics and heterospecifics), ant-mimicking jumping spiders, ants and flies (table 1). Conspecific non-ant-mimicking jumping spiders were the juvenile individuals of predator species used. Heterospecific jumping spiders were the juvenile individuals of species other than the predators. The non-ant-mimicking jumping spider prey species used in this study were the juvenile individuals of T. festiva, P. strupifer, Hasarius adansoni, Menemerus fulvus and Evarcha crassipes. Ant-mimicking jumping spider prey used in this study were the juveniles of M. magnus. The ant prey used were the workers of P. dives. House flies Musca sp. were used as fly prey. We collected the spiders by sweep-netting or by hand from the fields in southern Taiwan. All the spiders were reared individually in plastic tubes (diameter 2.7 cm, length 9.5 cm) in the laboratory in Tunghai University, Taiwan. The laboratory was kept at 25 ± °C and 12 L : 12 D light condition. The spiders were fed three to four fruitflies (Drosophila melanogaster) twice per week. In addition, sugar water placed in a micro-centrifuge vial (300 µl) was given occasionally. To ensure that the predators were hungry enough, they were kept without food for 4 days before the tests were conducted. Ants and house flies were collected as needed on the campus of Tunghai University and were fed sugar water during the experimental period.
Table 1.
Organisms used in this study, their roles in the experiments and acronyms.
| acronyms | roles in experiments | organisms used |
|---|---|---|
| CJ | conspecific non-ant-mimicking jumping spider prey | T. festiva, P. strupifer, M. magnus |
| HJ | heterospecific non-ant-mimicking jumping spider prey | T. festiva, P. strupifer, M. magnus, H. adansoni, M. fulvus, E. crassipes |
| AJ | ant-mimicking jumping spider prey | M. magnus |
| A | ant prey | P. dives |
| F | fly prey | Musca sp. |
(b). Experimental design and analysis
(i). Do large jumping spiders treat small ones as prey?
In this part of the study, we tested whether large jumping spiders readily preyed on small non-ant-mimicking jumping spiders no matter whether they were con- or heterospecifics. Adult or subadult females of T. festiva, P. strupifer and M. magnus (body length greater than 7 mm) were used as predators. The juvenile conspecific and heterospecific non-ant-mimicking jumping spiders were used as prey (abbreviated as CJ and HJ, respectively; table 1). When the predators were M. magnus only HJ prey were used, because in this predator the ant-mimicking jumping spider prey were also CJ prey. In each 10 min test period, one predator interacted freely with one prey. The test arena comprised a Petri dish (diameter 14 cm × height 1.5 cm) with a plastic tube (diameter 1 cm × height 3 cm) on the top to introduce the prey. To quantify the relative size of predator and prey, the prey/predator size-difference index R was calculated using the following equation:
where Py and Pd are the body length of prey and predators, respectively. A large R-value meant that the prey/predator size difference was large. To standardize the test conditions, the R-values of various prey and predator pairs were set to range from 0.4 to 0.5. Before the test, the chosen predators and prey were anaesthetized by CO2 and then photographed by a digital camera (Ricoh R8, Ricoh Co., Ltd, Japan) with a grid paper as background. The digital images were used to measure their body length with ImageJ software (ImageJ 1.42q, National Institutes of Health, USA). After being photographed, the predator was placed into the Petri dish directly and the prey was placed into the plastic tube. A piece of cardboard was inserted on the bottom of the tube and a cotton swab was used to block the top of the tube to prevent the prey from escaping. When the predator revived from anaesthetization, it was allowed to habituate to the test arena for 10 min. The behavioural test began when the cardboard was removed and the prey was then gently pushed by the cotton swab to drop into the test arena. The test lasted 10 min or was terminated when the predation event occurred. If no attack or predation occurred during the test period, the original prey was removed and a house fly prey was then introduced into the arena for another 10 min to check the hunting motivation of the predator. If the predator also showed no interest in the house fly, the event was discarded from the analysis. All the tests were recorded by a digital video camera (Sony DCR-SR100) for later behavioural analysis. All predator and prey spiders were used only once. For each predator species, the sample size was 30. After all the 30 tests were completed, we counted how many prey were eaten by predators and the mortalities of all prey types were calculated. We also estimated the rate of attacks experienced by all prey types by viewing the video footage. The attack rate was defined as the proportion of prey being attacked by predators. Chi-square tests for independence were performed to test whether the mortality and attack rates of CJ and HJ prey differed significantly.
(ii). Can ant mimicry provide protection from predation?
In this part of the study, we tested whether ant mimicry could reduce the attack and mortality rates of small ant-mimicking jumping spiders when they interacted with large jumping spiders. We used juvenile ant-mimicking jumping spiders (M. magnus) and ants (P. dives) as prey (abbreviated as AJ and A; table 1). The R-values of prey and predator pairs were also set to range between 0.4 and 0.5. The test procedures were similar to those of the previous experiment. All the tests were also videotaped for later behavioural analysis. The attack and mortality rates of A and AJ prey were compared with each other and with those of HJ and CJ prey using χ2-tests for independence.
(iii). Predators' behavioural responses to different prey
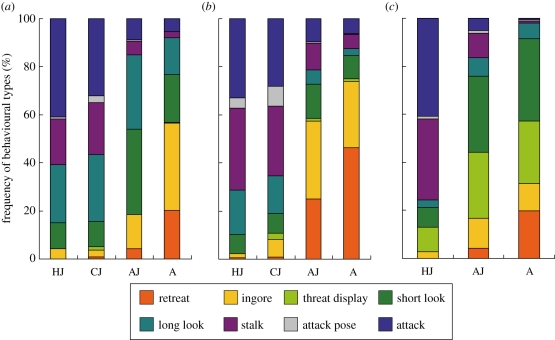
To determine whether jumping spider predators adopt different behaviours when encountering different prey types, we analysed the predators' responses recorded during the tests by video cameras. The predators' behavioural responses while encountering prey could be categorized into eight types (table 2). ‘Retreat’ is defined as when, on encountering the prey, the predator changes its direction of motion immediately or moves backwards right away to avoid it. If the predator does not change its direction of motion or does nothing when encountering the prey, this behaviour is designated as ‘ignore’. ‘Threat display’ is the predator raising its front legs to warn the prey. ‘Short look’ is defined as the predator looking at the prey briefly (for less than 5 s) and then leaving without doing anything. If the predator looks at the prey for more than 10 s without doing anything, that behaviour is designated as ‘long look’. ‘Stalk’ is a specific predation behaviour of the jumping spider in which the predator follows the prey or approaches the prey slowly. When the predator stops and pulls all legs close to its body, such a condition is defined as ‘attack pose’. This behaviour is jumping spiders' preparation pose for attacking prey from a certain distance. ‘Attack’ is defined as the predator attacking the prey. While viewing the video footages, we recorded the number of these eight behavioural types performed by the predators during the test period. To explore whether the behavioural compositions of predators' responses to various prey types differed, multivariate analyses were performed using the PRIMER 5 program [35]. The PRIMER routine is originally designed to analyse multivariate biodiversity data. Such data are characterized by having numerous species each with different abundance in various replicates. We consider behavioural compositions of various predator responses as being similar to species compositions of various habitats, so that the PRIMER routine can be applied. The basic principle of this routine is to use species composition of replicates (considering the abundance of each species) to calculate between-replicate similarities, then use such data to assess relationships between replicates in different habitats. We can treat behavioural type as species and use frequencies of designated behavioural types to calculate similarities between different predator responses. In our analysis, first the Bray–Curtis similarities between each pair of predator behavioural responses were calculated using the frequencies of eight behavioural types. The similarity data matrix was then ranked. All subsequent analyses were based upon such ranking, and therefore no assumption about the data distribution pattern was needed. The analysis of similarities (ANOSIM [35]) function was used to test whether jumping spider predators' behavioural responses to different prey types differed significantly. If any significant difference in predators' responses to different prey types was detected, then an analysis of similarity percentages (SIMPER function [35]) was conducted to determine the major behavioural types that were responsible for the observed behavioural response differences.
Table 2.
The types of behavioural responses of jumping spider predators to prey, their definitions and abbreviations.
| types | definition |
|---|---|
| retreat (Re) | change moving direction immediately or avoid when encountering prey |
| ignore (Ig) | ignore or pass when encountering prey |
| threat display (TD) | adopt threat display when encountering prey |
| short look (SL) | stop and fix its eyes on prey then leave |
| long look (LL) | fix its eyes on prey more than 10 s |
| stalk (St) | adopt stalking behaviour but does not adopt attack posture |
| attack pose (AP) | adopt the attack posture but does not attack |
| attack (At) | attack the prey |
(iv). Does prey/predator size difference affect prey survival?
In this part of the study, how prey/predator size difference affected the outcome of their interactions was tested. The predators used here were also adult and subadult individuals of T. festiva, P. strupifer and M. magnus, with body size ranging from 0.6 to 1.2 cm. Prey were the juveniles of HJ (T. festiva, P. strupifer, H. adansoni, M. fulvus, E. crassipes), with size ranging from 0.2 to 1.0 cm. Predators and prey were randomly selected from laboratory populations. We paired large and small jumping spiders according to their body length and created the following five R-value (size difference) groups: less than 0.3, 0.3–0.39, 0.4–0.49, 0.5–0.59 and greater than 0.6. The test procedures were identical to the aforementioned experiments, but the behavioural responses of predators were not videotaped. We recorded the survivorship of prey after 10 min of free interaction and calculated the mortality rate of each size-difference group. Logistic regression was used to test whether size difference significantly affected the interaction outcomes of large and small jumping spiders. The risk odds ratio was estimated to determine how the increase in mortality followed that of size differences.
(v). Simultaneous choice test
We performed simultaneous choice tests to reveal whether salticid predators preferred to prey on juvenile non-ant-mimicking jumping spiders (palatable prey) more than on ants and ant-mimicking jumping spiders (dangerous models and mimics). Large salticid predators were given three prey spiders simultaneously (ants, ant-mimicking and ordinary juvenile jumping spiders), so we could evaluate predators' responses to them. The R-values of prey and predator were set to range between 0.4 and 0.5. Different types of prey used in each test were similar in size (body length differences less than 0.5 mm) and were simultaneously presented to the predator T. festiva. The testing arena was a plastic box (15 cm in diameter and 8.5 cm in height) with three plastic tubes (1 cm in diameter and 3 cm in height) on top of the cover. The predator and prey were first anaesthetized by CO2 and then the three prey types were placed in tubes, respectively. In each tube, there was a piece of cardboard below and cotton swab on the top to prevent prey from falling into or escaping the test arena. The predator was placed into the arena directly and was allowed to habituate to the test arena for 10 min after it recovered from anaesthetization. After habituation, the cardboard was removed and three prey items were pushed gently into the test arena simultaneously. The test started when the three prey items entered the test arena. The prey and predator interacted freely for 30 min, and we recorded which prey was attacked and consumed during the test period. A total of 30 trials were performed, and the predation rates of the three prey types were compared by a χ2-test for goodness of fit to detect whether a predator exhibited a preference for a particular prey type.
3. Results
(a). Mortality and attack rate of different prey types
When large jumping spider predators encountered non-ant-mimicking jumping spiders, they readily launched the attack. In all three species of jumping spider predators, more than 80 per cent of individuals would launch at least one attack attempt on non-ant-mimicking jumping spiders, no matter whether they were con- or heterospecifics (figure 1 and table 3). In all three jumping spider predator species, the attack rate on non-ant-mimicking jumping spiders was significantly higher than that on A and AJ prey (figure 1 and table 3). Three jumping spider predators differed in their attack rates on A and AJ prey. In P. strupifer and T. festiva, the attack rates on ants and ant-mimicking jumping spiders did not differ significantly (figure 1a,b and table 3a,b). However, the attack rate of M. magnus on A differed significantly from that on AJ prey (figure 1c and table 3c). In general, for a particular prey type, the mortality rate it experienced was lower than the attack rate because not all attack attempts would lead to a successful kill. The mortality rates of non-ant-mimicking jumping spiders (no matter whether they encountered con- or heterospecific predators) were significantly higher than those of A and AJ prey (figure 1 and table 3). When the predators were T. festiva, no matter whether they interacted with CJ or HJ prey, the mortality rates were similar (figure 1b and table 3b). However, when the predators were P. strupifer, they caused a significantly higher mortality rate in HJ than in CJ prey (figure 1a and table 3a). The mortality rates of A and AJ prey caused by P. strupifer did not differ significantly (figure 1a and table 3a). Although the attack rates of T. festiva on A and AJ prey were similar, this predator caused a significantly higher mortality rate in the latter (figure 1b and table 3b). The mortality rate of AJ prey was significantly higher than that of A prey when the predator was M. magnus (figure 1c and table 3c).
Figure 1.
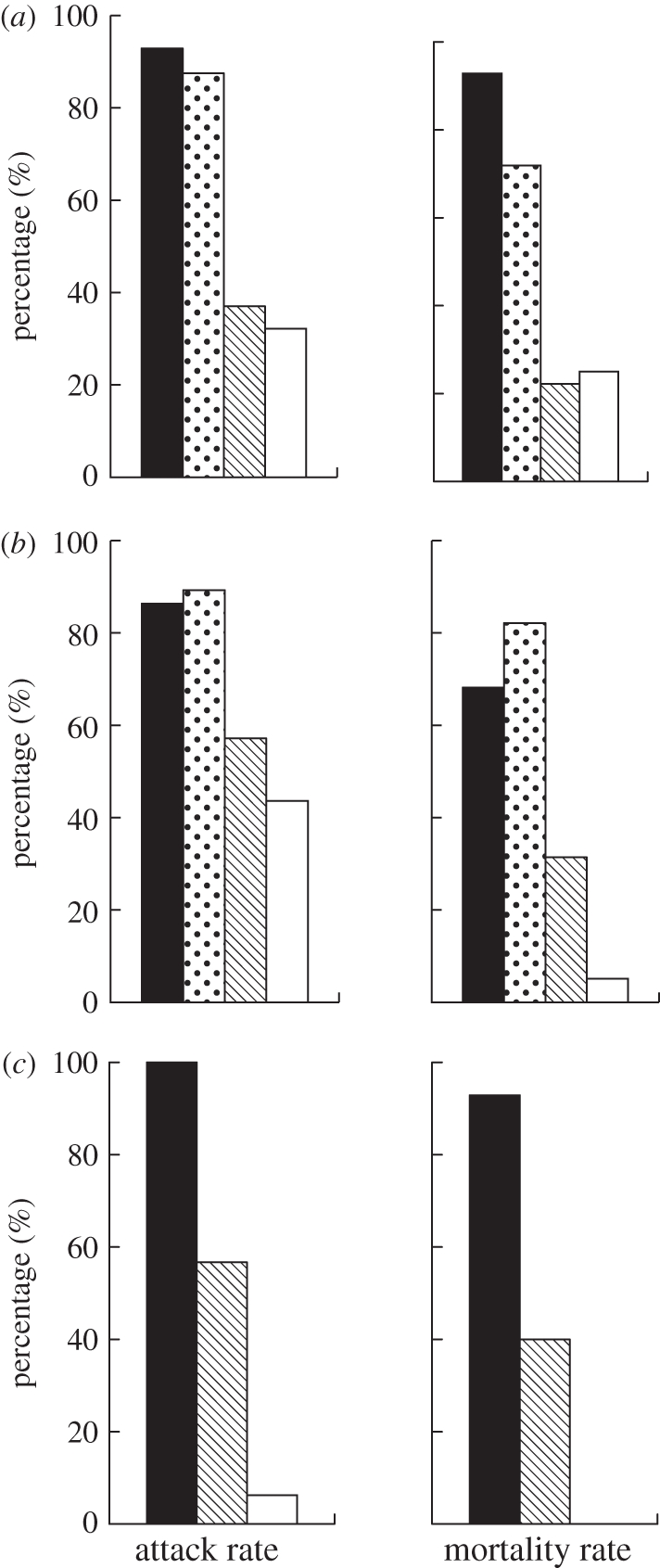
The attack rate (percentages of prey receiving at least one attack) and mortality of four prey types interacting with large jumping spiders (a) P. strupifer, (b) T. festiva and (c) M. magnus. (HJ, heterospecific non-ant-mimicking jumping spiders; CJ, conspecific non-ant-mimicking jumping spiders; AJ, ant-mimicking jumping spiders; A, ants). Black bars, HJ; dotted bars, CJ; striped bars, AJ; white bars, A.
Table 3.
Results of χ2-tests for independence comparing attack rates (upper diagonal) and mortality rates (lower diagonal) of different types of prey interacting with three species of large jumping spider predators (HJ, heterospecific non-ant-mimicking jumping spider prey; CJ, conspecific non-ant-mimicking jumping spider prey; AJ, ant-mimicking jumping spider prey; A, ant prey; n.s., non-significant at the α = 0.05 level).
| HJ | CJ | AJ | A | |
|---|---|---|---|---|
| (a) P. strupifer | ||||
| HJ | — | 0.468n.s. | 18.597*** | 21.626*** |
| CJ | 4.318* | — | 15.387*** | 19.036*** |
| AJ | 27.674*** | 14.201*** | — | 0.143n.s. |
| A | 26.159*** | 12.906*** | 0.058n.s. | — |
| (b) T. festiva | ||||
| HJ | — | 0.132n.s. | 8.421** | 16.717*** |
| CJ | 1.693n.s. | — | 7.75** | 14.333*** |
| AJ | 10.415** | 15.849*** | — | 1.337n.s. |
| A | 34.279*** | 40.712*** | 8.692** | — |
| (c) M. magnus | ||||
| HJ | — | 15.369*** | 51.625*** | |
| AJ | 17.601*** | — | 18.219*** | |
| A | 51.563*** | 15.616*** | — | |
* p < 0.05.
** p < 0.01.
*** p < 0.001.
(b). Predators' behavioural responses to different prey types
When large jumping spider predators encountered different prey types, they not only caused different mortality rates, but also adopted quite different behavioural responses. This could be reflected by the variations in relative frequencies of eight behavioural components in predators' responses to different prey types (figure 2). Results of ANOSIM global tests showed that in each salticid predator, their responses to different prey types differed significantly (table 4). Further pair-wise ANOSIM tests showed that for all three predator species, their responses to four prey types were significantly different. The only exceptions were the responses of P. strupifer (table 4a) and T. festiva (table 4b) to CJ and HJ prey. Results of SIMPER tests showed that the percentages of dissimilarity between most pair-wise comparisons were larger than 60 per cent, except those between CJ and HJ prey (table 4). The major behavioural components responsible for the observed variations in predators' responses differed from species to species. In T. festiva, the major contributors to the observed differences in responses to A and AJ prey were retreat and ignore. However, in M. magnus, the major contributors were ‘long look’ and ‘threat display’. In P. strupifer, the major contributors varied among different pair-wise comparisons, indicating that salticid predators adopted quite versatile behavioural responses to different prey types. It is traditionally believed that ant mimicry can effectively protect myrmecomorphic arthropods because the predators treat these organisms as ants. If this is the case, we would expect the predators to respond similarly to A and AJ prey. However, large salticids used in this study responded differently from A and AJ prey, indicating that at least in some salticid predators they do not simply treat Myrmarachne juveniles as ants and therefore reduce their attacks. Large salticid predators might be confused by the ant-like morphology and/or behaviours of small Myrmarachne spiders and consequently adopt a response different from that to ants or ordinary salticid prey. Congruent with such a hypothesis, the higher frequencies of ‘short look’ and ‘long look’ behaviours performed by P. strupifer and T. festiva when they faced AJ prey indicated that they spent more time inspecting this prey type.
Figure 2.
Relative frequencies (in %) of behavioural types of three jumping spider predators' responses to different prey types: (a) P. strupifer, (b) T. festiva and (c) M. magnus (HJ, heterospecific non-ant-mimicking jumping spider; CJ, conspecific non-ant-mimicking jumping spider; AJ, ant-mimicking jumping spider; A, ant). See table 2 for definitions of various behavioural types.
Table 4.
Results of ANOSIM tests comparing the responses of jumping spider predators to different prey types. For those significant pair-wise comparisons, the results of SIMPER tests are provided. See table 2 for abbreviations of behavioural types. (A, ants; AJ, ant-mimicking jumping spiders; CJ, conspecific non-ant-mimicking jumping spiders; HJ, heterospecific non-ant-mimicking jumping spiders; n.s., non-significant at the α = 0.05 level).
|
R-values of ANOSIM |
% of dis-similarity | major contributors of observed variation (in %) | |
|---|---|---|---|
| (a) P. strupifer | |||
| global R | 0.339*** | ||
| A, AJ | 0.176*** | 62.02 | SL (24.6), Ig (22.1), LL (21.4) |
| A, CJ | 0.478*** | 76.64 | Ig (30.7), SL (16.2), Re (16) |
| A, HJ | 0.481*** | 76.38 | Ig (30.8) SL (16.1), Re (16.1) |
| AJ, CJ | 0.457*** | 75.55 | SL (30.7), LL (25.1), Ig (14.2) |
| AJ, HJ | 0.477*** | 75.90 | SL (30.4), LL (25), At (15.3) |
| HJ, CJ | −0.028n.s. | 53.54 | — |
| (b) T. festiva | |||
| global R | 0.396*** | ||
| A, AJ | 0.117*** | 62.67 | Re (35.4), Ig (25.8), SL (11.5) |
| A, CJ | 0.685*** | 81.70 | Re (35.2), Ig (24.4), At (10.9) |
| A, HJ | 0.777*** | 85.45 | Re (36), Ig (25.8), At (10.2) |
| AJ, CJ | 0.201*** | 69.98 | Ig (26.6), Re (18.5), At (13.3) |
| AJ, HJ | 0.371*** | 71.48 | Ig (27.8), Re (19.3), SL (13.2) |
| HJ, CJ | 0.037n.s. | 49.83 | — |
| (c) M. magnus | |||
| global R | 0.449*** | ||
| A, AJ | 0.116*** | 67.20 | SL (24.9), TD (23.9), Re (15.6) |
| A, HJ | 0.846*** | 89.61 | SL (24.9), TD (18.4), Re (14.6) |
| AJ, HJ | 0.386*** | 72.74 | SL (25.8), TD (22.2), St (14.7) |
***p < 0.001.
(c). Effect of prey/predator size difference on mortality
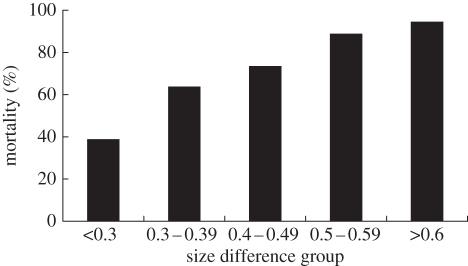
The mortality rate of non-ant-mimicking jumping spiders seemed to be strongly affected by prey/predator size differences. Results of logistic regression showed that mortality rates of prey in different prey/predator size-difference groups differed significantly. When prey and predator size difference was large, the non-ant-mimicking jumping spider prey suffered higher mortality from large jumping spider predators. The mortality more than doubled between the first (<0.3) and the last (>0.6) size-difference group (figure 3; odds ratio = 2.15; logistic regression, p < 0.001). The higher the prey/predator size difference, the greater the mortality rate of non-ant-mimicking jumping spiders.
Figure 3.
Mortality of non-ant-mimicking jumping spider prey in various size-difference groups (according to size-difference index R-values) while interacting with a large jumping spider T. festiva.
(d). Jumping spiders' preference for co-occurring prey types
When ant, ant-mimicking jumping spider and non-ant-mimicking jumping spider prey were simultaneously presented to a large T. festiva predator, usually the non-ant-mimicking jumping spiders were attacked first. They were attacked and consumed by large T. festiva in 25 out of 30 trials. An ant was never preyed upon by T. festiva in the simultaneous choice tests, while in five out of 30 trials, an ant-mimicking jumping spider was attacked and consumed. Telamonia festiva showed a significant preference for non-ant-mimicking jumping spider prey when presented with three prey types simultaneously (χ2-test for goodness of fit: χ2 = 23.86, p < 0.001, n = 30).
4. Discussion
Our findings indicate that ant resemblance can protect early-life-stage small jumping spiders from predation by large ones. In general, the large jumping spiders avoided ant-mimicking juveniles as they did with ants but readily preyed on con- or heterospecific small non-ant-mimicking juveniles. Ants are the major predators of many arthropods (including spiders) in tropical areas [2,10]. Ants significantly affect the spider communities (especially salticids [28]), and jumping spiders have been demonstrated to exhibit innate ant avoidance [33]. Only a few jumping spider species prey on ants, and ant mimicry is evidently able to provide protection against salticid predators. Our results also show that the size difference among two interacting jumping spiders will significantly affect the predatory outcomes. The larger the size difference, the greater the mortality of smaller ones. Such results indicate that smaller jumping spiders suffer high predation risk from larger ones. If a juvenile jumping spider can grow and survive into the next stage, it will be the potential predator of the earlier stages. Therefore, the juveniles of the early life stages will suffer higher predation risk than latter stages and the selection pressure will be very high in early-stage individuals. For this reason, we hypothesize that at least in some members of salticids the advantage of ant mimicry might be higher at early life stages. However, we cannot exclude the fact that in other salticids ant mimicry may benefit adults in contexts such as aggressive mimicry [1,5,36,37].
Since large salticid predators responded differently to A and AJ prey, being treated as ants by large salticids does not seem to be the major reason for the lower attack rate and mortality of small jumping spiders with ant-like appearance. Therefore, what might be the underlying mechanisms for the lower mortality of AJ prey? It is possible that AJ prey were unpalatable to jumping spiders, so they experienced a low attack rate. However, in the present study, the few AJ prey that were attacked by large ones were always consumed, as were other ordinary prey. This finding suggests that the ant-mimicking jumping spiders are palatable prey for large jumping spiders. Nelson & Jackson [33] also showed that although the jumping spiders displayed innate avoidance of ant and ant-mimicry jumping spiders, the latter were still acceptable prey. Another potential reason for the lower mortality of AJ prey might be their slender appendages and consequently more agile movements. The slender ant-like appearance and thin legs make ant-mimicking jumping spiders more agile than ordinary ones and therefore they might have better escaping ability. In this study, however, we used a small arena to conduct the experiments. In such a confined area, there was not enough space to allow the spiders to fully exercise their escaping ability and large spiders could easily catch the prey. The fact that ant-mimicking jumping spiders still had lower attack and mortality rates indicates that the higher survival was not related to a better escaping ability. We suggest that behavioural mimicry might play an important role in the anti-predation strategy of ant-mimicking jumping spiders. Ant-mimicry arthropods usually possess ant-like behaviours to enhance their morphological resemblance of ants. The behavioural mimicry of ant-mimicry spiders includes traits such as the ant-like zig-zag walking styles and the ‘antennal illusion’, in which the spiders wave their first or second pair of legs in the air to mimic the antenna of ants [38]. Ceccarelli [39] showed that the function of the ‘antennal illusion’ of ant-mimicking jumping spiders Myrmarachne might be regarded as a Batesian behavioural mimicry. Moreover, when ant-mimicking jumping spiders encountered large jumping spiders or were disturbed, they usually adopted a specific display posture resembling the threat display of ants (reviewed by [5]). This behaviour was also recorded in our study. The AJ prey usually faced the large jumping spiders and displayed the ant-like threat displays, unless the predators moved too close. In contrast, CJ and HJ prey did not exhibit such a display and usually ran away at once when encountering the predators. This phenomenon indicates that threat display might play an important role in the anti-predator strategy of ant-mimicking jumping spiders. However, we can not exclude the possibility that chemical cues might be involved in this system. Many jumping spiders are known to make considerable use of chemical cues [40–42]. Chemical cues from A and AJ prey might be different, thus salticid predators exhibited different behaviours against them based on the prey-specific chemical cues. Further study is needed to confirm such a proposition.
The nearly perfect morphological and behavioural ant resemblance in jumping spiders suggests that the selective agents involved in the evolutionary process must have good visual acuity. In the field, several potential predators with good visual abilities such as lizards and birds are usually found sympatric with jumping spiders. Many lizards prey on ants and in some reptile species ants are their main prey items [43–50]. For this reason, ant mimicry might not be able to provide protection for spiders when they encounter lizard predators. In tropical forests, various species of birds prey upon large orb web spiders, and field experiments showed that bird predation can significantly affect spider communities [51]. However, so far there is no direct evidence showing that bird predation serves as a strong predation force for wandering spiders such as salticids [52]. On the other hand, many hunting spiders possess good visual ability, especially the jumping spiders [3]. Jumping spiders have very keen visual ability and rely heavily on their vision to hunt for prey. The visual acuity of jumping spiders is the best among terrestrial arthropods [19]. Many predation strategies have evolved in jumping spiders and most of them are diurnal predators actively searching and hunting for their prey. In the field, it is common for jumping spiders to prey on other spiders and jumping spiders. As jumping spiders are among the most abundant spiders [8,53] and ants are the most dominant insects in the tropical and subtropical areas [2], the interactions among jumping spiders, their spider prey and ants should be quite common. Under the strong predation pressure of large jumping spiders, any mutations that result in ant-like appearance should be favoured by selection. The high visual acuity and resolution of jumping spiders might exert a strong selection pressure in favour of small salticids exhibiting nearly perfect resemblance of co-inhabiting ants. Besides, because jumping spiders are also the predators of many terrestrial arthropods, we suggest that the predation pressures from jumping spiders may also be one driving force of ant mimicry in other arthropod taxa. Congruent with such a proposition is the trend for the overall species numbers of myrmecomorphic arthropods to be higher in the tropics [1], where jumping spider diversity and abundance are high. We suggest multivariate behavioural analyses similar to those carried out in this study be conducted on other ant-like arthropods to determine whether being treated as ants or other mechanisms are responsible for the better survival of myrmecomorphic organisms. In addition, ecological studies quantifying predation pressures of jumping spiders on arthropod communities should be conducted to determine the selection strength of salticid predation in driving ant mimicry in jumping spiders and other organisms.
Acknowledgements
We thank T. C. Lin, W. M. Yuan, C. C. Hsiao and T. Y. Lin for all sorts of assistance. This work was supported by the National Science Council, Taiwan grants (NSC 97-2311-B-029-002-MY3, NSC 99-2621-B-029-002-MY3) to I.-M.T. and The Singapore Ministry of Education AcRF Tier 1 grant (R-154-000-355-112) to D.L.
References
- 1.McIver J. D., Stonedahl G. 1993. Myrmecomorphy: morphological and behavioral mimicry of ants. Annu. Rev. Entomol. 38, 351–379 [Google Scholar]
- 2.Hölldobler B., Wilson E. O. 1990. The ants . Cambridge, MA: Harvard University Press [Google Scholar]
- 3.Foelix R. F. 1996. Biology of spiders, 2nd edn. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Cloudsley-Thompson J. L. 1995. A review of anti-predator devices of spiders. Bull. Br. Arachnol. Soc. 10, 81–96 [Google Scholar]
- 5.Cushing P. E. 1997. Myrmecomorphy and myrmecophily in spiders: a review. Florida Entomol. 80, 165–193 10.2307/3495552 (doi:10.2307/3495552) [DOI] [Google Scholar]
- 6.Komárek S. 1998. Mimicry, aposematism and related phenomena in animals and plants—a bibliography 1800–1990. Prague: Vesmír [Google Scholar]
- 7.Nelson X. J., Jackson R. R. 2007. Complex display behaviour during the intraspecific interactions of myrmecomorphic jumping spiders (Araneae, Salticidae). J. Nat. Hist. 41, 1659–1678 10.1080/00222930701450504 (doi:10.1080/00222930701450504) [DOI] [Google Scholar]
- 8.Platnick N. I.The world spider catalog, v. 10.5. 2010. (online catalog). See http://research.amnh.org/iz/spiders/catalog/
- 9.Oliveira P. S. 1988. Ant-mimicry in some Brazilian salticids and clubionid spiders (Araneae: Salticidae, Clubionidae). Biol. J. Linn. Soc. 33, 1–15 10.1111/j.1095-8312.1988.tb00443.x (doi:10.1111/j.1095-8312.1988.tb00443.x) [DOI] [Google Scholar]
- 10.Edmunds M. 1974. Defense in animals: a survey of antipredator defenses. Harlow, UK: Longman Group Ltd [Google Scholar]
- 11.Edmunds M. 1978. Does mimicry of ants reduce predation by wasps on salticids spiders? Mem. Queensl. Mus. 33, 507–512 [Google Scholar]
- 12.Wanless F. R. 1978. A revision of the genera Belippo and Myrmarachne (Araneae: Salticidae) in the Ethiopian region. Bull. Br. Mus. Nat. Hist. 33, 1–139 [Google Scholar]
- 13.Oliveira P. S., Sazima I. 1984. The adaptive bases of ant-mimicry in a neotropical aphantochilid spider (Araneae: Aphantochilidae). Biol. J. Linn. Soc. 22, 145–155 10.1111/j.1095-8312.1984.tb01675.x (doi:10.1111/j.1095-8312.1984.tb01675.x) [DOI] [Google Scholar]
- 14.Oliveira P. S. 1986. Ant-mimicry in some spiders from Brazil. Bull. Soc. Zool. Fr. 111, 297–311 [Google Scholar]
- 15.Cutler B. 1991. Reduced predation on the antlike jumping spider Synageles occidentalis (Araneae: Salticidae). J. Insect Behav. 4, 401–407 10.1007/BF01048287 (doi:10.1007/BF01048287) [DOI] [Google Scholar]
- 16.Pekár S., Křál J. 2002. Mimicry complex in two central European zodariid spiders (Araneae: Zodariidae): how Zodarion deceives ants. Biol. J. Linn. Soc. 75, 517–532 10.1046/j.1095-8312.2002.00043.x (doi:10.1046/j.1095-8312.2002.00043.x) [DOI] [Google Scholar]
- 17.Blackledge T. A., Coddington J. A., Gillespie R. G. 2003. Are three-dimensional spider webs defensive adaptations? Ecol. Lett. 6, 13–18 10.1046/j.1461-0248.2003.00384.x (doi:10.1046/j.1461-0248.2003.00384.x) [DOI] [Google Scholar]
- 18.Snyder A. W. 1977. Acuity of compound eyes: physical limitations and design. J. Com. Physiol. A 116, 161–182 10.1007/BF00605401 (doi:10.1007/BF00605401) [DOI] [Google Scholar]
- 19.Land M. F., Nilsson D. E. 2002. Animal eyes. Oxford, UK: Oxford University Press [Google Scholar]
- 20.Harland D. P., Jackson R. R. 2000. Cues by which Portia fimbriata, an araneophagic jumping spider, distinguishes jumping-spider prey from other prey. J. Exp. Biol. 230, 3485–3494 [DOI] [PubMed] [Google Scholar]
- 21.Harland D. P., Jackson R. R. 2002. Influence of cues from the anterior medial eyes of virtual prey on Portia fimbriata, an araneophagic jumping spider. J. Exp. Biol. 205, 1861–1868 [DOI] [PubMed] [Google Scholar]
- 22.Jackson R. R. 1977. Prey of the jumping spider Phidippus johnsoni (Araneae: Salticidae). J. Arachnol. 5, 145–149 [DOI] [PubMed] [Google Scholar]
- 23.Horner N. V., Stangl F. B., Fuller G. K. 1988. Natural history observations of Salticus austinensis (Araneae, Salticidae) in North-Central Texas. J. Arachonol. 16, 260–262 [Google Scholar]
- 24.Bartos M. 2004. The prey of Yllenus arenarius (Araneae, Salticidae). Bull. Br. Arachnol. Soc. 13, 83–85 [Google Scholar]
- 25.Guseinov E. F. 2004. Natural prey of the jumping spider Menemerus semilimbatus (Hahn, 1827) (Araneae: Salticidae), with notes on its unusual predatory behaviour. In European Arachnology 2003. Proc. of the 21st European Colloquium of Arachnology, St Petersburg, Russia, 3 August (eds Logunov D. V., Penney D.). Moscow, Russia: KMK Scientific Press [Google Scholar]
- 26.Huseynov E. F. 2005. Natural prey of the jumping spider Menemerus taeniatus (Araneae: Salticidae). Eur. J. Entomol. 102, 797–799 [Google Scholar]
- 27.Nelson X. J., Jackson R. R., Pollard S. D., Edwards G. B., Barrion A. T. 2004. Predation by ants on jumping spiders (Araneae: Salticidae) in the Philippines. N. Z. J. Zool. 31, 45–56 [Google Scholar]
- 28.Halaj J. D., Ross W., Moldenke A. R. 1997. Negative effects of ant foraging on spiders in Douglas-fir canopies. Oecologia 109, 313–322 10.1007/s004420050089 (doi:10.1007/s004420050089) [DOI] [PubMed] [Google Scholar]
- 29.Blest A. D., O'Carrol D. C., Carter M. 1990. Comparative ultrastructure of layer I receptor mosaics in the principal eyes of jumping spiders: the evolution of regular arrays of light guides. Cell Tissue Res. 262, 445–460 10.1007/BF00305241 (doi:10.1007/BF00305241) [DOI] [Google Scholar]
- 30.Jackson R. R., Li D. 1998. Prey preferences and visual discrimination ability of Cyrba algerina, an araneophagic jumping spider (Araneae: Salticidae) with primitive retinae. Isr. J. Zool. 44, 227–242 [Google Scholar]
- 31.Jackson R. R. 2000. Prey preferences and visual discrimination ability of Brettus, Cocalus and Cyrba araneophagic jumping spiders (Araneae: Salticidae) from Australia. N. Z. J. Zool. 27, 29–39 [Google Scholar]
- 32.Cross F. R., Jackson R. R. 2009. Cross-modality priming of visual and olfactory selective attention by a spider that feeds indirectly on vertebrate blood. J. Exp. Biol. 212, 1869–1875 10.1242/jeb.028126 (doi:10.1242/jeb.028126) [DOI] [PubMed] [Google Scholar]
- 33.Nelson X. J., Jackson R. R. 2006. Vision-based innate aversion to ants and ant mimics. Behav. Ecol. 17, 676–691 10.1093/beheco/ark017 (doi:10.1093/beheco/ark017) [DOI] [Google Scholar]
- 34.Nelson X. J., Jackson R. R. 2007. Vision-based ability of an ant-mimicking jumping spider to discriminate between models, conspecific individuals and prey. Insectes Soc. 54, 1–4 10.1007/s00040-006-0901-x (doi:10.1007/s00040-006-0901-x) [DOI] [Google Scholar]
- 35.Clarke K. R., Warwick R. M. 2001. Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn Plymouth, UK: PRIMER-E [Google Scholar]
- 36.Nelson X. J., Jackson R. R. 2009. Prey classification by an araneophagic ant-like jumping spider (Araneae: Salticidae). J. Zool. 279, 173–179 10.1111/j.1469-7998.2009.00602.x (doi:10.1111/j.1469-7998.2009.00602.x) [DOI] [Google Scholar]
- 37.Nelson X. J., Jackson R. R. 2009. Aggressive use of Batesian mimicry by an ant-like jumping spider. Biol. Lett. 5, 755–757 10.1098/rsbl.2009.0355 (doi:10.1098/rsbl.2009.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson R. R. 1982. The biology of ant-like jumping spiders: intraspecific interaction of Myrmarachne lupata (Araneae, Salticidae). Zool. J. Linn. Soc. 76, 293–319 10.1111/j.1096-3642.1982.tb02185.x (doi:10.1111/j.1096-3642.1982.tb02185.x) [DOI] [Google Scholar]
- 39.Ceccarelli F. S. 2008. Behavioral mimicry in Myrmarachne species (Araneae, Salticidae) from North Queensland, Australia. J. Arachnol. 36, 344–351 10.1636/CSt07-114.1 (doi:10.1636/CSt07-114.1) [DOI] [Google Scholar]
- 40.Jackson R. R., Clark R. J., Harland D. P. 2002. Behavioural and cognitive influences of kairomones on an araneophagic jumping spider. Behaviour 139, 749–775 10.1163/156853902320262808 (doi:10.1163/156853902320262808) [DOI] [Google Scholar]
- 41.Jackson R. R., Nelson X. J., Sune G. O. 2005. A spider that feeds indirectly on vertebrate blood by choosing female mosquitoes as prey. Proc. Natl Acad. Sci. USA 102, 15 155–15 160 10.1073/pnas.0507398102 (doi:10.1073/pnas.0507398102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cross F. R., Jackson R. R., Pollard S. D. 2010. How blood-derived odor influences mate-choice decisions by a mosquito-eating predator. Proc. Natl Acad. Sci. USA 106, 19 416–19 419 10.1073/pnas.0904125106 (doi:10.1073/pnas.0904125106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamopoulou C., Valakos E. D., Pafilis P. 1999. Summer diet of Podarcis milensis, P. gaigeae and P. erhardii (Sauria: Lacertidae). Bonn. Zool. Beitr. 48, 275–282 [Google Scholar]
- 44.Haw J. M., Clout M. N., Powelsland R. G. 2001. Diet of moreporks (Ninox novaeseelandiae) in Pureora forest determined from prey remains in regurgitated pellets. N. Z. J. Ecol. 25, 61–67 [Google Scholar]
- 45.Zamprogno C., Zamprogno M. G. F., Teixeira R. L. 2001. Evidence of terrestrial feeding in the arboreal lizard Enyalius bilineatus (Sauria, Polychrotidae) of South-Eastern Brazil. Rev. Bras. Biol. 61, 91–94 [DOI] [PubMed] [Google Scholar]
- 46.Van Sluys M., Rocha C. F. D., Vrcibradic D., Aleksander C., Galdino B., Fontes A. F. 2004. Diet, activity, and microhabitat use of two syntopic Tropidurus species (Lacertilia: Tropiduridae) in Minas Gerais, Brazil. J. Herpetol. 38, 606–611 10.1670/218-03N (doi:10.1670/218-03N) [DOI] [Google Scholar]
- 47.Mesquita D. O., Colli G. R., Franca F. G. R., Vitt L. J. 2006. Ecology of a Cerrado lizard assemblage in the Jalapão region of Brazil. Copeia 2006, 460–471 10.1643/0045-8511(2006)2006[460:EOACLA]2.0.CO;2 (doi:10.1643/0045-8511(2006)2006[460:EOACLA]2.0.CO;2) [DOI] [Google Scholar]
- 48.Huang S. C., Norval G., Tso I. M. 2008. Predation by an exotic lizard, Anolis sagrei, alters the ant community structure in betelnut palm plantations in southern Taiwan. Ecol. Entomol. 33, 569–576 10.1111/j.1365-2311.2008.00995.x (doi:10.1111/j.1365-2311.2008.00995.x) [DOI] [Google Scholar]
- 49.Serrano-Cardozo V. H., Lemos-Espinal J. A., Smith J. R. 2008. Comparative diet of three sympatric Sceloporus in the semiarid Zapotitlán Valley, Mexico. Rev. Mex. Biodivers. 79, 427–434 [Google Scholar]
- 50.Molina-Borja M., Padrón-Fumero M., Alfonso-Martín T. 1998. Morphological and behavioural traits affecting the intensity and outcome of male contests in Gallotia gallotigalloti (family Lacertidae). Ethology 104, 314–322 10.1111/j.1439-0310.1998.tb00071.x (doi:10.1111/j.1439-0310.1998.tb00071.x) [DOI] [Google Scholar]
- 51.Wise D. H. 1993. Spiders in ecological webs. Cambridge, UK: Cambridge University Press [Google Scholar]
- 52.Gunnarsson B. 2008. Bird predation on spiders: ecological mechanisms and evolutionary consequences. J. Arachnol. 35, 509–529 10.1636/RT07-64.1 (doi:10.1636/RT07-64.1) [DOI] [Google Scholar]
- 53.Coddington J. A., Levi H. W. 1991. Systematics and evolution of spiders (Araneae). Annu. Rev. Ecol. Syst. 22, 565–592 10.1146/annurev.es.22.110191.003025 (doi:10.1146/annurev.es.22.110191.003025) [DOI] [Google Scholar]