Abstract
The evolutionary maintenance of cooperative breeding systems is thought to be a function of relative costs and benefits to breeders, helpers and juveniles. Beneficial effects of helpers on early-life survivorship and performance have been established in several species, but lifetime fitness benefits and/or costs of being helped remain unclear, particularly for long-lived species. We tested for effects of helpers on early- and late-life traits in a population of reintroduced red wolves (Canis rufus), while controlling for ecological variables such as home-range size and population density. We found that the presence of helpers in family groups was positively correlated with pup mass and survival at low population density, but negatively correlated with mass/size at high density, with no relation to survival. Interestingly, mass/size differences persisted into adulthood for both sexes. While the presence of helpers did not advance age at first reproduction for pups of either sex, females appeared to garner long-term fitness benefits from helpers through later age at last reproduction, longer reproductive lifespan and a greater number of lifetime reproductive events, which translated to higher lifetime reproductive success. In contrast, males with helpers exhibited diminished lifetime reproductive performance. Our findings suggest that while helper presence may have beneficial short-term effects in some ecological contexts, it may also incur long-term sex-dependent costs with critical ramifications for lifetime fitness.
Keywords: cooperative breeding, helpers, body mass, survival, age at first reproduction, lifetime reproductive success
1. Introduction
Cooperative breeding is a relatively rare but broadly distributed phenomenon in the animal kingdom, with the majority of cooperatively breeding species living in family units consisting of both breeding individuals and offspring that delay dispersal [1]. Though not all delayed dispersers play an active role in caring for young, in many species they may act as ‘helpers’ through juvenile provisioning, guarding, grooming and/or training [2,3]. The evolutionary stability of cooperative breeding is theoretically a complex function of costs and benefits of delayed dispersal to both the helper and those helped [4–6]. Beneficial effects of helpers on juvenile growth and survival have been documented in a wide array of cooperatively breeding species [2,3], and the benefits of large juvenile size have been shown to extend to the reproductive years. For instance, heavier prairie voles (Microtus ochrogaster) are preferred as social mates, and have increased fecundity [7,8]. Similarly, heavier banded mongoose (Mungos mungo) and meerkat (Suricata suricatta) females have earlier ages at first breeding as well as increased fecundity [6,9]. Moreover, helpers increase the probability that meerkat pups will become reproductive during their lifetimes [10].
Though studies are beginning to emerge showing favourable effects of helpers on early-life probability of reproduction and fecundity, evidence of lifetime fitness benefits of helpers—apart from increased probability of survival to breeding age—is minimal [11]. This deficiency may be due to challenges associated with obtaining complete longitudinal life-history data on individuals, particularly in long-lived species. Nevertheless, any definitive fitness benefits of helping behaviour can only be established by assessing the impact of helping on lifetime reproductive success (LRS) of those being helped. It is particularly critical to evaluate any early-life benefits of helping in the context of life-history theory, which predicts trade-offs between early- and late-life traits [12,13], as well as evidence that the long-term advantage of investing in certain traits such as larger body sizes may vary according to species and environmental context [14,15]. Furthermore, while some studies have demonstrated sex differences in LRS in relation to early-life factors such as population density and delayed dispersal [16,17], the possibility of sex differences in LRS driven by helpers has not previously been examined.
In this study, we examined sex-specific effects of cooperative breeding on components of LRS in a monogamous group-living canid, the red wolf Canis rufus. Helping behaviour is widespread among canids, as demonstrated by den-site attendance, provisioning, play and grooming by non-breeding pack members [18–20]. Studies in some canids have shown a positive relationship between helping behaviour and pup size and survival [18,21–23], although in other cases the relationship is less clear [24–26]. Furthermore, evidence suggests that the presence of helpers is not always beneficial and may in fact be harmful when population densities are high and food resources are limited [21,27,28]. Thus, ecological factors such as population density and home-range size are essential to bear in mind when assessing impacts of large family groups on offspring fitness.
We examined the effects of cooperative breeding on early- and late-life traits in red wolves reintroduced into North Carolina following extinction from their historical range across the southeastern United States [29]. Over 20 years, a large proportion (i.e. greater than 80%) of wild-born individuals were monitored intensively via radio-telemetry and genetic sampling, and we have information on individual home-range size, population density, pack social dynamics and life history of more than 400 free-ranging wolves. Drawing on this rich dataset, we address the following questions, critical to understanding the evolution and maintenance of a cooperative breeding strategy:
— Do helpers affect body mass, size and survival of offspring?
— Do helpers facilitate earlier age at first reproduction?
— Do helpers exert long-term effects on lifetime reproductive traits, such as age at last reproduction (ALR), reproductive lifespan (RLS), number of lifetime reproductive events (LREs) and—ultimately—LRS?
In addition, we test for sex differences in response to helpers for all three of these areas, and discuss the ramifications of our findings for the evolutionary maintenance of cooperative breeding in monogamous species.
2. Study System
Formerly distributed throughout the southeastern United States, the red wolf was declared extinct in the wild in 1980, as a result of systematic eradication and habitat loss following European settlement [29,30]. In 1973, the US Endangered Species Act resulted in the establishment of a captive breeding programme and in 1987 a reintroduction programme was launched in the Alligator River National Wildlife Refuge in North Carolina [29]. Since reintroduction, the red wolf population has grown to roughly 120 individuals ([31]; electronic supplementary material, figure S1), and from 1987 to 2007 463 animals were equipped with very high-frequency radio transmitters and monitored intensively for home-range and life-history attributes [32].
Paternity data reveal that the red wolf population largely exhibits both social and genetic monogamy, with reproduction in a pack limited almost exclusively to a single breeding pair [33]. All members of a red wolf pack, including the breeding pair and non-breeding individuals, are known to frequent dens after pups are born [32]. In this study, the majority (90%, n = 161) of non-breeding members of packs were offspring from a previous year that delayed dispersal, which we will refer to as ‘helpers’, though true helping behaviour, such as is present in all other members of the Canis genus, has not yet been documented in the (considerably less studied) red wolf, and non-breeders may not always provide help. In this population, 59 per cent (n = 321) of pups with known fate had at least one helper present for at least part of their first year. Packs had one or two helpers present on average.
3. Statistical analyses
In our analyses, we recognized three population density time intervals: Low Density I (1989–1993), Low Density II (1994–1998) and High Density (1999–2007) (electronic supplementary material, figure S1). During the High Density interval the population was stationary. Except where noted, time intervals were analysed separately to avoid difficulties of interpretation in testing for three-way interactions. Life-history traits were analysed with respect to adult mass and size, natal home-range size, natal population density and presence or absence (P/A) of helpers in the natal pack. Home-range size was calculated from the 95 per cent isopleths of utilization distributions, as estimated using kernel density estimators with fixed bandwidth estimated using the root-n bandwidth estimator, and ranged from 14 to 164 km2 [34]. Helper effects were analysed using a binomial variable denoting presence/absence owing to the relative paucity of packs with three or more helpers. All non-significant effects and their interactions (p > 0.1) were removed in a step-wise fashion to arrive at the final model. All analyses were conducted using SAS 9.1.3 (SAS Institute, Cary, NC, USA).
(a). Body mass, size and survival
Wolves were captured primarily via foothold traps, and weighed and measured upon capture [32]. Measurements of mass, ear, tail, hindfoot and body length were made on 127 pups (aged 6–8 months), and 199 adults. The four morphological measures were significantly correlated, and thus were reduced to a single structural size index (hereafter ‘size PC1’) using principal components analyses for pups and adults. The residuals of individual mass or size PC1 plotted against estimated age in months at time of weighing were used for all pup mass and size analyses, to compensate for growth in older pups. Though mass and size PC1 were positively correlated in both pups and adults (§3), we analysed them independently because mass of free-ranging canids can exhibit high seasonal variability and may be more indicative of condition than size per se [35]. For adults, we averaged individual mass and size PC1 over lifetime measures obtained after growth ceased (approx. 18 months).
We conducted simple linear regression analyses by sex to test for long-term within-individual relationships between pup and adult mass and size PC1. For both pup and adult mass and size PC1, stepwise analyses were conducted with sex and presence/absence of helpers as fixed effects, and natal home-range size and natal population density as covariates. Litter was included as a random effect to account for common environmental or maternal effects. We excluded the Low Density I interval from mass/size analyses owing to low sample size (n = 10).
To test for a role of helpers on survival for Low Density II and High Density intervals, we constructed logistic mixed models with survival to age 2 (yes or no) as the response variable, presence/absence of helpers and sex and their interaction as fixed effects, natal home-range size as a covariate and litter as a random effect. We were unable to test for an effect of helpers on survival to six to eight months of age, to mirror our analyses of effects of helpers on mass/size at that age, as the majority of pups were not radiocollared prior to six months. We chose age 2 as an index of survival to reproductive age, as this is the age at which many wolves begin to breed. Since the majority of mortalities prior to age 2 were due to anthropogenic causes, we assume for the purposes of this analysis that factors that make individuals more vulnerable to death from natural causes similarly increase risk from anthropogenic factors. This assumption is supported by qualitatively similar trends when confining the analysis to individuals dying of natural causes alone (n = 12 and n = 11 for Low Density II and High Density, respectively). Similarly, for censored individuals (lost owing to radiocollar failure), we assume that death of pups occurred at or around the time of censoring, as results with and without censored individuals were qualitatively similar.
(b). Age at first reproduction
Age at first reproduction was known for 105 individuals (52 males, 53 females). We analysed age at first reproduction with respect to sex, presence/absence of helpers, pup or adult mass or size PC1, natal home-range size and natal population density. Analyses were conducted by considering each population time interval both separately and pooled.
(c). Lifetime reproductive traits
Pearson correlation coefficients were calculated between all lifetime reproductive traits, including age at first reproduction, age at last reproduction, reproductive lifespan, lifetime number of reproductive events and LRS. LRS was defined as the total number of pups produced in an individual's lifetime that survived from birth in the spring until the following autumn, and was estimated as the sum of yearly counts of pups captured in the den and/or observed in a pack during the following months. Parental relationships were confirmed from genetic data, generated at 18 microsatellite loci, via genetic exclusion and the program CERVUS v. 2.0 [36] (for detailed genetic methods, see [37]). The Brown–Forsythe test was used to test for unequal variance in LRS between the sexes using both reproductive and non-reproductive individuals for whom complete lifetime information was known (23 females, 29 males).
Approximately 27 per cent (n = 489) of wolves monitored were known to be reproductive. Of these, complete lifetime reproductive information was known for 34 wild-born individuals (13 females, 21 males) that were either known to have died of natural causes or were presumed to have died after reproduction had ceased: 14 died of natural causes, two died of unknown causes but had lost dominance prior to death, four died of anthropogenic causes at advanced ages but had not reproduced in the previous year, and 14 were censored after 7 years of age, but had not reproduced for at least one year previously, or were sufficiently advanced in age as to be presumed dead. No individuals were included that appeared to cease reproduction primarily owing to the death of a mate by anthropogenic causes. An additional five individuals that died either naturally or of unknown causes at an advanced age for whom timing of reproduction was known, but not numbers of recruits, were used for all analyses of lifetime reproductive traits except LRS. Note that analyses using both more stringent (i.e. only those individuals known to die of natural causes; n = 14) and less stringent (i.e. including individuals that were either lost or known to die of unknown causes; n = 74) had similar outcomes for all four variables.
Age at last reproduction, reproductive lifespan, lifetime number of reproductive events and LRS were analysed with respect to sex, presence/absence of helpers, adult mass and size PC1, natal home-range size and natal population density. Sample sizes for lifetime reproductive traits were small when divided among time intervals, but because trends were similar among intervals we pooled samples from all three intervals for our final analysis. Furthermore, as all four variables were strongly positively correlated (§3), we conducted a MANCOVA using all four variables as the response variables, transformed into standardized z-scores (i.e. the difference between individual values for a given trait and the sample mean, divided by the sample standard deviation).
4. Results
(a). Body mass, size and survival
A red wolf structural size index (size PC1) was derived from the first principal component of a principal components analyses of ear, tail, hindfoot and body length for both pups and adults (pups: λ1 = 2.86, 57% of variation; adults: λ1 = 3.3, 66% of variation). Size PC1 had strong positive loadings for all four morphological traits. The second principal component (pups: λ2 = 0.84, 17% of variation; adults: λ2 = 0.70, 14% of variation) had a strong positive loading for tail length alone, but was not significantly related to any of our dependent variables, and thus was not considered further. Body mass and size PC1 were strongly correlated in both pups and adults (pups: F1,126=121.7, p < 0.0001, R2 = 0.49; adults: F1,198 = 678.5, p < 0.0001, R2 = 0.77). Long-term repeated measures of body mass and size PC1 were positively related for individuals captured both as pups and adults (female mass: F1,21 = 7.66, p = 0.012, R2 = 0.28; male mass: F1,23 = 4.76, p = 0.040, R2 = 0.18; female size: F1,13 = 16.51, p = 0.002, R2 = 0.58; male size: F1,23 =24.43, p < 0.0001, R2 = 0.52).
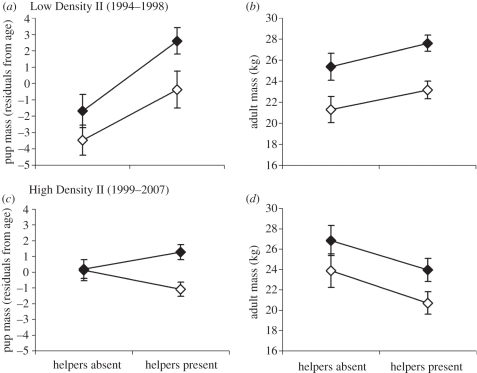
Natal home-range size and population density did not significantly affect either pup or adult mass or size PC1, and thus were dropped from the final analyses, which considered only sex, presence/absence of helpers and their interaction, and litter as a random effect. Presence of helpers was significantly related to greater pup mass in both males and females during the Low Density II interval, and pup size followed a similar trend (table 1 and figure 1a). During the High Density interval, there was a significant sex × P/A helper interaction for pup mass, with a weak positive relationship between helper presence and male mass, in contrast to a weak negative relationship for female mass (figure 1c). There was a significant negative relationship between the presence of helpers and pup size in both males and females. Results for adult mass/size were similar to those found for pup mass/size, with a significant positive relationship between size and the presence of helpers during the Low Density II interval, and a positive trend with mass (figure 1b). There was a significant negative relationship between mass/size and helpers in both sexes during the High Density interval (figure 1d).
Table 1.
ANCOVA models of pup and adult mass and size PC1 during two population intervals. Asterisks denote significant effects.
| Low Density II interval |
High Density interval |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mass |
size |
mass |
size |
|||||||||
| d.f. | F | p | d.f. | F | p | d.f. | F | p | d.f. | F | p | |
| pups | ||||||||||||
| sex | 1,19 | 30.8 | 0.02* | 1,16 | 0.6 | 0.45 | 1,73 | 25.9 | 0.03* | 1,73 | 24.4 | 0.00* |
| P/A helpers | 1,19 | 73.4 | 0.00* | 1,16 | 2.7 | 0.12 | 1,73 | 0.1 | 0.89 | 1,73 | 8.7 | 0.00* |
| sex × P/A helpers | 1,19 | 1.9 | 0.54 | 1,16 | 0.1 | 0.76 | 1,73 | 22.9 | 0.04* | 1,73 | 0.3 | 0.57 |
| adult | ||||||||||||
| sex | 1,42 | 179.2 | 0.00* | 1,41 | 23.4 | 0.00* | 1,32 | 5.6 | 0.02* | 1,27 | 16.9 | 0.00* |
| P/A helpers | 1,42 | 22.0 | 0.10 | 1,41 | 6.6 | 0.01* | 1,32 | 4.7 | 0.04* | 1,27 | 6.1 | 0.02* |
| sex × P/A helpers | 1,42 | 0.0 | 0.98 | 1,41 | 0.1 | 0.72 | 1,32 | 0.0 | 0.81 | 1,27 | 0.0 | 0.86 |
Figure 1.
Least-square means of red wolf mass in relation to the presence or absence of helpers in the natal pack in (a,c) pups and (b,d) adults during two population intervals. Similar patterns were observed for size PC1, with the exception of (c), which showed a negative relationship between helper presence and size PC1 for both sexes. Black diamonds, males; white diamonds, females.
The final model for survival to age 2 contained only P/A helpers and litter as a random effect. Neither sex nor home-range size significantly affected early-life survival. Furthermore, the effect of helpers was only significant for the Low Density II interval, with pup survival to age 2 increasing from 47 per cent in the absence of helpers to 78 per cent in the presence of helpers (n = 81, 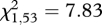 , p = 0.007). During the High Density interval, there was no effect of helpers on pup survival (n = 158,
, p = 0.007). During the High Density interval, there was no effect of helpers on pup survival (n = 158, 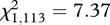 , p = 0.54).
, p = 0.54).
(b). Age at first reproduction
Neither pup or adult mass or size PC1, presence/absence of helpers, natal population density nor natal home-range size significantly affected age at first reproduction. Thus, the final model contained only sex as a main effect. Pooled across time intervals, there was a significant sex difference in age at first reproduction: 46 per cent (n = 52) of males produced their first litter within their first 2 years of life, while only 25 per cent (n = 53) of females reproduced at these ages (F1,102 = 10.79, p = 0.0014). However, while each time interval showed a trend for males to commence reproduction earlier than females (males: 2.69 ± 0.18 years; females: 3.47 ± 0.18 years), this relationship was significant only for Low Density II (Low Density I: F1,25 = 9.22, p = 0.19; Low Density II: F1,38 = 3.86, p = 0.006; High Density: F1,35 = 3.18, p = 0.22).
(c). Lifetime reproductive traits
Pairwise comparisons of lifetime reproductive traits showed significant positive correlations among age at last reproduction, reproductive lifespan, number of LREs and LRS (ALR versus RLS: r = 0.751, p < 0.0001; ALR versus LRE: r = 0.715, p < 0.0001; ALR versus LRS: r = 0.560, p < 0.0001; RLS versus LRE: r = 0.900, p < 0.0001; RLS versus LRS: r = 0.754, p < 0.0001; LRE versus LRS: r = 0.827, p < 0.0001). Furthermore, age at first reproduction (AFR) was significantly negatively correlated with RLS, and marginally significantly negatively correlated with lifetime number of reproductive events, indicating that early ages of reproduction are associated with longer RLSs and a higher number of LREs (AFR versus ALR: r = 0.155, p = 0.36; AFR versus RLS: r = −0.433, p = 0.0075; AFR versus LRE: r = −0.308, p = 0.064; AFR versus LRS: r = −0.238, p = 0.15). The variance in LRS did not differ significantly between males and females (females: s = 7.34; males: s = 5.32; F1,50 = 1.97; p = 0.12).
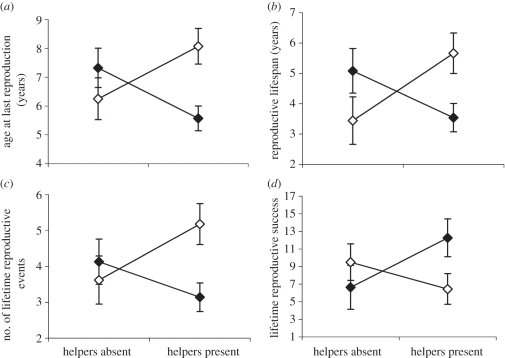
Adult mass, size PC1 and natal home-range size were not significantly related to any of the four lifetime reproductive traits, and thus were excluded from the final models. The final models for these traits contained sex, P/A helpers and their interaction, as well as natal population density (table 2). Population density was significantly negatively correlated to lifetime number of reproductive events and LRS, and marginally significantly negatively correlated to reproductive lifespan. Though highly non-significant, population density was also included in the final model for age at last reproduction, for consistency. All ANCOVA and MANCOVA models showed a significant sex × P/A helpers interaction, with females having a later age at last reproduction, longer reproductive lifespan, greater number of LREs and greater LRS than males in the presence of helpers (figure 2).
Table 2.
MANCOVA and ANCOVA models of age at last reproduction (ALR), reproductive lifespan (RLS), lifetime number of reproductive events (LRE) and lifetime reproductive success (LRS). Asterisks denote significant effects.
| effects | d.f. | F | p |
|---|---|---|---|
| MANCOVA | |||
| sex | 4,25 | 1.48 | 0.2372 |
| P/A helpers | 4,25 | 0.74 | 0.5738 |
| sex × P/A helpers | 4,25 | 3.36 | 0.0248* |
| density | 4,25 | 2.47 | 0.0710 |
| ALR | |||
| sex | 1,34 | 1.62 | 0.2120 |
| P/A helpers | 1,34 | 0.05 | 0.8248 |
| sex × P/A helpers | 1,34 | 10.18 | 0.0031* |
| density | 1,34 | 0.80 | 0.3769 |
| RLS | |||
| sex | 1,34 | 0.05 | 0.8255 |
| P/A helpers | 1,34 | 0.15 | 0.6969 |
| sex × P/A helpers | 1,34 | 9.56 | 0.0040* |
| density | 1,34 | 3.71 | 0.0626 |
| LRE | |||
| sex | 1,34 | 1.13 | 0.2962 |
| P/A helpers | 1,34 | 0.40 | 0.5297 |
| sex × P/A helpers | 1,34 | 6.94 | 0.0126* |
| density | 1,34 | 4.57 | 0.0398* |
| LRS | |||
| sex | 1,28 | 0.54 | 0.4686 |
| P/A helpers | 1,28 | 0.27 | 0.6096 |
| sex × P/A helpers | 1,28 | 4.76 | 0.0377* |
| density | 1,28 | 7.01 | 0.0132* |
Figure 2.
Least-square means from ANCOVAs on (a) age at last reproduction, (b) reproductive lifespan, (c) number of lifetime reproductive events and (d) lifetime reproductive success for male and females with helpers present or absent in their natal pack. Black diamonds, males; white diamonds, females.
5. Discussion
Early-life experiences can exert long-term effects on lifetime fitness [38,39]. In several ungulate [16,40–42] and one bird [43] species, for instance, high population density or poor habitat quality during the year of birth have been shown to negatively affect LRS. However, while there is abundant information on early-life effects of helpers in cooperatively breeding species, little is known regarding the long-term fitness effects of helping on juveniles and whether such effects are consistent across sexes. In this study, we present the first evidence of both social and environmental effects on LRS in a wild carnivore. In accordance with other studies, we found a negative relationship between population density and components of LRS in red wolves. The relationship between natal pack helpers on both early- and late-life traits, however, was complex and revealed intriguing sex differences in life history.
(a). Do helpers affect mass, size and survival of offspring?
During the Low Density II interval (1994–1998), pups with helpers present had larger body mass, as well as larger mass and size PC1 as adults, suggesting that packs with helpers can indeed be more capable of providing nourishment for pups (figure 1a). During the High Density interval (1999–2007), however, the positive effects of helper presence shifted: while male pups showed no difference in mass related to the presence of helpers, female pups with helpers actually exhibited lower mass, and both sexes exhibited smaller mass and size PC1 as adults (figure 1c,d). The greater susceptibility of female pups to negative effects of helper presence early in life suggests that females may be less successful than males when faced with competition from older siblings for food. As a social feeding hierarchy has been documented in a variety of other captive and wild carnivores (e.g. [44–46]), we propose that in red wolves this feeding hierarchy is further stratified between male and female pups, where larger and/or more aggressive males may be capable of excluding females from feeding—a hypothesis that should be tested in future behavioural research. Whatever the case, it appears that helpers in the natal pack can exert long-lasting effects on mass/size in red wolves, and that the direction of these effects is density-dependent.
The positive effects of helper presence during the Low Density II interval extended to a substantial increase in the proportion of pups surviving to reproductive age relative to those without helpers. While the effect of helpers on pup survival during their first year remains unknown, a positive effect on yearling survival during this interval suggests strong fitness benefit to having helpers, as has been documented in a variety of cooperatively breeding species [2,3]. However, this positive effect on survival was absent during the High Density interval. These findings are consistent with a study on grey wolves (Canis lupus), where helpers were shown to increase survival of young in a population where density was low and food was abundant, but had negative effects where density was high and food was scarce [27]. Our findings in red wolves differ only in that though helper presence is negatively related to lower pup mass/size at high density, survival to reproductive age did not differ between pups with or without helpers.
(b). Do helpers facilitate earlier age at first reproduction?
Helper-facilitated advancement of age at first reproduction has been reported in females of cooperatively breeding banded mongooses and meerkats [6,9]. Given that larger mass and size can facilitate earlier maturation, one would predict that red wolf pups with helpers in their natal pack should commence reproduction earlier than those without helpers during Low Density II, and vice versa during High Density. However, such was not the case: the presence of helpers did not affect age at first reproduction for either sex during either time interval. It is currently unclear what factors are most influential in determining age at first reproduction in this population, but other work suggests that one key to understanding this trait may lie in understanding what factors affect delayed dispersal, as females that disperse later are also more likely to commence reproduction at a later age [33].
(c). Do helpers exert long-term effects on lifetime reproductive traits?
Given that larger adult size has been associated with a greater probability of obtaining mates, achieving dominance and higher fecundity (e.g. [6–9]), one might predict that LRS would be higher in wolf pups receiving growth benefits in the presence of helpers. Thus, during the Low Density II interval, when helpers were associated with increased adult mass and size for both sexes, LRS should be higher for pups with helpers. Surprisingly, we found that long-term effects of helpers differed between the sexes. For both sexes, those that reproduced to later ages had longer reproductive lifespans, a greater number of LREs and higher LRS. When helpers were absent from the natal pack, males did not differ from females in expression of these traits, but when helpers were present, females actually fared better than males in that they had later age at last reproduction, longer reproductive lifespan, a greater number of LREs and ultimately higher LRS (table 2 and figure 2).
There are two main hypotheses that may explain this long-term correlation with natal social environment. First, it is possible that females that managed to survive early-life competition with male pups and older siblings and successfully reproduce were individuals of the highest quality and, on average, able to out-perform males (and females without helpers) upon whom selection was less strong. Second, the presence of helpers may trigger trade-offs between early- and late-life traits [12,13]. We found no evidence of a helper-mediated trade-off between age at first reproduction and late-life traits—though males did tend to commence reproduction earlier than females, this sex difference was not influenced by the presence of helpers. Nevertheless, it is possible that larger mass early in life may come at a cost to late-life reproductive performance for males. Faster early-life growth rates have been associated with physiological and/or pleiotropic trade-offs, resulting in faster rates of ageing and shorter lifespans [12,13]. The evidence from red wolves during both Low Density II and High Density suggests that males are larger in general than females (figure 2). While large mass/size has been demonstrated to have important advantages in many species, some studies indicate that large size can carry a cost during adult life, particularly in resource-limited environments [14,15]. Long-term metabolic costs of larger body mass in male red wolves with helpers may produce a trade-off with late-life traits, resulting in reduced age at last reproduction and associated traits. In other words, ‘help’ early in life may translate to ‘harm’ later in life. Conversely, when food is limited, females, which are smaller in general, may benefit from a certain level of caloric restriction, which has been shown to have beneficial late-life effects in model species (reviewed in [47]). Nevertheless, there are several weaknesses to this hypothesis—most critically, it cannot explain how differences within the sexes in relation to the presence of helpers should be manifest in the same way during both Low Density and High Density intervals, in spite of the reversal in the effects of helpers on mass and size between intervals. Unfortunately, we were restricted by sample size from determining whether the differences within and between the sexes in the presence or absence of helpers are identical across population density intervals (as similar trends would indicate), or differ in important regards. Whatever the case, additional research into the physiological and ecological variables influencing reproductive success is needed in order to evaluate the relative costs and benefits of large size, or other helper-influenced traits, in early- and late-life reproductive performance in red wolves.
(i). Ramifications for evolutionary stability of cooperative breeding in red wolves
In polygynous species, variance in LRS tends to be greater among males than among females, as some males may have very high reproductive success through breeding with multiple females, while others may have little or no opportunity to breed [48]. In contrast, monogamous species are predicted to have equal variance in reproductive success, though this hypothesis has rarely been tested in mammals. In this study, we did find equivalent variance in reproductive success between the two sexes; however, we also found a sex difference in mean LRS for those with helpers in their natal pack. This result is surprising given that greater pack stability (and therefore delayed dispersal) may be predicted to occur more frequently in a population experiencing lower levels of anthropogenically induced disturbance than the red wolf population currently experiences [49]. Our findings suggest that, historically, in the context of greater pack stability, males may have predominately exhibited lower LRS than females. This raises the question of how a monogamous, group-living social system has nevertheless been maintained among red wolves. To compensate for reduced LRS in males in the context of high pack continuity, one might predict increased selection for polygyny or extra-pair copulations, so that males could maximize their reproductive success during their short reproductive lifespans, and thereby increase mean lifetime reproduction for males in the population. Indeed, extra-pair copulations are common even in canids that exhibit social monogamy (e.g. [50–52]). However, we found only two instances of extra-pair matings and two of multiple paternity in red wolves among 90 breeding pairs and 174 reproductive events [33], suggesting that the selective forces and/or phylogenetic constraints responsible for the maintenance of a monogamous mating strategy are still strong. However, there are at least three non-exclusive mechanisms that may allow monogamous group-living to be maintained as a viable strategy in a population with greater pack stability. First, the existence of serial monogamy. While red wolves in our study were primarily genetically monogamous, death of a mate or male–male competition can lead to formation of a new breeding pair. Thus, males may be able to maintain a lower mean reproductive success on average through increased rates of male turnover, or serial monogamy, in the context of longer female reproductive lifespan. Second, the earlier mean age at first reproduction that we report for males regardless of helper presence may at least partially compensate for what might otherwise be an even greater disparity in reproductive lifespan relative to females. Third, though decreased anthropogenic disturbance results in increased proportion of kin-based wolf packs [49], there will inevitably be natural dissolution and formation of packs owing to death and abandonment, even in an undisturbed population. Thus, to some extent, a low frequency of males that do not experience helping behaviour and have higher LRS than those that do—and vice versa for females—will narrow the gap in mean fitness between the two sexes and help increase the viability of a monogamous, group-living social system.
6. Conclusion
Our findings regarding the effects of helpers on pup early-life traits suggests that helping behaviour in red wolves is facultative, and may vary with population density. The decreases in mass and size and absence of survival benefits for wolves raised in a pack with older siblings when the population was at carrying capacity suggest that siblings prioritize their own energetic needs. Even more intriguing is the evidence presented here that suggests life-history trade-offs may be socially mediated; males, which fared slightly better in the presence of helpers early in life, fared worse late in life, and vice versa for females, resulting in marked sex differences in LRS. While helping behaviour has been shown to favour such traits as larger body size and earlier reproduction in other species, the consequences of such help for lifetime fitness have not been previously demonstrated. Moreover, considered in the context of an integrated life history, it is not clear that such traits are always beneficial in the long run. In light of our findings regarding the complex relationship between helpers and pup LRS in the red wolf, we contend that while some forms of helping behaviour may indeed contribute to fitness, trade-offs may also come into play, which are strongly dependent on what constitutes the most fit strategy for both sexes in diverse social and ecological contexts.
Acknowledgements
The red wolf recovery programme is conducted by the USFWS, and we are grateful to Service personnel for their diligent efforts in the field and for access to the data. The study was funded by the USFWS. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the USFWS.
References
- 1.Emlen S. T. 1991. Evolution of cooperative breeding in birds and mammals. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 301–337 Oxford, UK: Blackwell Scientific [Google Scholar]
- 2.Solomon N. G., French J. A. 1997. Cooperative breeding in mammals. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Koenig W., Dickinson J. 2004. Ecology and evolution of cooperative breeding birds. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Emlen S. T., Wrege P. H. 1989. A test of alternate hypotheses for helping behavior in white-fronted bee-eaters of Kenya. Behav. Ecol. Sociobiol. 25, 303–319 10.1007/BF00302988 (doi:10.1007/BF00302988) [DOI] [Google Scholar]
- 5.Dickinson J. L., Hatchwell B. J. 2004. Fitness consequences of helping. In Ecology and evolution of cooperative breeding birds (eds Koenig W., Dickinson J.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Russell A. F. 2004. Mammals: comparisons and contrasts. In Ecology and evolution of cooperative breeding birds (eds Koenig W., Dickinson J.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 7.Solomon N. G. 1993. Body size and social preferences of male and female prairie voles, Microtus chrogaster. Anim. Behav. 45, 1031–1033 10.1006/anbe.1993.1122 (doi:10.1006/anbe.1993.1122) [DOI] [Google Scholar]
- 8.Solomon N. G. 1994. Effect of the preweaning environment on subsequent reproduction in prairie voles, Microtus ochrogaster. Anim. Behav. 48, 331–341 10.1006/anbe.1994.1246 (doi:10.1006/anbe.1994.1246) [DOI] [Google Scholar]
- 9.Hodge S. J. 2005. Helpers benefit offspring in both the short and long-term in the cooperatively breeding banded mongoose. Proc. R. Soc. B 272, 2479–2484 10.1098/rspb.2005.3255 (doi:10.1098/rspb.2005.3255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell A. F., Young A. J., Spong G., Jordan N. R., Clutton-Brock T. H. 2007. Helpers increase the reproductive potential of offspring in cooperative meerkats. Proc. R. Soc. B 274, 513–520 10.1098/rspb.2006.3698 (doi:10.1098/rspb.2006.3698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silk J. B. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559 10.1098/rstb.2006.1994 (doi:10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams G. C. 1957. Pleiotropy, natural selection and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 (doi:10.2307/2406060) [DOI] [Google Scholar]
- 13.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 14.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645 10.1098/rstb.2007.0011 (doi:10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy M. T., Sandercock B. K. 2009. Lifetime reproductive success of female eastern kingbirds (Tyrannus tyrannus): influence of lifespan, nest predation, and body size. Auk 124, 1010–1022 10.1642/0004-8038(2007)124[1010:LRSOFE]2.0.CO;2 (doi:10.1642/0004-8038(2007)124[1010:LRSOFE]2.0.CO;2) [DOI] [Google Scholar]
- 16.Kruuk L. E. B., Clutton-Brock T. H., Rose K. E., Guinness F. E. 1999. Early determinants of lifetime reproductive success differ between the sexes in red deer. Proc. R. Soc. Lond. B 266, 1655–1661 10.1098/rspb.1999.0828 (doi:10.1098/rspb.1999.0828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawn A. T., Radford A. N., du Plessis Morné A. 2007. Delayed breeding affects lifetime reproductive success differently in male and female green woodhoopoes. Curr. Biol. 17, 844–849 10.1016/j.cub.2007.03.036 (doi:10.1016/j.cub.2007.03.036) [DOI] [PubMed] [Google Scholar]
- 18.Moehlman P. D. 1986. Ecology and cooperation in canids. In Ecological aspects of social evolution: birds and mammals (eds Rubenstein D. I., Wrangham R. W.). Princeton, NJ: Princeton University Press [Google Scholar]
- 19.Mech L. D., Wolf P. C., Packard J. M. 1999. Regurgitative food transfer among wild wolves. Can. J. Zool. 77, 1192–1195 10.1139/cjz-77-8-1192 (doi:10.1139/cjz-77-8-1192) [DOI] [Google Scholar]
- 20.Packard J. M. 2003. Wolf behavior: reproductive, social and intelligent. In Wolves: behavior, ecology and conservation (eds Mech L. D., Boitani L.). Chicago, IL: University of Chicago Press [Google Scholar]
- 21.Malcolm J. R., Marten K. 1982. Naturals selection and the communal rearing of pups in African wild dogs (Lycaon pictus). Behav. Ecol. Sociobiol. 10, 1–13 10.1007/BF00296390 (doi:10.1007/BF00296390) [DOI] [Google Scholar]
- 22.Moehlman P. D. 1979. Jackal helpers and pup survival. Nature 277, 382–383 10.1038/277382a0 (doi:10.1038/277382a0) [DOI] [Google Scholar]
- 23.Creel S., Mills M. G. L., McNutt J. W. 2005. African wild dogs. In Biology and conservation of wild canids (eds MacDonald D. W., Sillero-Zubiri C.). Oxford, UK: Oxford University Press [Google Scholar]
- 24.Bekoff M., Wells M. C. 1986. Social ecology and behavior of coyotes. Adv. Study Behav. 16, 251–338 10.1016/S0065-3454(08)60193-X (doi:10.1016/S0065-3454(08)60193-X) [DOI] [Google Scholar]
- 25.Baker P. J., Robertson C. P. J., Funk S. M., Harris S. 1998. Potential fitness benefits of group living in the red fox, Vulpes vulpes. Anim. Behav. 56, 1411–1424 10.1006/anbe.1998.0950 (doi:10.1006/anbe.1998.0950) [DOI] [PubMed] [Google Scholar]
- 26.Kruchenkova E., Goltsman M., Sergeev S., Macdonald D. 2009. Is alloparenting helpful for Mednyi Island arctic foxes, Alopex lagopus semenovi? Naturwissenschaften 96, 457–466 10.1007/s00114-008-0494-5 (doi:10.1007/s00114-008-0494-5) [DOI] [PubMed] [Google Scholar]
- 27.Harrington F. H., David Mech L., Fritts S. H. 1983. Pack size and wolf pup survival: their relationship under varying ecological conditions. Behav. Ecol. Sociobiol. 13, 19–26 10.1007/BF00295072 (doi:10.1007/BF00295072) [DOI] [Google Scholar]
- 28.Gusset M., Macdonald D. W. 2009. Group size effects in cooperatively breeding African wild dogs. Anim. Behav. 79, 425–428 10.1016/j.anbehav.2009.11.021 (doi:10.1016/j.anbehav.2009.11.021) [DOI] [Google Scholar]
- 29.USFWS 1984. Red wolf recovery plan. Atlanta, GA: US Fish and Wildlife Service [Google Scholar]
- 30.McCarley H., Carley C. J. 1979. Recent changes in distribution and status of wild red wolves (Canis rufus). In Endangered Species Report 4, pp. 38 Albuquerque, NM: US Fish and Wildlife Service [Google Scholar]
- 31.USFWS 2007. Red Wolf (Canis rufus) 5-year status review: summary and evaluation. Manteo, NC: US Fish and Wildlife Service. [Google Scholar]
- 32.Phillips M. K., Henry V. G., Kelly B. T. 2003. Restoration of the red wolf. In Wolves: behavior, ecology and conservation (eds Mech L. D., Boitani L.). Chicago, IL: University of Chicago Press [Google Scholar]
- 33.Sparkman A. M., Adams J., Steury T. D., Waits L., Murray D. L. In review Direct fitness benefits of delayed dispersal in the cooperatively breeding red wolf (Canis rufus). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steury T. D., McCarthy J. E., Roth T. C., Lima S. L., Murray D. L. 2010. Evaluation of a root-n bandwidth selector for kernel home range estimation. J. Wildl. Manage. 74, 539–548 10.2193/2008-327 (doi:10.2193/2008-327) [DOI] [Google Scholar]
- 35.Poulle M. L., Crête M., Huot J. 1995. Seasonal variation in body mass and composition of eastern coyotes. Can. J. Zool. 73, 1625–1633 10.1139/z95-193 (doi:10.1139/z95-193) [DOI] [Google Scholar]
- 36.Marshall T. C., Slate J., Kruuk L. E. B., Pemberton J. M. 1998. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7, 639–655 10.1046/j.1365-294x.1998.00374.x (doi:10.1046/j.1365-294x.1998.00374.x) [DOI] [PubMed] [Google Scholar]
- 37.Adams J. R. 2006. A multi-faceted molecular approach to red wolf (Canis rufus) conservation and management. Dissertation, University of Idaho, Moscow, USA [Google Scholar]
- 38.Lindstrom J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 10.1016/S0169-5347(99)01639-0 (doi:10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 39.Metcalfe N. B., Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 10.1016/S0169-5347(01)02124-3 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 40.Coltman D. W., Smith J. A., Bancroft D. R., Pilkington J., MacColl A. D. C., Clutton-Brock T. H., Pemberton J. M. 1999. Density-dependent variation in lifetime breeding success and natural and sexual selection in Soay rams. Am. Nat. 154, 730–746 10.1086/303274 (doi:10.1086/303274) [DOI] [PubMed] [Google Scholar]
- 41.McLoughlin P. D., et al. 2007. Lifetime reproductive success and composition of the home range in a large herbivore. Ecology 88, 3192–3201 10.1890/06-1974.1 (doi:10.1890/06-1974.1) [DOI] [PubMed] [Google Scholar]
- 42.Hamel S., Gaillard J. M., Festa-Bianchet M., Cote S. D. 2009. Individual quality, early-life conditions, and reproductive success in contrasted populations of large herbivores. Ecology 90, 1981–1995 10.1890/08-0596.1 (doi:10.1890/08-0596.1) [DOI] [PubMed] [Google Scholar]
- 43.Korpimäki E. 1992. Fluctuating food abundance determines the lifetime reproductive success of male Tengmalm's owls. J. Anim. Ecol. 61, 102–111 [Google Scholar]
- 44.Zimen E. 1976. Regulation of pack size in wolves. Z. Tierpsychol. 40, 300–303 10.1111/j.1439-0310.1976.tb00939.x (doi:10.1111/j.1439-0310.1976.tb00939.x) [DOI] [PubMed] [Google Scholar]
- 45.Frank L. G. 1986. Social organization of the spotted hyena Crocuta crocuta 2. Dominance and reproduction. Anim. Behav. 34, 1510–1527 10.1016/S0003-3472(86)80221-4 (doi:10.1016/S0003-3472(86)80221-4) [DOI] [Google Scholar]
- 46.Gese E. M., Ruff R. L., Crabtree R. L. 1996. Social and nutritional factors influencing the dispersal of resident coyotes. Anim. Behav. 52, 1025–1043 10.1006/anbe.1996.0250 (doi:10.1006/anbe.1996.0250) [DOI] [Google Scholar]
- 47.Kenyon C. 2005. The plasticity of aging: insights from long-lived mutants. Cell 120, 449–460 10.1016/j.cell.2005.02.002 (doi:10.1016/j.cell.2005.02.002) [DOI] [PubMed] [Google Scholar]
- 48.Clutton-Brock T. H. (ed.) 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press [Google Scholar]
- 49.Rutledge L. Y., Patterson B. R., Mills K. J., Loveless K. M., Murray D. L., White B. N. 2010. Protection from harvesting restores the natural social structure of eastern wolf packs. Biol. Conserv. 143, 332–339 10.1016/j.biocon.2009.10.017 (doi:10.1016/j.biocon.2009.10.017) [DOI] [Google Scholar]
- 50.Girman D. J., Mills M. G. L., Geffen E., Wayne R. K. 1997. A molecular genetic analysis of social structure, dispersal, and interpack relationships of the African wild dog (Lycaon pictus). Behav. Ecol. Sociobiol. 40, 187–198 10.1007/s002650050332 (doi:10.1007/s002650050332) [DOI] [Google Scholar]
- 51.Baker P. J., Funk S. M., Bruford M. W., Harris S. 2004. Polygynandry in a red fox population: implications for the evolution of group living in canids? Behav. Ecol. 15, 766–778 10.1093/beheco/arh077 (doi:10.1093/beheco/arh077) [DOI] [Google Scholar]
- 52.Kitchen A. M., Gese E. M., Waits L. P., Karki S. M., Schauster E. R. 2006. Multiple breeding strategies in the swift fox, Vulpes velox. Anim. Behav. 71, 1029–1038 10.1016/j.anbehav.2005.06.015 (doi:10.1016/j.anbehav.2005.06.015) [DOI] [Google Scholar]