Abstract
Partial migration, in which a fraction of a population migrate and the rest remain resident, occurs in an extensive range of species and can have powerful ecological consequences. The question of what drives differences in individual migratory tendency is a contentious one. It has been shown that the timing of partial migration is based upon a trade-off between seasonal fluctuations in predation risk and growth potential. Phenotypic variation in either individual predation risk or growth potential should thus mediate the strength of the trade-off and ultimately predict patterns of partial migration at the individual level (i.e. which individuals migrate and which remain resident). We provide cross-population empirical support for the importance of one component of this model—individual predation risk—in predicting partial migration in wild populations of bream Abramis brama, a freshwater fish. Smaller, high-risk individuals migrate with a higher probability than larger, low-risk individuals, and we suggest that predation risk maintains size-dependent partial migration in this system.
Keywords: partial migration, predation risk, phenotypic variation, Abramis brama, behavioural polymorphism
1. Introduction
Migration is an exceptionally widespread phenomenon in nature that has long fascinated biologists. For many species there is a significant degree of variation in migratory tendency between individuals, such that only part of the population participates in migration. This is known as partial migration and has been reported for many species across taxa [1–5]. Understanding what drives individual differences in migratory propensity is critical if we are to understand the effects of partial migration upon population- and ecosystem-level processes. Yet despite this, the ultimate mechanisms that underlie differences in migratory propensity between individuals within populations remain controversial. Various theories to explain partial migration have been proposed, including those that invoke conditional differences between individuals, whereby the decision to migrate is related to age, sex, body condition or dominance status [6]. Secondly, differences between individuals may be driven by underlying genetic differences between migrants and residents [7], or thirdly partial migration may be a mixed evolutionary stable strategy maintained by frequency-dependent selection [8,9]. Despite the multiple suggestions that predation risk can be involved in partial migration (e.g. [1,3,10]), no studies have yet considered intraspecific variation in predation risk as a causal factor in partial migration. Here, we elaborate a conceptual model that was developed from Werner & Gilliam [11] by Brönmark et al. [1] to predict the timing of seasonal patterns of migration, to evaluate which individuals will participate in migration.
The Brönmark et al. model proposes that individuals trade off predation risk p (cost) and growth potential g (benefit), and that when the p/g ratio in a given habitat increases above a certain threshold, individuals should migrate to either increase g or decrease p [1]. The model as applied to understanding the timing of seasonal migration assumes equality between individuals in vulnerability to predation (p), an assumption that is seldom met in natural populations consisting of individuals with heterogeneous phenotypes. If, however, p differs between individuals, those facing higher p should be more inclined to migrate, given that g is comparable between individuals. We therefore here focus on the p in the p/g model, and evaluate the influence of individual vulnerability to predation on migration probability. Many fish populations are size structured and, in addition, exposed to gape-limited predators, so that individuals of different body sizes vary gradually in vulnerability to predation (p). Furthermore, growth rates over winter for many fish species are extremely low owing to extremely low food availability and a high temperature threshold for feeding and growth. Hence, partially migrating fish that migrate over winter when growth rates (g) are low, such as our model species common bream (Abramis brama L.), provide an excellent opportunity to empirically test the influence of individual variation in p on partial migration probability in wild populations.
Part of common bream populations migrate over winter from relatively high predation lakes into surrounding low predation streams. As the principal predators of bream, the piscivorous pike (Esox lucius L.), are gape-size limited, and both common bream and pike form size-structured populations, there should be a continuum of relative vulnerabilities to predation among individuals in common bream populations relating to individual sizes and the size composition of the piscivore population [12]. Assuming that the p/g model governs individual decisions to perform seasonal migration, we hypothesize that the probability of migration to low-predation habitats would increase with individual vulnerability to predation. This system allows us to test this as for common bream growth below 12°C is extremely low [13], which means that over the winter migratory period g is essentially zero in both the lake and stream habitats. The present study provides an empirical evaluation of this hypothesis in natural populations of animals, and highlights the importance of individual differences in predation risk for migratory dynamics in partially migrating animals.
2. Material and methods
(a). Study sites
Size-specific patterns of seasonal migration of common bream were evaluated in two shallow, Danish lakes. Lake Søgård (55°25′ N, 9°19′ E) is small, eutrophic and shallow (area 26 ha; average depth 1.6 m; mean summer Secchi depth 0.55 m), and the fish community is dominated by roach (Rutilus rutilus L.) and Eurasian perch (Perca fluviatilis L.), but also common bream, rudd (Scardinus erythrophthalmus L.), white bream (Blicca bjoerkna L.), pike and European eel (Anguilla anguilla L.) are present [14]. There is no submerged vegetation present, and the lake is surrounded by a 3–4 m wide margin of reed (Phragmites australis cav.; [14]). The lake has a well-defined inlet and outlet and previous investigations have shown that 40–70% of the cyprinid fish, and especially common bream, roach and white bream, use the inlet and outlet streams as overwintering habitats (C. Skov 2005–2007, unpublished results). Lake Loldrup (56°29′ N, 9°26′ E) is a small, shallow and slightly eutrophic lake (area 39 ha; average depth 1.2 m; mean summer Secchi depth 1.1 m) that has one inlet and one outlet stream. The fish community of Lake Loldrup is similar to that of Lake Søgård and is dominated by roach and common bream, but also includes Eurasian perch, pike and very few pikeperch (Sander lucioperca L.). Temperature data collected each hour from the lake, the inlet and the outlet (Temperature loggers; tidbit) revealed that during the migratory period (October–early May), monthly average temperatures in the lakes and streams did not exceed 12°, the growth threshold temperature for A. brama [13].
(b). Sampling and tagging of fish
Common bream and its principal potential predator, pike, were sampled and tagged during the periods 5–12 October and 13–18 October 2005 in Lake Loldrup and Lake Søgård, respectively. Fish were exclusively caught in the lake by both seining and electrofishing to ensure an accurate estimate of body size distributions of pike and bream, and individually weighed (nearest g) and measured (nearest mm, total length) before being tagged by surgically implanting a TIRIS Passive Integrated Transponder (PIT) tag (Texas Instruments, RI-TRP-RRHP, Plano, Texas, USA, half duplex, 134 kHz, 23.1 mm long, 3.85 mm diameter, 0.6 g in air) into the stomach cavity of the fish (see [15] for detailed methodology). PIT tags are passive telemetry tags that are activated when exposed to an electromagnetic field produced by antennae placed in streams (see below). When energized and activated, they emit a unique code enabling identification of tagged individuals. After a short recovery of approximately 30 min, tagged common bream and pike were released to the lake. An evaluation of PIT-tag marking techniques has shown that the method used results in no significant effect on fish well-being, including body condition [15]. A total of 221 and 237 bream (larger than 122 mm) and 91 and 90 pike, were captured, tagged and released in Lakes Søgård and Loldrup, respectively.
(c). Fish migration
Migration of tagged common bream and pike between the lake and the inlet and outlet streams was monitored by passive bio-telemetry using a modified PIT-tag antenna system [15,16]. When a tagged fish swims by an antenna, the PIT-tag is energized and emits a unique code that can be recorded and stored together with date and time on a memory card. Two loop-shaped antennas, each covering the entire cross section of the stream, were placed 3–5 m from each other along the inlet (15 m upstream the lake) and the outlet (150 m downstream the lake) streams. The use of two sequential antennas enables determination of fish swimming direction. The antenna recording frequency was 5 energize/receive cycles s−1 [17]. Migration data presented are based on data from time of tagging until June 1st 2006, when return migration to the lake had ended. Individual fish were defined as migrants if they were recorded by any antenna in the streams during the study period, and we calculated the time each individual spent residing in the streams. We only included migration patterns of fish that had been out of the lake for a minimum of 60 min during the study period, to exclude short, non-migratory visits to the antennal areas. To quantify relative predation risk between the lakes and the streams, we assessed the daily average percentage of the pike population that were present outside the lakes during the study period (from the time of tagging until 1st June 2006). Furthermore, extensive stretches of the streams were electrofished in the study period as well as regularly in consecutive years (both summer and winter).
(d). Individual predation vulnerability
To be able to predict individual migration probability from predation vulnerability, individual relative predation vulnerabilities were calculated using the index from Hambright et al. [18] which simply estimates the numerical proportion of the population of predators with gape sizes large enough to ingest a prey fish of a specific body depth. Our vulnerability calculations were based on the lake-specific length distributions of pike in the two lakes, estimated from the pike captured (and pit tagged) while seining and electrofishing. We calculated pike length-specific gape sizes and bream length-specific body depths according to the relationships in Nilsson & Brönmark [12]. From these measures we calculated bream individual vulnerability to pike predation as the proportion of pike with gape sizes larger than individual bream body depths [18]. Eurasian perch and pikeperch were excluded from the vulnerability calculations, since we captured no Eurasian perch large enough to predate on common bream of the sizes tagged, and since the catch of pikeperch was too small for reliable estimations of population length distribution, which indicates a pikeperch population too small to exert a notable predation pressure on common bream. In order to keep the PIT tag weight to body weight ratio below critical limits [19], here we tagged and monitored migration patterns of common bream more than 122 mm. We therefore only included pike large enough to be able to consume 123 mm common bream in the vulnerability calculations, meaning that 34 and 85 pike were included for Lakes Søgård and Loldrup, respectively.
(e). Statistical methodology
To investigate lake- and length-specific migration propensity in relation to individual predation vulnerabilities (IPV) we performed a logistic regression [20] with migration (yes or no) as dependent variable, and IPV and lake as independent variables, including the IPV * lake interaction in the model. Migration behaviours were further explored as migrating individuals' migration date, calculated as the number of days after October 1st when individuals migrated, as well as migration duration, the number of days individuals spent out of the lake. These variables were evaluated for dependence on IPV and between lakes by ANCOVA. As data could not be transformed to normality and homoscedasticity, we performed a non-parametric ANCOVA using rank transformation as described by Conover & Iman [21], where F-values are reduced to consider not meeting with the normality assumption.
3. Results
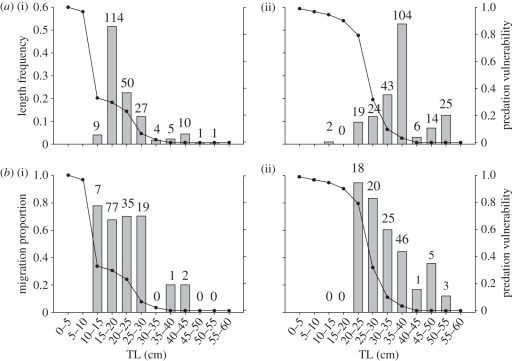
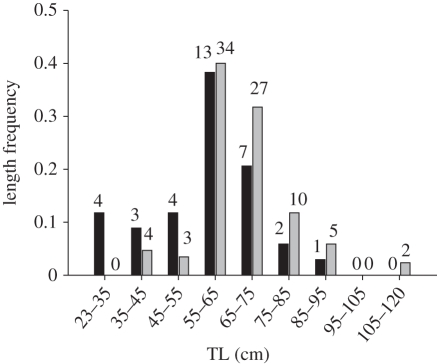
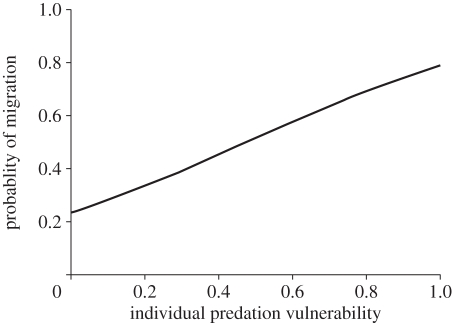
In total 64 and 50 per cent of the tagged common bream in Lake Søgård and Lake Loldrup, respectively, migrated over the study period. We found significant (paired t8 = 2.360, p = 0.046) interlake differences in the relative vulnerability of the size classes of common bream (figure 1). This was caused by differences in size distributions of the pike populations (figure 2). For instance, pike individuals longer than 70 cm constituted 30 and 14 per cent of the populations in Lakes Loldrup and Søgård, respectively. Consequently, common bream of e.g. 20–25 cm length had a higher vulnerability index in Lake Loldrup (0.80) than in Lake Søgård (0.23, figure 1). Migration probability in common bream increased significantly with increasing vulnerability to predation from pike (Logistic regression, Wald1 = 17.585, p < 0.0001, probability = e(α+β * IPV)/(1 + e(α+β * ;IPV)), α = −1.191 ± 0.448 (s.e.), β = 2.496 ± 0.595, n = 458, figure 3) irrespective of lake origin (no effect of lake: Wald1 = 1.486, p = 0.223; no significant lake * vulnerability interaction: Wald1 = 0.836, p = 0.361).
Figure 1.
(a) Length frequency distributions (bars) and size-specific predation vulnerabilities (lines) of bream in two Danish lakes. In both lakes larger fish are at lower risk of predation. (b) Individual migration propensity (bars) and size-specific predation vulnerabilities (lines) of bream in two Danish lakes. (a) The number of bream tagged per class of TL and (b) the number of bream that migrated per class of TL is given. Both lakes show the same pattern: predation risk and migratory propensity decrease with size (a,b) (i) Lake Søgård and (a,b) (ii) Lake Loldrup).
Figure 2.
Size frequency distribution of pike big enough to feed on the tagged bream (more than 122 mm) in Lake Loldrup (grey bars) and Lake Søgård (black bars). Numbers of individuals captured and tagged in each size group is also given.
Figure 3.
Best-fit prediction from the logistic regression for migration propensity regressed against predation vulnerability for both study lakes.
Individuals with high vulnerability to predation migrated sooner after October 1st in each lake (F1,340 = 15.725, p < 0.01), but IPV-dependent migration dates differed between lakes (F1,340 = 52.956, p < 0.01). There was no significant interaction term between lakes and IPV (F1,340 = 0.376, p > 0.1). Migration duration increased with IPV (F1,208 = 9.306, p < 0.01), and vulnerability-dependent migration duration did not differ between lakes (F1,208 = 0.786, p > 0.1). The median migration duration for bream with IPV more than 0.80 and less than 0.20 was 81 and 1 day, respectively. There was no significant lake * IPV interaction for migration duration (F1,208 = 0.952, p > 0.1). Finally, only few pike migrated into the outlet and inlets over the study period. The daily average percentage of the pike population residing in the inlet and outlets during the study period was 1 and 6.3 per cent for Lakes Loldrup and Søgård, respectively. Low densities of pike in the streams were also observed by electrofishing in the study year as well as in consecutive years. A total of more than 60 h of electrofishing resulted in the capture of one pike (38 cm).
4. Discussion
Our research provides correlational evidence consistent with the hypothesis that individual predation vulnerability explains patterns of partial migration. We show that individuals at high risk of predation have a higher probability of participating in migration. Common bream from both of our study populations show comparable patterns of risk-dependent migratory behaviour, which suggests that prey individuals in populations that vary in predation risk and predator size distribution can mediate their behaviour in a threat-sensitive manner. Our study highlights the central role predation risk plays in maintaining an ecologically significant behavioural polymorphism, migratory propensity. The results would be of general importance in explaining the evolution of partial migration in many animals, as heterogeneity in vulnerability against predation is commonplace in a wide range of partially migratory taxa. Numerous phenotypic traits related to individual vulnerability (p), such as morphometric differences [22,23] and behavioural traits like activity and boldness [24] show high degrees of within-population variation in many species. In support of the generality of our model, recent experimental work in artificial mesocosms showed that size-structured risk assessments governed partial migration in zooplankton [2]. It is important to note that as fish such as A. brama have indeterminate growth, and a number of fitness-related traits, including fecundity (e.g. [25]), are directly related to size, growth should be maximized to maximize fitness.
We show that common bream in specific size classes were at different risk of predation in the two lakes, and responded to this by displaying different migration propensity. The higher the vulnerability to predation, the higher was the propensity for individuals to migrate. Hence, individuals in each lake exhibited migratory behaviour coupled to a lake-specific predation threat for their particular body size. Whether this individual response to lake-specific predation risk is driven by environmental assessment by fish (i.e. it is highly flexible), or whether this is an evolved, population-level trait is not clear. Future work, for example by translocating individuals between lakes with divergent predation regimes, may shed light on the mechanisms that underlie the link between individual migratory behaviour and site-specific predation risk.
The risk of predation also related to other migration characteristics. The duration of the migration declined with predation risk, indicating that larger migrants with lower IPV spent longer periods in the lake during the winter period. This is in line with our data showing that individuals with lower IPV migrated later in the season than fish with high IPV.
Although our data are of correlational nature, we argue that it implies that predation risk is a parsimonious explanation for the dynamics of partial migration in bream. Alternative explanations such as that smaller bream are competitively excluded from the lake overwinter, or that smaller individuals benefit from higher stream temperatures to maximize growth rates do not apply in our system. Growth rates g are severely depressed at temperatures of less than 12°C in this species, and indeed in other cyprinid fish [13]. Hence differences in g between habitats do not occur during winter, making this system ideal for assessing the role of predation risk upon individual migratory tendency. As IPV predicts migration probability, and is a general effect across two lakes that differ in predation pressure for different sizes of fish, we argue that this is consistent with the suggestion that predation risk maintains condition-dependent (i.e. size-dependent) partial migration in this species.
This study suggests that by migrating into the inlets and outlets, bream escape predation risk from the bulk of the pike population. The clear majority of pike did not migrate but overwintered in the lakes, and low densities of pike in the streams were confirmed by electrofishing. The lake habitat thus exposes bream to higher risks of pike predation. In addition to piscivorous fish, avian predators are also likely to exert predation pressure on bream, and endothermic predators may be especially important during winter. Predatory birds such as cormorants Phalacrocorax carbo could forage upon bream, and this additional predation pressure may have an additive effect upon risk p for the smaller size classes of bream that bird predators are able to catch. Quantifying avian predation pressure upon fish populations, along with its consequences, remains a challenge in many model systems.
To conclude, our study illustrates the importance of individual predation risk upon migratory tendency and indicates the role of predation risk in maintaining behavioural polymorphism. This suggests that changes to predator populations in systems with migratory prey could have important consequences for behavioural and community dynamics. As recent theoretical work suggests that the intensity of partial migration can have profound consequences upon ecosystem integrity, affecting both population dynamics and trophic interactions [26], it is crucial that we continue to investigate this puzzling and fascinating phenomenon.
Acknowledgements
PIT-tagging was performed under permission from the Danish Animal Experiments Inspectorate.
Financial support was received from the Swedish Research Council, The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning, the Centre for Animal Movement Research (CAnMove) and the Danish Angling License funds. We would like to thank two anonymous reviewers, Neil Metcalfe and Daniel Ruzzante for helpful advice on a previous version of this paper.
References
- 1.Brönmark C., Skov C., Brodersen J., Nilsson P. A., Hansson L.-A. 2008. Seasonal migration determined by a trade-off between predator avoidance and growth. PLoS ONE 3, e1957. 10.1371/journal.pone.0001957 (doi:10.1371/journal.pone.0001957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson L.-A., Hylander S. 2009. Size-structured risk assessments govern Daphnia migration. Proc. R. Soc. B 276, 331–336 10.1098/rspb.2008.1088 (doi:10.1098/rspb.2008.1088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebblewhite M., Merrill E. H. 2009. Trade-offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 90, 3445–3454 10.1890/08-2090.1 (doi:10.1890/08-2090.1) [DOI] [PubMed] [Google Scholar]
- 4.Nilsson A. L. K., Sandell M. I. 2009. Stress hormone dynamics: an adaptation to migration? Biol. Lett. 5, 480–483 10.1098/rsbl.2009.0193 (doi:10.1098/rsbl.2009.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swingland I. R. 1984. Intraspecific differences in movement. In The ecology of animal migration (eds Swingland I. R., Greenwood P. J.), pp. 102–105 Oxford, UK: Clarendon Press [Google Scholar]
- 6.Brodersen J., Nilsson P. A., Hansson L.-A., Skov C., Brönmark C. 2008. Condition-dependent individual decision-making determines cyprinid partial migration. Ecology 89, 1195–1200 10.1890/07-1318.1 (doi:10.1890/07-1318.1) [DOI] [PubMed] [Google Scholar]
- 7.Biebach H. 1983. Genetic determination of partial migration in the European robin (Erithacus rubecula). Auk 100, 601–606 [Google Scholar]
- 8.Kaitala A., Kaitala V., Lundberg P. 1993. A theory of partial migration. Am. Nat. 142, 59–81 10.1086/285529 (doi:10.1086/285529) [DOI] [Google Scholar]
- 9.Lundberg P. 1988. The evolution of partial migration in birds. Trends Ecol. Evol. 3, 172–175 10.1016/0169-5347(88)90035-3 (doi:10.1016/0169-5347(88)90035-3) [DOI] [PubMed] [Google Scholar]
- 10.Jonsson B., Jonsson N. 1993. Partial migration—niche shift versus sexual-maturation in fishes. Rev. Fish. Biol. Fish. 3, 348–365 10.1007/BF00043384 (doi:10.1007/BF00043384) [DOI] [Google Scholar]
- 11.Werner E. E., Gilliam J. F. 1984. The ontogenetic niche and species interactions in size structured populations. Ann. Rev. Ecol. Syst. 15, 393–425 10.1146/annurev.es.15.110184.002141 (doi:10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- 12.Nilsson P. A., Brönmark C. 2000. Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88, 539–546 10.1034/j.1600-0706.2000.880310.x (doi:10.1034/j.1600-0706.2000.880310.x) [DOI] [Google Scholar]
- 13.Mann R. H. K. 1991. Growth and production. In Cyprinid fishes (eds Winfield I. J., Nelson J. S.), pp. 456–482 London, UK: Chapman and Hall [Google Scholar]
- 14.Grünfeld S. 2003. Nutrients, nutrient loading anf biology of Lake Søgård 2002. Report from the County of Vejle, Denmark. [In Danish.] [Google Scholar]
- 15.Skov C., Brodersen J., Brönmark C., Hansson L.-A., Hertonsson P., Nilsson P. A. 2005. Evaluation of PIT-tagging in cyprinids. J. Fish. Biol. 67, 1195–1201 10.1111/j.1095-8649.2005.00814.x (doi:10.1111/j.1095-8649.2005.00814.x) [DOI] [Google Scholar]
- 16.Skov C., Brodersen J., Nilsson P. A., Hansson L.-A., Brönmark C. 2008. Inter- and size-specific patterns of fish seasonal migration between a shallow lake and its streams. Ecol. Freshw. Fish 17, 406–415 10.1111/j.1600-0633.2008.00291.x (doi:10.1111/j.1600-0633.2008.00291.x) [DOI] [Google Scholar]
- 17.Castro-Santos T., Haro A., Walk S. 1996. A passive integrated transponder (PIT) tag system for monitoring fishways. Fish. Res. 28, 253–261 10.1016/0165-7836(96)00514-0 (doi:10.1016/0165-7836(96)00514-0) [DOI] [Google Scholar]
- 18.Hambright K. D., Drenner R. W., McComas S. R., Hairston N. G. J. 1991. Gape-limited piscivores: planktivore size refuges, and the trophic cascade hypothesis. Arch. Hydrobiol. 121, 389–404 [Google Scholar]
- 19.Jepsen N., Koed A., Thorstad E. B., Baras E. 2002. Surgical implantation of telemetry transmitters in fish: how much have we learned? Hydrobiologia 483, 239–248 10.1023/A:1021356302311 (doi:10.1023/A:1021356302311) [DOI] [Google Scholar]
- 20.Quinn G. P., Keough M. J. 2002. Experimental design and data analysis for biologists. Cambridge, UK: University Press [Google Scholar]
- 21.Conover W. J., Iman R. L. 1982. Analysis of covariance using the rank transformation. Biometrics 38, 715–724 10.2307/2530051 (doi:10.2307/2530051) [DOI] [PubMed] [Google Scholar]
- 22.Nilsson P. A., Brönmark C., Pettersson L. B. 1995. Benefits of a predator-induced morphology in crucian carp. Oecologia 104, 291–296 10.1007/BF00328363 (doi:10.1007/BF00328363) [DOI] [PubMed] [Google Scholar]
- 23.Tollrian R., Dodson S. I. 1999. Inducible defenses in Cladocera: constraints, costs, and multipredator environments. In The ecology and evolution of inducible defences (eds Tollrian R., Harvell C. D.), pp. 177–202 Princeton, NJ: Princeton University Press [Google Scholar]
- 24.Dugatkin L. A. 1992. Tendency to inspect predators predicts mortality risk in the guppy (Poecilia reticulata). Behav. Ecol. 3, 124–127 10.1093/beheco/3.2.124 (doi:10.1093/beheco/3.2.124) [DOI] [Google Scholar]
- 25.Wooton R. J. 1998. Ecology of teleost fishes. Dordrecht, The Netherlands: Kluwer Academics [Google Scholar]
- 26.Brodersen J., Ådahl E., Brönmark C., Hansson L.-A. 2008. Ecosystem effects of partial fish migration in lakes. Oikos 117, 40–46 10.1111/j.2007.0030-1299.16118.x (doi:10.1111/j.2007.0030-1299.16118.x) [DOI] [Google Scholar]