Abstract
Assassin bugs (Stenolemus bituberus) hunt web-building spiders by invading the web and plucking the silk to generate vibrations that lure the resident spider into striking range. To test whether vibrations generated by bugs aggressively mimic the vibrations generated by insect prey, we compared the responses of spiders to bugs with how they responded to prey, courting male spiders and leaves falling into the web. We also analysed the associated vibrations. Similar spider orientation and approach behaviours were observed in response to vibrations from bugs and prey, whereas different behaviours were observed in response to vibrations from male spiders and leaves. Peak frequency and duration of vibrations generated by bugs were similar to those generated by prey and courting males. Further, vibrations from bugs had a temporal structure and amplitude that were similar to vibrations generated by leg and body movements of prey and distinctly different to vibrations from courting males or leaves, or prey beating their wings. To be an effective predator, bugs do not need to mimic the full range of prey vibrations. Instead bugs are general mimics of a subset of prey vibrations that fall within the range of vibrations classified by spiders as ‘prey’.
Keywords: aggressive mimicry, Stenolemus bituberus, web-building spiders, vibration, lure
1. Introduction
Predators commonly rely on cryptic morphology and behaviour to avoid detection by the prey they are stalking or preparing to ambush. However, predators belonging to a less-studied minority actively advertise their presence and use deceptive communication to lure their prey into range of attack. These predators tap into existing sensory and perceptual biases in their prey, exploiting their tendency to approach certain stimuli. When the lure resembles another organism and solicits a similar response, the luring tactic is called ‘aggressive mimicry’ [1,2]. Some aggressive mimics, especially those exploiting sexual signals, are highly specific (sensu [3]), bearing close resemblance to a readily identifiable model. For example, Photuris fireflies mimic the visual courtship signals of female Photinus to attract males as prey [4–6]. Portia fimbriata jumping spiders lure female Euryattus sp. by mimicking male courtship vibrations [7]. Bolas spiders use chemical aggressive mimicry, imitating the pheromones from female moths to attract males [8–11]. Other aggressive mimics are more general, having limited resemblance to models and perhaps bearing similarity to a broader class of models rather than a specific model. For example, anglerfish use a general food-mimicking lure in front of their head to attract small prey [12,13].
Web-building spiders rely on vibrations in their webs to detect, identify and locate intercepted prey, and respond in a characteristic and predictable manner [14–17]. However, reliance on vibratory cues and predictable responses leaves web-building spiders vulnerable to exploitation by predators that aggressively mimic prey stimuli to gain control over their behaviour. Spiders from at least five different families routinely invade the webs of other spiders and lure them as prey with vibratory signals (e.g. Pholcus ‘cellar’ or ‘daddy long-leg’ spiders [18]; salticid ‘jumping’ spiders from the genera Portia, Brettus, Cyrba and Gelotia [19,20]). Several jumping spiders have been reported to use trial and error to derive effective signals [7,19–23]. To date, only one previous study has demonstrated a resemblance between vibratory signals used by web-invading spiders to manipulate prey spider behaviour and those generated by prey struggling in the prey spider's web [23].
As web-builders themselves, or as predators descended from web-building ancestors, it is perhaps not surprising to discover web invasion and vibratory deception in aranaeophagic spiders. A much more unexpected example comes from the aranaeophagic insect Stenolemus bituberus Stål (Emesinae, Reduviidae; electronic supplementary material), an assassin bug (hereafter ‘bug’) that uses two distinct predatory strategies, stalking and luring, to hunt web-building spiders [24,25] (electronic supplementary material). When stalking, they slowly approach the spider until within striking range and when luring they use their forelegs to pluck the silk threads, which lures the resident spider to within striking range [24–26]. Qualitative observations suggest that spiders approach luring bugs in a manner similar to how they approach prey struggling in the web, leading to the hypothesis that the plucking behaviours of bugs generate vibrations that mimic the vibrations generated by prey struggling in the web [24,25]. We test this hypothesis by quantitatively comparing behavioural responses of spiders to bugs and prey. Other vibrations in spider webs include mates and debris, and these are also potential models for aggressive mimicry. Hence, we also compare spider behaviour in response to courting males and leaves falling into the web. Further, to ascertain the resemblance between bug signals and each of the potential models, we quantify and compare the frequency, temporal and amplitude structure of vibrations generated by bugs to the vibrations generated by prey, mates and debris.
2. Material and methods
(a). Study animals
While bugs hunt web-building spiders from several genera they most often hunt spiders from the genus Achaearanea (Theridiidae [24]) and use luring to bring these spiders within range of attack [25]. Hence, we selected Achaearanea sp. as prey in our trials, using juveniles and adults (males and females) collected from the grounds of Macquarie University (Sydney, Australia). Individual spiders were housed in the laboratory inside wooden frames (20 × 20 × 3 cm) for at least 2 days before being used in a trial, allowing them to build webs. Male Achaearanea sp. (n = 5) were used to record courtship behaviour in the webs of adult females. We maintained males in individual 10 ml plastic vials. The laboratory was maintained at 24–26°C and 60–70 per cent RH under full spectrum lighting on a 12 L : 12 D cycle. We collected juvenile and adult bugs from Macquarie University grounds on the day before testing.
Two potential prey were used: Drosophila melanogaster (hereafter referred to as ‘vinegar fly’), maintained in laboratory cultures, and an unidentified species of Aphididae (hereafter referred to as ‘aphid’) that is often found in the webs of Achaearanea sp. in nature (A. Wignall 2007, personal observation). Specimens of spiders and aphids have been lodged at the Australian Museum, Sydney. We used sweep-netting to obtain live specimens of the aphids from standing grass immediately before each trial. To simulate debris falling into a spider web, we collected fresh leaves from plants (mean weight: 0.014 g, s.d.: ±0.003 g, range: 0.008 g−0.02 g). We recorded 20 examples each of hunting by bugs, struggles of vinegar flies and aphids, and leaves falling into the web. We also recorded five examples of courting male spiders. All vibrations from each recording were analysed.
(b). Recording techniques
Experiments were conducted inside a sound-attenuating chamber (1.8 × 1.8 × 2 m) on a vibration-isolating table (Kinetic Systems, USA) under full spectrum lighting. Video recordings were made using a 540TVL GoVideo camera (Digital Products International Inc., USA) and digitized to hard drive through a Digital Rapids DC 1500 A to D board using Stream v. 1.5.23 software (Digital Rapids, Canada) run on a computer with Microsoft Windows XP operating system (Dual 3.0 GHz Xeon, 4 GB RAM). Vibrations were recorded using a digital laser vibrometer (Polytec PDV100, Germany). The AES (Audio Engineering Society) output of the laser vibrometer was converted to EBU (CO3, Midiman, M-Audio, USA) and synchronized to the audio track of the video recording. Vibrations were recorded at 44.1 kHz per 16 bits. To ensure the laser was adequately focused, we monitored output of the laser vibrometer during the trials using headphones from a Eurorack UB 802 soundboard (Behringer, Germany) that was connected to the laser vibrometer's analogue output.
(c). Experimental procedure
To record vibrations generated by hunting bugs, a frame containing an Achaearanea sp. in its web was placed on the vibration-isolating table the day before testing, along with a bug in a 10 ml plastic vial containing clean paper as substrate. Four vinegar fly wings, cut in half, were carefully placed at regular intervals in the spider web, each approximately 5 cm from the spider's resting place. These wings were used as focal points for the laser vibrometer to record transverse vibrations, while minimizing the loading on the web [17]. The wing closest to the spider was selected for recordings. Care was taken not to damage the web. If damage did occur, the web was set aside and was not used in trials until the following day, allowing the spider time to repair the damage. Recordings began 5 min after placing wings in the web, during which time the spider could settle down if it had been disturbed during the set-up of equipment.
The piece of paper that the bug stood on inside its vial was removed from the vial along with the bug and placed adjacent to the periphery of the spider web. The bug was allowed to make its own way onto the web. Trials were aborted if hunting did not begin within 30 min. Hunting was defined as antennal tapping of the substrate or antennal waving (up and down movement of the distal segment of the antennae) in the direction of the spider, both of which are commonly observed during predation [26]. We continued recording until the spider was caught by the bug, or until the bug stopped hunting (defined as no predatory behaviours observed for 30 min).
To record the vibrations generated by prey struggling in the web, we used a pipette to collect prey (either a vinegar fly or aphid) and gently propel it into the web from a distance of 5 cm. If the prey did not stick in the web, we aborted the trial and set up a new spider. We continued recording until the spider oriented to or approached the prey. If no spider response was observed within 10 min, we ended the trial. To record the courtship vibrations generated by male spiders, we released a male spider onto the frame and allowed 30 min for courtship to begin (defined by the male either approaching and tapping the female or cutting silk threads). Recordings of courting males continued until the male copulated with the female. To record the vibrations generated by leaves falling into a web, we dropped the leaf into the web from a distance of 5 cm, and continued recording for 10 min. Trials in which the leaf did not stick to the web were aborted.
(d). Analyses
To assess spider responses to each of our treatments, we defined five general sequences of spider response: (i) no response; (ii) orient, no approach to stimulus; (iii) orient, pause, approach stimulus; (iv) orient, pause, approach stimulus, adopt copulatory position; and (v) approach stimulus, no pause. We also measured the latency until the spider responded to the vibrations by counting the number of vibrations before a response and by measuring the latency until response from the start of the vibration immediately preceding the spider's response. We analysed vibrations using Autosignal v. 1.7 (SeaSolve Software, Inc., USA) and Peak v. 4.13 (BIAS, Inc., USA), which allows synchronized analysis of video and acoustic recordings. We measured the frequency and duration of each vibration generated by luring bugs, prey, courting males and leaves falling into the spider web. Vibrations were often of very short duration (less than 0.006 s), which does not allow spectrograms to be calculated. We used fast Fourier transform to calculate peak waveform frequencies (similar results were obtained when analysed with discrete Fourier transform and peak-to-peak analyses). When data deviated significantly from a normal distribution, we used a Box-Cox transformation [27]. The sample sizes for analyses varied slightly depending on whether a particular variable could be calculated from a vibration. Statistical analyses were performed using JMP v. 5.0.1.2 for Apple Macintosh (SAS Institute, Cary, NC, USA).
3. Results
(a). Differential responses of spiders to stimuli
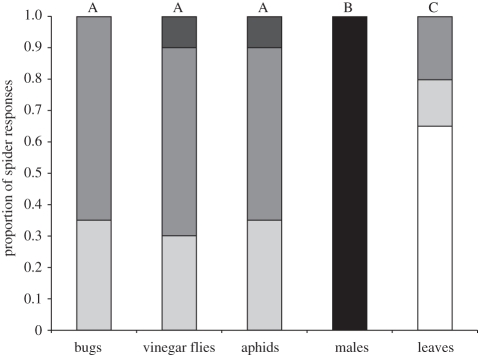
We found significant differences in the responses of spiders to the different vibration sources (Fisher's exact test p < 0.01; figure 1). How spiders responded to bugs was extremely similar to how spiders responded to prey (Fisher's exact tests, vinegar flies versus bugs: p = 0.56; aphids versus bugs: p = 0.65; vinegar flies versus aphids, p = 1.00). For both types of prey there were only two instances in which the spider made a direct approach, and each of these four instances was within 1 s of the initial impact in the web. Spider responses to other vibration sources were significantly different (all p < 0.01; figure 1). Spiders tended not to respond to leaves (65% no response) and female spiders responded to male courtship by entering a characteristic copulatory position.
Figure 1.
Responses of spiders to each vibration source. Significant differences are indicated by different letters (Fisher's exact tests). Black bar: orient, pause, copulatory position. Dark grey bar: direct approach. Grey bar: orient, pause, approach. Light grey bar: orient, no approach. White bar: no response.
There were no significant differences among vibration sources in the response latency (i.e. time elapsing from the last vibration until the spider responded; whole model F4,67 = 1.84, p = 0.13) or in the number of vibrations preceding the spider's response (F4,67 = 1.11, p = 0.36).
(b). Vibratory characteristics of stimuli
Linear discriminant analysis showed that there was significant variation in frequency, amplitude and duration of vibrations generated by hunting bugs, struggling vinegar flies and aphids, courting male spiders and leaves falling into webs (Wilks's lambda: approx. F12,12 610 = 328.07, p < 0.01). There was substantial overlap in the frequency, amplitude and/or duration of vibrations generated by each source, with 33.08 per cent of vibrations misclassified (i.e. resembling more than one vibration source; electronic supplementary material).
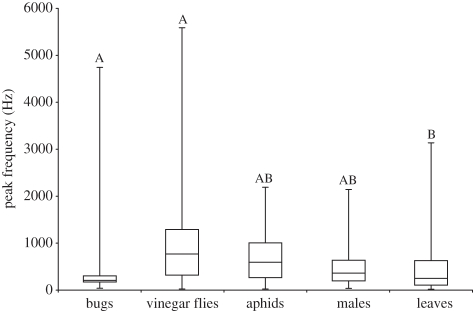
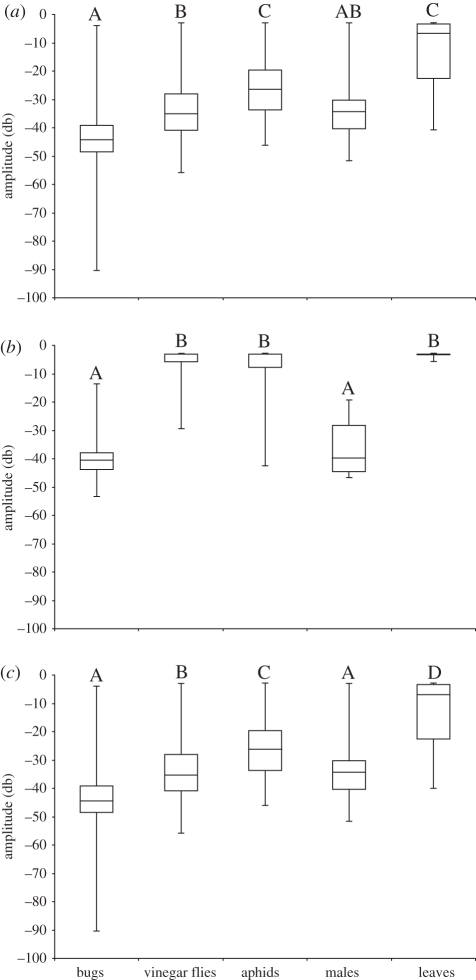
Repeated-measures MANOVA revealed significant differences in the frequency, amplitude and duration of vibrations generated by each source (whole model: F4,4768 = 48.26, p < 0.01). Repeated-measures ANOVA on the peak frequencies showed that the vibrations generated by bugs plucking silk and vinegar flies struggling in the web were of significantly higher peak frequency than those generated by leaves falling in the web (F84,4704 = 58.89, p < 0.01; see figure 2 for Tukey's tests). However, there were no significant differences in the peak frequency of vibrations generated by bugs and the two prey types or courting males. Repeated-measures ANOVA on vibration amplitude showed that bugs generated lower amplitudes than prey or leaves in webs (F84,4706 = 64.51, p < 0.01; see figure 3a for Tukey's tests). We also compared amplitudes of the first vibration generated in the web of each recording, as these include the initial ‘impact’ with the web. Initial amplitudes generated by bugs and courting males were lower than those from prey or leaves (F4,81 = 96.15, p < 0.01; see figure 3b for Tukey's tests). When these initial impact vibrations were excluded from the analyses, the amplitudes generated by bugs only overlapped those of courting males (F71,4634 = 69.96, p < 0.01; see figure 3c for Tukey's tests). However, amplitudes generated by courting males also overlapped with those generated by the two prey. Repeated-measures ANOVA on vibration duration generated a significant overall model (F84,4714 = 30.38, p < 0.01) but found no evidence of differences among the sources (F4,80 = 2.25, p = 0.07). A repeated-measures ANOVA on interval between vibrations also generated a significant overall model (whole model: F71,4625 = 16.78, p < 0.01) but again found no evidence of differences among the sources (F4,67 = 1.14, p = 0.34).
Figure 2.
Peak frequency (back-transformed medians and inter-quartile ranges) of vibrations generated by hunting S. bituberus, struggling D. melanogaster and aphids, courting males, and leaves falling into the web. Significant differences are indicated by different letters (Tukey's tests).
Figure 3.
Amplitude (back-transformed medians and inter-quartile ranges) of (a) all vibrations, (b) the first vibration of each trial and (c) all vibrations except for the first generated, by hunting bugs, struggling vinegar flies and aphids, courting males and leaves falling into the web. Significant differences are indicated by different letters (Tukey's tests).
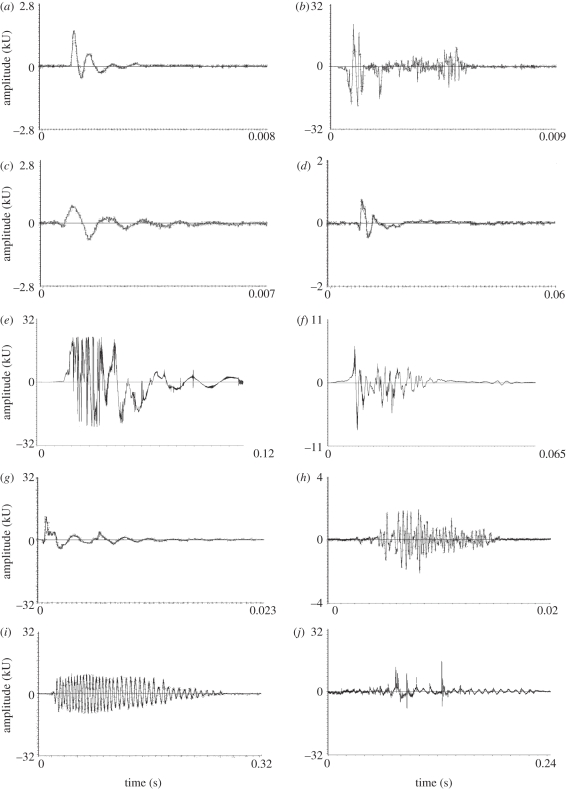
Global differences in frequency and duration provide limited discrimination among the stimuli, so we also examined the fine temporal structure and relative amplitude of vibrations produced by the different sources. Vibrations generated by bugs (figure 4a) were most similar to the short, low-amplitude vibrations generated by prey moving their body or limbs (figure 4c,d) and courting males (figure 4g). In addition to these vibrations, male spiders also generated a repeated, distinctive vibration (figure 4h) that was never observed from any of the other sources. This distinctive vibration would provide females with the means to discriminate males from other stimuli. The initial vibrations generated by both prey and leaves were characterized by a single high-amplitude vibration generated by impact with the web (figure 4b,e,f), and such vibrations were never observed in bugs or males. Wing beats of struggling prey were of much higher amplitude, of longer duration, and of both more variable and higher frequency than body and limb movements; again, such vibrations were never observed from bugs (figure 4i,j). After the initial impact, leaves made few, if any, subsequent vibrations in the web. In contrast, prey continued generating high-amplitude vibrations from wing beating and low-amplitude vibrations from movements of the limbs and body.
Figure 4.
Representative waveforms of vibrations generated by (a) bug plucking web, (b) leaf impacting web, (c) single aphid vibration making a small leg or body movement, (d) single vinegar fly vibration making a small leg or body movement, (e) aphid impacting web, (f) vinegar fly impacting web, (g) male spider making a small movement or step, (h) male spider ‘stridulating’ in web, (i) aphid wing beats in web and (j) vinegar fly wing beats in web. Note differences in time and amplitude scales.
4. Discussion
How spiders responded to signalling bugs was similar to how they responded to prey (vinegar flies and aphids) caught in the web. These responses were characterized by orientation to the source, or orientation and intermittent approach. This is in sharp contrast to the responses of female spiders to courting males, which were characterized by the adoption of a distinctive copulatory posture and the lack of detectable responses to leaves (figure 1). This tendency supports the interpretation of luring behaviour of bugs as aggressive mimicry of prey. Such an interpretation leads naturally to questions of vibratory features by which spiders classify vinegar flies and aphids, and misclassify luring bugs, as prey.
The vibrations generated by bugs showed clear structural similarities to those generated by prey struggling in the web. While there were some similarities to the vibrations generated by leaves and courting males, these vibrations also show clear differences in fine structure to those generated by bugs and prey. The distinctive responses of spiders to leaves and courting males indicate that they can readily discern differences between these stimuli. Courting males (the source that generated vibrations with the most overlap in peak frequency distribution with the prey) generate a temporally distinctive pattern that was not observed from any other source throughout interactions with females (figure 4h). Such vibrations are common in courting Achaearanea (e.g. observations of Lubin [28]) and may be generated by abdominal vibrating, tremulation or stridulation, as stridulatory devices are common in theridiid spiders [29]. Male spiders, on entering the female's web, also spend up to several minutes approaching the female and tapping her legs and body. Females may use these tactile stimuli and vibratory differences to identify males in the web. Leaves falling into the web generated a high-amplitude pulse of vibration on the initial impact with the web, but rarely generated any subsequent vibrations. While prey also generate an initial high-amplitude vibration upon impact with the web, they also produce numerous vibrations after impact as they struggle. The presence of subsequent vibrations may provide spiders with a means to discriminate between debris and prey.
Vibrations generated by bugs occupied a narrower band of frequencies compared with the much more variable and often higher-frequency vibrations from both types of prey. Given the similar size range of aphids and vinegar flies, and the similar challenge faced by struggling prey stuck in a web, it is not surprising that there is substantial overlap in characteristics of the vibrations produced. Rather than aggressively mimicking a specific prey model, bugs are best interpreted as general mimics that exploit a bias in spiders to approach prey-like vibrations in the web. In addition to wing-beating, prey ensnared in the web also make smaller-amplitude, slower movements such as pulling a leg or moving the head or thorax, which generate much lower-frequency and -amplitude vibrations. Bugs pluck, stretch and cut silk threads very slowly with their forelegs, using low-amplitude movements [26]. These behaviours do not generate the higher-frequency and -amplitude vibrations observed in impact or wing beating of prey or courtship vibrations of courting males. To a spider, the vibrations generated by bugs may resemble small or exhausted prey that cannot mount high-amplitude or high-frequency struggles.
The responses of spiders to prey in the web were occasionally characterized by a direct and rapid approach towards prey without pauses. This response was never observed towards bugs or the other sources, and may be elicited by the presence of higher-frequency and -amplitude vibrations in the struggles of prey. Flying prey that is ensnared by a web will usually beat its wings while struggling to escape. Vinegar flies can beat their wings at up to 185 times per second during flight [30]. In a three-dimensional web such as that of Achaearanea, this can generate vibrations in the high-frequency range observed, whereas in two-dimensional webs the frequencies generated by prey appear to be much lower (compare [31] with [17]). Although the physics of vibration propagation in three-dimensional webs is not well understood, the behaviour of prey is able to generate higher-frequency vibrations in the web than is observed from bugs. The tendency of bugs to generate vibrations in the lower-frequency and -amplitude range produced by prey may be adaptive. Spiders are dangerous prey, and we have observed bugs being counter-attacked, killed and eaten by the spider they were hunting [24,25]. This risk may be considerably increased when the spider approaches rapidly and without pause. The only occasions when we observed spiders directly approaching prey were immediately after high-amplitude vibrations. Bugs hence appear to aggressively mimic a broad class of prey-like vibrations that are effective at soliciting approaches but are also unlikely to elicit highly aggressive, dangerous responses.
Acknowledgements
We would like to thank F. Barth, C. Evans, A. Harmer, J. Hemmi, M. Herberstein, R. Jackson, D. Kane, R. Mankin, C. Schaber and S. Wilcox for their assistance and useful discussions throughout the period of this study. This work was supported by a grant from the Australian Research Council.
References
- 1.Wickler W. 1968. Mimicry in plants and animals. London, UK: Weidenfeld and Nicolson [Google Scholar]
- 2.Vane-Wright R. I. 1980. On the definition of mimicry. Biol. J. Linn. Soc. 13, 1–6 10.1111/j.1095-8312.1980.tb00066.x (doi:10.1111/j.1095-8312.1980.tb00066.x) [DOI] [Google Scholar]
- 3.Edmunds M. 2000. Why are there good and poor mimics? Biol. J. Linn. Soc. 70, 459–466 10.1006/bijl.1999.0425 (doi:10.1006/bijl.1999.0425) [DOI] [Google Scholar]
- 4.Lloyd J. E. 1975. Aggressive mimicry in Photuris fireflies: signal repertoires by femmes fatales. Science 187, 452–453 10.1126/science.187.4175.452 (doi:10.1126/science.187.4175.452) [DOI] [PubMed] [Google Scholar]
- 5.Lloyd J. E. 1984. Occurrence of aggressive mimicry in fireflies. Flor. Entomol. 67, 368–376 10.2307/3494715 (doi:10.2307/3494715 ) [DOI] [Google Scholar]
- 6.Lewis S. M., Cratsley C. K. 2008. Flash signal evolution, mate choice, and predation in fireflies. Ann. Rev. Entomol. 53, 293–321 10.1146/annurev.ento.53.103106.093346 (doi:10.1146/annurev.ento.53.103106.093346) [DOI] [PubMed] [Google Scholar]
- 7.Jackson R. R., Wilcox R. S. 1990. Aggressive mimicry, prey-specific predatory behaviour and predator-recognition in the predator–prey interactions of Portia fimbriata and Euryattus sp., jumping spiders from Queensland. Behav. Ecol. Sociobiol. 26, 111–119 10.1007/BF00171580 (doi:10.1007/BF00171580) [DOI] [Google Scholar]
- 8.Eberhard W. B. 1977. Aggressive chemical mimicry by a bolas spider. Science 198, 1173–1175 10.1126/science.198.4322.1173 (doi:10.1126/science.198.4322.1173) [DOI] [PubMed] [Google Scholar]
- 9.Gemeno C., Yeargan K. V., Haynes K. F. 2000. Aggressive chemical mimicry by the bolas spider Mastophora hutchinsoni: identification and quantification of a major prey's sex pheromone components in the spider's volatile emissions. J. Chem. Ecol. 26, 1235–1243 10.1023/A:1005488128468 (doi:10.1023/A:1005488128468) [DOI] [Google Scholar]
- 10.Haynes K. F., Yeargan K. V., Gemeno C. 2001. Detection of prey by a spider that aggressively mimics pheromone blends. J. Insect. Behav. 14, 535–544 10.1023/A:1011128223782 (doi:10.1023/A:1011128223782) [DOI] [Google Scholar]
- 11.Haynes K. F., Gemeno C., Yeargan K. V., Millar J. G., Johnson K. M. 2002. Aggressive chemical mimicry of moth pheromones by a bolas spider: how does this specialist predator attract more than one species of prey? Chemecology 12, 99–105 10.1007/s00049-002-8332-2 (doi:10.1007/s00049-002-8332-2) [DOI] [Google Scholar]
- 12.Gudger E. W. 1946. The angler-fishes, Lophius piscatorius et americanus, use the lure in fishing. Am. Nat. 79, 542–548 10.1086/281289 (doi:10.1086/281289) [DOI] [Google Scholar]
- 13.Pietsch T. W., Grobecker D. B. 1978. The compleat angler: aggressive mimicry in an antennariid anglerfish. Science 201, 369–370 10.1126/science.201.4353.369 (doi:10.1126/science.201.4353.369) [DOI] [PubMed] [Google Scholar]
- 14.Parry D. A. 1965. The signal generated by an insect in a spider's web. J. Exp. Biol. 43, 185–192 [DOI] [PubMed] [Google Scholar]
- 15.Suter R. B. 1978. Cyclosa turbinata (Araneae, Araneidae): prey discrimination via web-borne vibrations. Behav. Ecol. Sociobiol. 3, 283–296 10.1007/BF00296314 (doi:10.1007/BF00296314) [DOI] [Google Scholar]
- 16.Barth F. G. 1982. Spiders and vibratory signals: sensory reception and behavioural significance. In Spider communication: mechanisms and ecological significance (eds Witt P. N., Rovner J. S.). Princeton, NJ: Princeton University Press [Google Scholar]
- 17.Landolfa M. A., Barth F. G. 1996. Vibrations in the orb web of the spider Nephila clavipes: cues for discrimination and orientation. J. Comp. Physiol. A 179, 493–508 10.1007/BF00192316 (doi:10.1007/BF00192316) [DOI] [Google Scholar]
- 18.Jackson R. R., Brassington R. J. 1987. The biology of Pholcus phalangioides (Araneae, Pholcidae): predatory versatility, araneophagy and aggressive mimicry. J. Zool. Lond. 211, 227–238 10.1111/j.1469-7998.1987.tb01531.x (doi:10.1111/j.1469-7998.1987.tb01531.x) [DOI] [Google Scholar]
- 19.Jackson R. R. 1990. Predatory and silk utilisation behaviour of Gelotia sp. indet. (Araneae: Salticidae: Spartaeinae), a web-invading aggressive mimic from Sri Lanka. NZ J. Zool. 17, 475–482 [Google Scholar]
- 20.Jackson R. R. 2002. Trial-and-error derivation of aggressive-mimicry signals by Brettus and Cyrba, spartaeine jumping spiders (Araneae: Salticidae) from Israel, Kenya, and Sri Lanka. NZ J. Zool. 29, 95–117 10.1080/03014223.2002.9518294 (doi:10.1080/03014223.2002.9518294) [DOI] [Google Scholar]
- 21.Jackson R. R., Wilcox R. S. 1993. Spider flexibly chooses aggressive mimicry signals for different prey by trial and error. Behaviour 127, 21–36 10.1163/156853993X00407 (doi:10.1163/156853993X00407) [DOI] [Google Scholar]
- 22.Jackson R. R. 1995. Cues for web invasion and aggressive mimicry signalling in Portia (Araneae, Salticidae). J. Zool. Lond. 236, 131–149 10.111/j.1469-7998.1995.tb01789.x (doi:10.111/j.1469-7998.1995.tb01789.x) [DOI] [Google Scholar]
- 23.Tarsitano M., Jackson R. R., Kirchner W. H. 2000. Signals and signal choices made by the araneophagic jumping spider Portia fimbriata while hunting the orb-weaving web spiders Zygiella x-notata and Zosis geniculatus. Ethology 106, 595–615 10.1046/j.1439-0310.2000.00570.x (doi:10.1046/j.1439-0310.2000.00570.x) [DOI] [Google Scholar]
- 24.Wignall A. E., Taylor P. W. 2008. Biology and behaviour of an araneophagic assassin bug Stenolemus bituberus including a morphometric analysis of the instars (Heteroptera, Reduviidae). J. Nat. Hist. 42, 59–76 10.1080/00222930701825150 (doi:10.1080/00222930701825150) [DOI] [Google Scholar]
- 25.Wignall A. E., Taylor P. W. 2009. Alternative predatory tactics in an araneophagic assassin bug. Acta Ethol. 12, 23–27 10.1007/s10211-008-0049-y (doi:10.1007/s10211-008-0049-y) [DOI] [Google Scholar]
- 26.Wignall A. E., Taylor P. W. 2010. Predatory behaviour of an araneophagic assassin bug. J. Ethol. 28, 437–445 10.1007/s10164-009-0202-8 (doi:10.1007/s10164-009-0202-8) [DOI] [Google Scholar]
- 27.Box G. E. P., Cox D. R. 1964. An analysis of transformations. J. R. Stat. Soc. B 26, 211–252 [Google Scholar]
- 28.Lubin Y. D. 1986. Courtship and alternative mating tactics in a social spider. J. Arachnol. 14, 239–257 [Google Scholar]
- 29.Agnarsson I. 2004. Morphological phylogeny of cobweb spiders and their relatives (Araneae, Araneoidea, Theridiidae). Zool. J. Linn. Soc. 141, 447–626 10.1111/j.1096-3642.2004.00120.x (doi:10.1111/j.1096-3642.2004.00120.x) [DOI] [Google Scholar]
- 30.Zanker J. M., Gotz K. G. 1990. The wing beat of Drosophila melanogaster. II. Dynamics. Phil. Trans. R. Soc. Lond. B 327, 19–44 10.1098/rstb.1990.0041 (doi:10.1098/rstb.1990.0041) [DOI] [Google Scholar]
- 31.Walcott C. 1969. A spider's vibration receptor: its anatomy and physiology. Am. Zool. 9, 133–144 10.1093/icb/9.1.133 (doi:10.1093/icb/9.1.133) [DOI] [PubMed] [Google Scholar]