Abstract
The endosymbiotic bacterium Wolbachia pipientis manipulates host reproduction by rendering infected males reproductively incompatible with uninfected females (cytoplasmic incompatibility; CI). CI is believed to occur as a result of Wolbachia-induced modifications to sperm during maturation, which prevent infected sperm from initiating successful zygote development when fertilizing uninfected females' eggs. However, the mechanism by which CI occurs has been little studied outside the genus Drosophila. Here, we show that in the sperm heteromorphic Mediterranean flour moth, Ephestia kuehniella, infected males transfer fewer fertile sperm at mating than uninfected males. In contrast, non-fertile apyrene sperm are not affected. This indicates that Wolbachia may only affect fertile sperm production and highlights the potential of the Lepidoptera as a model for examining the mechanism by which Wolbachia induces CI in insects.
Keywords: endosymbiont, sperm heteromorphism, Lepidoptera, spermatogenesis
1. Introduction
The endosymbiotic bacterium Wolbachia pipientis is potentially the most common symbiont on Earth [1]. The key to its success is its ability to manipulate host reproduction to increase its transmission. Commonly Wolbachia induces cytoplasmic incompatibility (CI), which causes embryo death when sperm from an infected male fertilizes the ova of an uninfected female; all other crosses are viable. These manipulations lead to an increase in the number of infected females, which is beneficial to Wolbachia as it is maternally transmitted.
CI is believed to occur as a result of Wolbachia-induced modifications to maturing sperm [2]. These modifications must occur in the early stages of spermatogenesis, as the bacteria are shed from mature sperm cells. The mechanism by which CI occurs is unknown, although it may occur via Wolbachia-induced modifications of cytoskeletal activity [2]. Sperm modifications by Wolbachia also affect host fertility—in Drosophila simulans, infected males produce fewer sperm than uninfected males [3] and have reduced paternity in sperm competition [4]. However, it is unknown whether similar effects are seen in other non-drosophilid–Wolbachia host systems.
Here, we examine whether Wolbachia is associated with reductions in sperm transfer in the Mediterranean flour moth, Ephestia kuehniella. The effect of CI-inducing Wolbachia on sperm production is particularly interesting in the Lepidoptera, as males are sperm heteromorphic, producing both fertile and non-fertile sperm [5]. Both are transferred during copulation, and non-fertile sperm can constitute up to 95 per cent of an ejaculate [6]. Non-fertile sperm appear to suppress the female receptivity by filling their sperm storage organ [7]. It is unknown whether Wolbachia modifies both sperm types, or if it selectively modifies fertile sperm, the only type relevant to CI induction. Examining the effect of Wolbachia on lepidopteran heteromorphic sperm may increase our understanding of how Wolbachia induces CI in insects. In Lepidoptera, male mating capacity is directly related to sperm production (e.g. [8]). If Wolbachia infection is detrimental to male fertility, then we predict that infected males will transfer fewer sperm than uninfected males and, as a consequence, achieve fewer matings in their lifetime.
2. Material and methods
Moths were obtained from a laboratory population, singly infected with group A Wolbachia inducing high levels of CI (greater than 80%), collected from the wild in Yokohama, Japan [9]. Both infected and uninfected individuals were maintained in a large interbreeding laboratory population (derived from approx. 100 matrilines; electronic supplementary material, S1). By combining the offspring of approximately 60 infected and approximately 40 uninfected matrilines, we established separate infected and uninfected populations prior to the onset of the experiment. All moths were reared following a standard protocol [9]; see electronic supplementary material, S1 for further details on the populations used. Following eclosion, 50 virgin males from each population were individually coupled with virgin Wolbachia-infected females. For every subsequent day of his life, each male was allowed access to a fresh virgin female for 6 h. The first two females to mate with each male were removed immediately following copulation, and used to estimate sperm transfer. Males that failed to mate were isolated, and presented with a fresh female the following day. This continued until death and adult longevity noted. Lifetime mating success was measured as the total number of matings. Male body size was estimated by measuring the length of the forewing [10]. The total number of fertile and non-fertile sperm present in the male's first and second spermatophores was measured following a standard protocol [6]. Infection status was determined by PCR for the universal Wolbachia-specific primers wsp81F and wsp691R [11].
In order to further test our hypothesis that Wolbachia is detrimental to male fertility, we also repeated the first part of our experiment using a population of E. kuehniella produced via antibiotic curing (see electronic supplementary material, S2). Comparison with our Wolbachia-infected population allowed us to examine the impact of Wolbachia on male fertility while excluding potential genetic differences between the two populations initially used.
Analyses were performed in R (v. 2.0.1). The impact of Wolbachia infection on sperm transfer, and the total number of matings, was analysed using generalized linear models [12], specifying Poisson error distributions (data corrected for over-dispersion) with male size as a covariate. The effect on longevity was analysed using Cox's proportional hazards analyses on uncensored data, again with male size as a covariate. Model simplification was carried out with interactions removed before main effects.
3. Results
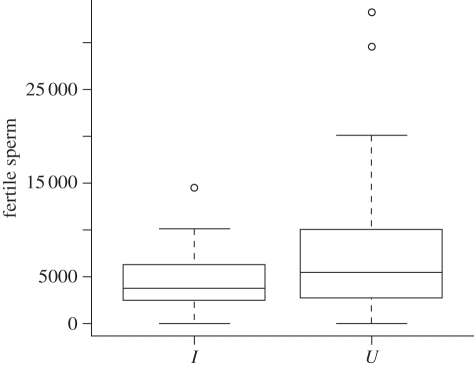
There was no difference with respect to infection status in the number of sperm transferred on a male's first mating, for either fertile (uninfected (U): mean 7873 ± 961 s.e.; infected (I): 6641 ± 939, F = 0.097, p > 0.7, d.f. = 53), or non-fertile sperm (U: 25 751 ± 2200; I: 26 178 ± 3386, F = 0.002, p > 0.9, d.f. = 47). Wolbachia infection did, however, affect fertile sperm transfer on males' second mating; infected males transferred approximately 38 per cent fewer fertile sperm compared with uninfected males (F = 5.80, p = 0.0199, d.f. = 49, figure 1; fertile sperm transfer was also reduced on a male's third mating) (F = 5.25, p = 0.026, d.f. = 46). In contrast, infection status did not affect the number of non-fertile sperm transferred on a male's second mating (U: 27 042 ± 3633; I: 27 756 ± 4858, F = 0.065, p > 0.8, d.f. = 49). This result was confirmed using antibiotically cured males (see electronic supplementary material, S2).
Figure 1.
The number of fertile eupyrene sperm transferred by Wolbachia-infected (I, n = 24) and uninfected (U, n = 38) males to females on their second mating. Median ± upper and lower quartile.
There was no effect of Wolbachia infection on lifetime number of matings: infected and uninfected males mated on average five times (range 1–7; s.e. ±0.345 and 0.266, respectively; F = 0.772, p > 0.3, d.f. = 59). In addition, there was no difference in size between uninfected and infected males (mean ± s.e. mm, U: 6.44 ± 0.035; I: 6.46 ± 0.054, F = 0.038, p > 0.8, d.f. = 79). There was also no effect of Wolbachia infection on adult male longevity (χ2=2.5, p > 0.1), although uninfected males tended to live longer (10.7 ± 0.285 days) than infected males (9.4 ± 0.707 days).
4. Discussion
We found no effect of Wolbachia infection status on the number of fertile or non-fertile sperm transferred on a male's first mating. Infected males may eclose with sufficient sperm supplies to produce a ‘standard’-sized ejaculate on their first mating. In contrast, Wolbachia-infected males transferred fewer fertile sperm on their second mating. The difference in sperm numbers could be the result of different genetic backgrounds of the infected and uninfected females used to establish the matrilines. However, this is unlikely as moths were maintained in a large interbreeding population, and the infected and uninfected lines were maintained separately for only one generation prior to the start of the experiment (electronic supplementary material, S1). In addition, this finding was subsequently corroborated in a sample of 15 infected and 15 tetracycline-cured males derived from the same population (electronic supplementary material, S2). Similar results have been found in D. simulans, where infected males produce 40 per cent fewer spermatocysts and have slower sperm maturation than uninfected males, but this was revealed only in non-virgin males [3]. Our finding thus mirrors that of Drosophila and is the first evidence that Wolbachia affects sperm production in a non-drosophilid species.
The mechanism by which Wolbachia affects sperm is largely unknown. In D. simulans, Wolbachia is present in developing sperm cysts but removed during spermatid elongation [2]. The removal of the bacteria may be energetically costly, and place constraints on sperm production. The same could be the case in the Lepidoptera. The presence of Wolbachia may also directly damage the sperm. In the moth Philudoria potatoria, fertile spermatocytes harbouring large numbers of intracellular bacteria—likely to have been Wolbachia—were altered during the advanced stages of spermatogenesis, causing nuclear degeneration [13]. It is possible that a similar mechanism is responsible for the observed reduction in fertile sperm number in E. kuehniella.
In contrast to our results on fertile sperm, we found no effect of Wolbachia infection on non-fertile sperm transfer. To date, there has been no examination of the impact of Wolbachia infection on non-fertile sperm, although there are several possibilities why Wolbachia may exert a differential effect on fertile and non-fertile sperm. Wolbachia may specifically target fertile sperm because only they are involved in CI induction, or may affect both, but have only a detrimental effect on fertile sperm. There may be differences between fertile and non-fertile sperm in sensitivity to Wolbachia manipulation; fertile spermatogenesis is known to be more sensitive to both genetic and experimental manipulation (e.g. [14]). It could also be due to differences in the timing and duration of Wolbachia exposure during spermatogenesis. In the Lepidoptera, fertile spermatogenesis begins in the larvae and stops in the pupae, but non-fertile spermatogenesis begins close to pupation and continues in the adult [14]. In addition, the duration of meiosis differs markedly between non-fertile and fertile sperm, with non-fertile meiosis lasting a considerably shorter time [15]. Finally, the differential effect of Wolbachia may arise as a consequence of their dichotomous spermatogenesis. CI modification of sperm involves changes in sperm nuclear function and in Drosophila melanogaster at least, Wolbachia does not appear to disrupt any extra-nuclear sperm factors [16]. Therefore in the Lepidoptera, it is possible that Wolbachia cannot adversely affect apyrene sperm as they lack nuclei. These explanations are not necessarily mutually exclusive, and some or all of them may contribute to the observed differences.
We found no difference between infected and uninfected males in lifetime number of matings. In many insects including Lepidoptera, mating capacity is directly related to males' ability to produce ejaculates (e.g. [8]). Contrary to our findings, we predicted that Wolbachia-infected males would mate less frequently as a result of reduced fertile sperm production. Sperm production in the Lepidoptera is often positively associated with male body size (e.g. [17]). However, there was no difference in body size between infected and uninfected males and body size did not explain differences in sperm transfer between males. Body size, therefore, cannot explain the observed association between Wolbachia infection and lowered fertile sperm transfer by non-virgins. We also found no effect of Wolbachia infection on longevity. Reported effects of Wolbachia on male longevity in Drosophila are inconsistent (e.g. [18,19]), although in D. melanogaster the virulent Wolbachia popcorn strain causes prolific tissue degeneration, which significantly shortens lifespan [20].
This study is the first to show that Wolbachia affects sperm transfer in a non-drosophilid host; we have shown that male E. kuehniella moths infected with Wolbachia transfer fewer fertile sperm when remating than uninfected males. Intriguingly, no such effect was observed for non-fertile apyrene sperm. As a result of their sperm heteromorphism, the Lepidoptera potentially represent an ideal model system to unravel the mechanisms underlying Wolbachia-induced CI.
Acknowledgements
We thank Prof. Tetsuhiko Sasaki for providing the insects. This research was funded by a grant from the Leverhulme Trust. The authors also wish to thank the JSPS, the ESF, NERC and the Royal Society.
References
- 1.O'Neill S. L., Hoffmann A. A., Werren J. H. 1997. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Riparbelli M. G., Giordano R., Callaini G. 2007. Effects of Wolbachia on sperm maturation and architecture in Drosophila simulans Riverside. Mech. Dev. 124, 699–714 10.1016/j.mod.2007.07.001 (doi:10.1016/j.mod.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 3.Snook R. R., Cleland S. Y., Wolfner M. F., Karr T. L. 2000. Offsetting effects of Wolbachia infection and heat shock on sperm production in Drosophila simulans: analyses of fecundity, fertility and accessory gland proteins. Genetics 155, 167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champion de Crespigny F. E., Wedell N. 2006. Wolbachia infection reduces sperm competitive ability in an insect. Proc. R. Soc. B 273, 1455–1458 10.1098/rspb.2006.3478 (doi:10.1098/rspb.2006.3478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meves F. 1903. Ueber oligopyrene und apyrene spermien und uber ihre entstehung, nach beobachtungen an Paludina und Pygaera. Arch. Mikrosk. Anat. Ent. 61, 1–84 10.1007/BF02977916 (doi:10.1007/BF02977916) [DOI] [Google Scholar]
- 6.Cook P. A., Wedell N. 1996. Ejaculate dynamics in butterflies: a strategy for maximizing fertilization success? Proc. R. Soc. Lond. B 263, 1047–1051 10.1098/rspb.1996.0154 (doi:10.1098/rspb.1996.0154) [DOI] [Google Scholar]
- 7.Cook P. A., Wedell N. 1999. Non-fertile sperm delay female remating. Nature 397, 486. 10.1038/17257 (doi:10.1038/17257) [DOI] [Google Scholar]
- 8.Svärd L., Wiklund C. 1989. Mass and production rate of ejaculates in relation to monandry/polyandry in butterflies. Behav. Ecol. Sociobiol. 24, 395–402 10.1007/BF00293267 (doi:10.1007/BF00293267) [DOI] [Google Scholar]
- 9.Sasaki T., Ishikawa H. 1999. Wolbachia infections and cytoplasmic incompatibility in the almond moth and the Mediterranean flour moth. Zool. Sci. 16, 739–744 10.2108/zsj.16.739 (doi:10.2108/zsj.16.739) [DOI] [Google Scholar]
- 10.Reid J. (ed.)1976. Techniques. In The moths and butterflies of Great Britain and Ireland, vol. 1 (ed. Reid J.), pp. 117–132 London, UK: Curwen Press [Google Scholar]
- 11.Zhou W., Rousset F., O'Neill S. L. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 265, 509–515 10.1098/rspb.1998.0324 (doi:10.1098/rspb.1998.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawley M. J. 2005. Statistics: an introduction using R. Chichester, UK: John Wiley & Sons [Google Scholar]
- 13.Wolf K. W., Glatzel S. 1996. Intracytoplasmic bacteria in male germ cells of Philudoria potatoria L. (Lasocampidae, Lepidoptera, Insecta). J. Invert. Pathol. 67, 279–288 10.1006/jipa.1996.0043 (doi:10.1006/jipa.1996.0043) [DOI] [PubMed] [Google Scholar]
- 14.Friedländer M. 1997. Control of the eupyrene-apyrene dimorphism in Lepidoptera. J. Insect Physiol. 43, 1085–1092 10.1016/S0022-1910(97)00044-9 (doi:10.1016/S0022-1910(97)00044-9) [DOI] [PubMed] [Google Scholar]
- 15.Friedländer M., Benz G. 1981. The eupyrene-apyrene dichotomous spermatogenesis of Lepidoptera: organ culture study of the timing of apyrene commitment in the codling moth. Int. J. Invert. Reprod. 3, 113–120 [Google Scholar]
- 16.Presgraves D. C. 2000. A genetic test of the mechanism of Wolbachia-induced cytoplasmic incompatibility in Drosophila. Genetics 154, 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis Z., Wedell N. 2007. Effect of adult feeding on male mating behaviour in the butterfly, Bicyclus anynana (Lepidoptera: Nymphalidae). J. Insect Behav. 20, 201–213 10.1007/s10905-007-9075-2 (doi:10.1007/s10905-007-9075-2) [DOI] [Google Scholar]
- 18.Dobson S. L., Rattanadechakul W., Marsland E. J. 2004. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity 93, 135–142 10.1038/sj.hdy.6800458 (doi:10.1038/sj.hdy.6800458) [DOI] [PubMed] [Google Scholar]
- 19.Fry A. J., Palmer M. R., Rand D. M. 2004. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 93, 379–389 [DOI] [PubMed] [Google Scholar]
- 20.Min K., Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl Acad. Sci. USA 94, 10 792–10 796 10.1073/pnas.94.20.10792 (doi:10.1073/pnas.94.20.10792) [DOI] [PMC free article] [PubMed] [Google Scholar]