Abstract
Male movements serve as courtship signals in many animal species, and may honestly reflect the genotypic and/or phenotypic quality of the individual. Attractive human dance moves, particularly those of males, have been reported to show associations with measures of physical strength, prenatal androgenization and symmetry. Here we use advanced three-dimensional motion-capture technology to identify possible biomechanical differences between women's perceptions of ‘good’ and ‘bad’ male dancers. Nineteen males were recorded using the ‘Vicon’ motion-capture system while dancing to a basic rhythm; controlled stimuli in the form of avatars were then created in the form of 15 s video clips, and rated by 39 females for dance quality. Initial analyses showed that 11 movement variables were significantly positively correlated with perceived dance quality. Linear regression subsequently revealed that three movement measures were key predictors of dance quality; these were variability and amplitude of movements of the neck and trunk, and speed of movements of the right knee. In summary, we have identified specific movements within men's dance that influence women's perceptions of dancing ability. We suggest that such movements may form honest signals of male quality in terms of health, vigour or strength, though this remains to be confirmed.
Keywords: movement, courtship signal, dance quality, motion-capture
1. Introduction
In 1859, Charles Darwin proposed that certain male traits evolved owing to sexual selection via female mate choice [1]. Since then much emphasis has been placed on conspicuous male secondary sexual ornaments and associations between such ornaments and female mate preferences [2]. However, such ornaments are not present in all mammalian species, and some authors have suggested that females may evaluate males largely on the quality of their movements, especially those movements that contain elements of vigour and/or skill, because these are most likely to indicate health and genetic quality [3]. Evidence from birds, ungulates and crustaceans demonstrates that females detect subtle variations in male motor performance during ritualized courtship displays, and base subsequent reproductive decisions upon such differences [4–6]. In humans, dance is a set of intentional, rhythmic, culturally influenced, non-verbal body movements that are considered to be an important aspect of sexuality and courtship attraction; indeed, dancing often forms part of courtship and marriage celebrations [7,8]. Dancing ability, particularly that of men, may serve as a signal of male mate quality in terms of physical strength [9], prenatal androgenization [10] and symmetry [11], and thus affect women's perceptions of men's attractiveness (for review see Hugill et al. [12]).
Identifying the characteristics of attractive dance in natural settings is difficult because of the confounding effects of facial attractiveness [13], height [14], clothing and socioeconomic status [15], dominance [16], body morphology and shape [17]. Previous studies assessing women's perceptions of male dancing ability have attempted to control for these factors using blurred video clips [9,18] or simple motion-capture avatars [11]. Here, we improve this methodology further using more realistic three-dimensional avatars from which precise biomechanical measurements can be extracted. To our knowledge, no previous studies have actually identified specific movement components within a dance that may influence perceived dance quality, a gap we aimed to fill in our study. Males danced for 30 s to the same basic drum rhythm and their movements were mapped onto computer-generated avatars, which females rated for dance quality. Biomechanical analysis allowed the amplitude, speed, duration and variability of body movements to be calculated. Analysis was concentrated on three body regions: legs (ankle, hip and knee), arms (shoulder, elbow and wrist) and the central body (neck and trunk).
2. Material and methods
An initial sample of 30 men aged 18–35 (mean = 22.72, s.d. = 4.37) took part in the investigation, none of whom were professional dancers, or had any physical injuries or current health problems which could have affected their movements. Body height and mass were measured, in addition elbow, ankle and wrist widths were measured, as well as leg lengths in order to accurately calculate angle data in Vicon Workstation.
A 12-camera optical motion-capture system (Vicon 612, Vicon, Oxford) was used, running Vicon workstation v.4.6 software. Each camera captured at a constant rate of 100 Hz. Thirty-eight 14 mm reflective markers were attached to each participant in accordance with the Vicon Plug-In-Gait marker set to capture all the major structures of the body. Participants were requested to perform one static calibration capture along with one 30 s dance to a constant core drum beat to eliminate music likeability as a possible confound. Participants were not given any prior instruction on how they should dance.
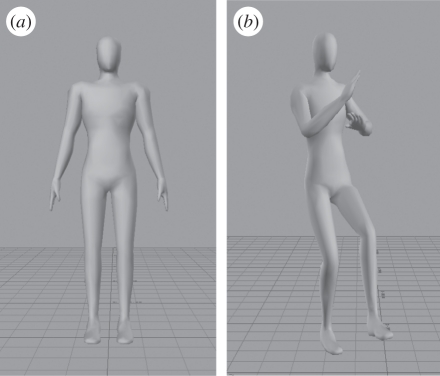
For avatar construction, it is vital that all the markers should be recognized, and that all optical data are complete and does not contain any gaps. Often, the reflective markers become detached, or are occluded by arm movements. If this occurs to any marker during a trial, the necessary joint angles cannot be calculated. Eleven participants were thus excluded from the study owing to incomplete marker capture, leaving 19 male dancers. Their motion-capture data were used to animate a virtual character (an avatar), using Autodesk MotionBuilder, 2010. The avatar chosen was a featureless, gender-neutral humanoid character that was included in the software package in order to put maximum emphasis on the biological movement (figure 1).
Figure 1.
Examples of an avatar created for the rating purposes. (a) Static pose, while (b) shows an avatar ‘dancing’.
Using these avatars, 37 heterosexual women aged 18–35 (mean = 22.30, s.d. = 6.22) rated dance quality for all 19 male dancers in a serial, randomized order based on a 15 s episode (middle section of each dance) shown in an 800 × 600 pixel window centred on a 15.4 inch laptop screen (1440 × 900 pixel resolution) using Medialab v. 1.33 (Empirisoft Inc., New York, NY, USA). The core audio track was not presented to raters. Immediately after each presentation of a dancer, participants made a judgement of dance quality on a seven-point Likert-type scale (from 1 = extremely bad dancer to 7 = extremely good dancer). After each rating, the participant was prompted to move on to the next rating segment. Subsequent analyses were based on mean ratings (M = 3.7, s.d. = 0.8, Cronbach's α = 0.94).
A kinematic model (Plug-In-Gait, Vicon, Oxford) was used to generate three-dimensional joint angles for the knees, hips, trunk, neck, shoulders and wrists. Ankle angle was calculated in two dimensions (flexion/extension and internal/external rotation) and for the elbow in one dimension (flexion/extension). Each joint angle was filtered using a second-order Butterworth low-pass filter with a cut-off frequency of 10 Hz. Simple visual inspection of the angles showed that they fluctuated in magnitude in a series of unidirectional movements. The amplitude and duration of each unidirectional movement were calculated as the angular displacement and time, respectively, between successive reversals in direction. The mean speed of each angular movement between direction reversals was calculated as the amplitude divided by the duration. The change in the magnitude of each joint angle from the mean joint position for the entire trial (angular offset) was determined at each movement reversal. Movement variability was then calculated as the standard deviation of all angular offsets for each joint angle. Because of the large number of joint angles produced (n = 38), movement amplitude, duration, speed and variability were each grouped by body region, after ensuring that the variance in variables within each body segment was similar. Three body regions were generated, including the legs (ankles, knees and hips), arms (shoulders, elbows and wrists) and central body (trunk and neck).
3. Results
Kolmogorov–Smirnov tests were used to check for normal distribution of each movement variable both for combined body regions and their constituent parts. For variables with normal distribution, two-tailed Pearson product–moment correlations were performed between mean ratings of dance quality and each of the three body regions for movement amplitude, movement variability, movement speed and movement duration. For variables that were not normally distributed, Spearman's rank correlations were used. If a significant correlation was found for any one body region, a further correlation was performed between ratings of dance quality and the constituent parts of that body region. A 95 per cent confidence level was used throughout. A summary of the significant correlations is presented in table 1.
Table 1.
Correlations between movement variables and ratings of dance quality. Correlations for sub-components are presented only when the principal component was significantly correlated with dance rating.
| movement amplitude | movement variability | movement speed | |
|---|---|---|---|
| arms | r = 0.45; p = 0.051 | r = 0.44; p = 0.057 | r = 0.34; p = 0.153 |
| right shoulder flexion/extension | n.a. | n.a. | n.a. |
| right shoulder abduction/adduction | n.a. | n.a. | n.a. |
| right shoulder internal/external rotation | n.a. | n.a. | n.a. |
| right elbow flexion/extension | n.a. | n.a. | n.a. |
| right wrist flexion/extension | n.a. | n.a. | n.a. |
| right wrist abduction/adduction | n.a. | n.a. | n.a. |
| right wrist internal/external rotation | n.a. | n.a. | n.a. |
| left shoulder flexion/extension | n.a. | n.a. | n.a. |
| left shoulder abduction/adduction | n.a. | n.a. | n.a. |
| left shoulder internal/external rotation | n.a. | n.a. | n.a. |
| left elbow flexion/extension | n.a. | n.a. | n.a. |
| left wrist flexion/extension | n.a. | n.a. | n.a. |
| left wrist abduction/adduction | n.a. | n.a. | n.a. |
| left wrist internal/external rotation | n.a. | n.a. | n.a. |
| central body | r = 0.55a; p < 0.05 | r = 0.81a; p < 0.001 | r = 0.41; p = 0.082 |
| neck flexion/extension | r = 0.47a; p < 0.05 | r = 0.68a,b; p < 0.01 | n.a. |
| neck abduction/adduction | r = 0.30; p = 0.219 | r = 0.66a; p < 0.05 | n.a. |
| neck internal/external rotation | r = 0.38; p = 0.113 | r = 0.73a; p < 0.001 | n.a. |
| trunk flexion/extension | r = 0.67a; p < 0.01 | r = 0.68a; p < 0.01 | n.a. |
| trunk abduction/adduction | r = 0.48a; p < 0.05 | r = 0.68a; p < 0.01 | n.a. |
| trunk internal/external rotation | r = 0.08; p = 0.734 | r = 0.51a,b; p < 0.05 | n.a. |
| legs | r = 0.24; p = 0.332 | r = 0.23; p = 0.334 | r = 0.47a; p < 0.05 |
| right hip flexion/extension | n.a. | n.a. | r = 0.21; p = 0.393 |
| right hip abduction/adduction | n.a. | n.a. | r = 0.02; p = 0.925 |
| right hip internal/external rotation | n.a. | n.a. | r = 0.23; p = 0.337 |
| right knee flexion/extension | n.a. | n.a. | r = 0.52a; p < 0.05 |
| right knee abduction/adduction | n.a. | n.a. | r = 0.24; p = 0.317 |
| right knee internal/external rotation | n.a. | n.a. | r = 0.70a; p < 0.01 |
| right ankle flexion/extension | n.a. | n.a. | r = 0.40; p = 0.100 |
| right ankle internal/external rotation | n.a. | n.a. | r = 0.24; p = 0.329 |
| left hip flexion/extension | n.a. | n.a. | r = −0.01; p = 0.963 |
| left hip abduction/adduction | n.a. | n.a. | r = 0.02; p = 0.944 |
| left hip internal/external rotation | n.a. | n.a. | r = 0.31; p = 0.200 |
| left knee flexion/extension | n.a. | n.a. | r = 0.31; p = 0.193 |
| left knee abduction/adduction | n.a. | n.a. | r = −0.03; p = 0.901 |
| left knee internal/external rotation | n.a. | n.a. | r = 0.34; p = 0.160 |
| left ankle flexion/extension | n.a. | n.a. | r = −0.04; p = 0.886 |
| left ankle internal/external rotation | n.a. | n.a. | r = −0.11; p = 0.646 |
aDenotes significant correlations.
bIndicates a correlation where the movement variable was not normally distributed.
For movement amplitude, all variables were normally distributed. We found significant positive correlations between dance ratings and the central body region. Key components comprised of: neck flexion/extension (head nodding), trunk flexion/extension (forward/backward bending) and trunk abduction/adduction (sideways bending).
For movement variability, all three body regions were normally distributed, although seven of their 38 constituent parts were not. We found significant positive correlations between dance ratings and central body region variability with all components being important, these were: neck flexion/extension; neck abduction/adduction (head sideways tilting); neck internal/external rotation (head shaking); trunk flexion/extension; trunk adduction/abduction; and trunk internal/external rotation (twisting).
Finally, for movement speed, all data were normally distributed. We found a significant positive relationship between speed of the legs and dance ratings, the relevant components being speed of right knee flexion/extension (bending) and speed of right knee internal/external rotation (twisting).
When the significant constituent parts were fed into a stepwise linear regression to predict dance ratings, neck internal/external rotation variability (β = 0.29), trunk adduction/abduction variability (β = 0.46) and right knee internal/external rotation speed (β = 0.38) contributed to the final model (F3,15 = 18.9, p < 0.001), which accounted for 79 per cent of the variance in the mean dance ratings.
4. Discussion
By using cutting-edge motion-capture technology, we have been able to precisely break down and analyse specific motion patterns in male dancing that seem to influence women's perceptions of dance quality. We find that the variability and amplitude of movements in the central body regions (head, neck and trunk) and speed of the right knee movements are especially important in signalling dance quality. A ‘good’ dancer thus displays larger and more variable movements in relation to bending and twisting movements of their head/neck and torso, and faster bending and twisting movements of their right knee. As 80 per cent of individuals are right-footed [19], greater movements of the right knee in comparison with the left are perhaps to be expected. In comparative research, there is extensive literature on the signalling capacities of movement (see Byers et al. [3]). Researchers have suggested that females prefer vigorous and skilled males; such cues are derived from male motor performance that provides a signal of his physical condition [3–6].
Our data indicate that in humans, certain aspects of movement amplitude, speed and variability are also important for female perceptions of male dancing ability. We suggest that human male movements could also form honest signals of traits such as health, fitness, genetic quality and developmental history [9–12], though this remains to be confirmed. By uncovering some specific movement parameters used in the assessments of dance quality, we are now in a much stronger position to further research the possible signalling mechanisms of dance in humans. Future studies should systematically manipulate the dance moves that we have identified as being most important, and assess the effects of such manipulations on female perceptions of dance quality.
Acknowledgements
We thank Alistair Ewen for his assistance in operating the Vicon system and three anonymous reviewers for their helpful comments. This research was supported by the German Research Foundation (DFG). B.F. is currently funded by an Emmy-Noether Fellowship of the DFG.
References
- 1.Darwin C. R. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, UK: J. Murray; [PMC free article] [PubMed] [Google Scholar]
- 2.Jennions M. D., Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327 10.1017/S0006323196005014 (doi:10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 3.Byers J., Hebets E., Podos J. 2010. Female mate choice based upon male motor performance. Anim. Behav. 79, 771–778 10.1016/j.anbehav.2010.01.009 (doi:10.1016/j.anbehav.2010.01.009) [DOI] [Google Scholar]
- 4.Byers J. A., Moodie J. D., Hall N. 1994. Pronghorn females choose vigorous mates. Anim. Behav. 47, 33–43 10.1006/anbe.1994.1005 (doi:10.1006/anbe.1994.1005) [DOI] [Google Scholar]
- 5.Clark C. J. 2009. Courtship dives of Anna's hummingbird offer insights into flight performance limits. Proc. R. Soc. B 276, 3047–3052 10.1098/rspb.2009.0508 (doi:10.1098/rspb.2009.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murai M., Backwell P. R. Y. 2006. A conspicuous courtship signal in the fiddler crab Uca perplexa: female choice based on display structure. Behav. Ecol. Sociobiol. 60, 736–741 10.1007/s00265-006-0217-x (doi:10.1007/s00265-006-0217-x) [DOI] [Google Scholar]
- 7.Hanna J. L. 2010. Dance and sexuality: many moves. J. Sex Res. 47, 212–241 10.1080/00224491003599744 (doi:10.1080/00224491003599744) [DOI] [PubMed] [Google Scholar]
- 8.Kaeppler A. L. 1978. Dance in anthropological perspective. Ann. Rev. Anthropol. 7, 31–49 10.1146/annurev.an.07.100178.000335 (doi:10.1146/annurev.an.07.100178.000335) [DOI] [Google Scholar]
- 9.Hugill N., Fink B., Neave N. 2009. Men's physical strength is associated with women's perceptions of their dancing ability. Personal. Individ. Diff. 47, 527–530 10.1016/j.paid.2009.04.009 (doi:10.1016/j.paid.2009.04.009) [DOI] [Google Scholar]
- 10.Fink B., Seydel H., Manning J. T., Kappeler P. M. 2007. A preliminary investigation of the associations between digit ratio and women's perception of men's dance. Personal. Individ. Diff. 42, 381–390 10.1016/j.paid.2006.07.019 (doi:10.1016/j.paid.2006.07.019) [DOI] [Google Scholar]
- 11.Brown W. M., Cronk L., Grochow K., Jacobson A., Liu C. K., Popović Z., Trivers R. 2005. Dance reveals symmetry especially in young men. Nature 438, 1148–1150 10.1038/nature04344 (doi:10.1038/nature04344) [DOI] [PubMed] [Google Scholar]
- 12.Hugill N., Fink B., Neave N. 2010. The role of human body movements in mate selection. Evol. Psychol. 8, 49–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham M. R., Barbee A. P., Pike C. L. 1990. What do women want? Facialmetric assessment of multiple motives in the perception of male facial physical attractiveness. J. Person. Soc. Psychol. 59, 61–72 10.1037/0022-3514.59.1.61 (doi:10.1037/0022-3514.59.1.61) [DOI] [PubMed] [Google Scholar]
- 14.Pawlowski B., Dunbar R. I. M., Lipowicz A. 2000. Evolutionary fitness: tall men have more reproductive success. Nature 403, 156. 10.1038/35003107 (doi:10.1038/35003107) [DOI] [PubMed] [Google Scholar]
- 15.Townsend J. M., Levy G. D. 1990. Effects of potential partners' costume and physical attractiveness on sexuality and partner selection. J. Psychol. 124, 371–389 [DOI] [PubMed] [Google Scholar]
- 16.Sadella E. K., Kenrick D. T., Vershure B. 1987. Dominance and heterosexual attraction. J. Personal Social Psychol. 52, 730–738 [Google Scholar]
- 17.Maisey D. S., Vale E. L. E., Cornelissen P. L., Tovée M. J. 1999. Characteristics of male attractiveness for women. Lancet 353, 1500. 10.1016/S0140-6736(99)00438-9 (doi:10.1016/S0140-6736(99)00438-9) [DOI] [PubMed] [Google Scholar]
- 18.Berry D. S., Kean K. J., Misovich S. J., Baron R. M. 1991. Quantized displays of human movement: a methodological alternative to the point-light display. J. Nonverb. Behav. 15, 81–97 10.1007/BF00998264 (doi:10.1007/BF00998264) [DOI] [Google Scholar]
- 19.Coren S. 1992. Left hander. London, UK: John Murray [Google Scholar]