Abstract
Increased investment in immunity is expected to be beneficial under crowded conditions because of the greater risk of pathogen and parasite transmission, but the evolution of this facultative response relies on the ability to accurately assess social cues in the environment and adjust immune defences accordingly. Because of their highly conspicuous nature, long-range sexual signals are prime candidates to be used in evaluating the social conditions likely to be experienced upon adulthood in continuously breeding species; however, their role in mediating immune responses is unknown. We tested whether exposure to acoustic sexual signals in the field cricket Teleogryllus oceanicus affects immunity by manipulating male juvenile experience of acoustic signals, and measuring the effect on adult immunity. Adult males exposed to song during rearing showed stronger immune responses than males reared in silence: they were better able to encapsulate artificial nylon implants and showed higher levels of antimicrobial lysozyme-like activity in their haemolymph. Experience of sexual signals thus translates into increased immunity, which suggests that such signals may play a role in conveying information about population demography and shaping density-dependent responses in unintended receivers.
Keywords: density-dependent prophylaxis, immunocompetence, insect immunity, predictive adaptive response, social experience, Teleogryllus oceanicus
1. Introduction
The risk of parasite or pathogen transmission increases when population density increases [1]. Increased investment in immunity under crowded conditions is expected to benefit individuals that can accurately assess social cues in their environment and adjust investment in immune defence accordingly [2,3]. However, little is known about the social cues individuals use to assess the presence of conspecifics. Physical contact is probably important [4,5], but the use of tactile cues is limited to situations in which population density is already high enough to permit frequent encounters between individuals. In continuously breeding species, long-range sexual signals could provide a conspicuous source of social information that developing individuals might use to evaluate the potential for future transmission risk. Here, we test whether experience of acoustic sexual signals in the Pacific field cricket, Teleogryllus oceanicus, mediates plasticity in immune defence.
Teleogryllus oceanicus is native to Australasia, and its range in the Hawaiian archipelago overlaps with that of an acoustically orienting parasitoid fly, Ormia ochracea [6]. Male crickets produce a long-range calling song to attract females for mating, but the song also attracts gravid female flies that deposit larvae on or around males. The larvae develop within the host and emerge 7–10 days later to pupate, at which point the host dies. On the island of Kauai, a recent wing mutation, flatwing, has rendered some males obligately silent, and it appears to be maintained by pressure from the parasitoid fly [6]. Male gryllids are also susceptible to a variety of other infectious agents, including gregarine parasites and viruses [7,8]. To test whether exposure to acoustic signals stimulates predictable increases in immune investment, and whether the two male morphs show intrinsic differences in immune investment, we manipulated male acoustic experience during rearing using playbacks. We established an acoustically rich treatment that simulated a population crowded with sexually mature males, and a silent treatment that simulated a population containing no sexually mature males. Upon maturity, we measured three immune parameters commonly assayed in insects: the ability to encapsulate a nylon implant, lysozyme-like activity and phenoloxidase activity. We also assessed whether any influence of acoustic experience changed with age. The supplemental section contains comprehensive details about all three assays.
2. Material and methods
(a). Colony origin
A laboratory colony was established in 2003 using eggs laid by approximately 12 females caught on the Hawaiian island of Kauai, and supplemented approximately once yearly. Crickets were reared at 25°C under a 12 L : 12 D cycle and provided water and Fluker's Cricket Chow ad libitum.
(b). Experimental design
When sex differences became apparent, juvenile male crickets were isolated from a laboratory colony into individual 118 ml containers provisioned with Purina Rabbit Chow, water and egg cartons for shelter. Our experiment consisted of a full 2 × 2 × 2 blocked design with acoustic experience, age and male morph as factors. The supplemental section contains further information, including sample sizes for each group. Isolated males were randomly separated into Song or No Song treatments. We started the treatment during juvenile stages because penultimate-instar nymphs of a closely related species, Gryllus bimaculatus, are capable of receiving acoustic signals [9], and T. oceanicus juveniles are exposed to calling song in the wild because this species breeds continuously. The Song treatment consisted of an incubator in which six Sony SRS-m30 speakers simultaneously played back male calling song during the dark portion of the light : dark cycle. The song was broadcast at 70–80 dB measured at the position of the crickets using an AZ Sound Meter (model 8922), which was designed to approximate the intensity an individual would experience in the wild when developing amid a dense population of calling males (approximate spacing of 50 cm). Bailey & Zuk [10] contains further details about song construction and playback. The No Song treatment consisted of an identical incubator but without the playbacks. We switched the treatments between the two incubators throughout the experiment to eliminate potential incubator effects.
Upon eclosion, the scraper (ca 2 mm2) was surgically removed from each male's lower elytrum to prevent males from singing and thereby confounding the treatments. Flatwing males were treated with a sham operation that removed a corresponding piece of the wing where the scraper would ordinarily develop. We assayed immunity in crickets at two ages: 6 days post-eclosion (young) and 13 days post-eclosion (old), which spans the range of ages documented in wild gryllids [8]. Crickets were weighed to the nearest 0.001 g and their pronotums measured to the nearest 0.01 mm.
(c). Encapsulation assay
The encapsulation assay estimated an individual's ability to encapsulate and melanize a foreign object introduced into its haemocoel. We inserted an abraded 2 mm nylon monofilament into a hole punctured between the second and third abdominal sternites of each cricket with a sterile insect pin. Crickets were sacrificed 24 h later, and the monofilament was removed and photographed under 3.2× magnification using an RC Colour Spot digital camera mounted on a Leica MZ75 stereomicroscope. Implants were rotated so that the darkest side was facing up and they were photographed blind to group identity.
Implant darkness indicates the extent of haemocyte adherence and melanization. Stronger immune responses produce darker implants. We quantified implant darkness using macros in NIH Image (v. 1.62), which outline an image of the implant and calculate a mean greyscale value ranging from 0 (lightest) to 256 (darkest). We also measured the darkness of ca 1 mm2 of the background next to the implant and subtracted this value from the implant darkness value to correct for background variation.
(d). Lysozyme assay
The lysozyme assay estimated antimicrobial activity by turbidometrically quantifying levels of haemolymph-bound lysozymes. We extracted 3 µl of haemolymph from the pierced cuticle prior to inserting implants. The haemolymph was added to 40 µl of 1X phosphate-buffered saline (PBS, 11.9 mM phosphates, 137 mM NaCl, 2.7 mM KCl, pH 7.4, Fisher) and stored at −80°C. In a 96-well plate, 10 µl of each haemolymph–PBS mixture was added to 10 µl of a 0.35 g ml−1 solution of Micrococcus luteus (lysodeikticus) (ICN Biomedicals cat. no. 159972). The change in absorbance of the solution was measured over the linear phase of the reaction (120 min) at 490 nm by subtracting the final absorbance reading from the initial absorbance reading (Δ absorbance). For both this and the following phenoloxidase assay, the first 88 samples were started in a Bio-Rad Model 550 microplate reader; owing to hardware failure, these and all remaining assays were completed using a Victor 2 microplate reader. Higher values indicate greater lysozyme-like activity. We ran several blank samples of 10 µl of the Micrococcus solution on each plate, yielding a total of 37 blanks. To control for possible settling of suspended particulates in the solution, which causes a natural decline in absorbance, the mean Δ absorbance of the blank samples was subtracted from each individual's Δ absorbance prior to analysis.
(e). Phenoloxidase assay
The phenoloxidase assay spectrophotometrically measures haemolymph levels of phenoloxidase, an enzyme necessary for melanin production and hence important in invertebrate immunity. Phenoloxidase is stored in haemolymph as an inactive precursor, pro-phenoloxidase. We estimated total phenoloxidase activity by first cleaving all pro-phenoloxidase with the proteolytic enzyme α-chymotrypsin. We added 5 µl of the haemolymph–PBS mixture to 7 µl of 1.3 mg ml−1 bovine pancreas α-chymotrypsin (Sigma–Aldrich C7762) and incubated it at room temperature for 20 min. We then added 90 µl of 15 mM l-dihydroxyphenylalanine (l-DOPA, ARCOS Organics AC167530050) to the solution. Phenoloxidase converts l-DOPA to dopachrome, causing the solution to darken. We measured the change in absorbance over the linear phase of the reaction (120 min) at 490 nm and subtracted the initial absorbance reading from the final one (Δ absorbance). Higher values indicate greater total phenoloxidase levels in the haemolymph. We ran 38 blank samples consisting of 7 µl α-chymotrypsin and 90 µl of l-DOPA as a check for contamination.
(f). Analysis
To determine the effects of acoustic experience on immunity, a general linear model (GLM) was performed for each immune parameter. Encapsulation and phenoloxidase data were transformed to the fourth power and by the square-root, respectively. Three outliers were identified in the lysozyme data using the criterion of Cook's distance. Their exclusion did not qualitatively change the results, so we did not remove them from our dataset (see electronic supplementary material for further details). Full models contained treatment, wing morph and age as factors, plus their interactions. Acoustic experience affects condition in this species [11], so to control for variation in condition, we included both mass and pronotum length as covariates in full models. Models were reduced using hierarchical removal of non-significant terms. Analyses were performed in Systat v. 10.
3. Results
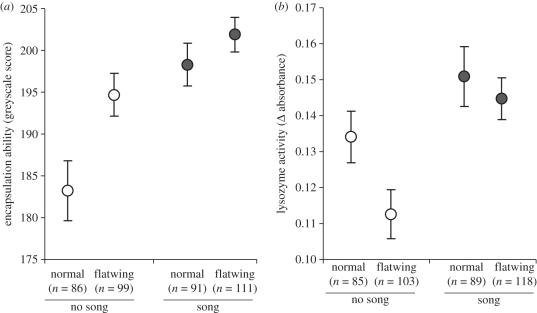
Teleogryllus oceanicus males that experienced song during rearing showed higher immune responses than crickets reared in silence: they had stronger encapsulation responses and greater lysozyme-like activity (table 1 and figure 1). Phenoloxidase activity did not differ between the treatments (table 1, ‘treatment’ was removed from the final phenoloxidase model). Flatwing males had stronger encapsulation responses but lower lysozyme-like activity than normal males (table 1). Older males had higher phenoloxidase activity than younger males (respectively, with s.e.: 0.441 ± 0.018 absorbance units versus 0.353 ± 0.016 absorbance units) but weaker encapsulation responses (respectively, with s.e.: 191.17 ± 2.00 greyscale units versus 198.45 ± 1.88 greyscale units), but age did not interact with acoustic treatment (table 1).
Table 1.
Final GLMs exploring the effects of acoustic experience, wing morph and age on encapsulation ability, lysozyme-like activity and phenoloxidase activity in T. oceanicus males. Significant p-values are indicated in bold.
| d.f. | F | pa | |
|---|---|---|---|
| encapsulation (r2 = 0.076) | |||
| acoustic experience | 1 | 15.42 | <0.001 |
| wing morph | 1 | 4.87 | 0.028 |
| age | 1 | 10.97 | 0.001 |
| error | 383 | ||
| lysozyme-like activity (r2 = 0.047) | |||
| acoustic experience | 1 | 9.93 | 0.002 |
| wing morph | 1 | 6.71 | 0.010 |
| age | 1 | 0 | 0.958 |
| wing morph × age | 1 | 4.12 | 0.043 |
| error | 390 | ||
| PO activity (r2 = 0.042) | |||
| age | 1 | 17.23 | <0.001 |
| error | 394 | ||
aBased on type III sums of squares.
Figure 1.
(a) The effect of acoustic experience and male morph on lysozyme-like activity and (b) encapsulation ability in T. oceanicus males. Immune strength increases with increasing values along the y-axis. Circles are means ± 1 s.e.
4. Discussion
Exposure to long-range sexual signals increased innate immunity in adult T. oceanicus males. Although immunity varied with adult age, the influence of acoustic experience remained consistent: juvenile experience of calling song led to increased encapsulation ability and lysozyme-like activity in adults. The acoustic effects that we found are distinct from other social influences that have been observed to mediate physiological responses in insects. In the desert locust (Schistocerca gregaria), for example, tactile contact on the hind legs of developing juveniles stimulates the expression of gregarious behaviour [4]. Long-range acoustic signals might be only a subset of many sorts of acoustic input that could, in principle, influence immunity. However, the fact that they are sufficient to enhance immune defence in male T. oceanicus in the absence of any other social cues suggests a novel mechanism by which prevailing social conditions might regulate immune investment in species that do not necessarily come into physical contact until adulthood, yet produce signals that can be perceived by unintended receivers during development.
The use of sexual signals not only depends on the ability of developing individuals to perceive and integrate information, but also on the honesty of information produced by signallers. A limitation of our experiment is that it is unclear whether intermediate levels of calling song experience during rearing would induce intermediate increases in immunity, or whether any exposure to sexual signals at all suffices to trigger a plastic response. Increased immunity after exposure to song might also reflect a more general response to noisy or disturbed conditions, which could confer advantages or disadvantages depending on the context, such as parasitoid defence or competition for food resources.
The adaptive value of finely tuning any density-dependent response to the social cues present in their environment is contingent upon those cues accurately representing the demographic constitution of the population. If they do not, then the disadvantage of upregulating the production of costly immunity compounds based on false information might outweigh the advantages of mounting a costly plastic response in the first place. In species that show density-dependent polyphenisms, social information gained from physical interactions is likely to be highly reliable. However, in the population we studied, the male-silencing flatwing mutation's rapid spread has altered the acoustic environment on Kauai dramatically: silence now predominates, as over 90 per cent of males are flatwings [6]. This mismatch between the true population density and the perceived population density may exacerbate the relative risk of mortality that normal-wing calling males experience. The lack of a significant experience × morph interaction in our results suggest that all males will be less likely to invest in strong encapsulation responses when developing amid relatively silent conditions of Kauai, which might decrease their defences against parasitoids during early stages of infection. However, flatwings are already protected from attack by this acoustically orienting parasitoid because they cannot sing [6]. We currently do not know why the male morphs exhibit different immune responses; possibilities worth pursuing include pleiotropic effects of the flatwing mutation or differential selection on immunity. Regardless of the reason, normal-wing males experience a double disadvantage, which may have contributed to their rapid replacement by flatwing mutants on Kauai: they attract parasitoids and they have weaker encapsulation responses.
Acknowledgements
We thank C. Clark, D. De La Riva, Y. Eck, K. Simester, C. Trinh, G. Woss and B. Zelimkhanian for assistance. This work was funded by the NSF, the UCR Academic Senate and the UCR Graduate Research Mentorship Program.
References
- 1.Anderson R. M., May R. M. 1978. Regulation and stability of host–parasite population interactions: I. Regulatory processes. J. Anim. Ecol. 47, 219–247 [Google Scholar]
- 2.Elliot S. L., Hart A. G. 2010. Density-dependent prophylactic immunity reconsidered in the light of host group living and social behaviour. Ecology 91, 65–72 10.1890/09-0424.1 (doi:10.1890/09-0424.1) [DOI] [PubMed] [Google Scholar]
- 3.Wilson K., Reeson A. F. 1998. Density-dependent prophylaxis: evidence from Lepidoptera–baculovirus interactions? Ecol. Entomol. 23, 100–101 10.1046/j.1365-2311.1998.00107.x (doi:10.1046/j.1365-2311.1998.00107.x) [DOI] [Google Scholar]
- 4.Simpson S. J., Despland E., Häegele B. F., Dodgson T. 2001. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc. Natl Acad. Sci. USA 98, 3895–3897 10.1073/pnas.071527998 (doi:10.1073/pnas.071527998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson K., Thomas M. B., Blanford S., Doggett M., Simpson S. J., Moore S. L. 2002. Coping with crowds: density-dependent disease resistance in desert locusts. Proc. Natl Acad. Sci. USA 99, 5471–5475 10.1073/pnas.082461999 (doi:10.1073/pnas.082461999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuk M., Rotenberry J. T., Tinghitella R. M. 2006. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2, 521–524 10.1098/rsbl.2006.0539 (doi:10.1098/rsbl.2006.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinganum C., O'Loughlin G. T., Hogan T. W. 1970. A nonoccluded virus of the field crickets Teleogryllus oceanicus and T. commodus (Orthoptera: Gryllidae). J. Invert. Pathol. 16, 214–220 10.1016/0022-2011(70)90062-5 (doi:10.1016/0022-2011(70)90062-5) [DOI] [Google Scholar]
- 8.Zuk M. 1987. Parasite load, body size, and age of wild-caught male field crickets (Orthoptera: Gryllidae): effects on sexual selection. Evolution 42, 969–976 10.2307/2408912 (doi:10.2307/2408912) [DOI] [PubMed] [Google Scholar]
- 9.Staudacher E. M. 2009. The auditory system of last instars in Gryllus bimaculatus DeGeer. Physiol. Entomol. 34, 18–29 10.1111/j.1365-3032.2008.00647.x (doi:10.1111/j.1365-3032.2008.00647.x) [DOI] [Google Scholar]
- 10.Bailey N. W., Zuk M. 2008. Acoustic experience shapes female mate choice in field crickets. Proc. R. Soc. B 275, 2645–2650 10.1098/rspb.2008.0859 (doi:10.1098/rspb.2008.0859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey N. W., Gray M., Zuk M. 2010. Acoustic experience shapes alternative mating tactics and reproductive investment in field crickets. Curr. Biol. 20, 845–849 10.1016/j.cub.2010.02.063 (doi:10.1016/j.cub.2010.02.063) [DOI] [PubMed] [Google Scholar]