Abstract
Madagascar and the Seychelles are Gondwanan remnants currently isolated in the Indian Ocean. In the Late Cretaceous, these islands were joined with India to form the Indigascar landmass, which itself then split into its three component parts around the start of the Tertiary. This history is reflected in the biota of the Seychelles, which appears to contain examples of both vicariance- and dispersal-mediated divergence from Malagasy or Indian sister taxa. One lineage for which this has been assumed but never thoroughly tested is the Seychellean tiger chameleon, a species assigned to the otherwise Madagascar-endemic genus Calumma. We present a multi-locus phylogenetic study of chameleons, and find that the Seychellean species is actually the sister taxon of a southern African clade and requires accomodation in its own genus as Archaius tigris. Divergence dating and biogeographic analyses indicate an origin by transoceanic dispersal from Africa to the Seychelles in the Eocene–Oligocene, providing, to our knowledge, the first such well-documented example and supporting novel palaeocurrent reconstructions.
Keywords: biogeography, overseas dispersal, Indian Ocean, Chamaeleonidae, Archaius
1. Introduction
Once forming the eastern half of the southern supercontinent Gondwana, the landmasses of Madagascar, Seychelles, India, Antarctica and Australia had completely separated en bloc from Africa and South America by the start of the Cretaceous [1]. Although there is recent debate on the length of contact between Indigascar (India, Madagascar and Seychelles [2]; also see electronic supplementary material) and South America via Antarctica [3,4], by the start of the Late Cretaceous South America and Africa were separated, thus severing all subaerial connections between Indigascar and Africa. India–Seychelles completed a gradual separation from Madagascar around 88 Ma [1], and India and the Seychelles separated about 65 Ma [5]. This left the Seychelles as a string of granitic and coral islands centred off the northeast coast of Madagascar approximately 1600 km east of Africa.
The famously endemic biota of Madagascar, previously ascribed largely to Gondwanan vicariance, appears in fact to owe much to overseas dispersal from Africa [6]. Along with some Asian and African ties, the equally endemic Seychelles biota is characterized by multiple affinities to both India and Madagascar. Based on data from partly dated molecular phylogenies (see electronic supplementary material), several taxa show clear signatures of Gondwanan vicariance with their sister species occurring in India (caecilians, sooglossid frogs). Other taxa have originated possibly by vicariance (aplocheiloid fishes) or probably by dispersal (hyperoliid frogs, day geckos) from Madagascar.
Chameleons are a lizard group almost entirely confined to Old World Gondwanan fragments, with centres of diversity in East Africa and Madagascar. Although their distribution superficially suggests Gondwanan vicariance, shallow molecular divergences among the major clades are instead most compatible with multiple overseas dispersals [7–10]. Seychelles harbours one species of chameleon, Calumma tigris, on the three largest granitic islands (Mahé, Praslin and Silhouette). As the name implies, non-molecular phylogenetic analyses place this species within the otherwise Madagascar-endemic genus Calumma [8]. This placement dictates a solidly Tertiary divergence from its Malagasy congeners [10] and thus suggests trans-oceanic dispersal northward to Seychelles. However, molecular data needed to test this hypothesis are lacking. Using a dated phylogenetic analysis of chameleons based on sequence data from multiple mitochondrial and nuclear loci, we recovered an unexpected sister-taxon relationship of this species to the African genus Rieppeleon, thus providing evidence for overseas dispersal from Africa to Seychelles that was probably favoured by currents and river discharges in the Palaeogene.
2. Material and methods
Taxon sampling comprised 42 species (43 individuals) of chameleons, spanning at least the deepest divergences within all previously recognized major clades, and including the type species of each genus. To provide calibration nodes for divergence-dating analyses, we also included as outgroups the tuatara (Sphenodon punctatus) and 13 extra-chamaeleonid squamate reptiles (see electronic supplementary material). We obtained DNA sequence data for the mitochondrial genes 16S, ND2 and ND4, and the three nuclear protein-coding genes CMOS, PRLR and RAG1, for a total alignment length of 5129 base pairs (bp) and 147 new sequences, deposited in GenBank under the numbers HQ130509–HQ130655. See electronic supplementary material for voucher information and GenBank numbers of all sequences used in our analyses. We performed maximum-likelihood (ML) best-tree and bootstrap analyses using RAxML v.7.2.5 [11,12], and unrooted Bayesian (UB) analyses using MrBayes v.3.1.2 [13]. We also used a relaxed-clock Bayesian (RCB) approach using Beast v.1.5.3 [14] with fossil constraints to estimate divergence times. Tests of alternative topologies were performed in an ML framework using the Shimodaira–Hasegawa test [15]. Biogeographic analyses were conducted in an ML framework using ancestral state reconstruction in Mesquite [16] and dispersal-extinction-cladogenesis analysis in Lagrange [17]. See electronic supplementary material for all analysis details.
3. Results and discussion
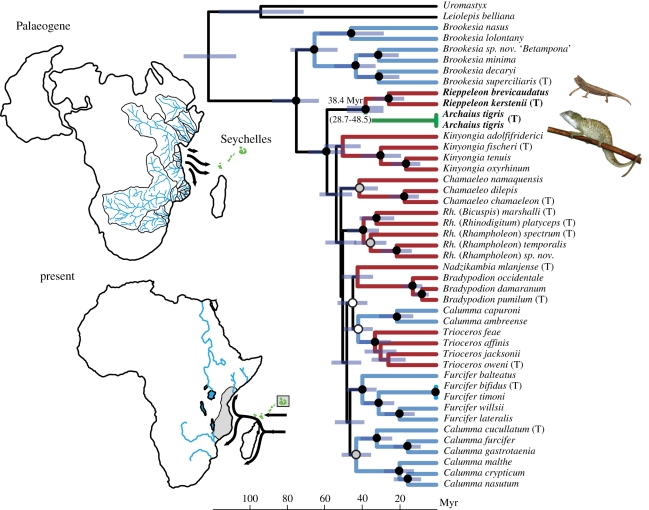
Our topology is consistent with results of recent DNA sequence-based studies [7–10,18–20] vis à vis assignment of species to major clades within Chamaeleonidae. The ML topology is generally similar to the UB and RCB topologies, which have greater support across most nodes; no conflicting nodes receive bootstrap support less than 31 per cent. In all analyses, the Seychelles-endemic C. tigris is maximally supported as the sister taxon of the southern African genus Rieppeleon (figure 1). Tests of several alternative topologies (e.g. monophyly of African ground chameleons, monophyly of Calumma) rejected all alternatives at the p = 0.01 level (see electronic supplementary material). The estimated mean divergence time between C. tigris and Rieppeleon is 38.4 Myr, with a 95 per cent highest probability density of 28.7–48.5 Myr, thus placing the split sometime in the Middle Eocene to Early Oligocene. The biogeographic analyses reconstructed Africa as the ancestral area for the Rieppeleon–Archaius clade with probabilities of 94 per cent (Mesquite) and 87 per cent (Lagrange) (see electronic supplementary material).
Figure 1.
Bayesian chronogram of chameleon phylogenetic relationships inferred from the full dataset (5129 bp). For clarity, only the two nearest outgroups are pictured. Type species of genera and subgenera are marked with a (T). Branch colours reflect distribution of taxa: blue, Madagascar; red, Africa; green, Seychelles. Black circles represent Bayesian posterior probabilities (PP) ≥95% and maximum-likelihood bootstrap (MLBS) values greater than 90%. Grey circles represent PP ≥ 95% and MLBS ≥70%, and white circles represent PP ≥ 95%. Bars represent 95% highest probability densities on divergence times. Top left, relevant Palaeogene oceanic currents and major eastward drainages from the Late Cretaceous-Palaeogene of Africa, modified from Ali & Huber [22] and Markwick & Valdes [25], respectively. Note that these studies are based on independent data, and the freshwater outflow has no causative influence on depicted ocean currents. Bottom left, present-day oceanic currents and major eastward-flowing African rivers. Shaded mainland area denotes approximate distribution of Rieppeleon, and shaded box encompasses distribution of A. tigris.
The genetic results are compatible with morphological evidence. Although the Seychelles chameleon shares with other Calumma two long, flexible bifid papillae on the hemipenis, no other morphological synapomorphies are known [21]. Calumma tigris differs strikingly from its closest relatives, Rieppeleon, in microhabitat usage (arboreal versus ground-shrub dwelling), tail length (long versus short) and general appearance (larger and more colourful versus small and drab) (figure 1, electronic supplementary material). However, it shares with one representative of Rieppeleon (Rieppeleon brevicaudatus), the presence of scaly skin flaps on the chin. Given the ecological, biogeographic and morphological distinctiveness of C. tigris and Rieppeleon, and because the phylogenetic depth of the separation between these two taxa is comparable to that between other chameleon genera, we propose to resurrect the genus Archaius Gray 1865, and designate the Seychellean species as A. tigris (see electronic supplementary material).
Evidence from fossils and molecular-dating studies clearly indicate repeated Caenozoic dispersals eastward from Africa to Madagascar in invertebrates, amphibians, reptiles and most famously, mammals [6]. This general pattern predicts dispersal eastward to the Seychelles as well, and chameleons provide, to our knowledge, the first documented example of such a colonization event. One perplexing aspect of this ‘eastward from Africa’ scenario is that present-day ocean currents flow decidedly westward from Madagascar and Seychelles (figure 1). The key to this incongruence apparently lies in the changing position of Madagascar and other landmasses relative to the large Indian Ocean gyre (rotating current) over time. Ali & Huber [22] used palaeo-oceanographic modelling to demonstrate that throughout the Palaeogene, the prevailing current was actually eastward towards Madagascar and the Seychelles and should have thus facilitated colonization of the islands (figure 1).
Another factor that may facilitate overseas dispersal is the presence of large freshwater outflows. Such systems are known to create large reduced-salinity plumes that can extend considerable distances from the mainland and even strengthen offshore currents (e.g. [23]), thus enhancing opportunities for colonization of oceanic islands via rafting [24]. The southeastern African coast is drained by the large Limpopo and Zambezi systems; at present, the central coast (i.e. northern Mozambique, Tanzania, Kenya) lacks a comparable system (figure 1). However, Late Cretaceous African palaeodrainage reconstructions [25] show that a large area now drained to the north via the Nile formerly drained to the east through what is now Kenya (figure 1), thus providing another major freshwater outflow. Although this specific reconstruction predates the Seychelles chameleon colonization, this outflow tract should have remained basically stable until the Miocene uplift of the East African Rift System led to the formation of the southern Nile tributaries [26].
Vicariance seems the best explanation for relationships among some groups with Gondwanan distributions. However, recent studies show that dispersal, especially from Africa, has played a major role in assembling the terrestrial biota of the western Indian Ocean. Improvements to palaeogeological and palaeoclimatic models are now helping to explain just how these seemingly unlikely events have occurred. The synergistic effects of eastern-directed oceanic currents and a large freshwater outflow during the Palaeogene probably facilitated oceanic dispersal of multiple taxa, including the Seychelles chameleon.
Acknowledgements
We are indebted to the Malagasy ministry for environment, water and forests for issuing permits for research and export of specimens and samples.
We thank Shelley Edwards and Piotr Wisniewski for laboratory assistance, and Tod Reeder for laboratory workspace and discussions. We are also grateful to Jason R. Ali and Matthew Huber for constructive discussions. For generous loans and provision of tissues and specimens, we thank Markus Grimm, Trip Lamb, Colin Tilbury, Bill Branch, Simon Van Noort and the California Academy of Sciences. Bärbel Koch contributed a photo of A. tigris. This work was supported by Deutsche Forschungsgemeinschaft grant VE247/3-1 to M.V. and by National Science Foundation grant EF 0334923 to Tod Reeder, and the South African National Biodiversity Institute.
References
- 1.Seward D., Grujic D., Schreurs G. 2004. An insight into the breakup of Gondwana: identifying events through low-temperature thermochronology from the basement rocks of Madagascar. Tectonics 23, TC3007. 10.1029/2003TC001556 (doi:10.1029/2003TC001556) [DOI] [Google Scholar]
- 2.Vidal N., et al. 2010. Blindsnake evolutionary tree reveals long history on Gondwana. Biol. Lett. 6, 558–561 10.1098/rsbl.2010.0220 (doi:10.1098/rsbl.2010.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali J. R., Aitchison J. C. 2009. Kerguelen Plateau and the Late Cretaceous southern-continent bioconnection hypothesis: tales from a topographical ocean. J. Biogeogr. 36, 1778–1784 10.1111/j.1365-2699.2009.02105.x (doi:10.1111/j.1365-2699.2009.02105.x) [DOI] [Google Scholar]
- 4.Krause D. W., Rogers R. R., Forster C. A., Hartman J. H., Buckley J. H., Sampson S. D. 1999. The Late Cretaceous vertebrate fauna of Madagascar: implications for Gondwanan paleobiogeography. GSA Today (Publ. Geol. Soc. Am.) 9, 1–7 [Google Scholar]
- 5.Plummer P. S., Belle E. R. 1995. Mesozoic tectonostratigraphic evolution of the Seychelles microcontinent. Sediment. Geol. 96, 73–91 10.1016/0037-0738(94)00127-G (doi:10.1016/0037-0738(94)00127-G) [DOI] [Google Scholar]
- 6.Yoder A. D., Nowak M. D. 2006. Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell. Annu. Rev. Ecol. Evol. Syst. 37, 405–431 10.1146/annurev.ecolsys.37.091305.110239 (doi:10.1146/annurev.ecolsys.37.091305.110239) [DOI] [Google Scholar]
- 7.Matthee C. A., Tilbury C. R., Townsend T. 2004. A phylogenetic review of the African leaf chameleons: genus Rhampholeon (Chamaeleonidae): the role of vicariance and climate change in speciation. Proc. R. Soc. Lond. B 271, 1967–1975 10.1098/rspb.2004.2806 (doi:10.1098/rspb.2004.2806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raxworthy C. J., Forstner M. R. J., Nussbaum R. A. 2002. Chameleon radiation by oceanic dispersal. Nature 415, 784–787 [DOI] [PubMed] [Google Scholar]
- 9.Townsend T., Larson A. 2002. Molecular phylogenetics and mitochondrial genomic evolution in the Chamaeleonidae (Reptilia, Squamata). Mol. Phylogenet. Evol. 23, 22–36 10.1006/mpev.2001.1076 (doi:10.1006/mpev.2001.1076) [DOI] [PubMed] [Google Scholar]
- 10.Townsend T. M., Vieites D. R., Glaw F., Vences M. 2009. Testing species-level diversification hypotheses in Madagascar: the case of microendemic Brookesia leaf chameleons. Syst. Biol. 58, 641–656 10.1093/sysbio/syp073 (doi:10.1093/sysbio/syp073) [DOI] [PubMed] [Google Scholar]
- 11.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 10.1093/bioinformatics/btl446 (doi:10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 12.Stamatakis A., Hoover P., Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57, 758–771 10.1080/10635150802429642 (doi:10.1080/10635150802429642) [DOI] [PubMed] [Google Scholar]
- 13.Ronquist F., Huelsenbeck J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 10.1093/bioinformatics/btg180 (doi:10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 14.Drummond A. J., Rambaut A. 2007. Beast: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214. 10.1186/1471-2148-7-214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimodaira H., Hasegawa M. 1999. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 16, 1114–1116 [Google Scholar]
- 16.Maddison W. P., Maddison D. R. 2006. Mesquite: a modular system for evolutionary analysis. See http://mesquiteproject.org
- 17.Ree R. H., Smith S. A. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 57, 4–14 10.1080/10635150701883881 (doi:10.1080/10635150701883881) [DOI] [PubMed] [Google Scholar]
- 18.Tilbury C. R., Tolley K. A. 2009. A re-appraisal of the systematics of the African genus Chamaeleo (Reptilia: Chamaeleonidae). Zootaxa 2079, 57–68 [Google Scholar]
- 19.Tilbury C. R., Tolley K. A., Branch W. R. 2006. A review of the systematics of the genus Bradypodion (Sauria: Chamaeleonidae), with the description of two new genera. Zootaxa 1363, 23–38 [Google Scholar]
- 20.Tolley K. A., Tilbury C. R., Branch W. R., Matthee C. A. 2004. Phylogenetics of the southern African dwarf chameleons, Bradypodion (Squamata: Chamaeleonidae). Mol. Phylogenet. Evol. 30, 354–365 10.1016/S1055-7903(03)00211-2 (doi:10.1016/S1055-7903(03)00211-2) [DOI] [PubMed] [Google Scholar]
- 21.Klaver C. J. J., Böhme W. 1986. Phylogeny and classification of the Chamaeleonidae (Sauria) with special reference to hemipenis morphology. Bonn. Zool. Monogr. 22, 1–64 [Google Scholar]
- 22.Ali J. R., Huber M. 2010. Mammalian biodiversity on Madagascar controlled by ocean currents. Nature 463, 653–680 10.1038/nature08706 (doi:10.1038/nature08706) [DOI] [PubMed] [Google Scholar]
- 23.Cherubin L. M., Richardson P. L. 2007. Caribbean current variability and the influence of the Amazon and Orinoco freshwater plumes. Deep Sea Res. (I Oceanogr. Res. Pap.) 54, 1451–1473 10.1016/j.dsr.2007.04.021 (doi:10.1016/j.dsr.2007.04.021) [DOI] [Google Scholar]
- 24.Measey G. J., Vences M., Drewes R. C., Chiari Y., Melo M., Bourles B. 2007. Freshwater paths across the ocean: molecular phylogeny of the frog Ptychadena newtoni gives insights into amphibian colonization of oceanic islands. J. Biogeogr. 34, 7–20 [Google Scholar]
- 25.Markwick P. J., Valdes P. J. 2004. Palaeo-digital elevation models for use as boundary conditions in coupled ocean-atmosphere GCM experiments: a Maastrichtian (Late Cretaceous) example. Palaeogeogr. Palaeoclimatol. Palaeoecol. 213, 37–63 [Google Scholar]
- 26.Talbot M. R., Williams M. A. J. 2009. Cenozoic evolution of the Nile Basin. In Nile: origin, environments, limnology and human use (ed. Dumont H. J.), pp. 37–60 Berlin, Germany: Springer [Google Scholar]