Abstract
Many introduced species engage in intraguild predation (IGP), the consumption of species with which they compete for shared resources. While the factors influencing local persistence of IG predator and prey species are well-understood, using these factors to predict the invasion speed of an introduced IG predator has received less attention. Existing theory predicts that native competitors slow invasions via depletion of shared resources, but this fails to account for additional resources acquired when an invader consumes competitors. Here, I outline a general framework for understanding the effect of IGP on invasion speeds. I find that invaders that consume native competitors may be able to spread where invasion by pure competitors would fail, and that invasion speed increases with increasing levels of IGP. Notably, if the benefit from consuming competitors outweighs the loss of shared resources to competitors, invasion proceeds faster than invasion in the absence of competitors. This may explain empirical observations of rapid spread rates of invaders that feed at multiple trophic levels.
Keywords: intraguild predation, invasion, reaction–diffusion, travelling wave
1. Introduction
Intraguild predation (IGP) refers to predation among species that compete for shared resources. If IGP is primarily unidirectional, the consumer and consumed species are referred to as the IG predator and IG prey, respectively. The IGP interaction has been well-studied, both because of its ubiquity in natural communities [1], and as the simplest module for studying food web complexity. Theory has sought to explain coexistence mechanisms for coevolved IGP systems through competitive superiority of the IG prey [2], regulation by parasites [3], incomplete resource overlap [4], or temporal or spatial refugia [5,6]. However, introduced IG predators may profoundly alter community structure through displacement of native predators and prey. High-profile invasions by IG predators include the rapidly spreading harlequin ladybird Harmonia axyridis [7] and ‘killer shrimp’ Dikerogammarus villosus [8] in Europe, brown tree snakes (Boiga irregularis) in Guam [9] and barred owls (Strix varia) colonizing endangered spotted owl territories [10]. A mechanistic understanding of the factors influencing the spread rates of introduced IG predators is therefore of basic and applied importance.
For many introduced species, theory and observations support the existence of an invasion front that advances at approximately constant speed [11]. Okubo et al. [12] show that the presence of competitors slows invasions relative to invasion into a competitor-free environment. However, invaders may compensate for resources lost to competitors by direct consumption of competitors, which may in turn influence invasion speeds. Here, I outline some general results for the dependence of an introduced IG predator's invasion speed on resource competition and IGP, and illustrate their application for two different models of IGP.
2. Theoretical framework
Suppose the introduced IG predator has density NI(x,t) at position x and time t, and per capita growth rate fI(N,S), where the vector S contains the local densities of interacting native species. I define the initial invasion fitness as the IG predator's per capita growth rate when introduced at low density to the native community at its pre-invasion equilibrium (S = S*):
| 2.1 |
I will show that this can be written
| 2.2 |
where E0 is the IG predator's initial invasion fitness in the absence of native competitors, Epred represents the invader's fitness gain from consuming competitors and Ecomp is the fitness reduction associated with resources lost to competitors. For an invader whose spatial spread is described by diffusion (with coefficient DI) and a pre-invasion community that is susceptible to invasion (i.e. E > 0), the classic result of Fisher [13] states that the invasion eventually advances with speed
| 2.3 |
By equation (2.2), this can be expressed as
| 2.4 |
3. Results
For two models of IGP describing competition for multiple resources (model 1) or a single resource (model 2), I show that the IG predator's initial invasion fitness can indeed be expressed as additive components describing competitor-free fitness, fitness gains from IGP and fitness reductions through competition. Therefore, from expression (2.4), it follows that:
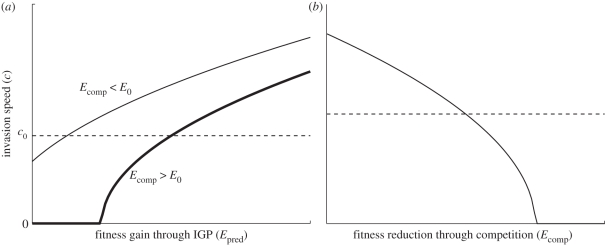
— Provided the invader receives some benefit from consuming competitors (Epred > 0), its invasion speed will always be faster than under pure interspecific competition. Conversely, killing but not consuming competitors does not increase the invasion speed beyond that predicted under pure competition.
— IGP can allow the spatial propagation of an invader that would be excluded under pure competition (i.e. when E0 < Ecomp).
— If the IG predator experiences a net benefit from its interaction with the native competitor (Epred > Ecomp), the invasion proceeds faster than if competitors were absent.
Let NR(x,t) and NI(x,t) denote the densities of the resident IG prey and introduced IG predator at location x and time t, respectively. When they share multiple resources, their dynamics decouple from the dynamics of individual resource species, and competition can be described by Lotka–Volterra competition. If both species move diffusively with diffusion coefficients DR and DI, respectively, the dynamics are described by model 1,
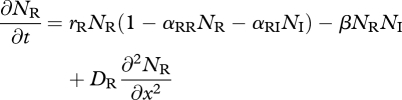 |
3.1 |
and
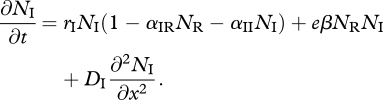 |
3.2 |
The parameter rj(j = R,I) is the intrinsic growth rate of species j, αjk is the competitive effect on species j of species k, β is the IGP rate and e is the conversion efficiency of IG prey into predators. In the absence of the invader, the resident attains its carrying capacity 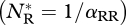 . This equilibrium is invasible provided the initial invasion fitness,
. This equilibrium is invasible provided the initial invasion fitness,
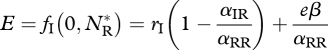 |
3.3 |
is positive. Consistent with equation (2.2), E splits into components describing the invasion fitness in the absence of competitors,
| 3.4 |
the fitness reduction through competition,
| 3.5 |
and fitness benefit from consuming competitors,
| 3.6 |
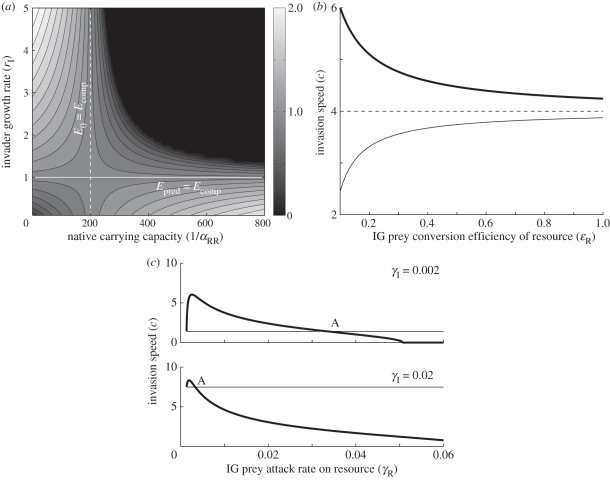
The dependence of the corresponding invasion speed (equation (2.4)) on invader life history and species interactions is understood by considering how the model parameters affect equations (3.4–3.6) (electronic supplementary material, appendix table S1). Increasing parameters relating directly to IGP (predation rate, β, and conversion efficiency, e) increases Epred and therefore the invasion speed (figure 1a), while increasing the competitive effect of the native on the invader (αIR) increases Ecomp, reducing the invasion speed (figure 1b). The invader's growth rate (rI), and the native competitor's carrying capacity (1/αRR) antagonistically affect the invasion speed (figure 2a). A native competitor with a low carrying capacity has a weak competitive effect on the invader, and the invasion speed is maximized for invaders with relatively high growth rates on basal resources. Conversely, a high native carrying capacity may exclude the invader from shared resources, and so the invasion speed is maximized for invaders whose growth rates on resources are low relative to attack rates on the competitor. The boundaries between these two strategies for maximizing invasion speed are delineated by the invasion threshold in the absence of IGP (E0 = Ecomp) and the point at which the net effect of the native on the IG predator density is zero (Epred = Ecomp).
Figure 1.
(a) Invasion speed of an introduced IG predator (c) as a function of the fitness gain from consuming competitors (Epred), for cases where the invader is able (thin line) or unable (thick line) to invade in the absence of IGP. The dashed line depicts the invasion speed in the absence of competitors (c0). (b) Invasion speed as a function of the fitness reduction through resource competition (Ecomp).
Figure 2.
(a) Invasion speed as a function of the native competitor's carrying capacity (1/αRR) and the invader's intrinsic growth rate (rI) for model 1. The bold and dashed white lines denote where Epred = Ecomp and E0 = Ecomp, respectively. Parameter values used are αIR = 0.005, e = 0.5, β = 0.01, DI = 0.25. Invasion speed for model 2 as a function of (b) the native's conversion efficiency of the resource (ɛR) for three values of the invader's attack rate on the resource (γI): 1/300 (thin line, Ecomp < Epred), 1/150 (dashed line, Ecomp = Epred) and 1/75 (thick line, Ecomp > Epred), and (c) the native's attack rate on the resource (γR) for two values of γI. The horizontal lines show the invasion speed in the absence of the native competitor, and A marks the point where Epred = Ecomp. Parameter values used are rH = 10, K = 1000, γR = β = 0.01, ɛR = ɛI = 0.75, μI = μR = DI = 1, e = 0.5.
When the IG prey and predator share one resource species, with local density H(x,t), the dynamics are described by model 2:
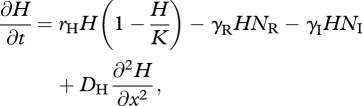 |
3.7 |
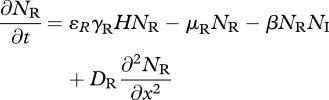 |
3.8 |
and
| 3.9 |
where rH, K and DH are the resource's intrinsic growth rate, carrying capacity and diffusion coefficient, and for species j (=R,I), γj and ɛj are the attack rate on, and conversion efficiency of, the resource, and μj is the per capita mortality rate (remaining parameters are as defined for model 1). Prior to invasion, the native resource and consumer attain equilibrium densities
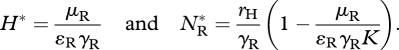 |
3.9 |
The IG predator can invade when its initial invasion fitness,
| 3.10 |
is positive. This again decomposes into terms describing the competitor-free fitness,
| 3.11 |
the fitness gain from consuming competitors,
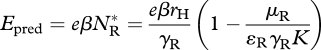 |
3.12 |
and fitness reduction through resource competition,
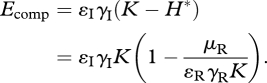 |
3.13 |
The effects of model parameters on the invasion speed are summarized in the electronic supplementary material, appendix table S2. The resource growth rate (rH) and IGP parameters (e, β) affect Epred only and their relationship to the invasion speed is as depicted in figure 1a. Increasing the resource carrying capacity (K), and parameters describing invader fitness on the resource (attack rate γI, conversion efficiency ɛI and longevity 1/μI) increase the invasion speed. The effect on the invasion speed of the analogous parameters for the native competitor's fitness (ɛR, γR, 1/μR) depends on the net effect of IGP on the invader: if the invader experiences a net benefit from the presence of the competitor (Epred > Ecomp), the invasion speed increases with increasing conversion efficiency and longevity, and decreases otherwise (figure 2b). The invasion speed initially increases, then decreases, with the competitor's attack rate on the resource; the value of γR for which Epred = Ecomp separates the regions in which invasion proceeds faster or slower than if the competitor were absent (figure 2c).
The robustness of the analytically derived invasion speed (equation (2.4)) was verified through numerical solution of each model and comparison to the simulated speed, calculated as the distance of a minimum detection density of the invader from the source over an ecologically relevant timescale (electronic supplementary material, appendix 2). Overall, agreement between simulated and analytical speeds is high (<10% difference); they only substantially diverge when parameter values are close to the invasion threshold 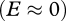 , where the predicted speed is close to zero.
, where the predicted speed is close to zero.
4. Discussion
The importance of considering invasions in the community context, through trophic and competitive interactions with native species, has been increasingly appreciated in invasion ecology [14]. I have presented theory for understanding the spread of invaders that feed on native competitors, outlining conditions under which IGP permits invasion where an introduced competitor would fail, and shown that invasion speeds always increase with increasing levels of IGP. This work yields two important general predictions: (i) invasion success of species feeding at multiple trophic levels should be higher, and (ii) their invasion speeds faster, than for introduced species feeding only on lower trophic levels. Prediction (i) is supported by recent theoretical and empirical studies [15,16]. Anecdotal evidence exists for prediction (ii); for example, estimated spread rates for the harlequin ladybird in Britain (58–144.5 km yr−1 [7]) are an order of magnitude higher than those of another notorious invader, the grey squirrel (7.66 km yr−1 [12]).
Decomposing invasion speeds into expressions describing invader fitness when competitors are absent, fitness reductions through resource depletion by competitors and fitness gains from consuming competitors illuminates how aspects of invaders' and native species' life-history shape spread rates. For example, when a native and invader compete for multiple shared resources, the invader may maximize its invasion speed by preferentially attacking either shared resources or competitors, depending on the strength of interspecific competition. In the single resource model, the native competitor's fitness may positively or negatively affect the invasion speed, depending on its relative quality as a food resource for the invader. Such traits may help explain altered dietary preferences of invaders in their native and introduced ranges.
One other study [17] investigated diffusive spread of an invasive IG predator using a model similar to model 1 (implicit resource dynamics), but with saturating predation on the native competitor. Focusing on the case where the IG prey-only and coexistence equilibrium are bistable, they derive conditions under which local invader removal can reverse the invasion (i.e. the point at which the travelling wavespeed is zero), and solve numerically to find threshold parameter values at which this occurs. However, neither the invasion speed itself is calculated, nor is it clear if the results generalize to multiple competitors or explicit consideration of resource dynamics. Assuming a linear functional response for predation, this manuscript presents for the first time simple expressions for the qualitative dependence of the invasion speed on competition and predation. That Bampfylde & Lewis [17] find travelling wave solutions suggest that the framework presented here is amenable to incorporating saturating predation. An intriguing but unexplored possibility is that a long handling time associated with attacking IG prey relative to basal resources might reduce the benefit of IGP and slow invasion; however, the lack of empirical support for this may indicate that such invaders fail to establish locally before spread is possible.
References
- 1.Arim M., Marquet P. A. 2004. Intraguild predation: a widespread interaction related to species biology. Ecol. Lett. 7, 557–564 10.1111/j.1461-0248.2004.00613.x (doi:10.1111/j.1461-0248.2004.00613.x) [DOI] [Google Scholar]
- 2.Holt R. D., Polis G. A. 1997. A theoretical framework for intraguild predation. Am. Nat. 149, 745–764 10.1086/286018 (doi:10.1086/286018) [DOI] [Google Scholar]
- 3.Hatcher M. J., Dick J. T. A., Dunn A. M. 2008. A keystone effect for parasites in intraguild predation? Biol. Lett. 4, 534–537 10.1098/rsbl.2008.0178 (doi:10.1098/rsbl.2008.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daugherty M. P., Harmon J. P., Briggs C. J. 2007. Trophic supplements to intraguild predation. Oikos 116, 662–677 [Google Scholar]
- 5.Amarasekare P. 2008. Coexistence of intraguild predators and prey in resource-rich environments. Ecology 89, 2786–2797 10.1890/07-1508.1 (doi:10.1890/07-1508.1) [DOI] [PubMed] [Google Scholar]
- 6.Finke D. L., Denno R. F. 2006. Spatial refuge from intraguild predation: implications for prey suppression and trophic cascades. Oecologia 149, 265–275 10.1007/s00442-006-0443-y (doi:10.1007/s00442-006-0443-y) [DOI] [PubMed] [Google Scholar]
- 7.Brown P. M. J., Roy H. E., Rothery P., Roy D. B., Ware R. L., Majerus M. E. N. 2008. Harmonia axyridis in Great Britain: analysis of the spread and distribution of a non-native coccinellid. BioControl 53, 55–68 10.1007/s10526-007-9124-y (doi:10.1007/s10526-007-9124-y) [DOI] [Google Scholar]
- 8.Dick J. T. A., Platvoet D. 2000. Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc. R. Soc. Lond. B 267, 977–983 10.1098/rspb.2000.1099 (doi:10.1098/rspb.2000.1099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiles G. J., Bart J., Beck R. E., Aguon C. F. 2003. Impacts of the brown tree snake: patterns of decline and species persistence in Guam's avifauna. Conserv. Biol. 17, 1350–1360 10.1046/j.1523-1739.2003.01526.x (doi:10.1046/j.1523-1739.2003.01526.x) [DOI] [Google Scholar]
- 10.Gutierrez R. J., Cody M., Courtney S., Franklin A. B. 2007. The invasion of barred owls and its potential effect on the spotted owl: a conservation conundrum. Biol. Invasions 9, 181–196 10.1007/s10530-006-9025-5 (doi:10.1007/s10530-006-9025-5) [DOI] [Google Scholar]
- 11.Shigesada N., Kawasaki K. 1997. Biological invasions: theory and practice. Oxford, UK: Oxford University Press [Google Scholar]
- 12.Okubo A., Maini P. K., Williamson M. H., Murray J. D. 1989. On the spatial spread of the grey squirrel in Britain. Proc. R. Soc. Lond. B 238, 113–125 10.1098/rspb.1989.0070 (doi:10.1098/rspb.1989.0070) [DOI] [PubMed] [Google Scholar]
- 13.Fisher R. A. 1937. The wave of advance of advantageous genes. Ann. Eugen. 7, 355–369 [Google Scholar]
- 14.Noonburg E. G., Byers J. E. 2005. More harm than good: when invader vulnerability to predators enhances impact on native species. Ecology 86, 2555–2560 10.1890/05-0143 (doi:10.1890/05-0143) [DOI] [Google Scholar]
- 15.Crowder D. W., Snyder W. E. 2010. Eating their way to the top? Mechanisms underlying the success of invasive insect generalist predators. Biol. Invasions 12, 2857–2876 10.1007/s10530-010-9733-8 (doi:10.1007/s10530-010-9733-8) [DOI] [Google Scholar]
- 16.Romanuk T. N., Zhou Y., Brose U., Berlow E. L., Williams R. J., Martinez N. D. 2009. Predicting invasion success in complex ecological networks. Phil. Trans. R. Soc. B 364, 1743–1754 10.1098/rstb.2008.0286 (doi:10.1098/rstb.2008.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bampfylde C. J., Lewis M. A. 2007. Biological control through intraguild predation: case studies in pest control, invasive species and range expansion. Bull. Math. Biol. 69, 1031–1066 10.1007/s11538-006-9158-9 (doi:10.1007/s11538-006-9158-9) [DOI] [PubMed] [Google Scholar]