Abstract
Passerine birds have an extensive repertoire of olfactory receptor genes. However, the circumstances in which passerine birds use olfactory signals are poorly understood. The aim of this study is to investigate whether olfactory cues play a role in natal nest recognition in fledged juvenile passerines. The natal nest provides fledglings with a safe place for sleeping and parental food provisioning. There is a particular demand in colony-breeding birds for fledglings to be able to identify their nests because many pairs breed close to each other. Olfactory orientation might thus be of special importance for the fledglings, because they do not have a visual representation of the nest site and its position in the colony when leaving the nest for the first time. We investigated the role of olfaction in nest recognition in zebra finches, which breed in dense colonies of up to 50 pairs. We performed odour preference tests, in which we offered zebra finch fledglings their own natal nest odour versus foreign nest odour. Zebra finch fledglings significantly preferred their own natal nest odour, indicating that fledglings of a colony breeding songbird may use olfactory cues for nest recognition.
Keywords: olfaction, olfactory recognition, fledglings, scent, passeriformes
1. Introduction
Although it has long been thought that birds have a poor sense of olfaction [1], avian olfaction has recently become an expanding area of interest [2–4]. Olfactory signals play an important role in orientation (pigeons: [3]; catbirds: [5]; Antarctic prions: [6]; blue petrels: [7–8]) and social communication, especially in procellariiformes [9–11].
In passerine birds, however, olfaction has only rarely been studied (e.g. [6,12–15]), which is probably because it was assumed that, owing to their small olfactory bulbs [16], olfaction is an unimportant sensory mode. However, the total number of olfactory receptor genes in passerines such as canaries (Serinus canaria) and blue tits (Cyanistes caeruleus) is as high as in procellariformes [17], and zebra finches (Taeniopygia guttata) have an extensive repertoire of olfactory receptor-like gene sequences [18]. The circumstances in which passerines make use of these faculties, however, have not been well identified. Determining whether olfactory cues also play a role in nest recognition in fledged juveniles is the aim of the present study.
It has been proposed that diurnal bird species seem to recognize their nest sites mainly based on visual cues [19,20]. However, visual cues might not be reliable for the orientation of fledglings for two reasons. First, in contrast to their parents, fledglings cannot have a visual representation of the colony and the position of their natal nest when leaving the nest for the first time. Second, visual cues might be insufficient, since colonies are often located in dense, dark bushes [21], and zebra finches have limited visual skills in crepuscular light [22]. Thus, olfactory cues might be potentially more reliable for natal nest recognition.
Here, we investigated whether juvenile zebra finches use olfactory cues to identify their natal nest. Zebra finches breed in dense colonies of up to 50 pairs where nests may rest in contact [21]. During the first days after fledging, juveniles spend only a few hours outside of the nest [21], and the nest is still used for feeding and sleeping. We experimentally tested juvenile zebra finches shortly after fledging, using an odour preference test in which we gave individuals the choice between their own natal nest odour and a foreign conspecific nest odour. We expected zebra finch fledglings to prefer their own nest odour if olfactory cues play a role in nest recognition.
2. Material and methods
(a). Breeding conditions
Randomly paired zebra finches (the birds have been bred longer than 10 generations in captivity) were allowed to breed pairwise in three-compartment cages (115 × 40 × 30 cm) at Bielefeld University. In the central compartment of each cage, a clean wooden nest-box (15 × 15 × 15 cm) was attached to the front central area (figure 1a). We provided to all birds the same ad libitum food (seeds and, three times a week, egg food, germinated seeds and fresh greens) and water on both sides of the cage, to ensure that birds did not develop a side preference owing to the food source. We provided coconut fibres, hay and moss as nest-building material in the middle part of the cage. Breeding cages and nest-boxes were checked daily, and the hatching dates and fledging dates of juveniles were recorded.
Figure 1.
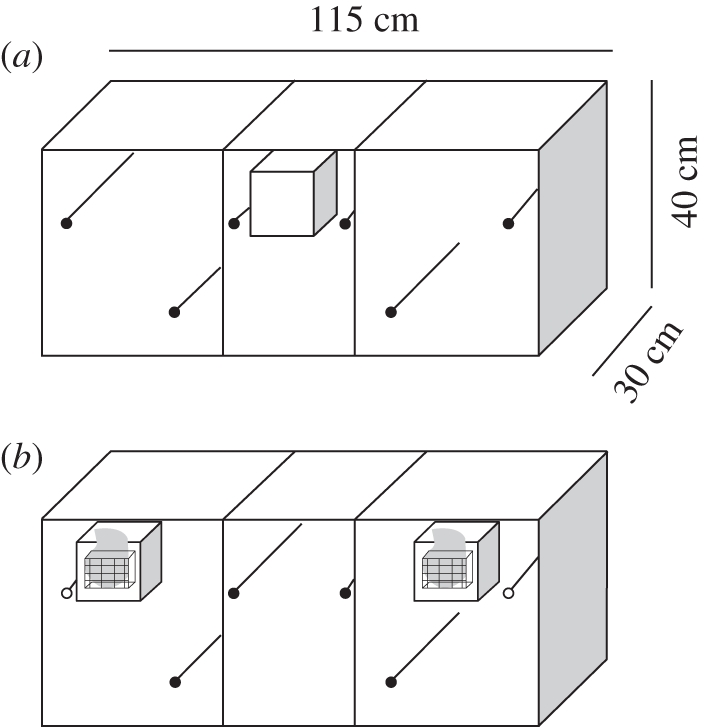
Breeding cage in (a) non-test situation and (b) during odour preference test. During the experiment the nest-box was removed and test nest-boxes with the odour stimuli were attached. Preference zones were defined as the test nest-boxes and the perch in front of it (indicated by blank circles).
(b). Odour preference tests
We performed our experiments from August 2009 until February 2010 with zebra finch fledglings (n = 24) from nine different broods. Juveniles of the same brood were tested at the median age of 23 days. All birds used were already fledged. Tests were conducted in the home cages. During the experiments, parents and siblings were removed from the breeding cage. Prior to the test, we removed the natal nest-box from the central compartment of the cage and attached two test nest-boxes to each side compartment of the cage (figure 1b). The test nest-boxes were filled with fresh coco fibres, imitating the structure of a nest. In the back wall of the test nest-boxes was a round hole (diameter, 7.5 cm) covered by a wire mesh basket, in which the odour samples were placed (figure 1b). Samples were not visible to subjects. A fan (LogiLink, Fan 102, DC 12V, 0.18A) was placed behind the basket to pass air through the odour sample into the test nest-box. In each test, two different olfactory stimuli were tested simultaneously. Each fledgling was tested once individually. To obtain odour samples, we cut nest material that was partially soiled with faeces (approx. 2.5 g) from the home nest. These odour samples were collected into pouches of synthetic gauze. Nest material from each pair was used for only one brood as a foreign odour stimulus. Prior to each test, we placed the odour samples in the baskets and turned on the fan for 20 min. For familiarization, we placed the test birds into the central compartment at least 5 min prior to the test. Opaque dividers between the central compartment and the side compartments prevented test individuals from moving into the side compartments. After the time of familiarization, individuals were tested for 5 min.
To control for side preferences, odour samples were then exchanged, and the fan was turned on again for 20 min. The same individual was then tested for another 5 min. The starting sides of odour samples and sequences of the different test conditions were randomized. All experiments were observed using two video cameras (one-quarter inch colour mini camera, ELV Electronics) and a monitor (14 inch colour-quad-monitor, ELV Electronics). During tests, we recorded the location of the individual every 3 s, and whether it had moved or not. We defined the test nest-box and the perch in front of it as the preference zone (figure 1b) and counted the time intervals that each individual spent in each preference zone. To calculate the time spent at each preference zone, we scored 1.5 s if the test individual changed location within a 3 s interval and 3 s if the test individual stayed at the location [23].
(c). Statistical analysis
Two individuals did not move into either one of the preference zones and were removed from the following analyses. To test for odour preferences, we compared the time juveniles spent in the preference zone for each stimulus odour using a Wilcoxon signed-rank test.
To control for relatedness, we analysed the preference per brood. Each brood was scored for the olfactory stimulus preferred by the majority of juveniles. The brood level choice was tested against random expectation using a χ2-test. All statistical analyses were carried out with SPSS 18.0.0.
3. Results
Zebra finch fledglings preferred their own natal nest odour when their own nest odour and a foreign nest odour were presented simultaneously. Fledglings spent significantly more time in the preference zone of their own natal nest odour (times at own nest odour: median: 62.3 s; 1.quartile: 22.5 s; 3.quartile: 289.5 s; times at foreign nest odour: median 18.8 s; 1.quartile: 3.0 s, 3.quartile: 190.5 s; Wilcoxon test: n = 22, Z = 1.96; p = 0.049; figure 2). Controlling for relatedness and the use of olfactory stimuli, we analysed the preference per brood, revealing the same significant preference for the own natal nest odour (χ2-test: n = 8; χ2 = 4.5; p = 0.033).
Figure 2.
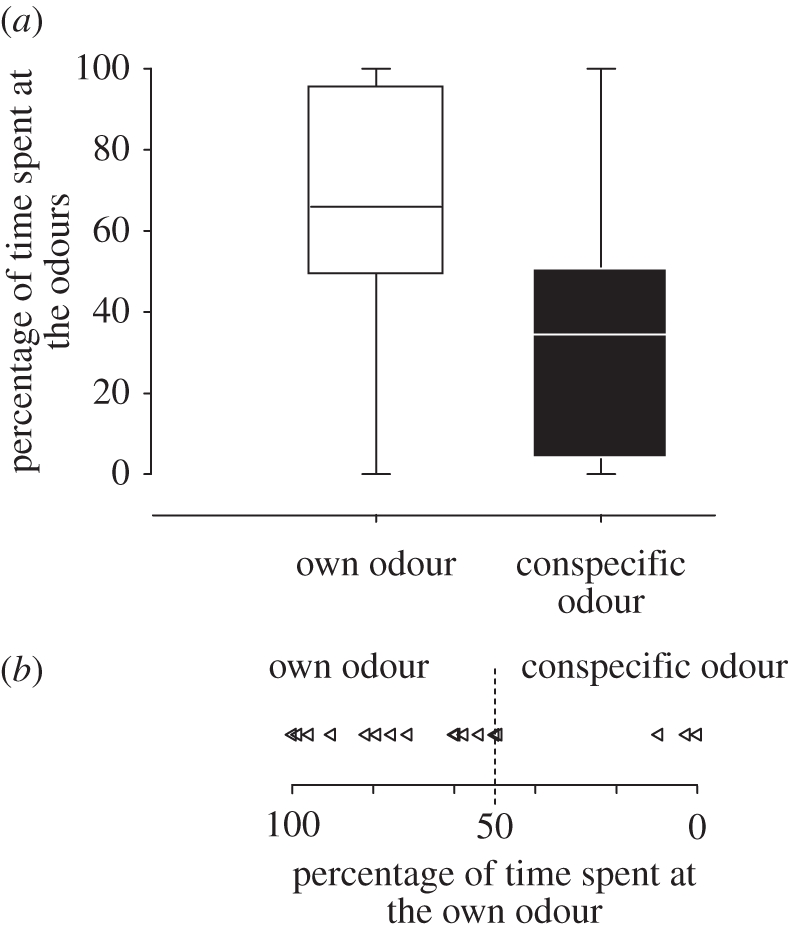
Results of the odour preference test. Preference is indicated by (a) percentage of time fledglings spent in each preference zone and (b) the individual choice.
4. Discussion
We showed that fledglings of a diurnal songbird species, the zebra finch, are able to recognize their own nest based on olfactory cues and that they prefer their own nest odour over a foreign, conspecific odour.
Our study is, to our knowledge, the first to demonstrate that not only nocturnal colony-breeding birds [7,9,24] but also the fledged juveniles of a diurnal passerine bird species use olfactory cues for nest recognition. It has been proposed that it is primarily nocturnal species that use olfactory cues for nest recognition [9] to compensate for limited visual options. However, the lack of a spatial representation of the colony area and the relative nest position within the colony increases the necessity of relying on a sensory modality other than vision.
Finding their own nest among a number of nests ensures parental feeding, which occurs, at least in part, in the natal nest after fledging [21]. A failure to find the nest in times of need may result in stress to fledglings because they may be left out at night, receive less food and lose weight. Fledglings are extremely sensitive to stress, which can potentially lead to lifelong costs [25]. These circumstances might lead to an even more pronounced use of olfaction in wild birds than in our laboratory born birds.
Yet, we can only speculate about the source of odours important in nest recognition. Since all birds had exactly the same food we can rule out the possibility that nest recognition is based on differences in food. Individual body odours from faeces, urine and/or preen oil secretion, which has been shown to have an effect in parental care [15] seem to be more likely involved. This raises the possibility that olfaction is also involved in social communication such as mate choice or inbreeding avoidance, as suggested for other bird species [11].
Our results reveal that even passerine birds, which are known to have small olfactory bulbs [16] can use olfactory cues for small-scale orientation tasks such as nest recognition. This finding suggests that the olfactory sense of diurnal passerine birds can fulfil important functions, and therefore, may not solely be used under extreme ecological pressure.
Acknowledgements
The research was carried out according to the German Laws for experimentation with animals.
We thank Nikolaus von Engelhardt and Edda Geissler for providing help and facilities. We further thank Fritz Trillmich, Anke Rehling, Franceso Bonadonna and an anonymous reviewer who provided helpful comments on the manuscript.
References
- 1.Remane A., Storch V., Welsch U. 1972. Kurzes Lehrbuch der Zoologie. Stuttgart, Germany: Gustav Fischer Verlag [Google Scholar]
- 2.Roper T. J. 1999. Olfaction in birds. Adv. Stud. Behav. 28, 247–332 10.1016/S0065-3454(08)60219-3 (doi:10.1016/S0065-3454(08)60219-3) [DOI] [Google Scholar]
- 3.Wallraff H. G. 2004. Avian olfactory navigation: its empirical foundation and conceptual state. Anim. Behav. 67, 189–204 10.1016/j.anbehav.2003.06.007 (doi:10.1016/j.anbehav.2003.06.007) [DOI] [Google Scholar]
- 4.Balthazart J., Taziaux M. 2009. The underestimated role of olfaction in avian reproduction. Behav. Brain. Res. 200, 248–259 10.1016/j.bbr.2008.08.036 (doi:10.1016/j.bbr.2008.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland R. A., Thorup K., Gagliardo A., Bisson I. A., Knecht E., Mizrahi D., Wikelski M. 2009. Testing the role of sensory systems in the migratory heading of a songbird. J. Exp. Biol. 212, 4065–4071 10.1242/jeb.034504 (doi:10.1242/jeb.034504) [DOI] [PubMed] [Google Scholar]
- 6.Bonadonna F., Hesters F., Jouventin P. 2003. Scent of a nest: discrimination of own-nest odours in Antarctic prions, Pachyptila desolata. Behav. Ecol. Sociobiol. 54, 174–178 [Google Scholar]
- 7.Bonadonna F., Villafane M., Bajzak C., Jouventin P. 2004. Recognition of burrow's olfactory signature in blue petrels, Halobaena caerulea: an efficient discrimination mechanism in the dark. Anim. Behav. 67, 893–898 10.1016/j.anbehav.2003.08.013 (doi:10.1016/j.anbehav.2003.08.013) [DOI] [Google Scholar]
- 8.Mardon J., Bonadonna F. 2009. Atypical homing or self-odour avoidance? Blue petrels (Halobaena caerulea) are attracted to their mate's odour but avoid their own. Behav. Ecol. Sociobiol. 63, 537–542 10.1007/s00265-008-0688-z (doi:10.1007/s00265-008-0688-z) [DOI] [Google Scholar]
- 9.Bonadonna F., Bretagnolle V. 2002. Smelling home: a good solution for burrow-finding in nocturnal petrels? J. Exp. Biol. 205, 2519–2523 [DOI] [PubMed] [Google Scholar]
- 10.Bonadonna F., Caro S. P., Brooke L. B. M. 2009. Olfactory sex recognition investigated in Antarctic prions. PLoS ONE 4, e4148. 10.1371/journal.pone.0004148 (doi:10.1371/journal.pone.0004148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonadonna F., Nevitt G. A. 2004. Partner-specific odor recognition in an Antarctic seabird. Science 306, 835–835 10.1126/science.1103001 (doi:10.1126/science.1103001) [DOI] [PubMed] [Google Scholar]
- 12.Amo L., Galvan I., Tomas G., Sanz J. J. 2008. Predator odour recognition and avoidance in a songbird. Funct. Ecol. 22, 289–293 10.1111/j.1365-2435.2007.01361.x (doi:10.1111/j.1365-2435.2007.01361.x) [DOI] [Google Scholar]
- 13.Mennerat A., Bonadonna F., Perret P., Lambrechts M. M. 2005. Olfactory conditioning experiments in a food-searching passerine bird in semi-natural conditions. Behav. Process. 70, 264–270 10.1016/j.beproc.2005.07.005 (doi:10.1016/j.beproc.2005.07.005) [DOI] [PubMed] [Google Scholar]
- 14.Mennerat A. 2008. Blue tits (Cyanistes caeruleus) respond to an experimental change in aromatic plant odour composition of their nest. Behav. Process. 79, 189–191 10.1016/j.beproc.2008.07.003 (doi:10.1016/j.beproc.2008.07.003) [DOI] [Google Scholar]
- 15.Whittaker D. J., Reichard D. G., Dapper A. L., Ketterson E. D. 2009. Behavioral responses of nesting female dark-eyed juncos Junco hyemalis to hetero- and conspecific passerine preen oils. J. Avian Biol. 40, 579–583 10.1111/j.1600-048X.2009.04813.x (doi:10.1111/j.1600-048X.2009.04813.x) [DOI] [Google Scholar]
- 16.Bang B. G., Cobb S. 1968. The size of olfactory bulb in 108 species of birds. Auk 85, 55–61 [Google Scholar]
- 17.Steiger S. S., Fidler A. E., Valcu M., Kempenaers B. 2008. Avian olfactory receptor gene repertoires: evidence for a well-developed sense of smell in birds? Proc. R. Soc. B 275, 2309–2317 10.1098/rspb.2008.0607 (doi:10.1098/rspb.2008.0607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren W. C., et al. 2010. The genome of a songbird. Nature 464, 757–762 10.1038/nature08819 (doi:10.1038/nature08819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peek F. W., Franks E., Case D. 1972. Recognition of nest, eggs, nest site, and young in female red-winged blackbirds. Wilson Bull. 84, 243–249 [Google Scholar]
- 20.Hughes B. O., Petherick J. C., Brown M. F., Waddington D. 1995. Visual recognition of key nest site stimuli by laying hens in cages. Appl. Anim. Behav. Sci. 42, 271–281 10.1016/0168-1591(94)00541-L (doi:10.1016/0168-1591(94)00541-L) [DOI] [Google Scholar]
- 21.Zann R. A. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press [Google Scholar]
- 22.Eckmeier D., Bischof H. J. 2008. The optokinetic response in wild type and white zebra finches. J. Comp. Physiol. A 194, 871–878 10.1007/s00359-008-0358-7 (doi:10.1007/s00359-008-0358-7) [DOI] [PubMed] [Google Scholar]
- 23.Witte K., Caspers B. 2006. Sexual imprinting on a novel blue ornament in zebra finches. Behaviour 143, 969–991 10.1163/156853906778623626 (doi:10.1163/156853906778623626) [DOI] [Google Scholar]
- 24.Minguez E. 1997. Olfactory nest recognition by British storm-petrel chicks. Anim. Behav. 53, 701–707 10.1006/anbe.1996.0308 (doi:10.1006/anbe.1996.0308) [DOI] [Google Scholar]
- 25.Krause E. T., Honarmand M., Wetzel J., Naguib M. 2009. Early fasting is long lasting: differences in early nutritional conditions reappear under stressful conditions in adult female zebra finches. PLoS ONE 4, e5015. 10.1371/journal.pone.0005015 (doi:10.1371/journal.pone.0005015) [DOI] [PMC free article] [PubMed] [Google Scholar]


