Abstract
Antarctic krill embryos and larvae were experimentally exposed to 380 (control), 1000 and 2000 µatm pCO2 in order to assess the possible impact of ocean acidification on early development of krill. No significant effects were detected on embryonic development or larval behaviour at 1000 µatm pCO2; however, at 2000 µatm pCO2 development was disrupted before gastrulation in 90 per cent of embryos, and no larvae hatched successfully. Our model projections demonstrated that Southern Ocean sea water pCO2 could rise up to 1400 µatm in krill's depth range under the IPCC IS92a scenario by the year 2100 (atmospheric pCO2 788 µatm). These results point out the urgent need for understanding the pCO2-response relationship for krill developmental and later stages, in order to predict the possible fate of this key species in the Southern Ocean.
Keywords: Antarctic krill, ocean acidification, early development, habitat pCO2, Southern Ocean
1. Introduction
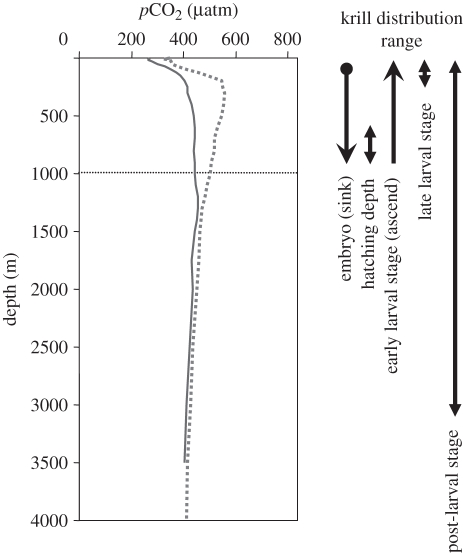
The ecosystems of the Southern Ocean are expected to be most severely affected by ocean acidification (OA) because of the higher solubilities of CO2 and CaCO3 in cold waters and because of regional upwelling of hypercapnic deep sea water [1,2]. Moreover, a future rise in surface water pCO2 may be augmented at great depths [3], where sea water pCO2 is already much higher than at the surface ([4]; figure 1). Hence, vertically migrating animals in the Southern Ocean will probably experience the most drastic changes in carbonate chemistry in future oceans. However, OA research has mainly dealt with tropical and temperate shallow-water calcifying organisms [1], and little attention has been paid to polar species [5]. Antarctic krill (Euphausia superba, hereafter krill) is the key species of the Southern Ocean ecosystem, and is found in a range of water depths. Krill spawn eggs at the surface which sink to 700–1000 m before larvae hatch to swim back to the surface [6]. The post-larval vertical distribution ranges from the surface to at least 3500 m ([7]; figure 1). Thus, krill are already exposed to high CO2 conditions at depth, which will probably become far more hypercapnic than surface waters (electronic supplementary material, S1).
Figure 1.
Vertical distribution range of krill and pCO2 vertical profile at Scotia Sea (59–30° S, 47–30° W; thick line) and Weddell Sea (64–30° S, 34–30° W; dotted line), the known main krill habitats around the Antarctic. The values of pCO2 were calculated from DIC and TA (GLODAP: [20]), and in situ temperature and salinity [21] using the CO2SYS program [22] with silicate and phosphate contents set to zero. Data on krill depths from Quetin & Ross [6] (embryo); [23] (larvae); and [7] (post-larvae).
The purpose of this study is to examine how elevated CO2 conditions affect krill. We focused on early developmental stages, since larvae and juveniles are generally more vulnerable to environmental perturbations, and their survival will largely determine population abundance, distribution and community structure [8].
2. Material and methods
The stock population of krill was collected from the Indian Ocean sector of the Southern Ocean between January and March in the 2005–2006 field season [9]. The krill were maintained in the Australian Antarctic Division's marine research aquarium, where they matured and spawned naturally [10].
(a). Experimental set-up
Experimental sea water was supplied from a 70 l header tank and equilibrated with air (control) or CO2-enriched air before being delivered to experimental jars (250 ml clear polycarbonate) containing krill eggs (see electronic supplementary material, S2). The CO2-enriched air was prepared with a mass flow controller (Horiba STEC SEC-E-40) and by an air valve, to regulate flow rates of pure CO2 and atmospheric air, respectively. The pCO2 levels of the CO2-enriched air and sea water were monitored by a CO2 monitor (Telaire 7001) and indirectly from pH measurement (Radiometer PHM 210 pH metre), respectively. Experimental temperature was set at 0.5°C. Effluent from each jar was drained into a 70 l sump, and recirculated through a degassing unit before returning back to the header tank via a filtration and cooling system. For details, see Kawaguchi et al. [10]. Total alkalinity was measured through a two-stage, potentiometric, open-cell titration. The carbonate chemistry of the experimental sea water is summarized in the electronic supplementary material, S3.
(b). Hatching experiment
Fertilized eggs were obtained in January 2008 and 2009. In the 2008 experiments, three batches of eggs originating from three different females were used. Each batch was randomly distributed into experimental jars, with approximately 20–30 eggs per jar. The embryos were incubated at one of the three target CO2 levels: control (380 µatm), medium (1000 µatm) or high (2000 µatm). In the 2009 experiments, four batches of eggs were incubated as in 2008. Hatch rates were determined for each jar after 7–10 days of spawning. The number of jars in each treatment is summarized in table 1. Embryonic stages were classified at the end of each 2009 experiment after George [11].
Table 1.
Summary of hatch rates from all experiments conducted in this study.
| batch ID | year | CO2 level | pCO2 (µatm) | hatch rate (%) | na |
|---|---|---|---|---|---|
| mean ± s.d. | |||||
| A1 | 2008 | control | 380 | 27.9 ± 14.5 | 12 |
| medium | 1000 | 32.0 ± 12.6 | 12 | ||
| high | 2000 | 0.6 ± 0.0 | 12 | ||
| B1 | 2008 | control | 380 | 21.0 ± 14.9 | 15 |
| medium | 1000 | 29.8 ± 11.4 | 9 | ||
| high | 2000 | 0.0±0.0 | 9 | ||
| C1 | 2008 | control | 380 | 22.4 ± 12.2 | 9 |
| medium | 1000 | 18.6 ± 12.2 | 9 | ||
| high | 2000 | 0.0 ± 0.0 | 9 | ||
| R2 | 2009 | control | 380 | 39.2 ± 9.8 | 3 |
| medium | 1000 | 45.0 ± 10.2 | 3 | ||
| high | 2000 | 0.0 ± 0.0 | 3 | ||
| S2 | 2009 | control | 380 | 16.7 ± 14.4 | 3 |
| medium | 1000 | 23.7 ± 5.5 | 3 | ||
| high | 2000 | 0.0 ± 0.0 | 3 | ||
| W2 | 2009 | control | 380 | 56.1 ± 4.4 | 3 |
| medium | 1000 | 50.0 ± 12.5 | 3 | ||
| high | 2000 | 0.0 ± 0.0 | 3 | ||
| Y2 | 2009 | control | 380 | 74.7 ± 22.5 | 3 |
| medium | 1000 | 66.5 ± 8.6 | 3 | ||
| high | 2000 | 0.0 ± 0.0 | 3 |
aNumber of replicates.
(c). Observation of larval swimming activity
Three of the four batches in the hatching experiments in 2009 (S2, W2 and Y2) were used for this observation. R2 was not used because of the limited capacity of the set-up. CO2 exposure started within 1 day of spawning and continued throughout the experimental period. Larval behaviour was observed on an average of 3 days after hatching, when they were in the nauplius stage. Since almost no eggs hatched in 2000 µatm (table 1), observations were made only for the 380 and 1000 µatm groups.
(d). Statistical tests
Statistical tests were performed using SPlus v. 8 software.
3. Results
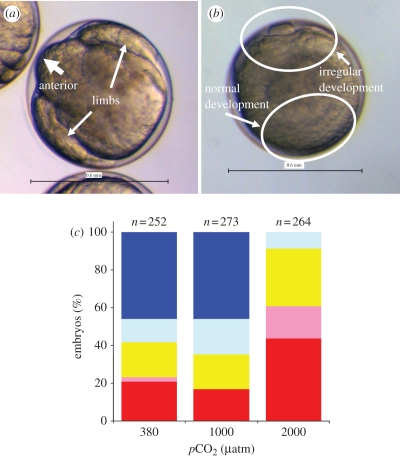
The hatch rates of control eggs used in our experiment were highly variable (16.7–74.7%, table 1) but the range is in fact comparable to the rates in field experiments (0–89% [12,13]). There were significant negative effects on hatch rates at 2000 µatm pCO2 but not at 1000 µatm (table 2). At 2000 µatm pCO2, development was disrupted by gastrulation stage in 90 per cent of embryos and no embryos survived to hatch except one (batch A1, 0.6%) (figure 2 and table 2). Egg batch, CO2 level and their interactions all significantly affected egg hatch rates when results from the three CO2 levels were compared. When the data from control and 1000 µatm groups were compared, CO2 level was not a significant factor, but the egg batch was (table 2). Neither egg batch, CO2 level nor their interactions had a significant effect on nauplius swimming at 380 and 1000 µatm (table 2).
Table 2.
Results of statistical tests (analysis of variance) assessing effects of egg batch, CO2 level, and their interaction on hatch rates and larval behaviour.
| factors | d.f. | statistics | p |
|---|---|---|---|
| hatch rates (3CO2 levels: control, 1000 and 2000 µatm) | |||
| egg batch | 6 | 14.2 | <0.00001 |
| CO2 level | 2 | 106.3 | <0.00001 |
| egg batch × CO2 level | 12 | 4.0 | <0.00001 |
| hatch rates (2CO2 levels: control and 1000 µatm) | |||
| egg batch | 6 | 16.5 | <0.00001 |
| CO2 level | 1 | 1.9 | >0.1 |
| egg batch × CO2 level | 6 | 0.5 | >0.5 |
| swimming behaviour of nauplius (2CO2 levels: control and 1000 µatm) | |||
| egg batch | 2 | 0.8 | >0.1 |
| CO2 level | 1 | 0.4 | >0.5 |
| egg batch × CO2 level | 2 | 0.8 | >0.1 |
Figure 2.
Effects of CO2 on krill development. (a) An embryo reared under current surface pCO2 developed into the limb bud stage; (b) an embryo reared at 2000 µatm pCO2. Embryonic development was disrupted during gastrulation with a larger portion of the ectoderm appearing irregular. (c) Comparison of embryonic development under 380, 1000 and 2000 µatm pCO2 conditions determined 7–10 days of spawning during 2009 experiments. Dark blue, hatched; light blue, limb formation; yellow, blastula–gastrula; pink, cleavage; red, no development. Scale bar, (a,b) 0.6 mm.
4. Discussion
Our results demonstrated that krill embryos develop normally under a pCO2 range of up to 1000 µatm but their development is almost totally inhibited at 2000 µatm (figure 2 and table 1). An important question is then whether the Southern Ocean pCO2 will reach the levels detrimental to krill or not. According to model projections with a model of the Ocean Carbon Model Intercomparison Project or OCMIP-2, forced by IPCC IS92a scenario (atmospheric pCO2 788 µatm by the year 2010 [14]), the sea water pCO2 is unlikely to reach 2000 µatm within this century even at depths, but may rise up to 1400 µatm (see electronic supplementary material, S1 for details). Currently, it is unclear whether CO2 exerts its impacts on krill in a pCO2-dependent manner or some threshold exists above which harmful CO2 effects will manifest suddenly. Krill apparently have evolved a certain level of resistance to increased pCO2, probably through their natural exposure from surface (380 µatm) to deep-sea pCO2 levels (figure 1), as a result of evolutionary adaptation, but they might be highly vulnerable to higher pCO2 levels.
The present study is merely the first step towards scientific understanding of krill's future in the era of OA and warming. The following topics are of primary importance for future studies. First, it is essential to develop a krill husbandry technique that makes larger number of krill available for laboratory investigations at different life-cycle stages. Even using the most advanced krill husbandry technique [10], the delicate nature of krill still poses a significant challenge to laboratory experiments. This has resulted in the limited number (3), and the coarse resolution on krill's CO2 sensitivity obtained in this study, both of which reduce our ability to predict krill's future. Second, we need to establish the finer CO2-and-effect relationship for the pCO2 range between 1000 and 2000 µatm, covering all developmental stages (particularly maturation stage because of its decisive influence on offspring population size). Third, the combined effects of OA and other environmental changes, such as warming, must be considered. Polar aquatic organisms are believed to live at near-stressful temperatures and could be vulnerable to warming [15]. The combined effects can be purely physiological but may also include various changes in krill habitats; e.g. reductions in sea-ice area, which have been regarded as a cause for recent declines in krill density in the South Atlantic [16], recent regional recovery of predators [17] and expanding fisheries [18] that have exerted increasing pressures on krill populations. Finally, mechanistic understanding of OA effects is also needed. Our experimental sea water at 2000 µatm pCO2 was undersaturated with regard to calcite (see electronic supplementary material, S3), the CaCO3 formed by crustaceans [19] and therefore the observed detrimental impacts could be brought about through undersaturation of sea water CaCO3. Further detailed investigation should be conducted using physiological, biochemical and molecular approaches.
Another important message of the present study relates to the importance of incorporating possible temporal and spatial ranges of ocean conditions into experimental protocols of OA research. This is particularly relevant to the prediction on animals that migrate vertically in their life cycle, such as krill.
Acknowledgements
We thank Hans-Otto Pörtner, Robin Ross and Langdon Quetin and the two anonymous reviewers for their useful comments. This research was supported by the Australian Antarctic Division and by the Australian Government Co-operative Research Centres Program through the Antarctic Climate & Ecosystems Co-operative Research Centre (ACE CRC).
References
- 1.Doney S. C., Fabry V. J., Richard A., Feely R. A., Kleypas J. A. 2009. Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192 10.1146/annurev.marine.010908.163834 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 2.Sabine C. L., et al. 2004. The oceanic sink for anthropogenic CO2. Science 305, 367–371 10.1126/science.1097403 (doi:10.1126/science.1097403) [DOI] [PubMed] [Google Scholar]
- 3.Brewer P. G., Peltzer E. T. 2009. Limits to marine life. Science 324, 347–348 10.1126/science.1170756 (doi:10.1126/science.1170756) [DOI] [PubMed] [Google Scholar]
- 4.Feely R. A., Doney S. C., Cooly S. R. 2009. Present conditions and future changes in a high-CO2 world. Oceanography 22, 36–47 [Google Scholar]
- 5.Fabry V. J., McClintock J. B., Mathis J. T., Grebmeier J. M. 2009. Ocean acidification at high latitudes: the bellwether. Oceanography 22, 160–171 [Google Scholar]
- 6.Quetin L. B., Ross R. M. 1984. Depth distribution of developing Euphausia superba embryos, predicted from sinking rates. Mar. Biol. 79, 47–53 10.1007/BF00404984 (doi:10.1007/BF00404984) [DOI] [Google Scholar]
- 7.Clarke A., Tyler P. 2008. Adult Antarctic krill feeding at abyssal depths. Curr. Biol. 18, 282–285 10.1016/j.cub.2008.01.059 (doi:10.1016/j.cub.2008.01.059) [DOI] [PubMed] [Google Scholar]
- 8.Kurihara H. 2008. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 373, 175–284 10.3354/meps07802 (doi:10.3354/meps07802) [DOI] [Google Scholar]
- 9.Nicol S., Meiners K., Raymond B. 2010. BROKE-West, a large ecosystem survey of the South West Indian Ocean sector of the Southern Ocean 30–80° E (CCAMLR Division 58.4.2). Deep-Sea Res. II 57, 693–700 10.1016/j.dsr2.2009.11.002 (doi:10.1016/j.dsr2.2009.11.002) [DOI] [Google Scholar]
- 10.Kawaguchi S., King R., Meijers R., Osborn J. E., Swadling K. M., Ritz D. A., Nicol S. 2010. An experimental aquarium for observing the schooling behaviour of Antarctic krill (Euphausia superba). Deep-Sea Res. II 57, 683–692 10.1016/j.dsr2.2009.10.017 (doi:10.1016/j.dsr2.2009.10.017) [DOI] [Google Scholar]
- 11.George R. Y. 1984. Ontogenetic adaptation in growth and respiration of Euphausia superba in relation to temperature and pressure. J. Crustacean Biol. 4, 252–262 [Google Scholar]
- 12.Harrington S. A., Ikeda T. 1986. Laboratory observations on spawning, brood size and egg hatchability of the Antarctic krill Euphausia superba from Prydz Bay, Antarctica. Mar. Biol. 92, 231–235 [Google Scholar]
- 13.Kikuno T. 1981. Spawning behaviour and early development of the Antarctic krill, Euphausia superba Dana, observed on board R.V. Kaiyo Maru in 1979/80. Nankyoku Shiryo (Antarctic Record) 73, 93–102 [Google Scholar]
- 14.Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L. (eds) 2007. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Tewksbury J. J., Huey R. B., Deutsch C. A. 2008. Putting the heat on tropical animals. Science 320, 1296–1297 10.1126/science.1159328 (doi:10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 16.Atkinson A., Siegel V., Pakhomov E., Rothery P. 2004. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432, 100–103 10.1038/nature02996 (doi:10.1038/nature02996) [DOI] [PubMed] [Google Scholar]
- 17.Trathan P. N., Reid K. 2009. Exploitation of the marine ecosystem in the sub-Antarctic: historical impacts and current consequences. Papers Proc. R. Soc. Tasmania 143, 9–14 [Google Scholar]
- 18.Kawaguchi S., Nicol S., Press A. J. 2009. Direct effects of climate change on the Antarctic krill fishery. Fish. Manag. Ecol. 16, 424–427 10.1111/j.1365-2400.2009.00686.x (doi:10.1111/j.1365-2400.2009.00686.x) [DOI] [Google Scholar]
- 19.Wilt F. H., Killian C. E., Livingston B. T. 2003. Development of calcareous skeletal elements in invertebrates. Differentiation 71, 237–250 [DOI] [PubMed] [Google Scholar]
- 20.Key R. M., et al. 2004. A global ocean carbon climatology: Results from GLODAP. Global Biogeochem. Cycles 18, GB4031. 10.1029/2004GB002247 (doi:10.1029/2004GB002247) [DOI] [Google Scholar]
- 21.Conkright M. E., Locarnini R. A., Garcia H. E., O'Brien T. D., Boyer T. P., Stephens C., Antonov J. I. 2002. World Ocean Atlas 2001: Objective Analyses, Data Statistics, and Figures, CD-ROM Documentation. Silver Spring, MD: National Oceanographic Data Center [Google Scholar]
- 22.Pierrot D., Lewis E., Wallace D. W. R. 2006. MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105 Oak Ridge, TN: US Department of Energy [Google Scholar]
- 23.Daly K. L. 2004. Overwintering growth and development of larval Euphausia superba: an interannual comparison under varying environmental conditions west of the Antarctic Peninsula. Deep-Sea Res. II 51, 2139–2168 10.1016/j.dsr2.2004.07.010 (doi:10.1016/j.dsr2.2004.07.010) [DOI] [Google Scholar]