Abstract
Insect societies are well-known for their advanced cooperation, but their colonies are also vulnerable to reproductive parasitism. Here, we present a novel example of an intraspecific social parasitism in a highly eusocial bee, the stingless bee Melipona scutellaris. In particular, we provide genetic evidence which shows that, upon loss of the mother queen, many colonies are invaded by unrelated queens that fly in from unrelated hives nearby. The reasons for the occurrence of this surprising form of social parasitism may be linked to the fact that unlike honeybees, Melipona bees produce new queens in great excess of colony needs, and that this exerts much greater selection on queens to seek alternative reproductive options, such as by taking over other nests. Overall, our results are the first to demonstrate that queens in highly eusocial bees can found colonies not only via supersedure or swarming, but also by infiltrating and taking over other unrelated nests.
Keywords: social parasitism, reproductive conflict, stingless bees, Melipona scutellaris
1. Introduction
Insect societies are well-known for their advanced cooperation, but their colonies can also be exploited by interspecific and intraspecific social parasites which can benefit from the resources stored within the nest, and get directly cared for by their hosts. Recently, social bees have become a major focus in the study of social parasitism in insect societies, after the discovery of several novel, highly unusual cases of intraspecific worker parasitism in this group [1]. For example, it was shown that both bumblebee [2] and queenless honeybee colonies [1,3] are occasionally parasitized by workers from other nests that fly in and lay male-producing eggs, which are then reared by the victim colony. In addition, in the Cape bee Apis mellifera capensis, where workers can produce female offspring via thelytokous parthenogenesis, a single clonal lineage of worker bees was found to reproductively parasitize and kill colonies of African honeybees, Apis mellifera scutellata [1,4]. One study even found the Cape bees to occasionally lay female-destined eggs directly into queen cells, thereby reincarnating themselves as queens [5].
In contrast to these varied forms of intraspecific social parasitism reported for bumblebees and honeybees, as yet little is known about the occurrence of such social parasitism in the other major group of eusocial bees, the stingless bees. Recently, however, Sommeijer et al. [6,7] speculated that intraspecific queen parasitism might perhaps occur in the stingless bee genus Melipona, after observing that in Melipona favosa, a large percentage of the virgin queens (57%) left their natal nest and that lone queens apparently tried to enter and take over other unrelated colonies nearby. Indeed, there are good a priori reasons for expecting such intraspecific queen parasitism in Melipona. In contrast to other highly eusocial bees, queens in Melipona are reared in great excess of colony needs, with ca 5–25% of all females developing as queens [8–10]. This phenomenon is linked to the fact that in Melipona, queens and workers develop in identical, sealed brood cells on a similar provision mass, thereby allowing females to control their own caste development and causing many to develop as queens, with the hope of being able to head a new swarm or replace a failing mother queen [11,12]. Nevertheless, chances of doing so successfully are slim, and the majority of all queens are normally killed by the workers soon after emergence [13–15], or are dispelled out of the colony [7,16]. With such low chances of any one queen succeeding in founding or inheriting a nest, penetrating and taking over other unrelated colonies nearby would thus be expected to be a profitable alternative to gain reproductive benefits [6].
The aim of this study was to carry out the first formal genetic test of whether or not intraspecific queen parasitism occurs in stingless bees, and whether queens could indeed succeed in entering unrelated hives to opportunistically rear their own brood. In order to do so, we carried out a long-term genetic study on the Brazilian stingless bee, Melipona scutellaris, and sampled female brood before and after queen replacement events to check whether newly established queens were either the daughter of the previous queen, or instead were unrelated queens that had flown in from elsewhere.
2. Material and methods
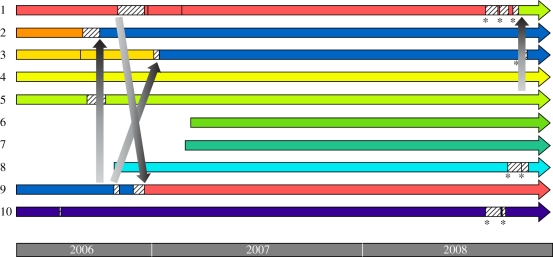
To study the incidence of intraspecific queen parasitism, we monitored 10 free-foraging M. scutellaris colonies in the bee laboratory at the University of São Paulo for a period of ca 3 years and regularly checked all colonies for queen replacement events (figure 1). Via genotyping at three microsatellite loci, we then determined whether the replacement queens were daughters of the previous queen or alien queens that had flown in from other hives nearby (detailed methods can be found in the electronic supplementary material). This was straightforward, given that in all cases, genotypes were consistent with the mother queens being singly mated (cf. [17]), as is typical for stingless bees [18]. Indeed, the probability of misclassification was very low, 0.004 (see the electronic supplementary material). We also artificially induced eight queen replacement events and obtained data on a further six queen replacement events from colonies kept in an apiary at São Simão, 200 miles north. In all cases, the multi-locus genotypes allowed alien queens to be unambiguously traced back to a particular source colony (figure 1; electronic supplementary material, table S1).
Figure 1.
Intraspecific colony takeover by unrelated queens in the 10 M. scutellaris colonies kept in São Paulo. Coloured blocks show the tenure of any one queen; hatched areas are periods during which the colonies became queenless, after which a new queen was adopted. Asterisks indicate experimental queen removals. Vertical arrows indicate cases where genotyping showed newly adopted queens to be unrelated queens that had flown in from other hives nearby. The order of the colonies on the figure corresponds to the relative positions at which they were placed in the laboratory.
3. Results
The queen genotypes (electronic supplementary material, table S1) show that intraspecific queen parasitism was common in M. scutellaris, with 25 per cent of all queen replacements (six out of 24) being with an alien queen that had flown in from another colony (95% binomial confidence limits: 9.8–46.7%). Colony takeovers by alien queens were detected both in the colonies kept in the laboratory in São Paulo (three out of ten natural queen replacements and one out of eight induced queen replacements, figure 1) as well as in the colonies kept in the apiary of São Simão (two out of six natural queen replacements; electronic supplementary material, table S1), where colonies were kept at a lower density (ca five colonies per hectare), akin to that found in nature. In all these cases, the new queens had genotypes that did not match with the genotype expected if they would have been the daughters of the superseded queens, and relatedness estimates show that the newly adopted alien queens were not related to either the adopting workers (mean r = –0.07, 95% C.L.: (−0.27, 0.14), n = 6; electronic supplementary material, table S1) or the superseded queens (mean r = –0.14, 95% C.L.: (−0.53, 0.24), n = 6). Alien queens also never came from neighbouring colonies (figure 1). This means that alien queen takeovers could not have been a mere by-product of queens accidentally returning to the wrong hive after their mating flight. In fact, in two out of four of the colonies in São Paulo, we inferred that the alien queens definitely came from queenright source colonies (the queen from colony 9 invading colony 2 and that of colony 5 invading colony 1, figure 1), and such colonies would normally not be expected to send out queens for mating.
The median queen life-expectancy in our study colonies was 175 days (Kaplan–Meier analysis, n = 20) and it took on average 15 days for a dead queen to be replaced (range 0–46, n = 18; electronic supplementary material, table S1). This means that at any one time, 8.6 per cent (15/175) of the colonies in the population would find themselves queenless, at which point they would be vulnerable to be invaded by alien queens. There was no significant difference between the time that it took for a dead queen to be replaced by a daughter queen (14.9 days, s.d. 13.7, n = 14) and by an alien queen (16.8 days, s.d. 9.3, n = 4; t-test, t = 0.25, p = 0.81). This means that colonies that had remained queenless for a long time were not more likely to adopt alien queens. Colonies also continuously produced a large number of virgin queens (ca 50 during the 15 days that it took for colonies to re-queen). Even considering the fact that workers may kill some of the virgin queens before allowing one to leave on a mating flight (ca seven out of eight in Melipona quadrifasciata [13]), it is therefore clear that colonies did not adopt unrelated queens merely out of a shortage of natal queens.
4. Discussion
Our results convincingly demonstrate, based on genetic data from two different localities, that M. scutellaris queens can leave the hive and infiltrate and successfully take over unrelated, queenless hives nearby. Given that Sommeijer et al. [6,7] collected behavioural evidence suggesting that the same phenomenon may be happening in another related species, M. favosa, it is probable that such queen parasitism occurs in many more Melipona species. This gives credibility to intraspecific queen parasitism being a specific reproductive strategy that evolved in response to the vast queen overproduction that occurs in this genus [8–10] and which selects queens to seek reproductive opportunities outside their natal colony. More generally, our data provide the first solid evidence that queens in highly eusocial bees can found colonies, not only via queen supersedure or swarming, but also by infiltrating and taking over unrelated nests nearby.
Previously, in social bees, anecdotal evidence for queens entering and taking over unrelated nests of the same species was found only in some species of primitively eusocial bumblebees, where late-emerging queens occasionally usurp and take over conspecific nests in the colony-founding stage [19–21], and in African honeybees, A. m. scutellata, where swarms sometimes usurp weaker colonies of the European honeybee [22]. Nevertheless, it is clear that both phenomena are quite different from the one we document, with the first being restricted to the colony-founding stage, and the second involving whole swarms of bees invading colonies of a related subspecies, as opposed to lone queens infiltrating and parasitizing conspecific nests in Melipona [6].
The occurrence of intraspecific queen parasitism in Melipona may well have important evolutionary consequences. For example, if queens are able to successfully invade unrelated nests, then producing new queens may become a profitable way for the adult workers to export copies of their own genes to the rest of the population. In fact, this may explain why in M. favosa and Melipona compressipes, workers have been observed to actively chase virgin queens out of the colony, resulting in about half leaving the colony alive [7,16]. That some gynes still end up being killed may be due to the workers perceiving the gynes as a threat to the current queen, since gynes have been found to occasionally attack the mother queen—presumably to try to kill and replace her [15]. The fact that producing many queens may genetically benefit colonies is in contrast to previous models [11,12], which argued that from a colony-level perspective, queen overproduction in Melipona always represents a great cost. On the other hand, it is true that there should also be strong selection for queen parasitism to be kept at a low level, given that workers should be selected to prevent unrelated queens from invading their colony. Providing such events are rare, however, accepting an occasional unrelated parasite queen may not entail a big cost, since the cost of accidentally rejecting a daughter queen would probably be much larger. In addition, it has been shown that M. scutellaris workers may keep on producing their own sons until many months after a new queen has become established, thereby providing them with direct fitness benefits even if they would accept an unrelated queen [17]. Given that virgin queens were continuously produced in high frequency, we consider it unlikely, however, that workers were adopting unrelated queens merely in situations when related queens did not happen to be available, that is, in order for them to be able to continue producing their own sons and ‘make the best of a bad situation’. Future work will have to determine how common intraspecific queen parasitism is in other species of stingless bees, including in natural, unmanaged populations.
Acknowledgements
We thank the FWO-Flanders, FAPESP (05/58093-8 to DAA; 04/15801-0 to VLIF) and CNPq (480957/2004-5) for financial support and Dr Paulo Nogueira-Neto for allowing us to collect data from his hives in São Simão. All work was carried out under permit nos 139311, 08BR001591/DF and 08BR002483/DF from the Brazilian Ministry of Environment.
References
- 1.Beekman M., Oldroyd B. P. 2008. When workers disunite: intraspecific parasitism by eusocial bees. Annu. Rev. Entomol. 53, 19–37 10.1146/annurev.ento.53.103106.093515 (doi:10.1146/annurev.ento.53.103106.093515) [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Vaamonde C., Koning J. W., Brown R. M., Jordan W. C., Bourke A. F. G. 2004. Social parasitism by male-producing reproductive workers in a eusocial insect. Nature 430, 557–560 10.1038/nature02769 (doi:10.1038/nature02769) [DOI] [PubMed] [Google Scholar]
- 3.Chapman N. C., Nanork P., Gloag R. S., Wattanachaiyingcharoen W., Beekman M., Oldroyd B. P. 2009. Queenless colonies of the Asian red dwarf honey bee (Apis florea) are infiltrated by workers from other queenless colonies. Behav. Ecol. 20, 817–820 10.1093/beheco/arp065 (doi:10.1093/beheco/arp065) [DOI] [Google Scholar]
- 4.Baudry E., Kryger P., Allsopp M., Koeniger N., Vautrin D., Mougel F., Cornuet J. M., Solignac M. 2004. Whole-genome scan in thelytokous-laying workers of the cape honeybee (Apis mellifera capensis): central fusion, reduced recombination rates and centromere mapping using half-tetrad analysis. Genetics 167, 243–252 10.1534/genetics.167.1.243 (doi:10.1534/genetics.167.1.243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan L. A., Allsopp M. H., Oldroyd B. P., Wossler T. C., Beekman M. 2008. Cheating honeybee workers produce royal offspring. Proc. R. Soc. B 275, 345–351 10.1098/rspb.2007.1422 (doi:10.1098/rspb.2007.1422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommeijer M. J., Bruijn L. L. M., Meeuwsen F. 2003. Reproductive behaviour of stingless bees: solitary gynes of Melipona favosa (Hymenoptera: Apidae, Meliponini) can penetrate existing nests. Entomol. Berichten 63, 31–35 [Google Scholar]
- 7.Sommeijer M. J., Bruijn L. L. M., Meeuwsen F., Slaa E. J. 2003. Reproductive behaviour of stingless bees: nest departures of non-accepted gynes and nuptial flights in Melipona favosa (Hymenoptera: Apidae, Meliponini). Entomol. Berichten 63, 7–13 [Google Scholar]
- 8.Kerr W. E. 1950. Genetic determination of castes in the genus Melipona. Genetics 35, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenseleers T., Ratnieks F. L. W. 2004. Tragedy of the commons in Melipona bees. Proc. R. Soc. Lond. B 271, S310–S312 10.1098/rsbl.2003.0159 (doi:10.1098/rsbl.2003.0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos-Filho P. S., Alves D. A., Eterovic A., Imperatiz-Fonseca V. L., Kleinert A. M. P. 2006. Numerical investment in sex and caste by stingless bees (Apidae: Meliponini): a comparative analysis. Apidologie 37, 207–221 10.1051/apido:2006015 (doi:10.1051/apido:2006015) [DOI] [Google Scholar]
- 11.Ratnieks F. L. W. 2001. Heirs and spares: caste conflict and excess queen production in Melipona bees. Behav. Ecol. Sociobiol. 50, 467–473 10.1007/s002650100388 (doi:10.1007/s002650100388) [DOI] [Google Scholar]
- 12.Wenseleers T., Ratnieks F. L. W., Billen J. 2003. Caste fate conflict in swarm-founding social Hymenoptera: an inclusive fitness analysis. J. Evol. Biol. 16, 647–658 10.1046/j.1420-9101.2003.00574.x (doi:10.1046/j.1420-9101.2003.00574.x) [DOI] [PubMed] [Google Scholar]
- 13.da Silva D. L. N., Zucchi R., Kerr W. E. 1972. Biological and behavioural aspects of the reproduction in some species of Melipona (Hymenoptera, Apidae, Meliponinae). Anim. Behav. 20, 123–132 10.1016/S0003-3472(72)80182-9 (doi:10.1016/S0003-3472(72)80182-9) [DOI] [PubMed] [Google Scholar]
- 14.Koedam D., Monge I. A., Sommeijer M. J. 1995. Social interactions of gynes and their longevity in queenright colonies of Melipona favosa (Apidae: Meliponinae). Neth. J. Zool. 45, 480–494 10.1163/156854295X00429 (doi:10.1163/156854295X00429) [DOI] [Google Scholar]
- 15.Wenseleers T., Hart A. G., Ratnieks F. L. W., Quezada-Euan J. J. G. 2004. Queen execution and caste conflict in the stingless bee Melipona beecheii. Ethology 110, 725–736 10.1111/j.1439-0310.2004.01008.x (doi:10.1111/j.1439-0310.2004.01008.x) [DOI] [Google Scholar]
- 16.Kerr W. E. 1996. Biologia e manejo da tiúba: a abelha do Maranhão. Editora da Universidade Federal do Maranhão, São Luís, Maranhão [Google Scholar]
- 17.Alves D. A., Imperatriz-Fonseca V. L., Francoy T. M., Santos-Filho P. S., Nogueira-Neto P., Billen J., Wenseleers T. 2009. The queen is dead—long live the workers: intraspecific parasitism by workers in the stingless bee Melipona scutellaris. Mol. Ecol. 18, 4102–4111 10.1111/j.1365-294X.2009.04323.x (doi:10.1111/j.1365-294X.2009.04323.x) [DOI] [PubMed] [Google Scholar]
- 18.Peters J. M., Queller D. C., Imperatriz-Fonseca V. L., Roubik D. W., Strassmann J. E. 1999. Mate number, kin selection and social conflicts in stingless bees and honeybees. Proc. R. Soc. Lond. B 266, 379–384 10.1098/rspb.1999.0648 (doi:10.1098/rspb.1999.0648) [DOI] [Google Scholar]
- 19.Paxton R. J., Thorén P. A., Estoup A., Tengo J. 2001. Queen–worker conflict over male production and the sex ratio in a facultatively polyandrous bumblebee, Bombus hypnorum: the consequences of nest usurpation. Mol. Ecol. 10, 2489–2498 10.1046/j.0962-1083.2001.01377.x (doi:10.1046/j.0962-1083.2001.01377.x) [DOI] [PubMed] [Google Scholar]
- 20.Goulson D. 2003. Bumblebees: behaviour and ecology. Oxford, UK: Oxford University Press [Google Scholar]
- 21.Carvell C., Rothery P., Pywell R. F., Heard M. S. 2008. Effects of resource availability and social parasite invasion on field colonies of Bombus terrestris. Ecol. Entomol. 33, 321–327 10.1111/j.1365-2311.2007.00961.x (doi:10.1111/j.1365-2311.2007.00961.x) [DOI] [Google Scholar]
- 22.Schneider S. S., Deeby T., Gilley D. C., DeGrandi-Hoffman G. 2004. Seasonal nest usurpation of European colonies by African swarms in Arizona, USA. Insectes Soc. 51, 359–364 10.1007/s00040-004-0753-1 (doi:10.1007/s00040-004-0753-1) [DOI] [Google Scholar]