Abstract
A number of social insect species have recently been shown to have genetically influenced caste determination (GCD), challenging the conventional view that caste determination should be strictly environmental. To date, GCD has been found in phylogenetically isolated species; examples of GCD being present in multiple species of a genus are lacking. Through crossing experiments of neotenic (juvenile) reproductives, we have recently provided the first evidence for a royal versus worker GCD in the termite Reticulitermes speratus. To elucidate whether this system is more widespread, we performed crossing experiments using three additional Reticulitermes species. Offspring caste and sex ratios were found to be highly similar to those found previously in R. speratus, raising the possibility that GCD was present in an ancestral lineage of Reticulitermes, and subsequently maintained throughout several episodes of speciation.
Keywords: worker, neotenic, genetically influenced caste determination
1. Introduction
Social insects dominate terrestrial ecosystems [1]. A pillar of this success is their caste system: the specialization of larvae into either royals or various forms of workers. Royals are specialist reproducers, while workers are usually sterile or subfertile. Kin selection theory provides an explanation for the existence of sterile worker castes: genes associated with sterility can be favoured by selection, given that they are also present in a royal relative, whose reproductive output is enhanced by the worker [2]. A prediction of kin selection is that caste should be determined by environmental factors acting on a totipotent genome [3].
Empirical studies of social insect caste differentiation performed in the twentieth century provided broad support for the importance of environmental factors in caste determination. It was demonstrated in numerous and diverse social insect species that ‘extrinsic’ factors, such as nutrition, temperature and pheromones, strongly influence differentiation into either the royal or worker caste, or different worker forms [4]. Until relatively recently, the potential for ‘intrinsic’ factors to influence this process remained underappreciated. Building on a few isolated reports in the twentieth century, a number of studies in the last decade from various social insect species have demonstrated the influence of genotype on caste determination [5,6]. A recent study in ants has also demonstrated that maternal effects can limit the caste potentiality of offspring [7].
Genetically influenced caste determination (GCD) can be considered a continuum, ranging from cases where genotype has a relatively weak influence on caste, to cases where caste is essentially hard-wired and based on genotype [5,6]. Among the proposed causes of GCD are the existence of queen-biasing ‘cheating alleles’ [8], repression of selfish offspring behaviour [9] and energetic advantages of wing-absence during claustral colony founding [10]. In the case of royal versus worker caste determination, the dozen or so cases of GCD thus far identified have come from phylogenetically isolated species; no cases of GCD being present in more than one member of a genus were found in a recent review ([5]; we note that Pogonomyrmex ‘dependent lineages’ appear to have arisen multiple times from hybridogenesis between Pogonomyrmex barbatus and Pogonomyrmex rugosus, rather than independently in these two species). The presence of GCD in multiple members of a genus would be suggestive of the existence of GCD in an earlier progenitor, and its maintenance over an extended evolutionary time frame.
We have recently provided the first evidence for a genetic effect on determination of laboratory-maintained workers versus royals in termites [11], via crossing of reproductives derived from the worker and royal lines of Reticulitermes speratus, followed by rearing of the offspring under uniform conditions. Under these experimental conditions, offspring caste and sex ratios fit a simple genetic model involving a single X-linked locus, worker (wk) with two alleles, A and B [11]. Female and male nymphs have the genotypes wkAA and wkBY, respectively, while workers have the genotypes wkAB and wkAY (figure 1).
Figure 1.
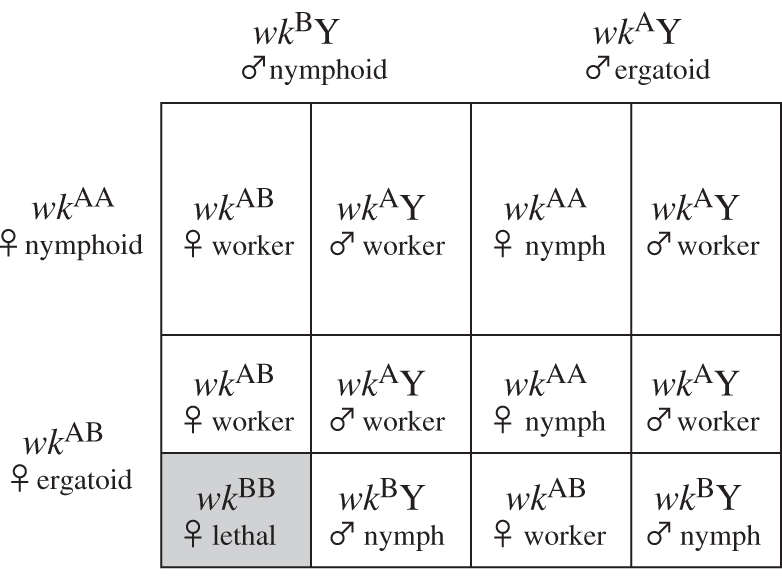
X-linked, one-locus-two-allele model for caste determination in R. speratus under laboratory conditions [11]. Offspring with wkAB and wkAY genotypes develop into workers; offspring with genotypes wkAA and wkBY into nymphs. The genotype wkBB is lethal. Female nymphoids and ergatoids also produce female nymphs parthenogenetically (wkAA; not shown). The genotype matches phenotype in the absence of reproductives.
To address the issue of whether the offspring patterns we found are unique to the R. speratus populations we studied, or whether they are more widespread, we have carried out crossing experiments on three additional species, Reticulitermes kanmonensis (hereafter, RK), Reticulitermes okinawanus (RO) and Reticulitermes yaeyamanus (RY).
2. Material and methods
In most termite species, female and male larvae undergo a bifurcation early in development, becoming either workers or nymphs. Workers are irreversibly wingless, while nymphs have wing-buds, and develop into alates (future kings and queens). In the absence of reproductives, Reticulitermes workers and nymphs can develop into juvenile ‘neotenic’ reproductives, respectively termed ‘ergatoids’ (E) and ‘nymphoids’ (N). The four possible crosses of neotenics (fNmN, fNmE, fEmN and fEmE) were set up for each of the three species, and offspring caste, sex and survival were determined as described previously for R. speratus ([11]; see the electronic supplementary material for details; unlike R. speratus, the three examined species do not reproduce parthenogenetically).
3. Results
(a). Offspring caste–sex ratios
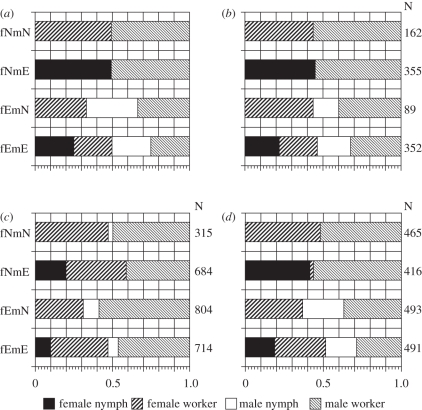
A total of 958, 2517 and 1865 third-instar offspring were, respectively, obtained from the experimental colonies of RK, RO and RY (electronic supplementary material, table S1). Offspring caste and sex ratios are shown in figure 2b–d. Almost all offspring from the fNmN cross (RK: 161/162; RO: 692/714; RY: 490/491) differentiated into the worker caste, with comparable numbers of each sex (female : male = 72 : 90; 339 : 353; 237 : 253). For fNmE, almost all female offspring of RK and RY (162/163; 204/215) differentiated into nymphs, but in RO, a considerable number of female workers also developed (nymph : worker = 156 : 315). Almost all male offspring from fNmE (RK: 190/192; RO: 333/333; RY: 278/278) became workers. For fEmN, female workers, male nymphs and male workers were produced in the ratios 39 : 15 : 35 (RK), 212 : 68 : 403 (RO) and 149 : 110 : 154 (RY); no female nymphs were produced (except for one in RO). For fEmE, female and male nymphs and workers were produced in comparable numbers in RK (81, 83, 76, 112). Each of the four offspring types was also produced in RO and RY; however, there was a bias towards workers of each sex (RO: 29, 118, 22, 146; RY: 86, 152, 93, 134; figure 2 and electronic supplementary material, table S1).
Figure 2.
Observed caste and sex ratios for R. kanmonensis, R. okinawanus and R. yaeyamanus, and expected ratios based on the GCD model for R. speratus (see figure 1 and text). (a) GCD model; (b) R. kanmonensis; (c) R. okinawanus; and (d) R. yaeyamanus.
Among replicates of the same crossing type in RK and RY, the offspring caste–sex ratios did not differ significantly (Fisher's exact tests with Holm's correction: p > 0.05). On the other hand, comparisons between different cross treatments revealed significant differences in a majority of cases (Fisher's exact tests with Holm's correction: p < 0.05; number of pairs with significant differences: RK, 98/179; RY, 133/150). Similar patterns were found in RO, but were less pronounced, owing to the increased worker ratios in each cross (numbers of significantly different pairs within a cross: 11/73; between different crosses: 150/227). In RK and RY, the offspring numbers in each caste–sex category of almost all the crosses did not differ significantly from the expected values of the R. speratus GCD model (exact goodness-of-fit test with Holm's correction, p > 0.05; figure 2).
(b). Egg-laying rates and third-instar offspring survival rates
Mean oviposition rates were significantly different among the different types of crosses (Poisson regression analysis; electronic supplementary material, table S2). In all of the three species, the mean oviposition rates were lowest in the fEmN cross (electronic supplementary material, figure S1). Logistic regression analyses indicated that the type of cross had a significant effect on the offspring survival rates in RK, but not in RO and RY (electronic supplementary material, table S3).
4. Discussion
The results of the RK and RY crossing experiments closely fit the X-linked single-locus two-allele model proposed for R. speratus (figures 1 and 2). This indicates that these species share the same GCD system. The results from the RO crosses were broadly similar to those of the aforementioned species; however, they did not fit the GCD model as closely, owing to the excess of workers produced in the crosses.
Survival rates of RK and RO offspring were lower (means 14% and 20%, respectively) than those found in experiments with R. speratus and RY (means 40% and 37%, respectively; electronic supplementary material, figure S1). Since egg-tending workers were from a different colony, the lower survival rates in the former two species may reflect enhanced nest-mate recognition. Unlike RK, R. speratus is more tolerant of non-nest-mates and undergoes colony fusion [12,13]; nest-mate recognition in RO and RY has not been examined. The sensitivity of eggs to laboratory conditions may also have reduced survival rate. According to the genetic model, the oviposition or survival rate of fEmN offspring is expected to be lower than that of the other three crosses, owing to the production of lethal wkBB genotypes. The oviposition rates of the fEmN cross were indeed the lowest in all the three species, supporting the idea that these species share a highly similar genetic system with R. speratus.
Our results represent the first evidence for GCD being present in multiple members of a genus. The four Reticulitermes species on which we have performed crossing experiments are all native to Japan; however, they are phylogenetically divergent among nine Asian Reticulitermes species, some being more closely related to species from China than to other Japanese species [14]. Our results therefore strongly suggest that the GCD mechanism we have identified was present in an ancestral Asian Reticulitermes species, having been maintained during the diversification of the genus in this area. Further experimentation on other taxa is required to test whether this system is shared by a broader range of termites.
In the crossing experiments in this study and in Hayashi et al. [11], we attempted to remove extrinsic effects on caste determination as much as possible, and test for the presence of intrinsic factors. How environmental influences interact with the proposed GCD mechanism to determine Reticulitermes caste compositions in the field remains to be determined. Matsuura et al. [15] recently discovered an extraordinary breeding structure in R. speratus field colonies, in which the primary king mates with numerous parthenogenetically produced female nymphoids (the daughters of a primary queen). In two colonies, both workers and nymphs had arisen from matings between the king and either the original queen, or her parthenogenetically produced daughters. This conflicts with the prediction of the GCD model shown in figure 1, which predicts that the primary pair (which is derived from nymphs) will produce only workers. One explanation for this mismatch is that environmental signals present in field colonies interact with the GCD mechanism described here in a complex manner.
The environmental factors that influence termite nymph versus worker differentiation have long remained mysterious. Hayashi et al. [11] reported that the presence of reproductives during egg-rearing resulted in development of workers from individuals with ‘nymph genotypes’. More recent studies have made significant progress in identifying key chemical signals involved in other caste determination processes [16,17]. Similar investigations should shed light on the environmental factors influencing the key nymph/worker dichotomy.
Acknowledgements
We thank H. Miyata for help in experimentation, and M. Shiyomi and T. Shigeta for useful discussion. This study was supported by the Grants-in-Aid for Scientific Research from JSPS (no. 19370009, 22370008), and the ARC (DP1097265).
References
- 1.Wilson E. O. 1990. Success and dominance in ecosystems: the case of social insects. Oldehdorf, Germany: International Ecology Institute [Google Scholar]
- 2.Hamilton W. D. 1963. The evolution of altruistic behavior. Am. Nat. 97, 354–356 10.1086/497114 (doi:10.1086/497114) [DOI] [Google Scholar]
- 3.Queller D. C., Strassmann J. E. 1998. Kin selection and social insects. BioScience 48, 165–175 10.2307/1313262 (doi:10.2307/1313262) [DOI] [Google Scholar]
- 4.Wheeler D. E. 1986. Developmental and physiological determinants of caste in social Hymenoptera—evolutionary implications. Am. Nat. 128, 13–34 10.1086/284536 (doi:10.1086/284536) [DOI] [Google Scholar]
- 5.Anderson K. E., Linksvayer T. A., Smith C. R. 2008. The causes and consequences of genetic caste determination in ants (Hymenoptera: Formicidae). Myrmecol. News 11, 119–132 [Google Scholar]
- 6.Schwander T., Lo N., Beekman M., Oldroyd B. P., Keller L. 2010. Nature vs nurture in social insect caste determination. Trends Ecol. Evol. 25, 275–282 10.1016/j.tree.2009.12.001 (doi:10.1016/j.tree.2009.12.001) [DOI] [PubMed] [Google Scholar]
- 7.Schwander T., Humbert J.-Y., Brent C. S., Cahan S. H., Chapuis L., Renai E., Keller L. 2008. Maternal effect on female caste determination in a social insect. Curr. Biol. 18, 265–269 10.1016/j.cub.2008.01.024 (doi:10.1016/j.cub.2008.01.024) [DOI] [PubMed] [Google Scholar]
- 8.Hughes W. O. H., Boomsma J. J. 2008. Genetic royal cheats in leaf-cutting ant societies. Proc. Natl Acad. Sci. USA 105, 5150–5153 10.1073/pnas.0710262105 (doi:10.1073/pnas.0710262105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strassmann J. E., Queller D. C. 2008. Social evolution: ant eggs lacking totipotency. Curr. Biol. 18, R299–R301 10.1016/j.cub.2008.02.032 (doi:10.1016/j.cub.2008.02.032) [DOI] [PubMed] [Google Scholar]
- 10.Crozier R. H. 1979. Genetics of Sociality. In Social insects, vol. 1 (ed. Hermann H. R.), pp. 223–286 New York, NY: Academic Press [Google Scholar]
- 11.Hayashi Y., Lo N., Miyata H., Kitade O. 2007. Sex-linked genetic influence on caste determination in a termite. Science 318, 985–987 10.1126/science.1146711 (doi:10.1126/science.1146711) [DOI] [PubMed] [Google Scholar]
- 12.Hayashi Y., Kitade O., Gonda M., Kondo T., Miyata H., Urayama K. 2005. Diverse colony genetic structures in the Japanese subterranean termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Sociobiology 46, 175–184 [Google Scholar]
- 13.Matsuura K., Nishida T. 2001. Colony fusion in a termite: what makes the society ‘open’? Insect. Soc. 48, 378–383 10.1007/PL00001795 (doi:10.1007/PL00001795) [DOI] [Google Scholar]
- 14.Park Y., Kitade O., Schwarz M., Kim J., Kim W. 2006. Intraspecific molecular phylogeny, genetic variation, and phylogeography of Reticulitermes speratus (Isoptera: Rhinotermitidae). Mol. Cell. 21, 89–103 [PubMed] [Google Scholar]
- 15.Matsuura K., Vargo E. L., Kawatsu K., Labadie P. E., Nakano H., Yashiro T., Tsuji K. 2009. Queen succession through asexual reproduction in termites. Science 323, 1687. 10.1126/science.1169702 (doi:10.1126/science.1169702) [DOI] [PubMed] [Google Scholar]
- 16.Hanus R., Vrkoslav V., Hrdy I., Cvacka J., Sobotnik J. 2010. Beyond cuticular hydrocarbons: evidence of proteinaceous secretion specific to termite kings and queens. Proc. R. Soc. B 277, 995–1002 10.1098/rspb.2009.1857 (doi:10.1098/rspb.2009.1857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuura K., Himuro C., Yokoi T., Yamamoto Y., Vargo E. L., Keller L. 2010. Identification of a pheromone regulating caste differentiation in termites. Proc. Natl Acad. Sci. USA 107, 12 963–12 968 10.1073/pnas.1004675107 (doi:10.1073/pnas.1004675107) [DOI] [PMC free article] [PubMed] [Google Scholar]