Abstract
The Red Queen hypothesis is based on the assumption that parasites must genetically match their hosts to infect them successfully. If the parasites fail, they are assumed to be killed by the host's immune system. Here, we tested this using sympatric (mostly susceptible) and allopatric (mostly resistant) populations of a freshwater snail and its trematode parasite. We determined whether parasites which do not infect are either killed or passed through the host's digestive tract and remain infectious. Our results show that parasites do not get a second chance: they either infect or are killed by the host. The results suggest strong selection against parasites that are not adapted to local host genotypes.
Keywords: host–parasite coevolution, Red Queen hypothesis, invertebrate immunity
1. Introduction
The Red Queen hypothesis posits that selection favours rare host genotypes in the presence of coevolving parasites [1–3]. In turn, parasites should be under strong selection to infect local host genotypes. Some degree of genetic matching is essential, such that a parasite genotype is recognized as ‘self’ by the host to evade the immune system [4–7]. Neither the consequences of failed infection nor selection on parasites are commonly examined (for an exception, see [8]), but strong selection is nonetheless an important assumption of the Red Queen hypothesis [9,10].
Host–parasite interactions can be highly specific. For example, invertebrates can be infected with and also resist specific parasite genotypes (e.g. [11,12]), as well as exhibit variation in these traits [13]. This specificity is clear from local adaptation experiments, wherein sympatric host–parasite combinations yield higher infection rates than allopatric combinations (e.g. [14,15]), as well as from long-term studies showing parasite tracking of common host genotypes [16–18]. It is known that allopatric parasites are less infective than sympatric parasites, but unknown whether unsuccessful parasites are killed.
For natural selection to favour parasite genotypes adapted to common host genotypes, parasites unable to infect should pay a high fitness cost [10]. Selection would be weak if parasites were not killed after an unsuccessful contact [19] and returned to the environment for a second try on another host, or if parasites were capable of reproducing without infecting a host. We tested whether unsuccessful parasites were killed by the host or released alive, using a freshwater snail and its trematode parasite. Parasite local adaptation and host–parasite coevolution have been demonstrated in this system [18,20–22]. We found little support for the idea that failed parasites pass through the snail unharmed. They either infect or die in the snail host, but do not get a second chance. This confirms that there is probably strong selection against parasites unable to infect the local host genotypes.
2. Material and methods
(a). Study system
Potamopyrgus antipodarum is the first intermediate host for Microphallus sp. This trematode parasite has a complex life cycle, and requires two host species to reproduce: snails and waterfowl. The parasite produces encysted larvae (i.e. metacercariae) and sterilizes the snail after three months. It develops to the adult stage after ingestion by the final waterfowl host, and then produces eggs passed in bird faeces. Snails are exposed upon ingesting the eggs. Trematode eggs hatch in the snail gut, and the parasite penetrates the snail tissues to develop in the gonads [23]. Hatched parasites not killed by snail digestive enzymes in the gut may be killed during tissue invasion [24]. If the parasite eggs fail to hatch, they may be released alive in the snail faeces [23].
(b). Experimental infections
We collected snails from shallow (<2 m) regions in Lakes Alexandrina and Poerua on the South Island of New Zealand. These natural populations are under negative frequency-dependent selection from Microphallus sp. parasites [18,25], and parasite local adaptation has been repeatedly found [22]. We collected parasites from waterfowl faeces along the lake margins.
Exposures were conducted at the Edward Percival Field Station in Kaikoura, New Zealand. Parasite eggs were obtained by washing bird faeces with water and filtering the mixture through 1 mm mesh. The experimental design is illustrated in figure 1. For each ‘field parasite’ treatment, we set-up three replicate containers (1 l water) of 50 snails from each host 1 source. Eggs from each population were equally divided among the replicate snail containers within each ‘field parasite’ treatment. Host 1 snails were exposed for 12 h and then moved to containers without parasites for 12 h; this was repeated over 3 days. Snail faeces were collected during the second 12 h interval for the ‘released parasite’ treatment. The host 1 snail-faeces mixture was divided into two equal volumes, one for each of the host 2 sources. Three replicate containers of 50 snails from each host 2 source were exposed to the ‘released parasite’ treatment for 7 days. Snail faeces degrade rapidly (personal observation), so host 2 snails would have encountered any parasite eggs released from host 1.
Figure 1.
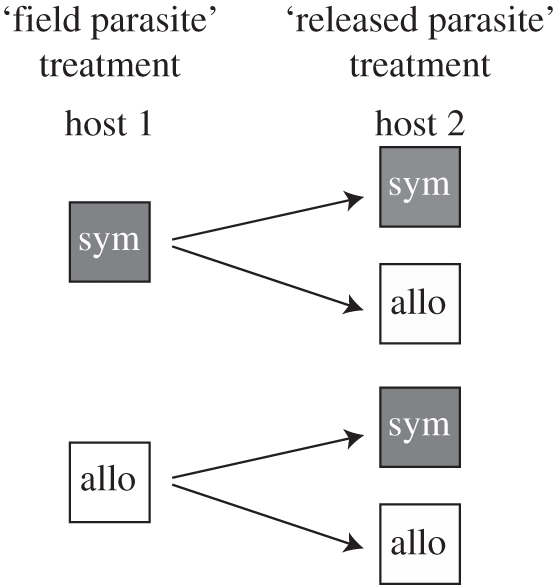
Infection experiment with sympatric and allopatric host–parasite populations. The first column represents the ‘field parasite’ treatment, where snails (host 1) were exposed to parasites from waterfowl faeces collected from the field. The second column represents the ‘released parasite’ treatment, where snails (host 2) were exposed to host 1 snail faeces. Sym, sympatric snails (dark grey) and allo, allopatric snails (white). Arrows indicate the potential movement of parasites.
All snails were transported to Indiana University. Snails were fed Spirulina, and the water was changed regularly. Three months post-exposure, we dissected all snails and recorded their infection status and the parasite stage.
(c). Analyses
Statistical analyses were conducted using SPSS 17.0. We considered only uninfected snails and experimentally infected snails (early-stage infections); natural infections are easy to identify as they are fully developed metacercariae.
We tested whether (i) parasite genotypes were better at infecting sympatric host 1 than allopatric host 1 in the ‘field parasite’ treatment (i.e. parasite local adaptation). We also wanted to determine whether failed sympatric parasite genotypes could pass through the snail gut unharmed by testing (ii) whether sympatric host 2 were more infected than allopatric host 2 in the ‘released parasite’ treatments (total of four contrasts), and (iii) whether sympatric host 2 exposed to allopatric host 1 snail faeces were more infected than those exposed to sympatric host 1 snail faeces (total of two contrasts). The latter hypothesis is derived from the idea that if infections establish in sympatric host 1, then the proportion of failed parasites will be lower than in allopatric host 1. Thus, sympatric host 2 groups may be exposed to different numbers of parasites.
To examine hypothesis (i), we used a two-way analysis of variance to test for differences in infection frequency among host and parasite sources in the ‘field parasite’ treatment. Parasite and host sources were treated as fixed factors. We used t-tests (assuming unequal variances) to contrast infection frequencies among host sources within each parasite source. In the ‘released parasite’ treatment, there were only eight infected snails out of 1021 host 2 individuals. Mean infection frequencies were less than 3 per cent. Therefore, we performed non-parametric Mann–Whitney tests on the six contrasts described in hypotheses (ii) and (iii) above. We were unable to control for ‘field parasite’ dose, and so we only compared infection frequencies within parasite sources.
3. Results
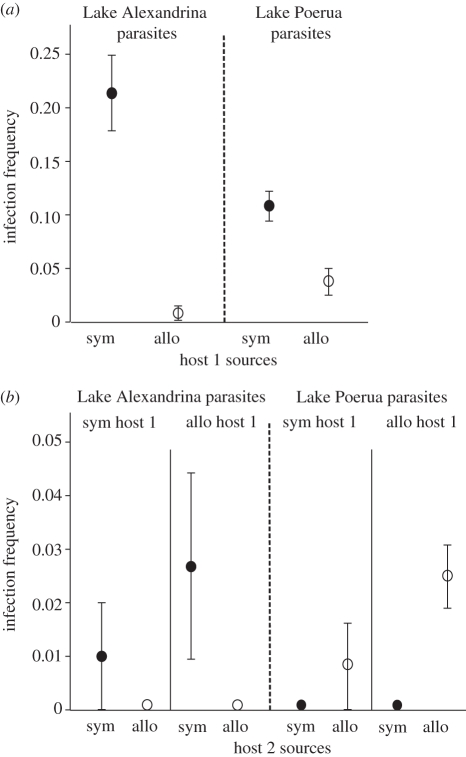
Infection frequencies were higher in the ‘field parasite’ treatments than in the ‘released parasite’ treatments. In the ‘field parasite’ treatments, sympatric host 1 was significantly more infected than allopatric host 1, consistently among parasite groups (table 1 and figure 2a). This indicates that the parasites were locally adapted, and thus better at infecting sympatric hosts than allopatric hosts (hypothesis (i)).
Table 1.
Analysis of variance (ANOVA) and contrasts of infection frequencies in the ‘field parasite’ (host 1) treatments. Lake Alexandrina parasites: sym, sympatric Lake Alexandrina snails; allo, allopatric Lake Poerua snails. Lake Poerua parasites: sym, Lake Poerua snails; allo, Lake Alexandrina snails.
| ANOVA | d.f. | M.S. | F | p |
|---|---|---|---|---|
| host 1 | 1 | 0.016 | 18.047 | 0.003 |
| parasites | 1 | 0.004 | 4.859 | 0.059 |
| host 1 × parasites | 1 | 0.058 | 66.502 | <0.001 |
| error | 8 | 0.001 | ||
| contrasts | diff. | t | p | |
| Lake Alexandrina parasites | ||||
| symhost 1 − allohost 1 | 0.21 | 6.678 | 0.017 | |
| Lake Poerua parasites | ||||
| symhost 1 − allohost 1 | 0.07 | 5.266 | 0.007 | |
Figure 2.
Experimental infection frequencies (mean +1s.e.) on (a) host 1 sources in the ‘field parasite’ treatment, and (b) host 2 sources in the ‘released parasite’ treatment from exposures to Lake Alexandrina and Lake Poerua parasites. Sym, sympatric snails (dark circles) and allo, allopatric snails (open circles).
In the ‘released parasite’ treatment, infection frequencies were very low, at less than 3 per cent on average (figure 2b). Each of the six contrasts was insignificant (table 2 and figure 2b). Contrasts of infection frequencies between the four sympatric–allopatric host 2 groups (hypothesis (ii)) were insignificant after we applied a Bonferonni correction (p < 0.0083). In addition, the infection frequencies between sympatric host 2 groups (hypothesis (iii)) were not significantly different. These results suggest that failed parasites are not regularly released alive.
Table 2.
Mann–Whitney tests of contrasts in the ‘released parasite’ (host 2) treatments. Lake Alexandrina parasites: sym, Lake Alexandrina snails; allo, Lake Poerua snails. Lake Poerua parasites: sym, Lake Poerua snails; allo, Lake Alexandrina snails. Hypotheses (ii) and (iii) are indicated as described in the §2. n.a. indicates that we could not statistically compare two host groups without infections. Differences in infection frequency are significant at p = 0.0083 (Bonferonni correction).
| hypothesis | contrast | diff. | U | p |
|---|---|---|---|---|
| Lake Alexandrina parasites | ||||
| (ii) | symhost 1, symhost 2 − allohost 2 | 0.010 | 3.0 | 0.317 |
| (ii) | allohost 1, symhost 2 − allohost 2 | 0.028 | 1.5 | 0.121 |
| (iii) | symhost 1, symhost 2 − allohost 1, symhost 2 | 0.017 | 3.0 | 0.487 |
| Lake Poerua parasites | ||||
| (ii) | symhost 1, symhost 2 − allohost 2 | 0.008 | 3.0 | 0.317 |
| (ii) | allohost 1, symhost 2 − allohost 2 | 0.026 | 0.0 | 0.037 |
| (iii) | symhost 1, symhost 2 − allohost 1, symhost 2 | 0.000 | n.a. | n.a. |
4. Discussion
Host-imposed selection on parasites is of central importance to the Red Queen [10]. Our data suggest strong selection against parasites that are not adapted to local host genotypes. First, in the ‘field parasite’ treatment, while many sympatric parasites were successful, fewer allopatric parasites infected, a finding consistent with local adaptation experiments using this system (e.g. [22]). Second, few snails in the ‘released parasite’ treatments were infected (figure 2b), suggesting that most parasites either infect or are killed, but not released. The benefits of being adapted to infect local hosts are substantial, much like the costs of failure. This is a requirement for parasites to evolve with host populations, and particularly to maintain sexual reproduction [5,7,10,26].
Coevolution with specific genetic interactions has been documented between several invertebrate species and their parasites, including Daphnia waterfleas with bacterial parasites [11], the bumble-bee Bombus terrestris with trypanosomes [27] and P. antipodarum snails with trematodes [17]. While parasite hatching may not be host-specific [28], the snail immune system may be specific. Few parasites were given a second chance. The presence of less than 3 per cent infections, on average, in the ‘released parasite’ treatment suggests that some parasites pass through the snail gut unharmed (as suggested in Byrd & Maples [23]). In the ‘field parasite’ treatment, snails were exposed to probably higher doses than in nature. Thus, the immune system of the snails may have been overwhelmed, although this remains speculative. Regardless, the percentage of released parasites is likely too low to weaken selection.
Under the Red Queen hypothesis, strong selection on parasites will promote adaptation to local host genotypes. Our results show that parasites will die if they cannot infect, and are therefore, under strong selection to infect hosts in the local population. Strong selection on parasites should be widespread in natural host–parasite systems. However, further empirical evidence is necessary to quantify the selection on parasites in natural populations, as well as determine host effects on parasite evolution [8].
Acknowledgements
We are grateful to Tom Little for helpful suggestions. Funding was provided by NSF (DEB-0640639 to C.M.L. and J.J.) and NSERC Canada (K.C.K.).
References
- 1.Bell G. 1982. The masterpiece of nature: the evolution and genetics of asexuality. Berkeley, CA: University of California Press [Google Scholar]
- 2.Hamilton W. D. 1980. Sex versus non-sex versus parasite. Oikos 35, 282–290 10.2307/3544435 (doi:10.2307/3544435) [DOI] [Google Scholar]
- 3.Jaenike J. 1978. An hypothesis to account for the maintenance of sex within populations. Evol. Theory 3, 191–194 [Google Scholar]
- 4.Agrawal A., Lively C. M. 2002. Infection genetics: gene-for-gene versus matching-alleles models and all points in between. Evol. Ecol. Res. 4, 79–90 [Google Scholar]
- 5.Engelstaedter J., Bonhoeffer S. 2009. Red Queen dynamics with non-standard fitness interactions. PLoS Comp. Biol. 5, e1000469. 10.1371/journal.pcbi.1000469 (doi:10.1371/journal.pcbi.1000469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton W. D., Axelrod R., Tanese R. 1990. Sexual reproduction as an adaptation to resist parasites (a review). Proc. Natl Acad. Sci. USA 87, 3566–3573 10.1073/pnas.87.9.3566 (doi:10.1073/pnas.87.9.3566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters A. D., Lively C. M. 1999. The Red Queen and fluctuating epistasis: a population genetic analysis of antagonistic coevolution. Am. Nat. 154, 393–405 10.1086/303247 (doi:10.1086/303247) [DOI] [PubMed] [Google Scholar]
- 8.Paterson S., et al. 2010. Antagonistic coevolution accelerates molecular evolution. Nature 464, 275–278 10.1038/nature08798 (doi:10.1038/nature08798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salathé M., Kouyos R. D., Bonhoeffer S. 2008. The state of affairs in the kingdom of the Red Queen. Trends Ecol. Evol. 23, 439–445 10.1016/j.tree.2008.04.010 (doi:10.1016/j.tree.2008.04.010) [DOI] [PubMed] [Google Scholar]
- 10.Salathé M., Kouyos R. D., Regoes R. R., Bonhoeffer S. 2008. Rapid parasite adaptation drives selection for high recombination rates. Evolution 62, 295–300 10.1111/j.1558-5646.2007.00265.x (doi:10.1111/j.1558-5646.2007.00265.x) [DOI] [PubMed] [Google Scholar]
- 11.Carius H. J., Little T. J., Ebert D. 2001. Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution 55, 1136–1145 [DOI] [PubMed] [Google Scholar]
- 12.Lively C. M., Dybdahl M. F. 2000. Parasite adaptation to locally common host genotypes. Nature 405, 679–681 10.1038/35015069 (doi:10.1038/35015069) [DOI] [PubMed] [Google Scholar]
- 13.Schmid-Hempel P., Ebert D. 2003. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 18, 27–32 10.1016/S0169-5347(02)00013-7 (doi:10.1016/S0169-5347(02)00013-7) [DOI] [Google Scholar]
- 14.Ebert D. 1994. Virulence and local adaptation of a horizontally transmitted parasite. Science 19, 1084–1086 10.1126/science.265.5175.1084 (doi:10.1126/science.265.5175.1084) [DOI] [PubMed] [Google Scholar]
- 15.Lively C. M. 1989. Adaptation by a parasitic trematode to local populations of its snail host. Evolution 43, 1663–1671 10.2307/2409382 (doi:10.2307/2409382) [DOI] [PubMed] [Google Scholar]
- 16.Decaestecker E., Gaba S., Raeymaekers J. A. M., Stoks R., Van Kerckhoven L., Ebert D., De Meester L. 2007. Host–parasite ‘Red Queen' dynamics archived in pond sediment. Nature 450, 870–874 10.1038/nature06291 (doi:10.1038/nature06291) [DOI] [PubMed] [Google Scholar]
- 17.Dybdahl M. F., Lively C. M. 1998. Host–parasite coevolution: evidence for rare advantage and time-lagged selection in a natural population. Evolution 52, 1057–1066 10.2307/2411236 (doi:10.2307/2411236) [DOI] [PubMed] [Google Scholar]
- 18.Jokela J., Dybdahl M. F., Lively C. M. 2009. The maintenance of sex, clonal dynamics, and host–parasite coevolution in a mixed population of sexual and asexual snails. Am. Nat. 174, S43–S53 10.1086/599080 (doi:10.1086/599080) [DOI] [PubMed] [Google Scholar]
- 19.Otto S. P., Nuismer S. L. 2004. Species interactions and the evolution of sex. Science 304, 1018–1020 10.1126/science.1094072 (doi:10.1126/science.1094072) [DOI] [PubMed] [Google Scholar]
- 20.King K. C., Delph L. F., Jokela J., Lively C. M. 2009. The geographic mosaic of sex and the Red Queen. Curr. Biol. 19, 1438–1441 10.1016/j.cub.2009.06.062 (doi:10.1016/j.cub.2009.06.062) [DOI] [PubMed] [Google Scholar]
- 21.Koskella B., Lively C. M. 2009. Evidence for negative frequency-dependent selection during experimental coevolution of a freshwater snail and a sterilizing trematode. Evolution 63, 2213–2221 10.1111/j.1558-5646.2009.00711.x (doi:10.1111/j.1558-5646.2009.00711.x) [DOI] [PubMed] [Google Scholar]
- 22.Lively C. M., Dybdahl M. F., Jokela J., Osnas E. E., Delph L. F. 2004. Host sex and local adaptation by parasites in a snail–trematode interaction. Am. Nat. 164, S6–S18 10.1086/424605 (doi:10.1086/424605) [DOI] [PubMed] [Google Scholar]
- 23.Byrd E. E., Maples W. P. 1964. Developmental stages in the Digenea. V. The egg, miracidium and brood mass in Dasymetra conferta Nicoll, 1911 (Trematoda: Plagiorchioidea: Ochetosomatinae). Parasitology 54, 295–312 10.1017/S0031182000067937 (doi:10.1017/S0031182000067937) [DOI] [Google Scholar]
- 24.Byrd E. E., Maples W. P. 1969. Intramolluscan stages of Dasymetra conferta Nicoll, 1911 (Trematoda: Plagiorchiidae). J. Parasitol. 55, 509–526 10.2307/3277291 (doi:10.2307/3277291) [DOI] [PubMed] [Google Scholar]
- 25.Dybdahl M. F., Lively C. M. 1995. Host–parasite interactions: infection of common clones in natural populations of a freshwater snail (Potamopyrgus antipodarum). Proc. R. Soc. Lond. B 260, 99–103 10.1098/rspb.1995.0065 (doi:10.1098/rspb.1995.0065) [DOI] [Google Scholar]
- 26.Howard S. R., Lively C. M. 1994. Parasitism, mutation accumulation and the maintenance of sex. Nature 367, 554–557 10.1038/367554a0 (doi:10.1038/367554a0) [DOI] [PubMed] [Google Scholar]
- 27.Schmid-Hempel P., Puhr K., Kruger N., Reber C., Schmid-Hempel R. 1999. Dynamic and genetic consequences of variation in horizontal transmission for a microparasite infection. Evolution 53, 426–434 10.2307/2640779 (doi:10.2307/2640779) [DOI] [PubMed] [Google Scholar]
- 28.Kopp K., Jokela J. 2007. Resistant invaders can convey benefits to native species. Oikos 116, 295–301 10.1111/j.0030-1299.2007.15290.x (doi:10.1111/j.0030-1299.2007.15290.x) [DOI] [Google Scholar]