Abstract
Large carnivores are highly threatened, yet the processes underlying their population declines are still poorly understood and widely debated. We explored how body mass and prey abundance influence carnivore density using data on 199 populations obtained across multiple sites for 11 carnivore species. We found that relative decreases in prey abundance resulted in a five- to sixfold greater decrease in the largest carnivores compared with the smallest species. We discuss a number of possible causes for this inherent vulnerability, but also explore a possible mechanistic link between predator size, energetics and population processes. Our results have important implications for carnivore ecology and conservation, demonstrating that larger species are particularly vulnerable to anthropogenic threats to their environment, especially those which have an adverse affect on the abundance of their prey.
Keywords: carnivore ecology, predator–prey relationships, abundance scaling, climate change, metabolism
1. Introduction
It is well recognized that large carnivores are highly threatened, owing to a combination of environmental change, biological factors and human pressures [1,2]. However, the main processes underlying global declines in large carnivores are still widely debated [3]. Body mass and prey abundance are known to influence average abundance across mammalian carnivores [4]. However, there is also evidence that larger carnivore species are rarer than expected based on typical abundance–mass relationships [5,6]. Carnivores are extremely wide ranging, with day ranges two- to threefold that of herbivores of the same size [7] and, across species, exhibit steeper scaling in day range and home range [8–10]. This increase in ranging behaviour would influence individual energetic rates and is consistent with the finding that energetics may place evolutionary constraints on body size in predators [11,12]. Ultimately, size and energetics may be linked with the intrinsic factors identified in a global analysis of the threat status of mammals [13]. The interplay between the environment, body size and the intrinsic factors driving this vulnerability remains poorly understood. Studies that identify causes of changes in species abundance in relation to size and ecology have the potential to greatly improve our understanding of population processes.
In this study, we present an analysis of predator–prey ratios obtained across multiple sites for 11 species of carnivores. We focus on a key environmental factor, food availability (prey abundance), in order to explore whether large carnivores show a greater population response to changes in the relative abundance of their food resources.
2. Material and methods
To compare carnivore abundance across species in relation to variation in prey biomass density (enabling a comparison across different species of carnivores that feed on prey of different sizes [4,14]), we explored how the logarithm (base 10) of carnivore density (logN) relates to log carnivore body mass (logM) and log prey biomass density (logP) for 199 predator–prey population estimates obtained from 11 species of carnivores (all with six or more population estimates; table 1; see also the electronic supplementary material). In our data analysis, we compared the explanatory power of four different linear combinations of these predictors using Akaike Information Criterion (AIC) [15,16]. We excluded data on the population densities of two species, the African wild dog (Lycaon pictus) and cheetah (Acinonyx jubatus), which are known to be poorly related to prey availability, owing to competition with other carnivores [17–20]. Whether or not wild dogs and cheetahs are included, our conclusions remain unaffected and the fitted models remain significant (electronic supplementary material, table S2); here, however, we focus on the results with wild dogs and cheetahs omitted.
Table 1.
Summary of carnivore density and prey biomass density used in this study, obtained from Carbone & Gittleman [4] and additional sources (see the electronic supplementary material); see text for details.
| species | scientific name | ave. weight (kg) | no. populations | carnivore density, N (km−2) |
prey biomass, P (kg km−2) range (min–max) | |||
|---|---|---|---|---|---|---|---|---|
| range (min–max) | slope | intercept | r2 | |||||
| least weasel | Mustela nivalis | 0.14 | 7 | 0.52–80.0 | 0.1615 | 3.49 | 0.02 | 23.9–832.5 |
| arctic fox | Alopex lagopus | 3.19 | 14 | 0.022–0.286 | 0.2385 | 0.0268 | 0.47 | 1.0–2810.9 |
| Canadian lynx | Lynx canadensis | 11.2 | 28 | 0.02–0.226 | 0.4954 | 0.0047 | 0.65 | 16.8–1386.0 |
| European badger | Meles meles | 13.0 | 9 | 0.79–8.4 | 0.3437 | 12.74 | 0.73 | 352.8–71 400.0 |
| coyote | Canis latrans | 13.0 | 19 | 0.023–0.444 | 0.508 | 0.0092 | 0.21 | 34.5–1485.0 |
| wolf | Canis lupus | 46.0 | 20 | 0.005–0.042 | 0.6661 | 0.0003 | 0.49 | 89.0–810.5 |
| leopard | Panthera pardus | 46.5 | 19 | 0.005–0.303 | 0.5079 | 0.0025 | 0.51 | 13.2–41 62.9 |
| spotted hyena | Crocuta crocuta | 58.6 | 19 | 0.005–1.842 | 0.7733 | 0.0004 | 0.52 | 126.0–17 262.6 |
| lion | Panthera leo | 142.0 | 40 | 0.008–0.52 | 0.5854 | 0.0011 | 0.66 | 35.0–14 198.4 |
| tiger | Panthera tigris | 181.0 | 16 | 0.006–0.168 | 0.7352 | 0.0002 | 0.72 | 171.0–5828.6 |
| polar bear | Ursus maritimus | 310.0 | 8 | 0.003–0.021 | 0.8806 | 00000.9 | 0.89 | 41.8–337.0 |
Most of the data used in this study were obtained from studies specifically focused on predator–prey relationships for a single carnivore species. Inevitably, the methods used in these studies somewhat varied. In some instances, data on prey density in one year were compared with predator density estimated in the next; in other instances, these data might be matched within the same year [4]. In addition, given the practical difficulties of getting such information, we found that most data were only available from different locations and periods across the species' ranges. Ideally, longitudinal data (from the same populations across years) should be used; nonetheless, we believe that these data have the potential to provide important insights into predator–prey relationships and a general understanding of consumer–resource relationships [21].
3. Results
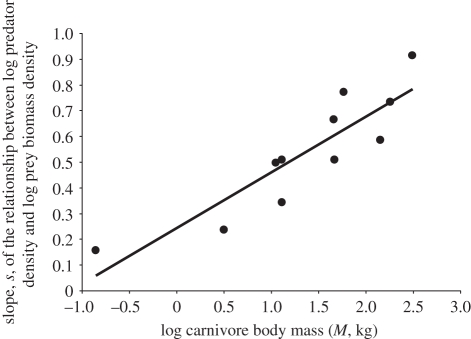
The model including all predictors (logP, logM and the interaction between them) explained 68 per cent of the variability in log carnivore densities, enjoying substantially more support than the next best alternative (ΔAIC = 11.24 between this and the next best model; table 2). This relationship is best described by a linear model of the form logN = 1.06 − 1.29 logM + 0.33 logP + 0.21 logM × logP (all predictors are significant with p < 0.001 and the full model is also significant with F3,195 = 140.9, p < 0.001, r2 = 0.68). The coefficients confirm that carnivore densities are negatively affected by body mass and positively affected by prey availability; crucially, the significant interaction term shows that the densities of the larger species of carnivores are disproportionately lower in areas of low prey density. Intriguingly, the slopes of the predator–prey responses seem to increase linearly with log carnivore body mass (figure 1).
Table 2.
Models fitted to empirical data on carnivore densities.
| fitted modela | estimated parameters | AIC | ΔAIC | w | r2 |
|---|---|---|---|---|---|
| lm(logN ∼ logM) | 3 | −168.22 | 168.79 | 0 | 0.25 |
| lm(logN ∼ logP) | 3 | −156.90 | 180.10 | 0 | 0.20 |
| lm(logN ∼ logM + logP) | 4 | −325.76 | 11.24 | 0 | 0.66 |
| lm(logN ∼ logM × logP) | 5 | −337.00 | 0.00 | 1.00 | 0.68 |
aModel specifications are compatible with R [16] and represent single predictor linear models in the first two cases, a two predictor linear model in the third case and a model containing both predictors and their interaction in the final case.
Figure 1.
The slope (s) of the predator–prey population responses plotted against log of carnivore mass (log M, in kilograms). Steeper slopes are related to a faster rate of change with changing prey. The best-fit line is s = 0.217 × log M + 0.245 (r2 = 0.79).
4. Discussion
Focusing on a common threat, that of declining food resources [22], this study confronts the important question of how mammalian carnivores of different size might respond to differing environmental conditions. Compared with the overall variation across the dataset, the carnivore mass–prey biomass interaction term explains only 2 per cent of the variation; nevertheless, slopes of the relationship between predators and prey vary substantially and carnivore mass explains nearly 80 per cent of the variation in these slopes (figure 1)—a result of great biological significance. A given reduction in prey abundance, leads to a five- to sixfold greater reduction in the larger carnivores when compared with the smallest carnivores.
What mechanisms could drive this apparent vulnerability? One possibility is that, because large carnivores consume large prey [12], which themselves may be vulnerable to threat processes [13], there may be an interaction across populations between predator and prey. However, our analysis of carnivore abundance controls for prey abundance and so does not support this argument unless more subtle processes, unrelated to abundance, are taking place. Alternatively, previous work has shown that energetic costs may limit body size in larger carnivores [11]. It is possible that similar physiological factors influence population processes as well. Physiologists have long been interested in metabolic costs under different levels of exercise [23,24]. Such studies have shown that, at maximum energy expenditure, large animals have relatively high metabolic rates [25–27]. Carnivores have larger home ranges [28–31] and hunt for longer [32,33] in areas of low prey density or productivity. Building on earlier physiological arguments, we might expect that when large carnivores work harder to maintain their energy budgets under conditions of low prey abundance, this in turn may influence their population density. If this is the case, predatory species with extremely high hunting costs will be particularly susceptible to changes in the environment that influence feeding ecology, because any increase in the time spent hunting greatly adds to overall energy expenditure [34]. In energetically stressful situations, both survival and reproduction are subject to reductions; this situation could be exacerbated in large carnivores by life-history attributes that already render them vulnerable to extinction [35]. Future work on this topic, using models of predator–prey dynamics to assess the influence of size and habitat productivity, might be particularly useful in providing specific testable predictions [36,37].
Understanding the links between physiology, behaviour and population phenomena remains one of the great challenges in ecology [38], and the current backdrop of declining environmental conditions, climate change and biodiversity loss makes that challenge particularly important [39]. Carnivores represent ideal species for exploring such relationships because, not only do we know a great deal about their behaviour and diets [40], but we also have good information on the abundance and distributions of many of their prey [4]. We believe that further research exploring the link between physiology, behaviour and carnivore population dynamics represents a valuable opportunity to establish clear relationships, from individual behaviour to population processes and macroecological patterns. This research also has important implications for the conservation of our largest carnivore species, which seem especially vulnerable to conditions influencing the abundance of their prey.
Acknowledgements
We thank Blaire Van Valkenburgh and Shai Meiri for their helpful comments on earlier drafts of the manuscript.
References
- 1.Cardillo M., Purvis A., Sechrest W., Gittleman J. L., Bielby J., Mace G. M. 2004. Human population density and extinction risk in the world's carnivores. PLoS Biol. 2, e197. (doi:10.1371/journal.pbio.0020197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Valkenburgh B., Wang X. M., Damuth J. 2004. Cope's rule, hypercarnivory, and extinction in North American canids. Science 306, 101–104 10.1126/science.1102417 (doi:10.1126/science.1102417) [DOI] [PubMed] [Google Scholar]
- 3.Chapron G., Miquelle D. G., Lambert A., Goodrich J. M., Legendre S., Clobert J. 2008. The impact on tigers of poaching versus prey depletion. J. Appl. Ecol. 45, 1667–1674 10.1111/j.1365-2664.2008.01538.x (doi:10.1111/j.1365-2664.2008.01538.x) [DOI] [Google Scholar]
- 4.Carbone C., Gittleman J. L. 2002. A common rule for the scaling of carnivore density. Science 295, 2273–2276 10.1126/science.1067994 (doi:10.1126/science.1067994) [DOI] [PubMed] [Google Scholar]
- 5.Damuth J. 1987. Interspecific allometry of population density in mammals and other animals: the independence of body mass and population energy-use. Biol. J. Linn. Soc. 31, 193–246 10.1111/j.1095-8312.1987.tb01990.x (doi:10.1111/j.1095-8312.1987.tb01990.x) [DOI] [Google Scholar]
- 6.Marquet P. A. 2002. Of predators, prey and power laws. Science 295, 2229–2230 10.1126/science.1070587 (doi:10.1126/science.1070587) [DOI] [PubMed] [Google Scholar]
- 7.Carbone C., Cowlishaw G., Isaac N. J. B., Rowcliffe J. M. 2005. How far do animals go? Determinants of day range in mammals. Am. Nat. 165, 290–297 [DOI] [PubMed] [Google Scholar]
- 8.Haskell J. P., Ritchie M. E., Olff H. 2002. Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature 418, 527–530 10.1038/nature00840 (doi:10.1038/nature00840) [DOI] [PubMed] [Google Scholar]
- 9.Kelt D. A., Van Vuren D. H. 2001. The ecology and macroecology of mammalian home range area. Am. Nat. 157, 637–645 [DOI] [PubMed] [Google Scholar]
- 10.Jetz W., Carbone C., Fulford J., Brown J. H. 2004. The scaling of animal space use. Science 306, 266–268 10.1126/science.1102138 (doi:10.1126/science.1102138) [DOI] [PubMed] [Google Scholar]
- 11.Carbone C., Teacher A., Rowcliffe J. M. 2007. The costs of carnivory. PLoS Biol. 5, e22. 10.1371/journal.pbio.0050022 (doi:10.1371/journal.pbio.0050022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone C., Mace G. M., Roberts S. C., Macdonald D. W. 1999. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288 10.1038/46266 (doi:10.1038/46266) [DOI] [PubMed] [Google Scholar]
- 13.Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 10.1126/science.1116030 (doi:10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 14.East R. 1984. Rainfall, soil nutrient status and biomass of large African savanna mammals. Afr. J. Ecol. 22, 245–270 10.1111/j.1365-2028.1984.tb00700.x (doi:10.1111/j.1365-2028.1984.tb00700.x) [DOI] [Google Scholar]
- 15.Whittingham M. J., Stephens P. A., Bradbury R. B., Freckleton R. P. 2006. Why do we still use stepwise modelling in ecology and behaviour? J. Anim. Ecol. 75, 1182–1189 10.1111/j.1365-2656.2006.01141.x (doi:10.1111/j.1365-2656.2006.01141.x) [DOI] [PubMed] [Google Scholar]
- 16.R Development Core Team 2005. R: a language and environment for statistical computing. Reference index v. 2.2.1 Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 17.Durant S. M. 2000. Predator avoidance, breeding experience and reproductive success in endangered cheetahs. Anim. Behav. 60, 121–130 10.1006/anbe.2000.1433 (doi:10.1006/anbe.2000.1433) [DOI] [PubMed] [Google Scholar]
- 18.Laurenson M. K. 1995. Dynamics implications of high offspring mortality for cheetah population dynamics. In Serengeti II: management, and conservation of an ecosystem (eds Sinclair A. C. E., Arcese P.), pp. 385–399 Chicago, IL: University of Chicago Press [Google Scholar]
- 19.Creel S., Creel N. M. 1996. Limitation of African wild dogs by competition with larger carnivores. Conserv. Biol. 10, 526–538 10.1046/j.1523-1739.1996.10020526.x (doi:10.1046/j.1523-1739.1996.10020526.x) [DOI] [Google Scholar]
- 20.Kelly M. J., Durant S. M. 2000. Viability of the Serengeti cheetah population. Conserv. Biol. 14, 786–797 10.1046/j.1523-1739.2000.98329.x (doi:10.1046/j.1523-1739.2000.98329.x) [DOI] [Google Scholar]
- 21.Carbone C., Pettorelli N. 2009. Testing relationships between energy and vertebrate abundance. Int. J. Ecol. 2009, 1–6 10.1155/2009/496175 (doi:10.1155/2009/496175) [DOI] [Google Scholar]
- 22.Karanth U., Stith B. M. 1999. Prey depletion as a critical determinant of tiger population viability In Riding the tiger: tiger conservation in human-dominated landscapes. (eds Seidensticker J., Christie S., Jackson P.), pp. 100–113 Cambridge, UK: Cambridge University Press [Google Scholar]
- 23.Taylor C. R., Heglund N. C., Maloiy G. M. 1982. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 1–21 [DOI] [PubMed] [Google Scholar]
- 24.Fedak M. A., Heglund N. C., Taylor C. R. 1982. Energetics and mechanics of terrestrial locomotion. 2. Kinetic energy changes of the limbs and body as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 23–40 [DOI] [PubMed] [Google Scholar]
- 25.Weibel E. R., Hoppeler H. 2005. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J. Exp. Biol. 208, 1635–1644 10.1242/jeb.01548 (doi:10.1242/jeb.01548) [DOI] [PubMed] [Google Scholar]
- 26.Suarez R. K., Darveau C. A. 2005. Multi-level regulation and metabolic scaling. J. Exp. Biol. 208, 1627–1634 10.1242/jeb.01503 (doi:10.1242/jeb.01503) [DOI] [PubMed] [Google Scholar]
- 27.Glazier D. S. 2009. Activity affects intraspecific body-size scaling of metabolic rate in ectothermic animals. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 179, 821–828 [DOI] [PubMed] [Google Scholar]
- 28.Creel S., Creel N. M. 2002. The African wild dog: behavior, ecology, and conservation. Princeton, NJ: Princeton University Press [Google Scholar]
- 29.Nilsen E. B., Herfindal I., Linnell J. D. C. 2005. Can intra-specific variation in carnivore home-range size be explained using remote-sensing estimates of environmental productivity? Ecoscience 12, 68–75 10.2980/i1195-6860-12-1-68.1 (doi:10.2980/i1195-6860-12-1-68.1) [DOI] [Google Scholar]
- 30.Van Orsdol K. G., Hanby J. P., Bygott J. D. 1985. Ecological correlates of lion social organisation (Panthera leo). J. Zool. Lond. Ser. A 206, 97–112 [Google Scholar]
- 31.Karanth K. U., Nichols J. D., Kumar N. S., Link W. A., Hines J. E. 2004. Tigers and their prey: predicting carnivore densities from prey abundance. Proc. Natl Acad. Sci. USA 101, 4854–4858 10.1073/pnas.0306210101 (doi:10.1073/pnas.0306210101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalewski A., Jedrzejewski W., Jedrzejewska B. 2004. Mobility and home range use by pine martens in a Polish primeval forest. Ecoscience 11, 113–122 [Google Scholar]
- 33.Schmidt K. 2008. Behavioural and spatial adaptation of the Eurasian lynx to a decline in prey availability. Acta Theriol. 53, 1–16 [Google Scholar]
- 34.Gorman M. L., Mills M. G., Raath J. P., Speakman J. R. 1998. High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 391, 479–481 10.1038/35131 (doi:10.1038/35131) [DOI] [Google Scholar]
- 35.Cardillo M. 2003. Biological determinants of extinction risk: why are smaller species less vulnerable? Anim. Conserv. 6, 63–69 10.1017/S1367943003003093 (doi:10.1017/S1367943003003093) [DOI] [Google Scholar]
- 36.Dieckmann U., Marrow P., Law R. 1995. Evolutionary cycling in predator–prey interactions: population dynamics and the Red Queen. J. Theoret. Biol. 176, 91–102 10.1006/jtbi.1995.0179 (doi:10.1006/jtbi.1995.0179) [DOI] [PubMed] [Google Scholar]
- 37.Abrams P. A. 2000. The evolution of predator–prey interactions: theory and evidence. Ann. Rev. Ecol. Syst. 31, 79–105 10.1146/annurev.ecolsys.31.1.79 (doi:10.1146/annurev.ecolsys.31.1.79) [DOI] [Google Scholar]
- 38.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 39.Collen B., Loh J., Whitmee S., McRae L., Amin R., Baillie J. E. M. 2009. Monitoring change in vertebrate abundance: the living planet index. Conserv. Biol. 23, 317–327 10.1111/j.1523-1739.2008.01117.x (doi:10.1111/j.1523-1739.2008.01117.x) [DOI] [PubMed] [Google Scholar]
- 40.Gittleman J. L. 1985. Carnivore body size: ecological and taxonomic correlates. Oecologia 67, 540–554 10.1007/BF00790026 (doi:10.1007/BF00790026) [DOI] [PubMed] [Google Scholar]