Abstract
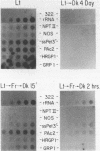
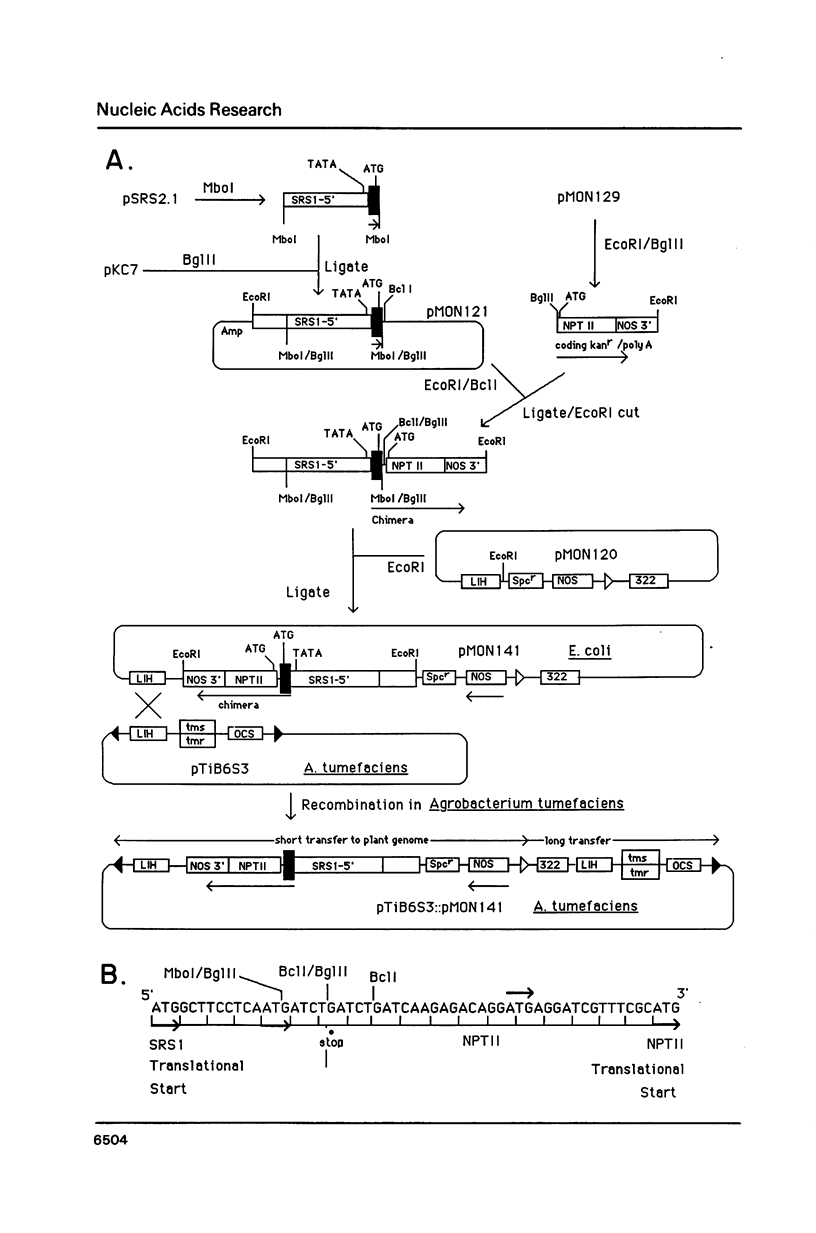
Two closely related ribulose-1,5-bisphosphate carboxylase small subunit (SSU) genes, SRS1 and SRS4, are transcribed at high levels in soybean plants in response to light. Transgenic petunia plants containing 5' sequences from SRS1 or SRS4 fused to the polypeptide encoding region of a neomycin phosphotransferase (NPTII) gene exhibit selectable kanamycin resistance. Deletion of three ATG codons from the region preceding the normal NPTII translation start site has little effect on the levels of kanamycin resistance in transformed plants. Run-on transcription assays in isolated nuclei demonstrate that transcription of the SRS1/NPTII chimera and the native petunia SSU11A gene subfamily is light regulated and under phytochrome control in leaves of transgenic plants. In young expanding leaves of fully light grown plants, transcription of these genes is markedly reduced within minutes of far-red treatment, while ribosomal DNA and actin gene transcription remains unchanged. This is analogous to the transcriptional response we observed for SRS1 and SRS4 in soybean seedlings. These data suggest (1) that transcription of SSU genes in both soybean and petunia require the continued presence or synthesis of phytochrome in the Pfr form and (2) that 5' sequences are sufficient to direct the phytochrome controlled transcriptional response of the SRS1 gene. In fully expanded mature leaves we found the transcription rates of the native SSU11A gene subfamily, the chimeric SRS1/NPTII gene, the rDNA genes, and several other control genes to be reduced markedly after far-red treatment or after extended periods of darkness. The contrast between results in young and mature leaves is discussed.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Berry-Lowe S. L., Meagher R. B. Transcriptional regulation of a gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean tissue is linked to the phytochrome response. Mol Cell Biol. 1985 Aug;5(8):1910–1917. doi: 10.1128/mcb.5.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., van den Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Linkage and homology analysis divides the eight genes for the small subunit of petunia ribulose 1,5-bisphosphate carboxylase into three gene families. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4964–4968. doi: 10.1073/pnas.82.15.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Rogers S. G., Horsch R. B., Sanders P. R., Flick J. S., Adams S. P., Bittner M. L., Brand L. A., Fink C. L., Fry J. S. Expression of bacterial genes in plant cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch R. B., Fraley R. T., Rogers S. G., Sanders P. R., Lloyd A., Hoffmann N. Inheritance of functional foreign genes in plants. Science. 1984 Feb 3;223(4635):496–498. doi: 10.1126/science.223.4635.496. [DOI] [PubMed] [Google Scholar]
- Jacobson L. A., Jen-Jacobson L., Wnek A. P. The relationship between translational initiation and messenger RNA inactivation in down-shifted Escherichia coli. Arch Biochem Biophys. 1985 Aug 15;241(1):118–131. doi: 10.1016/0003-9861(85)90368-6. [DOI] [PubMed] [Google Scholar]
- Kawashima N., Wildman S. G. Studies on fraction I protein. IV. Mode of inheritance of primary structure in relation to whether chloroplast or nuclear DNA contains the code for a chloroplast protein. Biochim Biophys Acta. 1972 Feb 23;262(1):42–49. doi: 10.1016/0005-2787(72)90217-1. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Nagy F., Kay S. A., Boutry M., Hsu M. Y., Chua N. H. Phytochrome-controlled expression of a wheat Cab gene in transgenic tobacco seedlings. EMBO J. 1986 Jun;5(6):1119–1124. doi: 10.1002/j.1460-2075.1986.tb04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Rowland L. J., Strommer J. N. Insertion of an unstable element in an intervening sequence of maize Adh1 affects transcription but not processing. Proc Natl Acad Sci U S A. 1985 May;82(9):2875–2879. doi: 10.1073/pnas.82.9.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C. H., Sanders D. M., Bates M. R., Shaw C. H. Light regulation of a SSRubisco-nos chimaeric gene: photoregulatory control sequences from a C3 plant function in cells of a CAM plant. Nucleic Acids Res. 1986 Aug 26;14(16):6603–6612. doi: 10.1093/nar/14.16.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko M. P., Kausch A. P., Castresana C., Fassler J., Herrera-Estrella L., Van den Broeck G., Van Montagu M., Schell J., Cashmore A. R. Light regulation of plant gene expression by an upstream enhancer-like element. Nature. 1985 Dec 12;318(6046):579–582. doi: 10.1038/318579a0. [DOI] [PubMed] [Google Scholar]
- Tumer N. E., Clark W. G., Tabor G. J., Hironaka C. M., Fraley R. T., Shah D. M. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Res. 1986 Apr 25;14(8):3325–3342. doi: 10.1093/nar/14.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]