Abstract
The immune system of an organism is an essential component of the defense mechanism aimed at combating pathogenic stress. Age-associated immune dysfunction, also dubbed “immune senescence,” manifests as increased susceptibility to infections, increased onset and progression of autoimmune diseases, and onset of neoplasia. Over the years, extensive research has generated consensus in terms of the phenotypic and functional defects within the immune system in various organisms, including humans. Indeed, age-associated alterations such as thymic involution, T cell repertoire skewing, decreased ability to activate naïve T cells and to generate robust memory responses, have been shown to have a causative role in immune decline. Further, understanding the molecular mechanisms underlying the generation of proteotoxic stress, DNA damage response, modulation of ubiquitin proteasome pathway, and regulation of transcription factor NFκB activation, in immune decline, have paved the way to delineating signaling pathways that cross-talk and impact immune senescence. Given the role of the immune system in combating infections, its effectiveness with age may well be a marker of health and a predictor of longevity. It is therefore believed that a better understanding of the mechanisms underlying immune senescence will lead to an effective interventional strategy aimed at improving the health span of individuals. Antioxid. Redox Signal. 14, 1551–1585.
-
Interventional Strategies for Reversal of Immune Dysfunction Accompanying Advancing Age
I. Introduction
Longevity is determined by an effective cross-talk between deleterious processes that act on an organism over its lifetime and the physiological responses that promote effective homeostasis. Aging represents the progressive functional decline in a variety of organ systems that occurs with advancing time. While the accumulation of damage to macromolecules is considered as a marker of conserved aging phenotype across species (75), the type of damage most crucial in initiating the aging process, the driving force for maintaining and sustaining these damages, and the underlying basis of the damage resulting in physiological aging is an area of intense research. As depicted in Figure 1, age-associated degenerative processes resulting in the loss of function impacts immune responses. In fact, one of the well-documented physiological declines of immense importance in response to stress or damage, which impacts negatively on the health span of individuals, is the decline in function of the immune system (73, 230, 396). Immune system, by virtue of its property as a sensor and defender of pathogenic signals, is a key sentinel in detecting declines that manifest during aging (76, 103, 104). As stressors often define the boundaries in a system, a failure to defend against impinging noxious and infectious agents often defines immune dysfunction that accompanies aging (314). Figure 2 compares the immune function observed in young versus elderly humans, and outlines challenges posed by declining immunity in the elderly. In fact, owing to its central role in defense, together with the increased susceptibility to infectious diseases with advancing age, studies regarding aging of the immune system have been the focus of research in a great number of laboratories. As recent projections estimate that the proportion of aged population (65 years and older) will double from 7% of the total world population now to 14% in 2040 (Fig. 3), understanding and ameliorating immune senescence will likely contribute to improved health span of the elderly population.
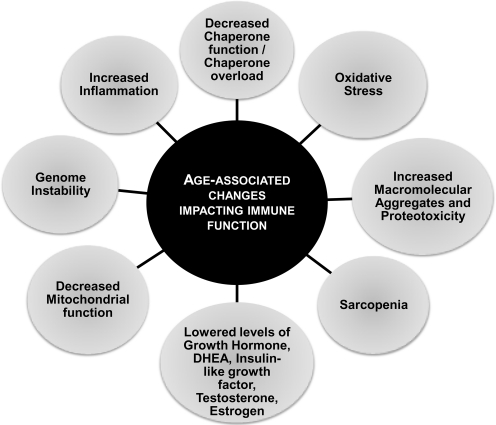
FIG. 1.
An overview of alterations impacting immune status in humans with advancing age. Key changes that have been reported to occur in humans during advancing age, affecting the immune status and resulting in overall decline in immune function, are depicted. DHEA, dehydroepiandrosterone.
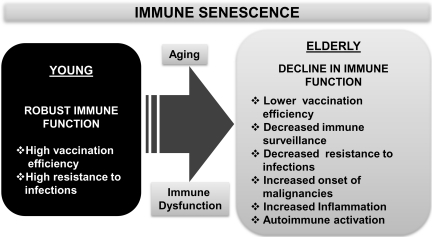
FIG. 2.
A synopsis of immune senescence and its role in host defense. A summary of observed pathological manifestations attributed to altered immune function during advancing age is depicted. Immune dysfunction in the elderly that often results in increased morbidity and mortality due to infections is contrasted with robust immune function in the young that includes an effective response to vaccination and an enhanced ability to resist infections.
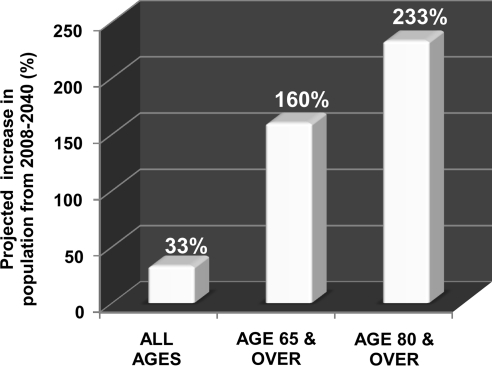
FIG. 3.
Projected increase (%) in aged population during the period 2008–2040. The proportion of elderly individuals aged 65 years and older is expected to increase during the next three decades. Projected increase in the total world population is contrasted with that of the elderly population (65 years and older or 80 years and older) during the next three decades using data obtained from the U.S. Census Bureau report. During the period from 2008 to 2040, the population of individuals aged 65 years and above is projected to increase by 160%, whereas that of individuals aged 80 years and older will be over 230%.
II. Aging and Immunity
It has been well established that the immune system is compromised in aged individuals. While changes occur in both arms of immunity, innate and adaptive, studies have demonstrated that certain specific immune responses are diminished, leaving others unaffected or exacerbated. This decrease in immunity that occurs for the large part, often referred to as “immune senescence,” has been attributed to be the basis of increased frequency and severity of infections, lowered immune surveillance of malignant cells, and decreased efficacy of vaccination in the elderly (72, 194, 232, 394). The focus of a large amount of research in immune senescence has centered on T cells largely due to their role in mediating and regulating antigen-specific responses. The central role of T cells is further underscored by fewer age-associated deficiencies in the antigen presenting cell (APC) compartment. As robust adaptive immunity relies on effective communication among T cells, APCs, and B cells, dysfunctional T cells significantly impact adaptive immune responses. In this review we have attempted to provide an overview of the defects in the immune system with regard to both innate and adaptive immunity that accompanies advancing age, and discuss the molecular mechanisms that contribute to declining function in the aged. We have also addressed some of the potential interventions that may forestall, retard, or reverse the onset of degeneration and enhance vaccination efficacy in the elderly.
Functional and effective immunity to a plethora of pathogenic insults that are encountered over the lifetime of an individual is dependent on the well-orchestrated interplay between innate and adaptive immune systems. Age-related malfunction and/or dysregulation has been documented in both the innate (79, 104, 276, 287, 335, 383, 384) and adaptive immune systems (194, 327, 397), which ultimately impact resistance to disease, onset of malignancies, and recognition of autoimmune specificities.
III. Aging of the Innate Immune System
As the front line of defense, the cells and cellular factors (cytokines, chemokines, and antimicrobial peptides) of the innate immune system are the sentinels of the body, standing guard against the onslaught of microbes. As we live in a microbial world and are continuously exposed to microorganisms, the effectiveness of antimicrobial defense mechanisms takes a front seat in defining the wellness of an individual. Failure to mount an effective response often can prove to be fatal as seen in victims of severe burn injuries. Invasions by microorganisms are initially countered by innate defense mechanisms that preexist in all individuals. This response manifests rapidly within minutes of the encounter with invading organisms. The cells and cellular factors (cytokines, chemokines, and antimicrobial peptides) of the innate immune system, as first defenders, provide the basis for an adequate response to pathogens. As aging is often associated with a decrease in function of epithelial barriers such as those of the skin, lung, and gastrointestinal tract (90, 122, 264), demands placed on the innate immune cells are significantly increased and are constantly on vigil to defend against the manifestation of a full blown infection. Although significant changes in antimicrobial peptide generation have not been well documented, an indirect correlation appears to exist between altered epithelial surfaces and the protection afforded by layers of these epithelial cells, as mechanical barriers to infection. Reports of definitive alterations in other innate immune cells and/or factors have been rather controversial, with some laboratories reporting little or no change, and yet others reporting a significant decline in functional status, with advancing age (112, 180, 218, 246, 342, 356, 382). In the light of increased bacterial infections and chronic inflammatory conditions that often accompany aging, defects in the functional response, but not in the numbers, of neutrophils has long been demonstrated in human aging.
Similarly, specific cytokines and chemokines, which are signal molecules produced by innate immune cells, have been reported to substantially alter with age, especially proinflammatory cytokines such as interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)α, and TGFβ, leading to chronic inflammation, and thus contributing to the inflamm-aging phenotype, often observed in the elderly (36, 93, 103, 266). Increases in proinflammatory cytokines have been attributed to be the underlying basis of the progression of degenerative geriatric diseases that often accompany advanced age.
A. Granulocytes: neutrophils, eosinophils, and basophils
Granulocytes are key participants in the earliest responses to microbial infections. As central and rapid mediators of antimicrobial defense system, they are endowed with the ability to generate reactive oxygen species (ROS), nitrogen species, a wide range of degradative enzymes, and significant number of antimicrobial peptides.
Neutrophils or polymorphonuclear neutrophils, referred to as polys or PMNs, are the most abundant leukocytes in the blood. They migrate rapidly to the sites of infection where they mount defense against the invading organisms, including ingestion of the microbes by phagocytosis and the release of microbicidal granules. Upon phagocytosis, neutrophils produce a range of toxic products that aid in the killing of the ingested microbe. The most important toxic products are nitric oxide, super oxide, oxy halides, and hydrogen peroxide. Neutrophils are short-lived leukocytes with a half-life of about 8–12 h, and their life span is extended by signals induced upon exposure to pro-inflammatory cytokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF), at the sites of inflammation. This enhanced life span of neutrophils, mediated by proinflammatory signals, is crucial for mounting an effective and persistent antibacterial activity during infection.
A detailed analysis of the literature with regard to a role for neutrophils in immune dysfunction in aged individuals demonstrates that these cells do not show any alteration in numbers, when assessed either as precursors or as mature effectors (50). Thus, while neutrophil counts in the blood of the elderly appear to be comparable to those in healthy young individuals, neutrophil function is significantly compromised in older humans and aged mice. The reported decline during aging in neutrophils appears to encompass major functional attributes and include lowered chemotaxis, decreased phagocytosis of microbes, and reduced generation of superoxide in response to pathogen-associated molecules such as lipopolysaccharide (LPS), formyl peptide receptor 1 (FMLP) or opsonized bacteria (102, 215, 333) (Fig. 4). Although a majority of studies demonstrate lower ROS production with age, some recent studies appear to indicate increased spontaneous ROS production by aged neutrophils, with little or no change in FMLP or LPS induced ROS production (267). However, impact of low-grade inflammation in these subjects could not be categorically ruled out. Additionally, in kinetic studies reported by Fulop et al. (112), lower ROS was demonstrated at early time points (2 and 24 h) but not at later time points (48 h). Response of neutrophils to GM-CSF has also been demonstrated to decline with age, resulting in an increase in apoptosis at the site of infection. This increased apoptosis of neutrophils observed in aged humans may account for the long-term persistence of inflammation in the elderly (100, 216, 373). The underlying basis for defects in neutrophil function in the elderly has been attributed either to modulation/s in cell membrane-mediated events or to defects in proximal signaling mechanisms, since stimulators such as phorbol esters are fully capable of inducing a relatively robust response in the elderly, primarily by overriding early receptor-mediated and membrane-associated activation events. Decline in priming and activation of neutrophils in response to LPS, granulocyte CSF (G-CSF), FMLP, and ligation of triggering receptor expressed on myeloid cell 1 has also been reported in elderly humans (101, 112, 158). Recent studies have implicated defective recruitment of signaling intermediaries into lipid rafts as a potential unifying defect in the signals generated at the membrane (9, 343). Future studies will likely establish whether these defects are attributable to altered membrane fluidity, cytoskeletal changes, or altered protein domains.
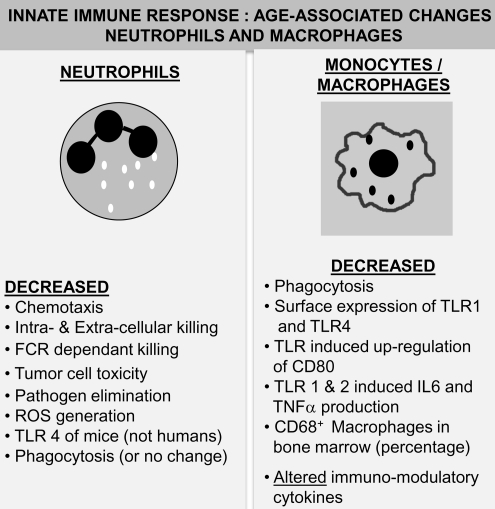
FIG. 4.
Age-associated alterations in innate immunity: I. Neutrophils and macrophages. Neutrophils: A summary of reported changes in neutrophil function resulting in alterations in innate immune responses during advanced age in humans and mice is presented. Age-associated alteration in neutrophils manifests as a decline in most functional responses with a consistent and significant increase in pro-inflammatory cytokine production. Macrophages: A summary of age-associated alterations in macrophages is depicted. While a minimal decline in numbers is reported in some studies, consistent functional deficits ranging from decreased TLR expression to phagocytosis is observed. Additionally, TLR-induced pro-inflammatory cytokine production has been demonstrated to decline with age. FCR, Fc receptor; ROS, reactive oxygen species; TLR, toll-like receptor.
Neutrophils obtained from aged mice appear to demonstrate distinct alterations in numbers and is in stark contrast to that described in older humans (27). There is an effective decrease in numbers of neutrophils recruited to infection site in aged mice. This, combined with altered neutrophil generation in the aged mouse microenvironment, appears to indicate that both extrinsic as well as intrinsic factors impact on effective immunity mediated by neutrophils, by altering their numbers in mouse models (180). However, in contrast to declining neutrophil numbers, age-associated alterations in the functional responses of neutrophils in mice are relatively minor, and as suggested by Kovacs et al. (180) in their excellent review, studies in mice might not reflect neutrophil immune senescence in humans, since human neutrophils demonstrate functional deficits with no contraction in numbers during aging.
Eosinophils and Basophils are derived from precursors that are similar to that of neutrophils however unlike neutrophils they are present in smaller numbers in blood under normal conditions. They play a central role in the pathogenesis of airway inflammation and in the defense against parasites, for the most part.
As opposed to neutrophils, there is a paucity of information on cellular function with advancing age, with regard to other members of the granulocyte family, that is, eosinophils, basophils, and mast cells. A decline in eosinophil function with respect to degranulation, but not superoxide production in asthma, has been demonstrated in the elderly (228). Reports on basophil function, on the other hand, have been controversial with some data suggesting impaired activity in the aged, yet others reporting a high immunoglobulin E-mediated releasability/degranulation in older subjects (225, 334). In the light of recent information on a central role attributed to basophils in the presentation of protease-digested antigenic peptides (349), it is important that future studies focus on the role of basophils in allergen-induced T helper responses in the elderly, with an eye on dissecting its role in antigen presentation, during aging. This is especially important, given that basophils generate IL-4 and IL-13, cytokines that are important for T helper type 2 (Th2) polarization.
B. Monocytes and macrophages
Macrophages and monocytes function not only as phagocytic cells in innate immunity, but are also important defenders with specialized functions at various sites in the body such as bone, lung, liver, brain, and skin. Macrophages mature continuously from monocytes that leave the circulation to migrate into tissues throughout the body. They are found in large numbers in connective tissue, the sub-mucosal layer of the gastro-intestinal tract, lung, liver, and spleen. Owing to their distinct tissue localization, heterogeneity appears to exist not only in terms of their function, but also in age-dependent changes in their function. Along with neutrophils, macrophages form a large family of phagocytic cells. Macrophages, located in the sub-mucosal tissues, are the first cells to encounter invading pathogens, but they are soon reinforced by large numbers of recruited neutrophils. By virtue of expression of pathogen recognition receptors on their surface, macrophages function as “pathogen sensors” and play important roles in the initiation of inflammatory responses, eradication of infectious organisms, and as regulators and effectors in adaptive immunity (24, 270). Declines in macrophage surface receptor expression, such as major histocompatibility class (MHC)-II, have been reported in both mice and humans during advancing age (143, 350). Additionally, upon stimulation with interferon (IFN)γ, the number of MHC-II molecules expressed on the surface of macrophages from aged mice was reported to be lowered by 50% of that seen in young mice, potentially impacting their antigen presenting capacity (143). Further, decline in the ability to generate super-oxide anion and lowered bacterial killing due to decreased respiratory burst have also been reported to occur in both aged animals and elderly humans (283, 286). Combined with this, a decline in phagocytic ability (297) accompanies reduction in the generation of macrophage-specific chemokines, such as macrophage inflammatory protein (MIP)-1β, MIP-1α, Eotaxin, and MIP-2 during aging (363). With regard to inflammatory cytokines and chemokines, while studies in humans have demonstrated a significant increase in levels of IL-6, IL-1, TNFα, regulated upon activation normal T cell expressed and secreted (RANTES), MIP-1α, and IL-8 in monocytes obtained from peripheral blood of older subjects, studies from rodents appear to demonstrate an overall decrease in cytokine and chemokine production by macrophages with age [reviewed in (180, 283)].
A recent study evaluating the effect of age on surface expression of pathogen recognition receptor, toll-like receptor (TLR) 1, 2, and 4 in peripheral blood monocytes from human volunteers reported lower levels of TLR1 expression, with TLR2 and TLR4 only minimally affected by age (276). This observation is similar to previous studies that demonstrated no decrease in TLR4 expression on macrophages in mice, with age. Cytokine production after ligation of TLR1/2 demonstrated a significant decrease in the generation of TNFα and IL-6 with age (383), despite an increase in constitutive or basal proinflammatory cytokine production. This may well be attributed to increased IL-10 generation during aging. This result is in contrast to studies employing activation through ligands for TLR2/6, TLR4, and TLR5 receptors, which demonstrated no discernable effect of advancing age on activation, in humans. Given that an increase in basal levels of proinflammatory cytokines accompanies aging, alterations in the microenvironment, availability of other chronic stimulators, and the potential cross-talk need to be considered. Further, recent studies indicate that macrophages differentiate either into M1 and M2 type subsets, under appropriate cytokine milieu (221), or into professional APCs such as dendritic cells. Thus, the impact of microenvironment and the shortening of telomeres in the aged is likely to affect M1/M2 differentiation of macrophages, as also the presence of higher levels of circulating IL-6 and other proinflammatory factors (180).
Proximal signaling events after activation of macrophages have demonstrated either decreased or increased induction of nuclear factor kappa B (NFκB) (362, 406). A recent microarray analysis performed from resting and LPS-activated splenic macrophages, obtained from young and aged mice, demonstrated decreased expression of genes involved in the TLR-signaling pathway leading up to the activation of NFκB, including TNF receptor associated factor 6, myeloid differentiation primary response gene 88, and other members of the NFκB pathway. The negative regulator IL-1 receptor-associated kinase 3 was only expressed in cells from the aged but not in young mice (52). Overall, these observations suggest that TLR-dependent activation of downstream signaling is compromised in the macrophages from the aged, and may be attributable to the increase in intracellular ROS concentration within the aging macrophage. A summary of salient declines and alterations with age in monocytes/macrophages are depicted in Figure 4.
C. Natural killer and natural killer T cells
Natural killer (NK) cells, as mediators of innate host defense against viral and tumor targets, play an important role in immune surveillance. NK cells are heterogeneous and differ in their proliferative potential, homing properties, functional capabilities, and their responses to various cytokines. Based on their relative density of CD56 surface expression, they can be divided into two major subsets. In humans, peripheral blood NK cells are comprised of around 10% CD56bright and 90% CD56dim cells (39). While CD56dim NK cells are highly cytotoxic and show less proliferation and produce fewer cytokines, CD56bright NK cell subset are highly proliferative, produce a range of cytokines, such as IFNγ, TNFβ and IL-10, and chemokines, such as RANTES and MIP-1α, but show relatively minimal cytotoxic activity.
NK cytolytic activity appears to be preserved in human aging (181). Studies conducted in humans have demonstrated an increase in overall numbers of circulating NK cells, with little or no loss in cytolytic activity, either toward sensitive tumor targets or when non-MHC restricted activity was assessed (105, 106). A detailed phenotypic analysis of peripheral blood mononuclear cell (PBMC) demonstrated little or no age-related alteration in NK cell activity in healthy subjects recruited per strict SENIEUR protocol adherence (206). These studies for the first time also demonstrated that in centenarians, increased number of NK cells was observed, and that these NK cells were of comparable activity to that seen in young donors (19–36 years). Unexpectedly, this study found lowered NK activity in healthy middle-aged donors, but not in elderly donors. In contrast, in the elderly, disease states appeared to be associated with lowered NK activity, and were indeed predictive of increased morbidity and mortality (201). The increase in NK cell numbers observed in the elderly has been attributed to an increase in CD56+dim population of NK cells, a mature highly cytotoxic subset. Research has now demonstrated that this subset of NK cells (CD56+dim) compensates for the overall decrease in activity contributed by other subsets during aging (236). Analyses of NK cell function, on a per cell basis by cloning, however, demonstrated defects in both cytotoxicity and lymphokine-activated killer cells activity (224). Further examination led to the demonstration of defects in inositol phosphate metabolism, in the context of spontaneous cytotoxicity, but not antibody dependent cytotoxicity of the NK cell (222). These studies highlight the distinct and differential effect of aging on multiple signaling pathways in NK cells, which may be subset dependent and disease dependent, in the aging host.
As cytokines produced by NK cells act to bridge innate and adaptive immunity, any deficits in the generation of cytokines by NK cells during aging can ultimately impact and modulate the regulation of adaptive immunity. It has been previously reported that IL-2-induced IFNγ, IL-2- and IL-12-mediated secretion of chemokines such as MIP-1α, RANTES, and IL-8 are decreased in NK cells from the aged (182, 223), thus explaining the basis of skewed Th2 responses observed in elderly individuals.
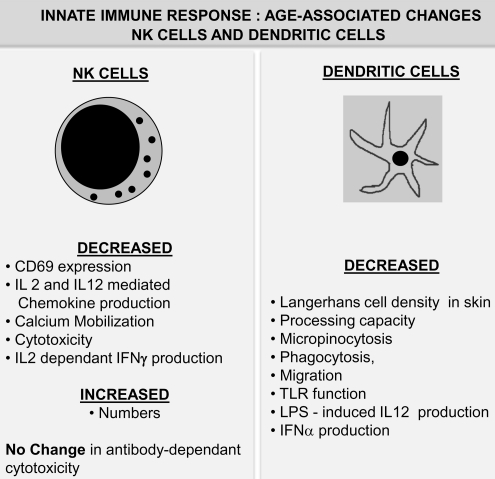
Natural killer T (NKT) cells are a subset of cells that exhibit properties of both T cells and NK cells. Analysis of NKT cells, a population that comprises of CD1d-restricted heterogenous T cell population expressing invariant T cell receptor (TCR) (iNKT) and responsive to α-Galactosyl Ceramide, has been carried out during aging and found to be decreased in the blood of elderly subjects (77). In addition, recent studies appear to indicate altered proliferation of these NKT cells and a skewing of the cytokine profile from Th1 to Th2 type in older individuals compared to those observed in NKT cells from young individuals (163). Thus, aging impacts both NK and NKT cell numbers and function. Figure 5 summarizes age-associated alterations in NK cell function. Further research is clearly warranted to elucidate their role and contribution to immune decline in the elderly.
FIG. 5.
Age-associated alterations in innate immunity: II. NK cells and dendritic cells. NK cells: Aging is not only accompanied by a significant increase in NK cell numbers, but significant functional deficits have also been reported to occur in these cells. Dendritic cells: A compilation of changes in function of the dendritic cell population during advanced age in humans is presented. Unlike other immune cells, numbers of dendritic cells remain relatively unchanged with age. However, dendritic cell functions, including antigen processing, antigen presentation, and phagocytosis, appear to be significantly affected by advanced age. IL, interleukin; NK, natural killer.
D. Dendritic cells
Dendritic cells (DCs) are specialized to take up antigens, process it, and present it for recognition by the T cells. Immature DC migrate from the blood to reside in tissues and are both phagocytic and micropinocytic. Upon encountering a pathogen they rapidly mature, express costimulatory molecules, and migrate to the lymph nodes. By virtue of their ability to phagocytose antigens and mature to interact with adaptive immune cells, DCs are considered as bridging innate and adaptive arms of immune response.
Given the variation of subsets of DC and their occurrence at different sites, such as myeloid DC (mDC) and plasmacytoid DC (pDC), reports on the effect of aging on the numbers and function of DCs have been rather inconsistent. Some studies have reported no change in pDC, and a concomitant increase in mDC, with the appearance of a more mature phenotype, whereas others have reported either an increase in pDC with no change in mDC, or no change in both the numbers or function, in cells derived from the elderly (78, 342). This discrepancy may be attributable to the protocols employed for generating DC ex vivo. However, tissue-specific DC populations, such as Langerhans cells observed in the epidermis of the skin, have been reported to consistently decline in density, with age (26). This decline appeared to accompany decreased branching and increased expression of maturation markers. However, in terms of antigen presenting function and ability of antigen-specific stimulation of T cell proliferation, DC remain unaffected by age (218, 356). In the context of specific response to infection and to TLR agonists such as LPS and single-stranded RNA, DC from aged individuals demonstrate decreased generation of some cytokines, including IFNα and IL-12, and an increased secretion of others such as IL-6 and TNFα (78, 342). An examination of TLR density as the basis for the decline revealed no change, implicating downstream signaling deficits after receptor activation.
Micropinocytosis, phagocytosis, and migration are decreased in DCs from the elderly. Phosphatidyl inositol 3 kinase, implicated in both micropinocytosis and phagocytosis, has been demonstrated to be impaired in its ability to phosphorylate its target serine/threonine protein kinase/PKB (AKT) in DC from the elderly. This defect may also contribute to the altered innate immune response of DC from elderly humans (4). While scant information is available about the impact of aging on antigen processing and presentation in DC from the elderly, alterations in proteasomal proteolysis and autophagy occurring during aging is likely to negatively impact antigen processing ability, and perhaps limit the repertoire of specificities generated and presented in the elderly (Fig. 5).
The previous section outlines the impact of aging on innate immune cells and their contribution to overall immune decline during senescence. However, one must also consider the role of adaptive immunity and its influence on overall defense capacity, given the constant cross-talk and regulation that occurs between innate and adaptive immune systems, which ultimately defines the outcome of host defense.
IV. Adaptive Immunity and Aging
Unlike the components of innate immunity that are present before the onset of infection and are not specific to any given pathogen, in adaptive immunity the specific response component is triggered only when there is a recognized antigenic challenge/threat. Thus, elements of the adaptive immune system respond to challenges with a high degree of specificity and memory, and are mediated by B cells and T cells within the immune apparatus. Innate and adaptive immunity do not operate independently, but function as a highly integrated and cooperative system.
The specificity and regulation of adaptive immunity is dependent on the interaction of fully matured DC with T cells, cytokines, and others signaling molecules. Aging appears to alter this interaction. A classic example of this is the potentiation observed in inflammatory cytokines, leading to an inflamm-aging phenotype. The increased rates of common infections in the elderly is a testimony to poorly orchestrated host defense, and has been attributed to alterations in both innate and adaptive arms of immune response and the cytokines that mediate the effects. The strength and balance of cytokine signals orchestrate immune outcomes. In general, activation of adaptive immunity that involves cell-mediated immune responses referred to as Th1 or type 1 response is predominated by the production of cytokines IL-2, and IFNγ, whereas Th2 or type 2 associated with allergic or parasitic infections is accompanied by high levels of IL-10, IL-4, and IL-5. The relative contribution of cytokines dictate final outcome by counter-regulating each other, such that either Th1 or Th2 type predominates (6).
A. B lymphocytes
B lymphocytes arising from hematopoietic stem cells in the bone marrow as pro-B cells differentiate into pre-B and then B cells after immunoglobulin gene rearrangement. Immunoglobulin expressing naïve B cells migrate to the periphery and form the nexus for the development of humoral immunity.
B lymphocytes are thus essential mediators of antibody responses. Declining antibody responses in B lymphocytes during aging has been well documented in both humans and mice [reviewed in (40, 107)]. While effects of a decline in T cell functional help have been attributed to lowered humoral immunity with age, B cell intrinsic defects contributing to declining antibody affinity with age have also been reported (20, 133).
Levels of serum immunoglobulin and secreted antibody have been analyzed during aging. In both mice and humans, although serum immunoglobulin levels remain relatively unchanged with age, antibodies generated in the aged are often of lower affinity (117). As antibody affinity is dictated by somatic hyper-mutation and isotype switching, decreased antibody affinity during aging has been attributed to lower isotype switching and diminished somatic hyper-mutation. Age-related decline in affinity maturation due to diminished germinal center reaction also contributes to this loss in affinity (308). There is also evidence suggesting a shift in B cell selection, resulting in higher frequency of auto-reactive clones in the aged. Additionally, recent results appear to demonstrate that the alteration in intrinsic properties of B cells in aged mice and elderly humans may also account for defects in the transcription factor E47 (108). E47, which regulates immunoglobulin class switch, is downregulated in murine splenic B cells during aging, due in part to reduced mRNA stability. Since specific targets such as activation-induced cytidine deaminase, E47, and defects in immunoglobulin class switch recombination have now been identified in B cells, it will be interesting to examine the extent to which modulation of these targets may account for reversal of B cell dysfunction in the aged.
In murine models of aging, the frequency of precursors capable of generating B cells, pre-B cells, appears to decline with age, resulting in fewer naïve B cells, without affecting the peripheral population of B cells. Thus, it appears that the B cell compartment in the elderly is composed largely of antigen-experienced memory B cells (358). This disproportionate increase in antigen-experienced B cells limits new repertoire expansion and skews it toward pre-existing specificities due to homeostatic proliferation. In addition, failure of memory B cells to undergo apoptosis allows for the accumulation and long-term survival of these aberrant cells at the expense of generating new specificities (153, 175). The inability of the elderly to effectively respond to vaccination, contributing to the immune compromised phenotype, could well be due in part to such alterations in the B cell compartment.
Recent studies in mice have also demonstrated that expression of genes vital to lineage commitment and differentiation are significantly reduced with age, impacting negatively on B cell generation (60, 189). Thus, alteration in hematopoietic stem cell potential to generate myeloid cells at the expense of lymphoid precursors affects early B cell progenitors. These age-associated defects have also been reported to be due both to microenvironmental alterations, that is, niche alteration, as well as cell intrinsic defects (60, 189). For instance, both the generation and response to IL-7 in the aged microenvironment and in B cell precursors appear compromised (60). Additionally, B cell signaling downstream of receptor activation is also significantly altered in the aged (354, 402). In humans, similar to these observations in mice, a pronounced decrease in peripheral B cell numbers and percentages and an increase in B cell intrinsic defects are known to occur with advancing age (107, 108) (Fig. 6).
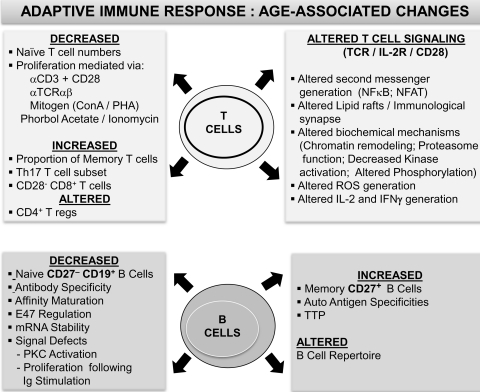
FIG. 6.
Summary of age-associated alterations in adaptive immunity: T lymphocytes and B lymphocytes. Observed alterations in B and T lymphocyte phenotypic and functional attributes during advancing age in humans that contribute to the immune senescence phenotype are presented. B lymphocytes: While both signaling within B cells and antibody affinity maturation decline in advanced age, increases in auto-antigen specificities as well as memory B cell numbers appear to accompany aging. T lymphocytes: Defects ranging from T cell emigration to the periphery to aberrant signaling within the TCR activation pathway have been demonstrated to be largely responsible for the reported age-associated functional deficits. IFN, interferon; NFAT, nuclear factor of activated T cells; NFκB, nuclear factor kappa B; TCR, T cell receptor.
B. T lymphocytes
As mediators of adaptive immunity, T cells take center stage as both regulators and effectors. It is because of this important role that they have been the focus of studies in immune senescence. Altered T cell function has been the most dramatic and consistent change reported during aging.
Being the primary site of T cell development, the thymus plays a crucial role in determining self-tolerance and MHC restriction. Therefore, it is not surprising that of all age-associated changes in the immune system, involution of the thymus is cited as being central to immune senescence.
A decrease in thymopoiesis and a progressive involution of thymus has been demonstrated to be the reason for naïve T cell decline during aging. In support of this key observation, studies have shown that systemic administration of cytokines and hormones or bone marrow transplantation increases thymic activity and naïve T cell output with advancing age (17, 219). Further, surgeries resulting in thymic ablation or thymectomy result in premature aging, in keeping with the contribution of the thymus in immune and naïve T cell decline associated with advancing age. A contraction of TCR diversity with age accompanies a concomitant appearance of oligoclonal specificities in the elderly (252, 381). Added to this skewing of repertoire, appearance of KIR (Killer inhibitory receptor) akin to that seen in NK cells has also been reported in T cells during aging (272, 273). Whether KIR expression is attributable to alterations in expression of receptor genes or due to epigenetic modification of regulatory silencing, or truly a compensatory mechanism for overall contraction in TCR diversity, remains to be investigated further.
An overview of T cell defects accompanying aging is provided in Figure 6 and summarizes the notable changes previously reported. Some of the studies in support of the noted changes are described in the following paragraphs.
In parallel with thymic involution, reduction in naïve T cell numbers occurs in the periphery of aged individuals, and is associated with an increase in the number of antigen-experienced memory and effector T cells, with little or no change in absolute numbers of total T cells (360). Expanded memory and effector cells accompany aging within both the CD4 and CD8 subsets, with greater number of dividing cells in the elderly contributing to greater antigen-independent basal proliferation (322). In studies of naïve CD4+ T cells in mice, there is increasing support for the observation that, while newly generated CD4+ T cells in aged animals manifest no defects, an age-associated increase in the life-span of naïve CD4+ T cells in contributing to T cell homeostasis, also drives functional deficits (378).
Within the expanded memory T cell subpopulations are the CD8+CD28− subset having shortened telomeres, which is suggestive of previous replicative history. The loss of CD28 costimulatory molecule on the surface of CD8+ T cells with age has been observed consistently, and is now employed as a key predictor or prognostic indicator of immune competence in the elderly (322). These CD8+ CD28− T cells also appear to have receptor specificities for cytomegalovirus (CMV) antigens, implicating chronic antigenic stimulation as a basis for repertoire exhaustion in the elderly. A recent microarray analysis of the T cell population obtained from elderly subjects demonstrated that the gene expression profiles were similar between CD8+ CD28− T cells obtained from young and elderly donors, but differed considerably from the young, when CD8+CD28+ T cells were compared. Hierarchical clustering of the data between the subsets identified three major groups, with Young CD8+CD28+ T cells being at one end of inexperienced naïve expression profiles, and the CD8+CD28− T cells from young and the elderly being at the other end of experienced stimulated profile, and the CD8+CD28+ T cells obtained from the elderly, being intermediate (197).
With the significant increase in the number of memory and effector T cells with age, there is a concomitant decline in functional responsiveness to new antigens, as evidenced by decreased responses to vaccination (145, 327, 394). As cytokines modulate immune responses, it has been postulated that alterations in their induction may dictate functional defects in the T cell compartment. Reports from both aged mice and elderly humans indicate that there is a shift in cytokine induction profile to predominantly IL-4 and IL-10 (Th2 type profile), with increased production of TGFβ and IFNγ in the mix. This alteration in the bias of T cell cytokine response toward Th2 type, rather than Th1 (IL-2, IFNγ) type, may account for some of the decline in responsiveness to Th1 type activation during aging (42, 304, 400).
Evaluation of regulatory T cells (Tregs) during aging has provided some inconsistent results. In one recent study, naturally occurring CD4+ Tregs expressing high levels of CD25+ and Forkhead box p3 (Foxp3)+ were comparable in young and elderly subjects, however, the ability of these Tregs to inhibit IL-10 production in a coculture with CD4+ T cells, was significantly higher (156) in cells derived from elderly subjects. Similar observations were also reported in a study employing mouse cells (262). However, in another independent study using cells from elderly donors (70 years of age), the authors demonstrated a slight increase in frequency of Foxp3+ cells (191). Clearly, more studies are required to resolve these inconsistencies. Given the importance of Tregs under pathological conditions, it is believed that a clearer picture on the dynamics of these cells with age and their contribution to the overall immune status will only emerge from studies using cells either obtained from healthy elderly with minimal underlying disease or by employing protocols that adhere to strict donor recruitment guidelines.
Given that pro-inflammatory conditions accompany aging, another T cell subset of interest in immune senescence is the recently identified Th17 subset. Recent studies by Huang et al. (150) have demonstrated that Th17 induction and cytokine secretion is significantly higher in T cells from the aged than that from young mice. These studies implicated a role for NFκB in Th17 cytokine generation. Given the increased constitutive levels of NFκB during aging, regulation of IL-17 generation may be clearly impacted. Additionally, pro-inflammatory cytokine milieu present during aging may underlie the observed increase in Th17 induction. Since IL-6 is an important cytokine for Th17 subset skewing, increased IL-6 during aging may contribute to the amplification of Th17 subsets with age.
Analysis in the context of activation and signaling at the cellular level has been a subject of research for several years, since the first observation that IL-2 production decreases significantly with age in both mice and human T cells upon activation (3, 208, 247). Studies in mice have demonstrated that age-related decline in signaling events starts at the membrane, with decreased recruitment of TCR associated signaling molecules to the immunological synapse, the site of activation cluster (89). While such direct evidence for lowered signaling at the synapse during aging is lacking in human T cells, T cells exposed to oxidative stress manifest reduction in signaling, leading to lowered IL-2 production (71, 98). The decline in IL-2 production has been attributed to be one of the major factors underlying immune decline in T cell functional response (141, 370).
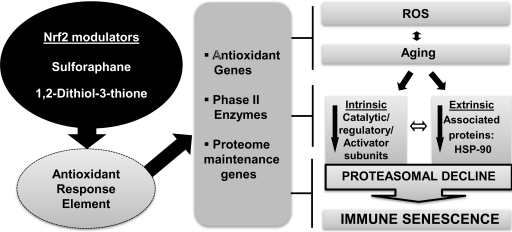
IL-2 generation and proliferation in T cells marks the culmination of a series of biochemical events that constitutes intracellular signaling. Our laboratory has demonstrated that irrespective of the activating stimuli, induction and regulation of the immune response gene inducer, NFκB, is significantly compromised. This is in clear contrast to increased nuclear levels of NFκB under basal conditions (93). Our recent studies delineating this paradox in aging have provided some mechanistic insight into the role of 26S proteasomal defects in NFκB -mediated pro-inflammatory cytokine induction during aging (66). Clearly, our studies and those of others have implicated a central role for the 26S proteasome in T cell functional response in the elderly (71, 294). Recent studies in flies, mice, and our own studies on human T cells ex vivo in culture indicate that restoration of proteome maintenance genes and antioxidant response element (ARE) activators, such as nuclear factor erythroid-2-related factor 2 (Nrf2) modulators, may be beneficial in retarding or restoring functional activity (199, 364) (unpublished data from our laboratory).
In evaluating T cell signal transduction machinery, investigators have demonstrated that cells from healthy aged mice and humans manifest several defects in early kinase activation, calcium mobilization, and transcription factor generation (142, 401). The molecular basis for the decline in signaling events is largely unresolved, although it is recognized that the contribution of ROS is rather high. It is hoped that future studies will likely shed more light on the contribution of signaling deficits on the generation of effectors and in immune decline.
V. Causes and Mechanisms Underlying Immune Senescence
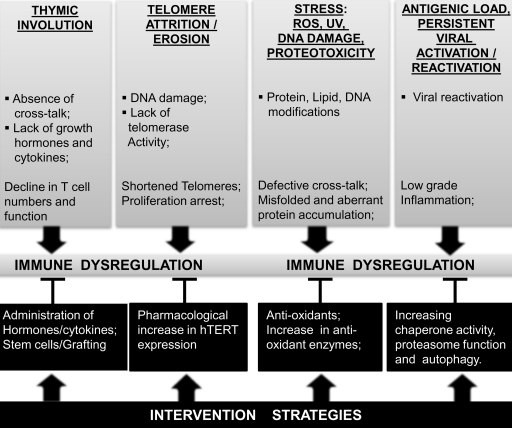
Complex physiological phenotypes of immune senescence that manifest during organismal aging are the result of cooperative as well as antagonistic changes in multiple pathways. The entire pathway underlying senescence remains to be fully delineated. Investigation of the mechanisms that contribute to and/or regulate senescence can be broadly attributed to thymic involution, oxidative stress, proteostasis, telomere attrition, DNA damage signaling, epigenetic alterations, increased inflammation, and transcriptional deviations. Figure 7 provides an outline of the triggers, mechanisms, and cross-talk that result in altered immune regulation during aging, and are discussed in the following sections.
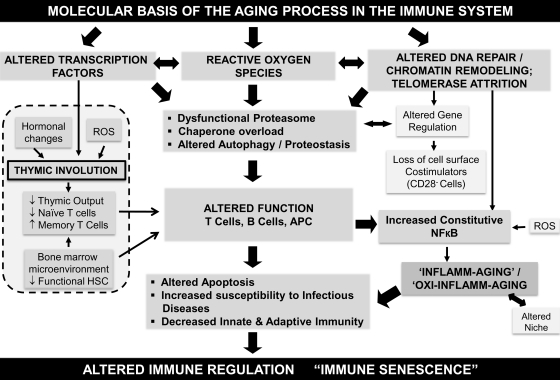
FIG. 7.
An outline of the biochemical and molecular mechanisms reported to impact immune function during advancing age, resulting in immune senescence. A schematic representation of the molecular basis of aging in the immune system is presented. Reported studies implicate a significant role for altered transcription factor induction, increased generation of ROS, altered DNA repair mechanisms, and telomere attrition in the dysfunctional immune system. Cross-talk and feedback regulation among these mechanisms appear to manifest as immune dysfunction, and impact a series of functional mechanisms and targets within the immune system that may account for increased susceptibility to infections and the development of an inflammatory phenotype. APC, antigen presenting cell.
A. Thymic involution in immune senescence
During aging, the integrity and function of the immune system declines progressively. This decline in innate and adaptive arms of immunity arises from alterations in almost every component of the immune apparatus, but the predominant, significant, and most consistent change manifests in the T cell compartment (86, 396). The underlying thymic involution and loss of T cell education and repopulation that occurs with age accounts for this decline (17, 219, 357).
The homeostatic maintenance of peripheral T cells is dependent on a regular and continuous supply of cells from the thymus. Thymus is a vital organ in which T cells develop and are schooled extensively and exquisitely to recognize antigens before they are exported to the periphery. This export of T cells ensures that a viable, effective, and functional immune system is ready and poised to fend off invaders. One of the well-documented features of the thymus is its ability to undergo atrophy fairly early in an individual's life. This atrophy, referred to as thymic “involution,” results in lowered naïve T cell output due to lowered development and emigration, ultimately impacting the maintenance of naïve T cells in the periphery in elderly subjects (10, 17, 86). While the impact of this loss in naïve T cell output is minimal in terms of previously encountered antigenic specificities, that is, recall antigens, its effect appears to be more significant on newly encountered infections, or when demands for repopulation are high, such as after major insults that deplete cells, that is, after exposure to rigors of chemotherapy and ionizing radiation (21, 59, 87, 132, 140, 219).
Early studies on the histology of the thymus clearly demarcated regions that are important for thymopoiesis and those, such as the nonepithelial peri-vascular spaces, that are not active participants in this process (99, 339). In fact, studies demonstrated a clear loss in epithelial spaces as early as the first year of one's life with >75% decline occurring up to middle age, and then a gradual decline for the rest of one's life (219). As a consequence, epithelial spaces are progressively lost and peri-vascular nonepithelial spaces fill up the elderly thymus, accounting for about 90% of the space (160, 219).
Due to thymic involution there is a direct impact on the generation of double positive thymocytes and in situ loss of TCR rearrangements essential for T cell repertoire diversity. Using TCR excision circles (179), laboratories of Sempowski and Aspinall have reported a steady decline in circulating T cells with advancing age (17, 338). However, an important question that has yet to be investigated is whether declining output in quality and/or quantity of the cells is responsible for the contracting space in the thymus or if it is vice-versa.
In searching for an underlying cause for the decline in thymic output of T lymphocytes with age, researchers have focused on mechanisms that range from depletion of thymic progenitors, loss of self/nonself recognition, and failure of selection process, decrease or an inherent inability for TCR-rearrangement, and a declining communication between thymic stroma/nurse cells in the thymic cortex and medulla with the developing thymocytes. Of these mechanisms, thymic milieu and thymopoietic factors have been intensely researched, and several studies appear to point to these as being key to the decline observed with age (160, 177).
Studies employing murine models have been useful in detailing the precise nature and type of T cell decline in the thymus. These studies have outlined a more dramatic decline in the CD8 subset as opposed to those of the CD4 subsets. It appears that while a constant level of TRECs (TCR excision circles: a readout for recent thymic emigrants) is maintained for up to about 1 year in CD4 subpopulations, a decline is noticed much earlier in the CD8 subpopulation in mice. While experimental models of thymic involution are well established in animals, a recent study documents reduced thymus activity and infection in a group of young adults who underwent thymic removal in their infancy, during corrective surgery for a congenital heart defect (124, 325). This study, for the first time, demonstrated that compared with age-matched adults, thymectomized subjects had immunologic abnormalities reminiscent of immune senescence observed in the elderly (325), providing the much needed proof of concept for the role of thymic involution in human aging.
Research has also demonstrated that thymopoiesis is regulated by cytokines produced by the cells of the thymic stroma (129, 339). This cytokine milieu is required and helps maintain and sustain effective thymopoiesis. However, changes in cytokine patterns accompanying age impact thymic environment, affecting maturation as well as thymic education. The cytokines demonstrated to be crucial are the cytokines central to hematopoiesis, including GM-CSF, G-CSF, IL-1, IL-13, IL-6, IL-7, stem cell factor, macrophage CSF (M-CSF), oncostatin M, and leukemia inhibitory factor (LIF). Studies evaluating and profiling cytokines in human thymus during aging have demonstrated a shift in cytokine expression patterns to predominantly LIF, oncostatin M, stem cell factor, IL-6, and M-CSF. Levels of thymic stimulatory cytokine such as IL-7 essentially remain unchanged with advancing age (339). Thus, alterations in IL-7 appear not to overtly contribute to thymic involution in the elderly, whereas IL-6 family of cytokines has been shown to play a role. Not only are patterns of IL-6 expression altered with age, but also exogenous administration of IL-6 family of cytokines induces thymic involution both acutely and rapidly, significantly reducing T cell output to the periphery (129, 328). In addition to IL-6, other cytokines such as LIF and hormones such as corticosteroids have been implicated in thymic involution (339). Recent studies in murine models demonstrate a direct role for TGFβ in regulating thymic involution (185). Whether the effects of these factors involve cross-talk remains to be established.
Increased adipogenesis in the thymic stroma and increased IL-6 production by the adipocytes in the thymic stroma of the aged have also been attributed to the increase in IL-6 in the thymic milieu and decline in thymic output and atrophy (412). Further investigations are clearly warranted to resolve the role of intrinsic and extrinsic cytokine production in the thymus and the impact of niche during aging.
While involution and atrophy are common place in the thymus during aging, more recent studies have demonstrated that thymopoiesis, nevertheless, continues to occur at very low rates in the remnants of functional tissue during advanced age. Additionally, studies have also demonstrated that caloric restriction (CR) is successful in retarding thymic involution by decreasing adipogenesis within the thymic milieu (412). These data are indicative that thymus is sensitive to manipulation by exogenous agents. Therefore, interventional therapies aimed at rejuvenating the immune system can be achieved by enhancing thymopoiesis or decreasing thymic involution.
B. ROS, aging, and immune dysfunction
The metabolism of oxygen is central to life in aerobic organisms; however, one of the unpleasant side effect is the production of ROS that has been implicated in deleterious effects ranging from cardiovascular disease to cancer and aging (97). The free radical theory of aging posits that a single modifiable process by genetics and environmental factors in cell, in which the accumulation of endogenous oxygen radicals occur, could be the basis for the deleterious effects observed during aging in living organisms (135, 136). However, to this date, it is unclear whether increased ROS is the cause or a consequence of aging, and the debate still rages on. More recently, however, it has come to be appreciated that not all ROS are deleterious, and a gradation of ROS induction occurs, with smaller and milder amounts acting as a mediator in cellular signaling, and excess or imbalance resulting in deleterious effects (58, 71, 157, 237, 279, 374). Corroborating this idea, recent evidence suggests that hematopoietic stem cells and early progenitors contain lower levels of ROS than their more differentiated progeny, and that these may be critical in maintaining their “stemcell-ness,” or stem cell potential (82). Thus, both the more differentiated effector cells and the naive stem cells appear to share a common principle, that is, ROS induction in the context of cellular signaling, but clearly differ in the extent or magnitude of ROS induction. Increasingly, age-associated accumulation of ROS appears to be attributable to an imbalance between the free radical generation and antioxidant defenses induced by the phase-II enzymes, with an increase in the former and a concomitant decline in the latter (71, 199). This accumulation of ROS during aging accounts for the generation of oxidatively modified proteins and DNA. Proteins undergo oxidative modifications upon exposure to ROS, and failure to remove oxidatively modified proteins impacts function. This is especially true for mitochondria, the site of ROS generation (380). Increased ROS also causes oxidative stress, which has been demonstrated to be the driving force of deleterious effects, including inflamm-aging, the chronic inflammatory condition accompanying aging in the immune system (66, 205, 410). As ROS serve important effector functions in cellular metabolism and signaling within the immune system ranging from apoptosis to cell proliferation, aberrant modulation appears to impact widely, affecting host defense [reviewed in (283)]. Aging of the immune system appears to accompany imbalance in reactive oxygen intermediates, which may skew lymphocyte repertoire, impact innate immunity, induce chronic inflammation, and be a forerunner of autoimmunity, as described below. Further, pathological mechanisms that accompany imbalance of reactive oxygen intermediates appear to have a larger impact on regulatory mechanisms within networks, more during advancing age than in young cohorts. In innate immune cells, this oxidative stress/imbalance can be observed as altered phagocytic capacity, due to altered intracellular signaling pathways, which impact pathogen killing capacity (111, 248, 340). Additionally, alterations in cell adhesion cascades and defective ROS metabolism appear to affect further the functional capacity of the cell during aging (51). Similarly, deficits attributable to ROS are also implicated in DC maturation, T cell-DC interaction, immunological synapse formation, intracellular signaling in T cells, and functional responses (46, 193, 301). Activation of T cell signaling is dependent on cognate antigen recognition and costimulatory signals, leading up to differentiation into effector and memory cells. Many of the parameters of T cell activation that decline with age are also downregulated significantly by exposure to oxidative stress (126, 127, 188), suggesting that the deleterious effects of these two salient processes on T cell functional responses are indeed comparable (217, 278). In fact, evidence suggests that alterations in intracellular as well as surface thiols may be vital to T cell dysfunction in senescence (47, 131, 195, 303, 403). Considering that T cell activation is exquisitely dependent on the initiation of biochemical events that are uniquely reliant on the mobilization of Ca2+ and phosphorylation of immune-tyrosine activation motifs on the signal transducing module, alteration in the availability of the kinase or phosphatase is critically important.
Oxidative stress, resulting from redox disequilibrium, has been demonstrated to play a significant role in the induction of lowered T cell responsiveness. ROS-mediated response is observed not only in aging but also in chronic pathological conditions (116). This seems to be contradictory in the face of recent reports that clearly outline T cell activation after receptor engagement, to be redox-dependent. In fact, rapid and acute production of ROS appears to be necessary for downstream activation of signals culminating in nuclear translocation of transcription factors and induction of gene expression (92, 403). Thus, regulated and exquisitely balanced generation of ROS is presumed to be vital in discriminating noxious pathological effects from physiological effects, in key cellular processes. Therefore, it seems that the immune system demonstrates very diverse response to ROS, depending on the cellular context, microenvironment, and their exposure to external stimuli. Thus, human T cell functions, like those demonstrated in mice, are equally sensitive to oxidative stress and sublethal levels of hydrogen peroxide (H2O2), which have been shown to be both inhibitory and deleterious (46), further emphasizing the impact of ROS imbalance in pathological outcomes. H2O2 released from monocytes after activation have also been shown to be suppressive in T cells, impacting IL-2 production after mitogenic activation (96, 414). A role for secreted antioxidant glutathione (GSH) and its regulation by Tregs have been implicated in T cell proliferation (411). Further, altered oxidative stress in T cells from acquired immunodeficiency syndrome patients has been shown to impact conformational alteration of p56lck in T cells accounting for lowered tyrosine kinase activity (123). Similarly, alterations in linker for activation of T cells and in TCR ζ chain expression have been demonstrated in pathological conditions such as rheumatoid arthritis and cancer, where a role for oxidative stress has been demonstrated, unequivocally (126, 271, 309).
Oxidative stress resulting from the depletion of intracellular stores of GSH is inhibitory for T cell proliferative response (131, 403), and more recently decreased GSH has been linked to displacement of linker for activation of T cells from membranes, interfering with T cell activation. Besides the impact of redox equilibrium on proximal steps in T cell signaling, redox-sensitive transcription factor NFκB that regulates T cell function appears to be a central mediator of transcription, including the induction of IL-2 and IL-2 receptor (120, 161, 293, 376). Redox conditions strongly influence CD4 T helper cell polarization. As T helper cells can be distinguished by their ability to mediate predominantly inflammatory responses (Th1 type) or anti-inflammatory responses (Th2 type), based on their cytokine generation profile, skewing of cell types to either predominantly Th1 or Th2 type can impact immune outcomes. Differentiation of CD4 T helper cells in the presence of low chronic oxidative stress modulates their cytokine profile and chemokine receptor expression skewing them toward a more Th2 type than Th1 type, similar to that observed during aging (274). In mice with reduced ROS generating capacity, Th1 type responses appear to predominate, whereas in humans, supplementing ROS scavenger vitamin E appears to increase Th1 responses in elderly individuals (407). However, reports of treatment with ROS scavengers, vitamins C and E, in human DC, induced Tregs with a Th2 cytokine profile (367). Clearly, more work is needed to confirm polarization to the Th2 type cytokine profile upon ROS exposure.
C. Inflammaging and the paradox of NFκB signaling in immune senescence
One of the consequences of ROS generation is the futile induction of proinflammatory cytokines such as TNFα, IL-6, and IL-1 and the ensuing chronic inflammation (283, 324), which may lead to autoimmunity. This low-grade chronic inflammation accompanying aging has been attributed to the cells of the innate immune system as well as nonimmune cells. Under physiological conditions, acute inflammation is a highly orchestrated, tissue-specific response to invasion by microbes or injuries. However, uncontrolled inflammation is injurious to the host cells that could result in pathology, including autoimmunity. Therefore, cells are endowed with mechanisms that permit the resolution of such injurious responses. It is now becoming increasingly clear that either such regulatory mechanisms or cross-talk, necessary for the resolution of inflammation, are dysfunctional during aging. While a host of factors such as activated protein-1, hypoxia-inducible factor-1, peroxisome proliferator-activated receptor (PPAR), NFκB, and signaling intermediaries such as p38-MAPK (mitogen activated protein kinase) have been implicated in the cross-talk that results in inflammation, we have largely restricted our discussion to NFκB, as NFκB pathway has been considered to be a prototypical proinflammatory signaling pathway. Additionally, studies identifying gene expression motifs identified NFκB across various organisms and tissue types, as detailed below.
Studies examining the cross-talk between PPAR and its impact on proinflammatory gene induction have been extensively studied in cells such as monocytes and adipocytes. These studies have demonstrated significant cross regulation between oxidative stress, PPARs, and NFκB (54). Given that PPARγ is a negative regulator of macrophage activation and with an observed decrease in the induction of PPARs during aging, it is not surprising that NFκB is dysregulated and may underlie increased inflammatory responses during aging. This, coupled with p38-MAPK-mediated inactivation of tristetraprolin, may also account for increased post-transcriptional induction of proinflammatory cytokines. In a recent meta-analysis of age-related expression profiles of 27 datasets from rats, mice, and humans, de Magalhaes et al. (75) found overexpression of inflammation and immune response genes as the most common age-related genetic signature. An independent study conducted by Adler et al. (2), employing cis-regulatory motif modules for identifying motifs that drive age-dependent gene expression across various tissues and organisms, identified the transcription factor NFκB. This observation is paradoxical, considering that induction of NFκB after antigenic exposure is clearly inhibited during aging. Studies reported from our laboratory have demonstrated that in T cells from the elderly, induction of NFκB is significantly compromised due to a decline in proteasomal proteolysis of the inhibitor IκB (294). Thus, it appears that while inducible NFκB is downregulated, constitutive low-grade activation of NFκB accompanies aging. However, the precise mechanism for the induction of constitutive NFκB induction in the elderly has remained unexplained. While several studies have implicated a role for oxidative stress in the induction of this redox-sensitive transcription factor, studies to date have failed to explain the basis of a robust NFκB induction under conditions of proteasomal functional loss or ablation. As the canonical pathway to NFκB induction involves both the proteasome and robust ubiquitination and deubiquitination activities (393), loss in proteasomal activity in the face of active NFκB induction has remained a paradox. Recent studies from our laboratory have provided evidence for the involvement of an atypical NFκB pathway in inflammatory cytokine induction, when mediated by oxidative stress through a tyrosine kinase dependent pathway (66). These studies, for the first time, outline a role for the inhibitory 26S proteasome in substantially augmenting the generation of proinflammatory cytokine generation, through the absence of negative regulation during aging. A similar impact of proteasomal regulation of NFκB at the transcriptional level has been demonstrated by Natoli's group in a cell culture system (313). Thus, while studies of transcriptional feed forward reactions have been the major focus in several laboratories, negative feedback regulation and regulatory molecules that are being discovered may also impact aging. Thus, under conditions of aging or when proteasomal proteolysis is compromised, induced accumulation of ROS due to proteotoxic stress may mediate the induction of NFκB and NFκB-dependent genes indiscriminately, due to a failure in the negative feedback regulation.
D. Telomere attrition in immune senescence
Telomeres are located at the ends of linear chromosomes, constituted by nucleoproteins consisting of short tandem repeats of hexa-nucleotides (TTAGGG) and associated proteins that protect the ends of chromosomes in eukaryotes. The length of telomeres shortens with each cell division. Studies have demonstrated that telomere length inversely correlates with age and occurs both in in vitro and in vivo aging (14, 134). Telomeric shortening has been demonstrated in numerous cell types, including hematopoietic stem cells, lymphocytes, keratinocytes, epithelial cells, endothelial cells, and human fibroblasts in culture (15). One of the mechanisms that overrides telomeric loss in malignant cells and germ cells has been attributed to telomerase, a ribonuclear enzyme that can generate telomeres based on an RNA template (TERC) and a reverse transcriptase (TERT) (12). While most somatic cells in humans do not express telomerase activity, in immune cells telomerase activity is induced upon activation (37, 399). One of the postulated mechanisms by which genomic instability at telomeres promotes aging is through the continuous activation of DNA damage (68). Mice deficient in TERC have dysfunctional telomeres and show premature aging. This premature aging phenotype is reversed in telomere-deficient mice due to the lack of 5′-3′ exonuclease 1 (29). Thus, telomeres, TERC, and TERT are all interconnected with cellular senescence and aging.
Analysis and comparison of telomerase activity and telomere length in the immune system, that is, human T and B cells, demonstrated that age is unrelated to low telomere length in B cells or CD4+ T cells. However, low telomere length and age is related in CD8+ T cells and PBMC. While B cells demonstrated the highest telomerase activity and longest telomere length, CD8+CD28− had the lowest telomerase activity. CD4+ T cells from the elderly showed higher telomerase activity than CD8+ CD28+ T cells, although their telomere lengths were similar. Low telomere length is now emerging as a biomarker of aging in most tissues. In T lymphocytes, it appears that stress-induced by proliferation may indeed dictate telomere length, as naïve T cells but not antigen-experienced T cells, have longer telomeres (207). Studies of x-linked lymphoproliferative syndrome further corroborate this link between proliferation stress and telomere length with younger individuals with the disease demonstrating shorter telomere lengths than the older normal subjects (288).
B lymphocytes show slower telomere loss than T lymphocytes, with no real difference between naïve and memory B cell subsets (351, 352, 398). Germinal center B cells demonstrate the highest telomerase activity. While the precise molecular mechanisms for the difference in T and B cell telomerase activity and telomere length regulation remain to be fully defined, it is unknown at this time if differential induction and regulation of NFκB in these two cell types may underlie the noted difference in telomeres and telomerase activity. As telomerase induction involves NFκB activation through PKCθ and PI-3kinase signaling pathways (5, 341), and AKT-mediated regulation is required for the phosphorylation of human TERT resulting in its activation, it is conceivable that agents such as ROS that regulate and impact NFκB, AKT, and PKCθ will likely influence telomerase activity and telomere length in cells. In fact, loss of telomerase activity in highly differentiated CD8+CD28− CD27− T cells has been demonstrated to be due to decreased AKT phosphorylation (289).
It is now established that telomere length in human peripheral blood is genetically determined (347), and that age-related erosion is proportional to baseline telomere length (19). However, the question of how original telomere length and life span are related remains unresolved. Studies demonstrate that nongenetic factors, such as oxidative stress or free radical exposure, clearly influence the outcome. ROS influences telomeric ends due to the susceptibility of the GGG site to 8-oxoguanine lesions (268, 284). Additionally, since redox regulates the transcription factor NFκB, ROS may also influence telomerase activity. Thus, ROS may participate in both driving aging and regulating telomere length maintenance mechanisms.
Besides activation mechanisms, it appears that hTERT transcriptional regulation may also account for the decline in telomerase activity, since transduction of hTERT in lymphocytes extends the proliferative potential of replicative senescent cells. However, some studies have demonstrated chromosomal abnormalities in these hTERT-transduced T cells (332). Additionally, long-term hTERT-transduced T cells show accumulation of cyclin-dependent kinase inhibitors such as p21 and p16ink4a. Thus, while interventions to stabilize telomere length may be a useful strategy, given the potential side effect mechanisms other than those employing genetic approaches may prove to be useful. In fact, recent studies by Effros's group demonstrating that pharmacologic enhancement of telomerase activity by TAT2, a small molecule activator obtained from a chemical screen, modestly retards telomere shortening and increases proliferation potential in CD8 T cells appear to be a step in the right direction (94).
E. Accelerated T cell aging due to repeated exposure to antigenic insults
Recent studies have attributed repeated antigenic insults and clonal repertoire skewing that occurs with age, to a preponderance of virally activated T cell clones (260, 396). Chronic activation of T cells due to lifelong viral persistence in immune-competent hosts appears to influence and shape the T cell repertoire (7, 220, 234). Although a variety of persistent infections occur, the most common pathogens often associated with a skewed expansion of T cells in the elderly are CMV, Herpes simplex virus, and Epstein-Barr virus (396). Evidence that chronic CMV infection may accelerate aging of the immune system, allowing subclinical infection to persist, is fast gaining credence in the field of immuno-gerontology. In fact, the similarity in the predominant phenotype of subsets of CD8+ T cells in individuals chronically infected with CMV and elderly subjects has been reported (213). Occurrence of CMV infection and aging share similarities in that both conditions lead to skewing toward CD8+ CD28− phenotype (119, 171). In CMV-infected sero-positive elderly individuals, about 25% of the CD8 population demonstrates specificity to CMV epitopes, and is similar to that seen in CMV-infected young adults. While aging of the immune system may accompany such expansions impacting responses to other commonly encountered antigens, whether such expansions are the sole cause of immune dysfunction observed during aging is hotly debated and warrants more research. If persistent viruses are indeed the driving force in immune senescence, antiviral therapy at an early age against common Herpes viruses may be a successful intervention strategy.
F. Proteostasis and aging in the immune system
Proteostasis or protein homeostasis is essential for the maintenance of cellular homeostasis. Deficiencies in protein homeostasis accompany metabolic, geriatric, cardiovascular, neurodegenerative, and oncological disorders (118). Recent studies reveal that cells possess proteostasis maintenance capacity and that challenges that result in demands exceeding this capacity lead to a functional decline (48). An age-associated breakdown or collapse in proteostasis, concomitant with an increase in protein oxidation, resulting from oxidative stress or other modifications, accounts for the accumulation of misfolded proteins during aging (38, 240). Additionally, alterations in ubiquitin proteasome pathway (UPP), chaperone overload, and aberrant autophagy may impact proteostasis, as depicted in Figure 8. Protein stability and resistance to stress-mediated accumulation of misfolded/aberrant proteins have been attributed to longevity determinants in mole rats (282). Additionally, aberrant protein accumulation, due to impaired proteostasis, may result in pro-inflammatory cytokine generation. As the breakdown in proteostasis impacts cells in both the adaptive and innate arms of immunity affecting the induction and effector arms of immune response to pathogenic insults, understanding proteostasis in the immune system is of import in delineating immune senescence.
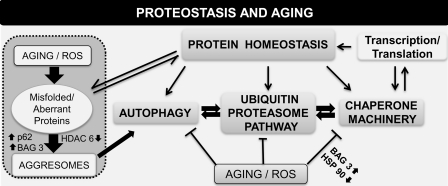
FIG. 8.
Schematic representation of mechanisms reported to modulate proteostasis during aging. Proteostatic mechanisms and the control of protein stability during aging are presented. Factors such as ROS, accumulation of aberrant proteins, failure of the ubiquitin proteasome pathway, chaperone overload, and decline in autophagy have all been reported to contribute to some extent to the decline in protein homeostasis and to overall functional alteration during advanced age. Recent studies highlight the important roles for BAG3, sequestosome, and HDAC6 in regulating aggresome formation. ↓, decreased; ↑, increased; HDAC6, histone deacetylase 6.
The major contributors to altered proteostasis during aging are the malfunction of the UPP, the chaperone machinery, and the modulation of autophagy. The following paragraphs provide a brief review of each of these pathways in immune senescence.
G. UPP in aging and immune senescence
Protein degradation by the UPP is an essential cellular mechanism mediated by the 26S proteasome, whose activity decreases with advancing age in several organisms, including humans (70, 166, 292). The proteasome is a conserved proteolytic machinery with a central 20S proteolytic barrel containing the catalytic sites, the capped 19S regulator, and a 11S activator complex. Proteins targeted to the proteasomes are for the most part tagged with multiple ubiquitin, a 8.5 kDa protein moiety (251). Impaired function of ubiquitin proteasome system (UPS) has been implicated in the late life onset of neurodegenerative diseases and proteotoxicity (69). In addition, age-related decline in the catalytic activity of the proteasome has been observed in tissues of humans and aged flies (110, 290, 294, 388). While the mechanisms for the decline in proteasomal activity are under investigation, a recent study has provided genetic evidence showing that age-related decline in proteasome activity causes accumulation of ubiquitinated proteins (372). These studies also demonstrated that 26S abundance attenuates with age, which is associated with impaired assembly of the 20S proteasome with the 19S regulator. Overexpression of this 19S regulator subunit (Rpn-11) suppressed age-associated reduction in proteolysis, with a resultant decrease in the accumulation of ubiquitinated proteins, and extension of lifespan in flies by 30%. As a corollary, loss of function of Rpn11 caused an early reduction in 26S activity and premature accumulation of ubiquitinated proteins, with shorter (60% decrease) life span. As histone deacetylase 6 (HDAC6) has been demonstrated to play a crucial role in the clearance of ubiquitinated protein deposits, further study of HDAC6 under these conditions of Rpn-11 over- and under-expression is warranted (32, 229).
Interest in the UPP for immunologists has been stimulated by the recognition that besides maintenance of proteostasis, the generation of antigenic peptides, the regulation of key proteins that regulate cell cycle (p27 kip, p21), apoptosis, cell transformation (p53), and gene transcription (NFκB), is also involved in the pathogenesis of inflammation (275) and in immune surveillance for virus and cancer (69, 307). Additionally, when cells are stimulated with pro-inflammatory cytokines, most of the constitutive proteasomes in the 20S catalytic core are replaced by immuno-proteasomes subunits β5i, β2i, and β1i, affecting the peptide production for antigen presentation. Recent reports demonstrate that cortical thymic epithelial cells selectively express a type of proteasome known as the thymoproteasome in place of β5i, called β5t (128, 245, 371). This subunit of the proteasome appears to be necessary for positive selection in the thymus. In addition, studies also appear to show that these specialized proteasomes in the thymus not only shape the T cell repertoire of cytotoxic T cells, but may also influence cytokine production, T cell differentiation, and T cell function.
Although our knowledge of the proteasomal system and regulation of intracellular protein turnover in the immune system, and in particular in T cells, is still emerging, several studies demonstrate that the function of the proteasome significantly declines with advancing age in T cells and subsets of T cells (290, 294). Similar declines in proteasome function have been demonstrated in other cell types such as rat liver hepatocytes, human dermal fibroblasts, skeletal muscles, and central nervous system during aging (43, 110, 154). While previous studies in cells other than T cells indicate that the defects occurring in the aged were related to overall decrease in specific subunit expression, recent studies from our laboratory indicate that a decrease in expression of specific proteasome subunits and alterations in the association of heat shock protein 90 (HSP90) may underlie some of the age-associated decline in activity (unpublished results). Given that immuno-proteasome subunits have been implicated in pro-inflammatory cytokine induction, autoimmunity, and induction of Th17 subsets, it is likely that age-associated alteration in immuno-proteasome will likely impact immune response to pathogens. In fact, studies by Pang et al. have demonstrated that deficiencies in immuno-proteasome subunits affect differentially two immuno-dominant influenza virus-specific CD8+ T cell responses (277). Given that alterations in CD8+ T cells accompany aging, it is likely that modulation of proteasomal subunit expression may well underlie this functional alteration in immune response.
Besides tagging substrates targeted for proteolytic degradation by the proteasome, ubiquitination is an important post-translational modification necessary for mediating protein–protein interaction and in endocytosis (70, 125). Recent studies demonstrate that both ubiquitination and deubiquitination play central roles in signal transduction, leading to the activation of NFκB in the immune system (346). Depending on the type of ubiquitin chain linkage (K63, K48, K11, etc.) and whether the targets are mono- or poly-ubiquitinated, various effector functions of protein substrates have been reported and range from receptor internalization, transcriptional regulation, to DNA repair. By virtue of their role in mediating NFκB induction and their involvement in DNA repair, regulation of ubiquitin levels and ubiquitination of substrates might be linked to the immune aging process. Additionally, since levels of free ubiquitin in the cell are limiting, changes in demands for ubiquitin placed by cells under stress will influence ubiquitin modifications. Studies by Salomons and colleagues have demonstrated that under proteotoxicity, polyubiquitination in the cells increase, impacting histone ubiquitination and altering chromatin remodeling, transcriptional fidelity, and DNA repair (320). Since aging in the immune system is accompanied by a loss in proteasomal proteolysis, accumulation of ubiquitinated proteins is often observed. Impact of such ubiquitinated protein accumulations during aging on histone ubiquitination and/or DNA repair remains to be delineated.
Studies now provide a link between autophagy and ubiquitination by virtue of the demonstration that autophagy receptors such as sequestosome and neighbor of BRCA1 gene 1, bind ubiquitin, and autophagy-specific ubiquitin modifiers (microtubule-associated protein 1 light chain 3/γ-aminobutyric acid type A receptor-associated protein), simultaneously (192). With both proteasome and autophagy mechanisms being involved in aging, it is likely that ubiquitin may take center stage in regulating protein cargo in normal and senescent conditions involving stress. The idea that the ubiquitination pathway is important in longevity and aging is supported by a study demonstrating a role for WW domain containing E3 ubiquitin protein ligase, a HECT E3 ubiquitin ligase, as a positive regulator of life span in C.elegans in response to dietary restriction (44). Thus, studies outlining a role for stress defense in aging bring to the forefront ubiquitination mechanisms that may regulate stress and thus impact longevity.
H. Autophagy, aging, and immune response
The lysosomal system, like the UPP, is an important facet of the cellular quality-control mechanism. These two systems, acting in concert, are responsible for most intracellular protein turnover (57).
Autophagy is a lysosomal-mediated cellular catabolic process that mediates the bulk destruction or turnover of long-lived proteins, organelles, and other components of the cytoplasm to sustain metabolism during starvation and metabolic stress (198, 200). In mammalian cells, three different autophagic pathways have been described, primarily based on the manner in which the intracellular materials targeted for degradation are delivered to the lysosomes. These are macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA) (62, 176, 238). In mammalian cells, microautophagy is the least characterized of the three forms (298).
In macroautophagy, subcellular double-membrane structures called autophagosomes are formed to sequester cytoplasmic constituents that are targeted for degradation and transported to the lysosomes for degradation. This type of autophagy is inducible under stress conditions. In microautophagy, materials targeted for degradation are sequestered and delivered to the lysosomes through the direct invagination of the lysosomal membrane (176). CMA, on the other hand, is highly selective and is restricted to proteins bearing a KFERQ-like sequence motif (81). Specific chaperone proteins, heat shock cognate protein 70, and its cochaperones bind to the penta-peptide motif expressing proteins and are transported to the lysosomal surface, where they bind lysosome-associated membrane protein type 2A (LAMP2A) (64, 139). With the assistance of other lysosomal chaperones, these proteins are individually transported into the lysosome for degradation (64).
Efficiency of protein synthesis and degradation systems determines the quality of cellular housekeeping. The efficiency of functioning of both the proteasomal and autophagic-lysosomal systems are compromised with age (65, 290, 291, 294, 390), leading to less efficient cellular housekeeping and accumulation of waste products. Several reasons have been suggested for the autophagic decline with age. These could be attributed in part to the decreased rate of autophagosome formation and lowered efficiency of fusion between the autophagosome and the lysosome, leading to a failure in the degradation of the cargo targeted to the lysosome [reviewed in (226, 298)]. Additionally, an increase in the concentration of free radicals (ROS) and the lipofuscin pigment within the lysosome with age (35) also leads to a decrease in the lysosomal degradation of proteins.
A decrease in the levels of LAMP2A has been attributed to decreased CMA with age (65, 173, 369). LAMP2A, in addition to facilitating the transfer of proteins across the lysosomal membrane into the lumen for degradation, is also required for the maturation of autophagosomes and their fusion with the lysosomes. Finally, age-associated dysregulation in macroautophagy has also been attributed to changes in hormonal regulation [reviewed in (63)].
Recent studies by Zhang and Cuervo (415) demonstrate that overexpression of LAMP2A in livers of aged mice not only increased CMA but also macroautophagy and the ubiquitin-proteasome system, thus improving overall organ function, underscoring the importance of the cross-talk among all protein quality-control systems (192) in the maintenance of overall cellular homeostasis with age.
Malfunction of autophagy has been shown to result in increased susceptibility to infections and premature aging. Recent studies have demonstrated that autophagy family members are suppressors of innate immunity and that NFκB signaling can inhibit autophagy (244, 317, 385). Absence of autophagy results in ROS-dependent amplification of RIG-I like receptor signaling. Autophagy-related gene (Atg)5−/− cells exhibit enhanced retinoic acid-inducible gene-like receptor signaling, increased IFN secretion, and resistance to infection by vesicular stomatitis virus, all of which have been attributed to ROS-associated dysfunctional mitochondria in Atg5−/− cells (366). Additionally, since significant involvement of autophagy members has been demonstrated in antigen presentation via MHC-II pathway (330), as well as during cross presentation (204), any alteration or malfunction in autophagy is likely to impact adaptive immune function. Evidence from Atg5−/− mice demonstrates that CD4+ and CD8+ T cell development is compromised (295, 296). Further, not only is there a decrease in T cell numbers, but their proliferation is also significantly affected in these mice. These studies underscore the importance of autophagy in T cell development and functional response. Studies also indicate that during starvation, Th2-polarized cells accumulate increased amounts of autophagosomes resulting in death, whereas Th1-polarized CD4 T cells are protected from death (202). As rapamycin treatment induces autophagy, a role for activation of autophagy in life span extension is implicated in recent studies employing rapamycin fed in late life, that is, beginning at 600 days of age in genetically heterogeneous mice (137). While the authors posit that this late life feeding with rapamycin may induce life span extension by a combination of antineoplastic effects and effects on cellular stress resistance and nutrient dynamics, its impact on autophagy or inflammatory cytokine induction was not evaluated. However, since rapamycin is a strong immunosuppressive drug (408) that, on the one hand, inhibits type-1 IFN (41), modulates T cell trafficking (345), and regulates expression of Foxp3 in Tregs (138, 326), but on the other, upregulates and improves the quality of CD8 memory T cell differentiation (13), it is not yet clear how this feeding regimen impacts immune senescence. Given the counter regulatory role of NFκB in inhibiting autophagy, a cross-talk between the inflammatory and autophagy pathway is likely to occur, since inducers of autophagy such as FOXO3a and sirtuin 1 (SIRT1) can inhibit NFκB signaling (28, 315–318) and enhance autophagic degradation. Increased inflammatory responses observed during advanced age and increased inflammatory disease onset and progression during aging may well be an outcome of this cross-talk.
I. Chaperone activity and immune senescence
Molecular chaperones are an evolutionarily conserved class of proteins that guard against protein misfolding and degradation in the cell (250). As sentinels of proteostasis in the cell, they fulfill an essential role during conditions that place severe demands, such as environmental insults (184), when their production is significantly upregulated. These proteins, often referred to as stress or HSPs, are increasingly being identified as key players in aging phenotypes, in a variety of species (22). HSPs are targets of the heat shock factor and their expression and function influences a range of activity in the cells of the immune system, from signaling to antigen presentation (80, 285, 331, 405). Given their broad range of activity, forced expression of HSPs has demonstrated increased life span and decreased proteotoxicity under conditions of chaperone overload in several model organisms (25, 38).
HSPs, as sensors of both extrinsic and intrinsic stress, are clearly essential for stress resistance. With advancing age, as one's ability to combat stress deteriorates, HSPs are significantly affected (240). In fact, lifespan has been positively correlated with stress resistance and thus HSP expression and functional activity (241). In the immune system, molecular chaperones have been reported to participate in signal transduction, antigen generation and presentation, and maturation of APCs. By virtue of their ability to bind both protein and nonprotein molecules, HSPs serve an important role in delivering antigen ligands and TLR ligands in the cell. This property, besides their role in chaperone activity and protein maintenance, accounts for their varied effects on immune function (250). Thus, age-associated decline in chaperone function is likely to impact antigen presentation, signaling, and innate immune responses. Studies carried out employing PBMC have demonstrated that HSP expression after heat shock is significantly decreased during aging (263). Repetitive mild heat shock appears to protect lymphocytes from stress (353). Additionally, by virtue of their central role in chaperoning the 26S proteasome, HSP90 chaperone protein appears to play a vital role in protein degradation in the cell and in the activation of the immune response gene regulator, NFκB (319). Thus, any modulation in the generation of molecular chaperone in response to stress or environmental or cellular insults will likely impact immune outcomes. Additionally, by virtue of their role in antigenic peptide generation and in cross presentation, chaperones also regulate the outcome of immune responses (80, 285, 331, 405). Reports of immune deficits in heat shock factor 1−/− mice, including lower antibody production, decreased IL-6 production, and inefficient cross priming of CD8+ cells, are indicative of the wide reaching effects of a decrease in molecular chaperones with age (196, 250, 417). Given the broad range of substrates regulated by various chaperones, any imbalance in molecular chaperones may have profound long-term consequences, and interventions designed to regulate these imbalances will likely affect diverse cellular activities during aging in the immune system.
J. Epigenetics in immune senescence
Epigenetic alterations such as DNA methylation and histone acetylation appear to accumulate with age and clearly impact immune function often contributing to unwanted side effects such as risks of auto immunity. Recent evidence suggests that epigenetic changes may be critical determinants of organismal and cellular senescence. While studies have demonstrated an accumulation of epigenetic changes with advancing age, total genomic–methylcytosine has been found to decrease in various organisms and appears to be inversely proportional to maximum life span (231, 302, 404). Repetitive element methylation, particularly in Alu sequences, decreases throughout aging. The decrease in global methylation during aging has been attributed to the reduction in DNA (cytosine-5-)-methyltransferase 1 activity with age (45, 214).
As opposed to DNA methylation, information on site-specific histone modification in aging is still emerging. The abundance of trimethylated form of histone H4 (H4K20Me) has been demonstrated to increase in liver and kidneys of aging rats, resulting in transcriptional repression (323). Histone acetylation (H3K9ac) was demonstrated to decrease and H3 ser phosphorylation appeared to increase, in concert with the accumulation of heterochromatin with age (170).
Growing evidence also supports the premise of epigenetic dysregulation as an underlying mechanism for increased autoimmune manifestations, along with a greater incidence of neoplasia in the elderly. Hyper-methylation of the promoter of tumor suppressor gene such as RB, p16, and wnt-associated factors, as well as aberrant DNA (cytosine-5-)-methyltransferase 1 activity, appears to link aging and cancer (95, 211, 306). Recent studies on micro-RNA (miRNA) implicate epigenetic modifications as being central to miRNA-induced lifespan alterations (30, 168). Treatment with HDACs appears to extend life span in several organisms (49, 416). An aging-like phenotype and shortened lifespan has been reported in SIRT6-deficient mice. Additionally, it was demonstrated that SIRT6 repressed NFκB-mediated transcriptional activity, and that the target genes involved were significantly similar to those associated with human aging (169). Taken together, these studies implicate a central role for NFκB transcriptional regulation in human aging and life span potential. Given that NFκB plays a central role in inflammatory cytokine induction in aging, epigenetic mechanisms that regulate NFκB may likely prove to be important targets not only in controlling inflammation, but also for devising interventional strategies to ameliorate immune dysregulation.
K. DNA damage and repair, genomic instability, and immune aging
Despite continuous surveillance and repair, a wide range of DNA damage accumulates with age, leading to genomic instability and contributing to the dysregulated phenotype that accompanies aging (115, 212, 389). The combined effects of alterations due to DNA damage affecting the sequence per se and the accompanying epigenetic changes in the chromatin structure clearly impact cellular function and may underlie age-associated functional declines (344). Studies of human progeroid syndromes and mouse models that have defects in DNA repair pathways have provided insight into understanding the role of DNA damage pathways (74, 91, 210, 281, 368). Mice engineered for defects in DNA damage response (DDR) genes demonstrate premature aging phenotypes comparable to that observed in aging wild-type litter mates (257, 336). As stress resistance and longevity are intimately linked, mechanisms to counter DDRs may well provide opportunities for intervention. As DNA damage repair is involved in genomic rearrangements during the development of the immune system, any defect in DDR would result in immunodeficiency (23, 329). For example, mouse model systems of the progeroid syndromes, Nijmegen breakage syndrome (NBS1) animals, demonstrate immunodeficiency and increased risk for cancer onset with age (159, 258). Cells from these NBS1 mice manifest defects in double-strand break repair and activate a major DNA damage checkpoint pathway kinase. Additionally, patients with NBS are prone to, sometimes fatal, infections due to defects in class switch recombination and impaired immunity. Thus, DDR defects impact immune function adversely (310). Additionally, many cancers arising in progeroid conditions have been demonstrated to be lymphomas or leukemias of B and T cell origin, which can result from impaired V(D)J recombination (159). Thus, defects in DDR may be a driving force in immune senescence during aging. Understanding details of how DDR proteins are regulated in the immune system will provide us with avenues for channeling DDR functions for delaying and managing functional impairment of the immune system during aging.
L. miRNAs in immune senescence
Noncoding miRNAs constitute an important class of regulators of cellular processes through their ability to regulate transcription and translation. Emerging studies in this nascent field show the importance of miRNA in fine-tuning immune responses. It is estimated that possibly more than 1000 miRNA exist in humans. miRNAs present a unique mechanism that can regulate diverse functions by impinging on multiple pathways simultaneously (114, 409). Recent studies suggest that miRNA may play a vital role in a variety of stress responses, making them a target during advancing age. In terms of immune responses, evidence for the role of miRNAs in cell-mediated immunity is depicted by T cell deficiency in conditional Dicer−/− mice (243). Expression of several miRNAs has also been demonstrated to be regulated by IFNα and IFNβ (265). Further, studies also demonstrate that some miRNA, such as mi146a/b, may be regulated by NFκB (67, 365). Given the central role of NFκB in immune dysfunction accompanying aging, it is envisioned that miRNA may well underlie immunomodulation in senescence. In fact, recent studies on pathway analysis of senescence associated miRNA targets revealed common processes to different senescence induction mechanisms (190). In the context of immune senescence, recent reports of miRNA-mediated regulation of the generation of memory cells, inflammatory gene induction, chromatin remodeling, and MHC expression (146, 377) implicate a role for miRNA in regulating immune decline in aging. Additionally, miR 17–92 cluster has recently been implicated during aging in various human cell types, including CD8+ T cell subsets (130). Future innovative and comprehensive studies in this field will help delineate specific miRNA in regulating pathways to senescence in the immune system.
VI. Interventional Strategies for Reversal of Immune Dysfunction Accompanying Advancing Age
Strategies that retard or reverse age associated dysfunction of the immune system will likely improve the health-span of individuals, with improved response to vaccination, decreased susceptibility to infectious diseases, and decreased onset and progression of autoimmunity. Some of the true and tested modulators of aging such as CR, antioxidant therapy, nutraceutical intervention, induction of telomerase activity, and cytokine and hormone administration have been attempted in retarding immune dysfunction with moderate success. Figure 9 outlines some of the interventions attempted and their effects on retarding immune dysregulation. Readers are directed to some recent reviews on intervention studies in overall aging and lifespan extension. A list of interventional strategies ranging from antioxidant administration to CR that have been attempted to modulate life span in various organisms and in human cells, ex vivo, and their ability to extend life span is provided in Table 1 (18, 33, 88, 137, 148, 149, 167, 172, 183, 209, 227, 321, 387, 392, 395). A few of these interventions, in the context of immune aging are discussed below.
FIG. 9.
Mechanisms contributing to immune dysregulation during aging and some intervention strategies reported to override age-associated immune dysfunction. The underlying causes, mechanisms, and functional impact of age-related immune dysregulation and potential interventional strategies reported to override specific age-related immune dysfunction are outlined.
Table 1.
Some Reported Interventional Strategies Aimed at Increasing Life Span
| Factor | Organism/cell origin | Life span extension (yes/no) | References |
|---|---|---|---|
| Exercise | Mus musculus | No | 321 |
| Rattus norvegicus | Yes | 148 | |
| Heat shock | Caenorhabditis elegans | Yes | 209 |
| Drosophila melanogaster | Yes | 172 | |
| Mild repeated heat shock | Human cells | No | 387 |
| Vitamin C | Human cells | Yes | 167 |
| Tea extract | D. melanogaster | Yes | 227 |
| N-acetyl cysteine | D. melanogaster | Yes | 33 |
| N-t-butyl hydroxylamine | Human cells | Yes | 18 |
| Resveratrol | Saccharomyces cereviseae | Yes | 149 |
| Rapamycin | Outbred mice | Yes | 137 |
| Spermidine | Human peripheral blood mononuclear cell | Yes | 88 |
| Caloric restriction | M. musculus | Yes | 183, 395 |
| Homo sapiens | Not assessed | 392 |
A. CR and its impact on immune aging
CR, which represents decreased dietary calorie intake at a maximum range of 30%–50%, has been shown to increase longevity in a multitude of organisms ranging from C. elegans to nonhuman primates and humans (355). However, the basis for this increase in longevity is a subject of intense research. For overall response to CR during aging, readers are referred to recent reviews in the field. We have restricted our survey to literature in the context of immune senescence, which is not very extensive. While studies in nonhuman primates have demonstrated a decline in the loss of naïve T cells with age upon CR, with a concomitant preservation of repertoire diversity, antigen presentation, and decreased proinflammatory cytokine production, they failed to evaluate its impact on the susceptibility to diseases or onset of autoimmunity (233). Additionally, recent studies in old rhesus monkeys demonstrated that when CR was initiated late in life, it produced lymphopenia, thus having an adverse effect (261). Therefore, while CR appears to retard the loss of naïve T cells in the aged, depending on the time of CR initiation, the proof of concept for retarding immune impairment, that is, improved efficacy to vaccination and capability to fight and fend off attack by pathogenic organisms, is still far from being established. Also needed are studies that dissect the underlying molecular basis for the retardation and improvement in immune function. Recent experiments by Yang et al. (412) have demonstrated that CR in aged mice either decreased or inhibited thymic adipogenesis, and this was postulated to be directly responsible for retarding thymic involution and enhancing naïve T cell output in murine models. Studies by Ritz and Gardner (305) called into question the extent of CR and its effect on energy restriction, since it resulted in increased mortality. These studies demonstrated that although CR, without malnutrition-restricted energy, was previously demonstrated to improve T cell functional correlates, exposure to influenza virus infection under these conditions resulted in increased mortality. This energy restriction during acute stress appeared to impact NK cell function and also increased the severity of the influenza infection. Thus, although it appears that CR is an effective antiaging strategy in several animal models and does demonstrate some beneficial effects on immune response, questions still linger with regard to its utility. Clinical studies have demonstrated that CR leads to attenuation of inflammatory responses (147, 239). More recent data implicate a role for CR on NFκB signaling pathways (56, 165). Thus, while it appears that CR positively impacts on stress response pathways in the aged, including pro-inflammatory cytokine generation, it is not clear if all the benefits of CR are due to a reversal in stress response pathway (355). Mechanisms underlying CR on mammalian immune senescence are clearly multifaceted, involving both cell autonomous as well as cell-dependent effects. In fact, CR has been demonstrated to exhibit protective action against cellular damage, while boosting antioxidative mechanisms (55). However, their precise mechanisms of action await further investigation. Attenuation of ROS production, inhibition of NFκB activity, and downregulation of inflammatory genes have all been demonstrated following CR, with SIRT1 being implicated as a mediator of these CR effects (61). As CR positively impacts SIRT1 in life span studies (31), the potential link between NFκB and SIRT1 may offer a more mechanistic insight into the role of pro-inflammatory cytokines in aging (317, 413). Clearly, a more thorough and controlled evaluation of the risk–benefit ratio is needed to determine if CR will be a mode of successful intervention for human health-span studies, in the context of the rejuvenation of the immune system. Additionally, given the length and degree of CR necessary to improve immune function, whether CR will emerge as an effective strategy in humans to preserve immune function to at least a modest degree necessary to fend off infections is still debatable.
B. Cytokines, growth factors, and hormones as interventions in immune senescence
As thymic involution is a well-established prelude to declining T cell numbers and functional activity in the elderly, rejuvenation of the thymus to a fully functional organ will likely override the loss in T cell emigration to the periphery. Some of the potential strategies would be to exploit the rapidly developing knowledge of stem cell biology and the aging niche, to introduce thymic progenitors for repopulating the thymus in the elderly. Growth factors such as keratinocyte growth factor (KGF) have been demonstrated to increase thymopoiesis in old mice and improve T cell-dependent antibody response (235, 311). As KGF receptor is present on thymic epithelial cells that secrete IL-7, treatment with KGF has been shown to augment IL-7 production within the thymus in these studies. In mice, KGF protein administration appears to impact thymopoiesis after bone marrow transplantation (8, 162). Recent studies indicate that treatment with KGF is associated with a bimodal increase in Tregs due to its effect on Treg proliferation and due to an impact on increased numbers exiting the thymus (34). In an elegant study conducted by Seggewiss et al. (337), beneficial impact of KGF on thymic restoration was demonstrated in a primate model. As some studies clearly pointed to a decline in intrathymic decline in IL-7 during aging, intervention studies with IL-7 were attempted. These studies provided mixed results, with some showing no effect, while others demonstrating that administration of IL-7 in old mice reversed thymic atrophy, resulting in increased thymic output and improved T cell responses (11). Recent studies using nonhuman primate models are not as equivocal, since they demonstrate a positive impact on thymic output after IL-7 adminstration (16). In a clinical trial, administration of IL-7 led to an expansion in CD4 and CD8 subset of T cells, but a contraction in CD4+Tregs (121). Studies with old rats showed that treatment with growth hormone resulted in thymic regeneration and reconstitution of hematopoetic cells in the bone marrow (109, 259). In addition, a clinical trial showed that growth hormone increased the size of thymus and stimulated robust peripheral immune responses in humans (249). Thus, strategies employed to enhance thymic output have proven to be useful in overriding thymic decline in aging.
Ghrelin is a polypeptide hormone that has been demonstrated to have a positive effect on immune restoration. Studies demonstrate the Ghrelin promotes secretion of growth hormone and can block the NFκB pathway, inducing anti-inflammatory effects and a decline in Th1 and Th17 cytokine secretions (83–85, 178, 348). Evidence that Ghrelin-deficient mice show thymic atrophy and peripheral proinflammatory cytokine induction and that exogenous administration of Ghrelin significantly improves thymopoiesis, thymic emigration of T cells to the periphery, and peripheral T cell numbers indicates a positive role for Ghrelin in immune restoration during aging (85).
Hormones such as melatonin and insulin-like growth factor 1 have been employed to rejuvenate immune system with mixed results. Other hormones with strong immunomodulatory effects are estrogen and insulin that essentially affect cell signaling. Dehydroepiandrosterone (DHEA) has also been used in immune restoration during aging in murine models. Studies involving humans have not yielded consistent results to date. Vitamin D administration has been demonstrated to improve immune function through its effect on APCs and in the regulation of APC–T cell interactions (144). Vitamin D deficiency has been recognized as a common correlate of aging in humans and has been attributed to underlie chronic inflammation in the elderly (152). Recent recommendations of Vitamin D supplementation may shed light on its role in immune reversal in the elderly in the not too distant future.
C. Immunotherapy as a potential intervention mechanism
As T cell proliferation and expansion significantly declines with age, there has been an effort underway to propagate autologous activated T cells in vitro, followed by suggested infusion in the elderly. While such a restorative therapy may be useful in the short-term, its long-term viability, however, needs to be validated. Alternatively, as chronic infection has been demonstrated to account for clonal expansion in the elderly, preventive strategies, such as vaccination, to decrease chronic infections with persistent viruses such as CMV may be an alternate mechanism (113).
D. Induction of proteome maintenance and antioxidant response genes as an intervention for ameliorating immune senescence
Nrf1, 2, and 3 are members of a subgroup of Cap ’n’ collar (Cnc) family of transcription factors that mediate adaptive responses to cellular stress. Nrf2 is the most studied of the family of Nrf proteins and coordinates the transcriptional response of cells to oxidative stressors and electrophiles. Several recent studies have implicated Nrf2 in defense against aging-associated disorders such as neurodegeneration, cancer, and oxidative stress (1, 164).
Homozygous disruption of Nrf2 in mice was demonstrated to abolish the induction of GSH-S-transferase and NAD(P)H dehydrogenase, quinone 1, demonstrating a central role in antioxidant induction and chemoprevention. Since the first observation, several recent studies have now established the increased susceptibility of Nrf2−/− mice to toxicity, DNA adduct formation, and cancer development to chemically induced carcinogenesis, further underscoring the contribution of Nrf2 in detoxification, chemoprevention, and augmentation of cellular antioxidant capacity (253).
The Nrf2-ARE signaling pathway regulates expression of genes involved in the detoxification and augmentation of cellular antioxidant capacity (253). Nrf2 controls both the inducible and constitutive gene expression of proteins mediated by the ARE (255, 256, 386). Alteration of the cellular redox status due to persistent or elevated ROS and/or a reduced antioxidant capacity are important triggers for the transcription of ARE-mediated genes (312). Nrf2 regulates the induction of about 200 ARE genes, including γ-glutamyl cysteine synthethase, heme oxygenase 1, NAD(P)H dehydrogenase, quinone 1, several proteasomal subunits, and phase-II enzymes (242, 269). Activity of Nrf2 is regulated by its interaction with its partner Kelch-like ECH-associated protein 1 (KEAP1). Nrf2 is highly unstable, with a half-life of about 15 min in unstressed cells, and being subjected to proteolysis by the UPS (254, 359). KEAP1 targets Nrf2 for degradation by promoting Nrf2 ubiquitination (253). While Nrf2−/− mice develop normally, they are highly susceptible to ROS and die prematurely (299, 300). Studies have implicated Nrf2 in the pathogenesis of multiple sclerosis (151). In a recent study in rats, it was demonstrated that agents that induce Nrf2 appear to induce GSH in aged animals (361). Increased Nrf2-ARE induction has also been suggested to contribute to the benefits of CR on cancer chemo-protection, but not life span extension (280). Although, C. elegans lacks Nrf2 regulator KEAP1, SKN-1 (the Nrf2 homolog in C. elegans) is necessary for diet restriction-induced longevity. Similarly, overexpression of SKN-1 was reported to increase life span through the disruption of insulin like signaling pathway (379). While these studies set the stage for identifying a role for Nrf2 in life span extension, absence of KEAP1 homolog makes the interpretation and extrapolation of these findings to mammals rather complex. A recent study in Drosophila showed that knock down of the Nrf2 regulator KEAP1 (KEAP1−/−) led to an increase in life span in males (364). In another study in mice, it was shown that knock-out of Nrf2 (Nrf2−/−) made the animals highly susceptible to oxidative stress and decreased their life span and demonstrated an autoimmune phenotype (203). KEAP1−/− mice, on the contrary, die within 3 weeks postnatal due to esophagal hyperkeratosis resulting from elevated keratin expression (391). Using skin-derived fibroblasts from Snell Dwarf long-lived mutant mice, a recent study demonstrated elevated Nrf2 levels with enhanced GSH levels and resistance to plasma membrane lipid peroxidation (199). Thus, it appears that Nrf2, an electrophile sensor, regulates antioxidant response and may underlie increased stress resistance in long-lived mutants.
To analyze the regulatory role of Nrf2 in immune senescence, studies have demonstrated that inducers of Nrf2, such as sulforaphane (SFN), are potent inducers of antigen presenting activities in old mice, restoring Th1 immunity (174). The authors in this study demonstrated that dendritic cells from old mice have lower thiol levels. SFN treatment of these cells resulted in an increase in thiol levels and restored antigen presentation. This restoration in functional activity was abrogated in cells from Nrf2−/− mice in an adoptive transfer experiment. The reversal of functional response by SFN suggests that, despite a decline in Nrf2 levels in the aged, exogenous activators of Nrf2 can be exploited to modulate antioxidant responses. Experiments from our laboratory have demonstrated that agents such as 1,2-dithiol-3-thione (D3T) and Oltipraz (Nrf2 activators) demonstrate beneficial effects on proteasomal functional activity (unpublished results) in T cells obtained from elderly human donors. As Kwak and his colleagues have demonstrated significant induction of proteasome subunits after activation of Nrf2-ARE pathway, we believe that inducers of Nrf2 may exert their beneficial effects by regulating the UPS (187). Since aging is accompanied by decline in UPS and alterations in UPS accompany immune senescence, it is possible that inducers of Nrf2 such as D3T (Fig. 10), oltipraz, and SFN (Fig. 10) (375) may be beneficial in restoring proteostasis, in addition to antioxidant signaling in aged cells within the immune system. Agents such as D3T and SFN perform their function by transcriptional induction of phase-2 detoxification enzymes, such as GSH-S-transferases, quinone reductase, and epoxide hydrolases, through the transcriptional activation of an enhancer element involving Nrf2. A common chemical property of all the compounds, including D3T and SFN, is the ability to react either covalently or through redox reactions with thiols in the cell. Targeted disruption of Nrf2 gene leads to lower constitutive expression of phase 2 enzyme genes and a significant loss of dithiolethione-mediated inducibility. The precise chemical basis by which D3T stimulates phase 2 enzyme induction is still unclear; however, studies appear to indicate the role of thiols in initiating this activity. Additional studies have attributed the generation of ROS, after interactions with the thiols in the cellular milieu, as an ARE induction event.
FIG. 10.
Chemical structure of Nrf2 inducers SFN and D3T. D3T and SFN are potent inducers of Nrf2, which through the activation of antioxidant response element leads to the induction of antioxidant genes. By virtue of their ability to bind cellular thiols, D3T and SFN act as antioxidant inducers of Nrf2, both in vivo and ex vivo. It has been proposed that the sulfur motifs of D3T and SFN interact with the sulfhydryls of the cysteine residues on Keap1, leading to its dissociation from Nrf2. This results in the stabilization and nuclear translocation of Nrf2, culminating in the induction of phase II enzyme genes. D3T, 1,2-dithiol-3-thione; SFN, sulforaphane; Nrf2, nuclear factor erythroid-2-related factor 2.
The precise mechanism for the upregulation and improvement in proteolytic activity after D3T treatment is still an active area of investigation in our laboratory. A schematic representation of potential targets affected by Nrf2 modulators in mediating their effects during immune senescence is depicted in Figure 11, and appears to involve activation of not only phase II enzymes but also proteome maintenance genes, thus overriding defects in proteostasis.
FIG. 11.
Nrf2 modulators and their mode of action in overriding immune dysfunction during aging. Nrf2 modulators such as D3T and SFN inhibit ROS generation and persistence by inducing phase II enzymes, proteome maintenance, and antioxidant genes through the antioxidant response element. Induction of these genes and enzymes aids in overriding defects due to aberrant accumulation of misfolded proteins, decreased proteasomal proteolytic activity, and increased pro-inflammatory activity, thus contributing to the reversal of immune senescence. ↓, decreased.
E. Induction of UPS as a mechanism for intervention in immune senescence
Aging in the immune system is accompanied by significant declines in proteasome function and assembly. In fact, ROS, which increases significantly in T cells from the elderly, may have a direct role in defective proteasome function (71). Given that several organisms demonstrate a decline in UPS with advancing age, overexpression of immunoproteasome subunits has resulted in the extension of cellular lifespan by about 20% (53). Dietary restriction, in extending life span, has been demonstrated to negatively impact the accumulation of ubiquitinated proteins in various organs, and in naked mole rats that are relatively long-lived, proteasomal proteolytic activity is preserved well into later stages in life (282). Thus, it appears that mechanisms that preserve or positively upregulate proteasomal proteolytic activity without any adverse side effects on protein homeostasis may be a successful strategy for intervention in aging. Given the role of proteasome in the generation of antigenic peptides, thymic-positive selection and NFκB induction in signaling, it is clear that mechanisms that reverse or retard proteasomal deterioration will be effective in rejuvenating immunity. Overexpression of catalytic subunits of the proteasome has demonstrated not only an effective improvement in proteolysis in the cells, but also confer resistance to oxidant-induced damage (186). Further, recent studies have shown that restoration of normal levels of proteasomal subunits and activity are responsible in the reduction of aging biomarkers in a cellular model of senescence (155). Kwak and colleagues have demonstrated that cancer chemopreventatives, such as oltipraz and D3T, are potent inducers of proteasome genes (187). In fact, promoters of proteasome subunits have AREs, indicating that they respond to ARE activators. Recent studies employing Nrf2 activators have successfully demonstrated increase in lifespan in flies and snell dwarf mutant mice (199, 364). However, these studies did not analyze the nature of proteasome reversal or the impact of Nrf2 on immuno-proteasome distribution and function. Studies initiated in our laboratory with Nrf2 activators implicate a positive role for these activators in T cells from the elderly. Further studies will be required to confirm these findings and in establishing Nrf2 activators as potent inducers of immune activation in the elderly.
VII. Conclusions and Future Perspectives
A number of important activators and signaling pathways that mediate immune senescence are now well defined. Nevertheless, newer signaling modules and pathways within the realm of miRNA, transcriptional regulation, chromatin remodeling, autophagy, Ubiquitin-Proteasomal system, etc., continue to emerge. Lately, the role of the aging-milieu in directly impacting immune cells, leading to loss of function, is also garnering more attention. However, questions still linger as to whether the observed decline in immune function is a cause or a consequence of cross-talk among various systems within the aging organism. For instance, is the pro-inflammatory phenotype accompanying aging a response to perpetual noxious stimuli or is it a result of cues gone awry?
Finding commonality between genes responsible for altering the aging milieu and those responsible for aging within the immune system may be important in addressing the complex process of aging of the immune system (immune senescence). Since genes that regulate longevity also modulate the ability to combat stress, it is believed that understanding the role of these genes in the immune system may shed new light on immune decline during aging, leading to the development of effective restorative strategies.
A consistent change across species implicates aberrant redox regulation as an important player in aging. Interventions designed to counter age-associated dysregulation in redox status have begun to show promise in improving health span in model systems.
A systematic review of cell autonomous mechanisms and those of the aging immune niche will help identify and delineate pathways that are modulated during aging. These pathways may well be suitable targets for manipulation in the immune system to either slow or reverse the deleterious effects of aging.
Overall, there still remain some of the important questions to be addressed: (a) What are the natural stressors that accelerate immune defects observed during aging and what are the molecular mechanisms and signatures that signal these changes? (b) What is the relationship between immune cell aging and immune-niche aging? (c) What is the relevance of animal models to the aging of the immune system in humans? (d) Can interventional strategies directed at reversing immune deficits also impact the niche alongside cells?
It is hoped that questions such as these will merit in depth analyses in the future.
Abbreviations Used
- AKT
serine/threonine protein kinase/PKB
- APC
antigen presenting cell
- ARE
Antioxidant response element
- Atg
autophagy related gene
- CMA
chaperone-mediated autophagy
- CMV
cytomegalovirus
- CR
caloric restriction
- D3T
1,2-dithiol-3-thione
- DC
dendritic cell
- DDR
DNA damage response
- FMLP
formyl peptide receptor 1
- Foxp3
Forkhead box p3
- G-CSF
granulocyte colony stimulating factor
- GM-CSF
granulocyte–macrophage CSF
- GSH
glutathione
- HDAC
histone deacetylase
- HSPs
heat shock proteins
- hTERT
human telomerase reverse transcriptase
- IFN
interferon
- IL
interleukin
- KEAP1
Kelch-like ECH-associated protein 1
- KGF
keratinocyte growth factor
- LAMP2A
lysosome-associated membrane protein type 2A
- LIF
leukemia inhibitory factor
- LPS
lipopolysaccharide
- M-CSF
macrophage CSF
- mDC
myeloid DC
- MHC
major histocompatibility class
- MIP
macrophage inflammatory protein
- miRNA
micro-RNA
- NFκB
nuclear factor kappa B
- NK
natural killer
- Nrf2
nuclear factor erythroid-2-related factor 2
- PBMC
peripheral blood mononuclear cell
- pDC
plasmacytoid DC
- PPAR
peroxisome proliferator-activated receptor
- RANTES
regulated upon activation normal T cell expressed and secreted
- ROS
reactive oxygen species
- SFN
sulforaphane
- SIRT1
sirtuin1
- TCR
T cell receptor
- TERC
telomerase RNA component
- TERT
telomerase reverse transcriptase
- Th
T helper type
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- Tregs
regulatory T cells
- UPP
ubiquitin proteasome pathway
- UPS
ubiquitin proteasome system
Footnotes
Reviewing Editors: Rommy von Bernhardi, Manuel Collado, Monica de la Fuente, Michele Goodhardt, Beatrix Grubeck-Loebenstein, Anonymous, and Michael Toledano
Acknowledgments
This study was supported by NIH Grants AG13081, AG030599, and AG025220.
References
- 1.Acharya A. Das I. Chandhok D. Saha T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler AS. Sinha S. Kawahara TL. Zhang JY. Segal E. Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adolfsson O. Huber BT. Meydani SN. Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J Immunol. 2001;167:3809–3817. doi: 10.4049/jimmunol.167.7.3809. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A. Agrawal S. Cao JN. Su H. Osann K. Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama M. Yamada O. Hideshima T. Yanagisawa T. Yokoi K. Fujisawa K. Eto Y. Yamada H. Anderson KC. TNFalpha induces rapid activation and nuclear translocation of telomerase in human lymphocytes. Biochem Biophys Res Commun. 2004;316:528–532. doi: 10.1016/j.bbrc.2004.02.080. [DOI] [PubMed] [Google Scholar]
- 6.Alberti S. Cevenini E. Ostan R. Capri M. Salvioli S. Bucci L. Ginaldi L. De MM. Franceschi C. Monti D. Age-dependent modifications of type 1 and type 2 cytokines within virgin and memory CD4+ T cells in humans. Mech Ageing Dev. 2006;127:560–566. doi: 10.1016/j.mad.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Almanzar G. Schwaiger S. Jenewein B. Keller M. Herndler-Brandstetter D. Wurzner R. Schonitzer D. Grubeck-Loebenstein B. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alpdogan O. Hubbard VM. Smith OM. Patel N. Lu S. Goldberg GL. Gray DH. Feinman J. Kochman AA. Eng JM. Suh D. Muriglan SJ. Boyd RL. van den Brink MR. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez E. Ruiz-Gutierrez V. Sobrino F. Santa-Maria C. Age-related changes in membrane lipid composition, fluidity and respiratory burst in rat peritoneal neutrophils. Clin Exp Immunol. 2001;124:95–102. doi: 10.1046/j.1365-2249.2001.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alves NL. Huntington ND. Rodewald HR. Di Santo JP. Thymic epithelial cells: the multi-tasking framework of the T cell “cradle.”. Trends Immunol. 2009;30:468–474. doi: 10.1016/j.it.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Andrew D. Aspinall R. Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- 12.Andrews NP. Fujii H. Goronzy JJ. Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki K. Turner AP. Shaffer VO. Gangappa S. Keller SA. Bachmann MF. Larsen CP. Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armanios M. Alder JK. Parry EM. Karim B. Strong MA. Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aspinall R. Pido-Lopez J. Imami N. Henson SM. Ngom PT. Morre M. Niphuis H. Remarque E. Rosenwirth B. Heeney JL. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007;10:5–17. doi: 10.1089/rej.2006.9098. [DOI] [PubMed] [Google Scholar]
- 17.Aspinall R. Pitts D. Lapenna A. Mitchell W. Immunity in the elderly: the role of the thymus. J Comp Pathol. 2010;142:S111–S115. doi: 10.1016/j.jcpa.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Atamna H. Paler-Martinez A. Ames BN. N-t-butyl hydroxylamine, a hydrolysis product of alpha-phenyl-N-t-butyl nitrone, is more potent in delaying senescence in human lung fibroblasts. J Biol Chem. 2000;275:6741–6748. doi: 10.1074/jbc.275.10.6741. [DOI] [PubMed] [Google Scholar]
- 19.Aviv A. Chen W. Gardner JP. Kimura M. Brimacombe M. Cao X. Srinivasan SR. Berenson GS. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aw D. Silva AB. Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aw D. Silva AB. Palmer DB. The effect of age on the phenotype and function of developing thymocytes. J Comp Pathol. 2010;142:S45–S59. doi: 10.1016/j.jcpa.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Balch WE. Morimoto RI. Dillin A. Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 23.Bassing CH. Alt FW. The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst) 2004;3:781–796. doi: 10.1016/j.dnarep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Bauer S. Muller T. Hamm S. Pattern recognition by toll-like receptors. Adv Exp Med Biol. 2009;653:15–34. doi: 10.1007/978-1-4419-0901-5_2. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Zvi A. Miller EA. Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhushan M. Cumberbatch M. Dearman RJ. Andrew SM. Kimber I. Griffiths CE. Tumour necrosis factor-alpha-induced migration of human Langerhans cells: the influence of ageing. Br J Dermatol. 2002;146:32–40. doi: 10.1046/j.1365-2133.2002.04549.x. [DOI] [PubMed] [Google Scholar]
- 27.Biasi D. Carletto A. Dell'Agnola C. Caramaschi P. Montesanti F. Zavateri G. Zeminian S. Bellavite P. Bambara LM. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation. 1996;20:673–681. doi: 10.1007/BF01488803. [DOI] [PubMed] [Google Scholar]
- 28.Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- 29.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 30.Boehm M. Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- 31.Bordone L. Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 32.Boyault C. Zhang Y. Fritah S. Caron C. Gilquin B. Kwon SH. Garrido C. Yao TP. Vourc'h C. Matthias P. Khochbin S. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21:2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brack C. Bechter-Thuring E. Labuhn M. N-acetylcysteine slows down ageing and increases the life span of Drosophila melanogaster. Cell Mol Life Sci. 1997;53:960–966. doi: 10.1007/PL00013199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruinsma M. van Soest PL. Leenen PJ. Lambrecht BN. Cupedo T. Lowenberg B. Cornelissen JJ. Braakman E. Keratinocyte growth factor induces expansion of murine peripheral CD4+Foxp3+ regulatory T cells and increases their thymic output. J Immunol. 2007;179:7424–7430. doi: 10.4049/jimmunol.179.11.7424. [DOI] [PubMed] [Google Scholar]
- 35.Brunk UT. Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 36.Bruunsgaard H. ndersen-Ranberg K. Hjelmborg JB. Pedersen BK. Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 37.Buchkovich KJ. Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calderwood SK. Murshid A. Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging—a mini-review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancro MP. Hao Y. Scholz JL. Riley RL. Frasca D. Dunn-Walters DK. Blomberg BB. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30:313–318. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao W. Manicassamy S. Tang H. Kasturi SP. Pirani A. Murthy N. Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlin LM. Yanagi K. Verhoef A. Nolte-'t Hoen EN. Yates J. Gardner L. Lamb J. Lombardi G. Dallman MJ. Davis DM. Secretion of IFN-gamma and not IL-2 by anergic human T cells correlates with assembly of an immature immune synapse. Blood. 2005;106:3874–3879. doi: 10.1182/blood-2005-03-0996. [DOI] [PubMed] [Google Scholar]
- 43.Carney JM. Starke-Reed PE. Oliver CN. Landum RW. Cheng MS. Wu JF. Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrano AC. Liu Z. Dillin A. Hunter T. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature. 2009;460:396–399. doi: 10.1038/nature08130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casillas MA., Jr. Lopatina N. Andrews LG. Tollefsbol TO. Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Mol Cell Biochem. 2003;252:33–43. doi: 10.1023/a:1025548623524. [DOI] [PubMed] [Google Scholar]
- 46.Cemerski S. Cantagrel A. van Meerwijk JP. Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem. 2002;277:19585–19593. doi: 10.1074/jbc.M111451200. [DOI] [PubMed] [Google Scholar]
- 47.Cemerski S. van Meerwijk JP. Romagnoli P. Oxidative-stress-induced T lymphocyte hyporesponsiveness is caused by structural modification rather than proteasomal degradation of crucial TCR signaling molecules. Eur J Immunol. 2003;33:2178–2185. doi: 10.1002/eji.200323898. [DOI] [PubMed] [Google Scholar]
- 48.Cenci S. Pengo N. Sitia R. Proteotoxic stress and cell lifespan control. Mol Cells. 2008;26:323–328. [PubMed] [Google Scholar]
- 49.Chang KT. Min KT. Regulation of lifespan by histone deacetylase. Ageing Res Rev. 2002;1:313–326. doi: 10.1016/s1568-1637(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 50.Chatta GS. Andrews RG. Rodger E. Schrag M. Hammond WP. Dale DC. Hematopoietic progenitors and aging: alterations in granulocytic precursors and responsiveness to recombinant human G-CSF, GM-CSF, and IL-3. J Gerontol. 1993;48:M207–M212. doi: 10.1093/geronj/48.5.m207. [DOI] [PubMed] [Google Scholar]
- 51.Chaves MM. Costa DC. Pereira CC. Andrade TR. Horta BC. Nogueira-Machado JA. Role of inositol 1,4,5-triphosphate and p38 mitogen-activated protein kinase in reactive oxygen species generation by granulocytes in a cyclic AMP-dependent manner: an age-related phenomenon. Gerontology. 2007;53:228–233. doi: 10.1159/000100960. [DOI] [PubMed] [Google Scholar]
- 52.Chelvarajan RL. Liu Y. Popa D. Getchell ML. Getchell TV. Stromberg AJ. Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 53.Chondrogianni N. Tzavelas C. Pemberton AJ. Nezis IP. Rivett AJ. Gonos ES. Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem. 2005;280:11840–11850. doi: 10.1074/jbc.M413007200. [DOI] [PubMed] [Google Scholar]
- 54.Chung HY. Cesari M. Anton S. Marzetti E. Giovannini S. Seo AY. Carter C. Yu BP. Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung HY. Kim HJ. Kim KW. Choi JS. Yu BP. Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc Res Tech. 2002;59:264–272. doi: 10.1002/jemt.10203. [DOI] [PubMed] [Google Scholar]
- 56.Chung HY. Sung B. Jung KJ. Zou Y. Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 57.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 58.Circu ML. Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clise-Dwyer K. Huston GE. Buck AL. Duso DK. Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- 60.Corcoran LM. Transcriptional control of B cell activation. Curr Top Microbiol Immunol. 2005;290:105–146. doi: 10.1007/3-540-26363-2_6. [DOI] [PubMed] [Google Scholar]
- 61.Csiszar A. Labinskyy N. Jimenez R. Pinto JT. Ballabh P. Losonczy G. Pearson KJ. de CR. Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuervo AM. Bergamini E. Brunk UT. Droge W. Ffrench M. Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 64.Cuervo AM. Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 65.Cuervo AM. Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 66.Cullen SJ. Ponnappan S. Ponnappan U. Proteasome inhibition up-regulates inflammatory gene transcription induced by an atypical pathway of NF-kappaB activation. Biochem Pharmacol. 2010;79:706–714. doi: 10.1016/j.bcp.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curtale G. Citarella F. Carissimi C. Goldoni M. Carucci N. Fulci V. Franceschini D. Meloni F. Barnaba V. Macino G. An emerging player in the adaptive immune response: microRNA-146a is a modulator of IL-2 expression and AICD in T lymphocytes. Blood. 2010;115:265–273. doi: 10.1182/blood-2009-06-225987. [DOI] [PubMed] [Google Scholar]
- 68.d'Adda di FF. Reaper PM. Clay-Farrace L. Fiegler H. Carr P. Von ZT. Saretzki G. Carter NP. Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 69.Dahlmann B. Role of proteasomes in disease. BMC Biochem. 2007;8(Suppl 1):S3. doi: 10.1186/1471-2091-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dantuma NP. Lindsten K. Stressing the ubiquitin-proteasome system. Cardiovasc Res. 2010;85:263–271. doi: 10.1093/cvr/cvp255. [DOI] [PubMed] [Google Scholar]
- 71.Das R. Ponnappan S. Ponnappan U. Redox regulation of the proteasome in T lymphocytes during aging. Free Radic Biol Med. 2007;42:541–551. doi: 10.1016/j.freeradbiomed.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davenport MP. Price DA. McMichael AJ. The T cell repertoire in infection and vaccination: implications for control of persistent viruses. Curr Opin Immunol. 2007;19:294–300. doi: 10.1016/j.coi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 73.De Benedictis G. Franceschi C. The unusual genetics of human longevity. Sci Aging Knowledge Environ. 2006;2006:e20. doi: 10.1126/sageke.2006.10.pe20. [DOI] [PubMed] [Google Scholar]
- 74.de Boer J. Andressoo JO. de WJ. Huijmans J. Beems RB. van SH. Weeda G. van der Horst GT. van LW. Themmen AP. Meradji M. Hoeijmakers JH. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 75.de Magalhaes JP. Curado J. Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Martinis M. Franceschi C. Monti D. Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 77.DelaRosa O. Tarazona R. Casado JG. Alonso C. Ostos B. Pena J. Solana R. Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol. 2002;37:213–217. doi: 10.1016/s0531-5565(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 78.Della BS. Bierti L. Presicce P. Arienti R. Valenti M. Saresella M. Vergani C. Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 79.Delpedro AD. Barjavel MJ. Mamdouh Z. Faure S. Bakouche O. Signal transduction in LPS-activated aged and young monocytes. J Interferon Cytokine Res. 1998;18:429–437. doi: 10.1089/jir.1998.18.429. [DOI] [PubMed] [Google Scholar]
- 80.Dengjel J. Schoor O. Fischer R. Reich M. Kraus M. Muller M. Kreymborg K. Altenberend F. Brandenburg J. Kalbacher H. Brock R. Driessen C. Rammensee HG. Stevanovic S. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990;15:305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 82.Diehn M. Cho RW. Lobo NA. Kalisky T. Dorie MJ. Kulp AN. Qian D. Lam JS. Ailles LE. Wong M. Joshua B. Kaplan MJ. Wapnir I. Dirbas FM. Somlo G. Garberoglio C. Paz B. Shen J. Lau SK. Quake SR. Brown JM. Weissman IL. Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dixit VD. Taub DD. Ghrelin and immunity: a young player in an old field. Exp Gerontol. 2005;40:900–910. doi: 10.1016/j.exger.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Dixit VD. Yang H. Cooper-Jenkins A. Giri BB. Patel K. Taub DD. Reduction of T cell-derived ghrelin enhances proinflammatory cytokine expression: implications for age-associated increases in inflammation. Blood. 2009;113:5202–5205. doi: 10.1182/blood-2008-09-181255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dixit VD. Yang H. Sun Y. Weeraratna AT. Youm YH. Smith RG. Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dorshkind K. Montecino-Rodriguez E. Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 87.Douek DC. McFarland RD. Keiser PH. Gage EA. Massey JM. Haynes BF. Polis MA. Haase AT. Feinberg MB. Sullivan JL. Jamieson BD. Zack JA. Picker LJ. Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 88.Eisenberg T. Knauer H. Schauer A. Buttner S. Ruckenstuhl C. Carmona-Gutierrez D. Ring J. Schroeder S. Magnes C. Antonacci L. Fussi H. Deszcz L. Hartl R. Schraml E. Criollo A. Megalou E. Weiskopf D. Laun P. Heeren G. Breitenbach M. Grubeck-Loebenstein B. Herker E. Fahrenkrog B. Frohlich KU. Sinner F. Tavernarakis N. Minois N. Kroemer G. Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 89.Eisenbraun MD. Tamir A. Miller RA. Altered composition of the immunological synapse in an anergic, age-dependent memory T cell subset. J Immunol. 2000;164:6105–6112. doi: 10.4049/jimmunol.164.12.6105. [DOI] [PubMed] [Google Scholar]
- 90.Enck P. Zimmermann K. Rusch K. Schwiertz A. Klosterhalfen S. Frick JS. The effects of ageing on the colonic bacterial microflora in adults. Z Gastroenterol. 2009;47:653–658. doi: 10.1055/s-0028-1109055. [DOI] [PubMed] [Google Scholar]
- 91.Eriksson M. Brown WT. Gordon LB. Glynn MW. Singer J. Scott L. Erdos MR. Robbins CM. Moses TY. Berglund P. Dutra A. Pak E. Durkin S. Csoka AB. Boehnke M. Glover TW. Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ermak G. Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 93.Ershler WB. Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 94.Fauce SR. Jamieson BD. Chin AC. Mitsuyasu RT. Parish ST. Ng HL. Kitchen CM. Yang OO. Harley CB. Effros RB. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feinberg AP. Ohlsson R. Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 96.Finke JH. Zea AH. Stanley J. Longo DL. Mizoguchi H. Tubbs RR. Wiltrout RH. O'Shea JJ. Kudoh S. Klein E. Loss of T-cell receptor zeta chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993;53:5613–5616. [PubMed] [Google Scholar]
- 97.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 98.Flescher E. Ledbetter JA. Schieven GL. Vela-Roch N. Fossum D. Dang H. Ogawa N. Talal N. Longitudinal exposure of human T lymphocytes to weak oxidative stress suppresses transmembrane and nuclear signal transduction. J Immunol. 1994;153:4880–4889. [PubMed] [Google Scholar]
- 99.Flores KG. Li J. Sempowski GD. Haynes BF. Hale LP. Analysis of the human thymic perivascular space during aging. J Clin Invest. 1999;104:1031–1039. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fortin CF. Larbi A. Dupuis G. Lesur O. Fulop T., Jr. GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology. 2007;8:173–187. doi: 10.1007/s10522-006-9067-1. [DOI] [PubMed] [Google Scholar]
- 101.Fortin CF. Lesur O. Fulop T., Jr. Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS Lett. 2007;581:1173–1178. doi: 10.1016/j.febslet.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 102.Fortin CF. McDonald PP. Lesur O. Fulop T., Jr. Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11:873–882. doi: 10.1089/rej.2008.0750. [DOI] [PubMed] [Google Scholar]
- 103.Franceschi C. Bonafe M. Valensin S. Olivieri F. De LM. Ottaviani E. De BG. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 104.Franceschi C. Capri M. Monti D. Giunta S. Olivieri F. Sevini F. Panourgia MP. Invidia L. Celani L. Scurti M. Cevenini E. Castellani GC. Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 105.Franceschi C. Monti D. Barbieri D. Salvioli S. Grassilli E. Capri M. Troiano L. Guido M. Bonafe M. Tropea F. Salomoni P. Benatti F. Bellesia E. Macchioni S. Anderlini R. Sansoni P. Mariotti S. Wratten ML. Tetta C. Cossarizza A. Successful immunosenescence and the remodelling of immune responses with ageing. Nephrol Dial Transplant. 1996;11(Suppl 9):18–25. doi: 10.1093/ndt/11.supp9.18. [DOI] [PubMed] [Google Scholar]
- 106.Franceschi C. Monti D. Sansoni P. Cossarizza A. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today. 1995;16:12–16. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 107.Frasca D. Blomberg BB. Effects of aging on B cell function. Current Opinion in Immunology. 2009;21:425–430. doi: 10.1016/j.coi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frasca D. Landin AM. Lechner SC. Ryan JG. Schwartz R. Riley RL. Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 109.French RA. Broussard SR. Meier WA. Minshall C. Arkins S. Zachary JF. Dantzer R. Kelley KW. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology. 2002;143:690–699. doi: 10.1210/endo.143.2.8612. [DOI] [PubMed] [Google Scholar]
- 110.Friguet B. Bulteau AL. Chondrogianni N. Conconi M. Petropoulos I. Protein degradation by the proteasome and its implications in aging. Ann N Y Acad Sci. 2000;908:143–154. doi: 10.1111/j.1749-6632.2000.tb06643.x. [DOI] [PubMed] [Google Scholar]
- 111.Fulop T., Jr. Foris G. Worum I. Leovey A. Age-dependent alterations of Fc gamma receptor-mediated effector functions of human polymorphonuclear leucocytes. Clin Exp Immunol. 1985;61:425–432. [PMC free article] [PubMed] [Google Scholar]
- 112.Fulop T. Larbi A. Douziech N. Fortin C. Guerard KP. Lesur O. Khalil A. Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 113.Fulop T. Larbi A. Hirokawa K. Mocchegiani E. Lesourds B. Castle S. Wikby A. Franceschi C. Pawelec G. Immunosupportive therapies in aging. Clin Interv Aging. 2007;2:33–54. doi: 10.2147/ciia.2007.2.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gantier MP. Sadler AJ. Williams BR. Fine-tuning of the innate immune response by microRNAs. Immunol Cell Biol. 2007;85:458–462. doi: 10.1038/sj.icb.7100091. [DOI] [PubMed] [Google Scholar]
- 115.Garinis GA. van der Horst GT. Vijg J. Hoeijmakers JH. DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gelderman KA. Hultqvist M. Holmberg J. Olofsson P. Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci U S A. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gibson KL. Wu YC. Barnett Y. Duggan O. Vaughan R. Kondeatis E. Nilsson BO. Wikby A. Kipling D. Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gidalevitz T. Kikis EA. Morimoto RI. A cellular perspective on conformational disease: the role of genetic background and proteostasis networks. Curr Opin Struct Biol. 2010;20:23–32. doi: 10.1016/j.sbi.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gillespie GM. Wills MR. Appay V. O'Callaghan C. Murphy M. Smith N. Sissons P. Rowland-Jones S. Bell JI. Moss PA. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ginn-Pease ME. Whisler RL. Redox signals and NF-kappaB activation in T cells. Free Radic Biol Med. 1998;25:346–361. doi: 10.1016/s0891-5849(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 121.Goldberg GL. Zakrzewski JL. Perales MA. van den Brink MR. Clinical strategies to enhance T cell reconstitution. Semin Immunol. 2007;19:289–296. doi: 10.1016/j.smim.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gomez CR. Boehmer ED. Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 123.Greenway A. Azad A. Mills J. McPhee D. Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J Virol. 1996;70:6701–6708. doi: 10.1128/jvi.70.10.6701-6708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gress RE. Deeks SG. Reduced thymus activity and infection prematurely age the immune system. J Clin Invest. 2009;119:2884–2887. doi: 10.1172/JCI40855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grillari J. Katinger H. Voglauer R. Aging and the ubiquitinome: traditional and non-traditional functions of ubiquitin in aging cells and tissues. Exp Gerontol. 2006;41:1067–1079. doi: 10.1016/j.exger.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 126.Gringhuis SI. Leow A. Papendrecht-Van Der Voort EA. Remans PH. Breedveld FC. Verweij CL. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J Immunol. 2000;164:2170–2179. doi: 10.4049/jimmunol.164.4.2170. [DOI] [PubMed] [Google Scholar]
- 127.Gringhuis SI. Papendrecht-Van Der Voort EA. Leow A. Nivine Levarht EW. Breedveld FC. Verweij CL. Effect of redox balance alterations on cellular localization of LAT and downstream T-cell receptor signaling pathways. Mol Cell Biol. 2002;22:400–411. doi: 10.1128/MCB.22.2.400-411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Groettrup M. Kirk CJ. Basler M. Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol. 2010;10:73–78. doi: 10.1038/nri2687. [DOI] [PubMed] [Google Scholar]
- 129.Gruver AL. Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84:915–923. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hackl M. Brunner S. Fortschegger K. Schreiner C. Micutkova L. Muck C. Laschober GT. Lepperdinger G. Sampson N. Berger P. Herndler-Brandstetter D. Wieser M. Kuhnel H. Strasser A. Rinnerthaler M. Breitenbach M. Mildner M. Eckhart L. Tschachler E. Trost A. Bauer JW. Papak C. Trajanoski Z. Scheideler M. Grillari-Voglauer R. Grubeck-Loebenstein B. Jansen-Durr P. Grillari J. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010;9:291–296. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hadzic T. Li L. Cheng N. Walsh SA. Spitz DR. Knudson CM. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J Immunol. 2005;175:7965–7972. doi: 10.4049/jimmunol.175.12.7965. [DOI] [PubMed] [Google Scholar]
- 132.Hale JS. Boursalian TE. Turk GL. Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han S. Yang K. Ozen Z. Peng W. Marinova E. Kelsoe G. Zheng B. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J Immunol. 2003;170:1267–1273. doi: 10.4049/jimmunol.170.3.1267. [DOI] [PubMed] [Google Scholar]
- 134.Harley CB. Futcher AB. Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 135.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 136.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 137.Harrison DE. Strong R. Sharp ZD. Nelson JF. Astle CM. Flurkey K. Nadon NL. Wilkinson JE. Frenkel K. Carter CS. Pahor M. Javors MA. Fernandez E. Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haxhinasto S. Mathis D. Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hayes SA. Dice JF. Roles of molecular chaperones in protein degradation. J Cell Biol. 1996;132:255–258. doi: 10.1083/jcb.132.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haynes BF. Markert ML. Sempowski GD. Patel DD. Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 141.Haynes L. Linton PJ. Eaton SM. Tonkonogy SL. Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haynes L. Maue AC. Effects of aging on T cell function. Current Opinion in Immunology. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herrero C. Marques L. Lloberas J. Celada A. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J Clin Invest. 2001;107:485–493. doi: 10.1172/JCI11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hewison M. Vitamin D and innate immunity. Curr Opin Investig Drugs. 2008;9:485–490. [PubMed] [Google Scholar]
- 145.Hodes RJ. Aging and the immune system. Immunol Rev. 1997;160:5–8. doi: 10.1111/j.1600-065x.1997.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 146.Hoefig KP. Heissmeyer V. MicroRNAs grow up in the immune system. Curr Opin Immunol. 2008;20:281–287. doi: 10.1016/j.coi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 147.Holloszy JO. Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holloszy JO. Smith EK. Vining M. Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59:826–831. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- 149.Howitz KT. Bitterman KJ. Cohen HY. Lamming DW. Lavu S. Wood JG. Zipkin RE. Chung P. Kisielewski A. Zhang LL. Scherer B. Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 150.Huang MC. Liao JJ. Bonasera S. Longo DL. Goetzl EJ. Nuclear factor-kappaB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 2008;22:2142–2150. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- 151.Hubbs AF. Benkovic SA. Miller DB. O'Callaghan JP. Battelli L. Schwegler-Berry D. Ma Q. Vacuolar leukoencephalopathy with widespread astrogliosis in mice lacking transcription factor Nrf2. Am J Pathol. 2007;170:2068–2076. doi: 10.2353/ajpath.2007.060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hunt KJ. Walsh BM. Voegeli D. Roberts HC. Inflammation in aging part 1: physiology and immunological mechanisms. Biol Res Nurs. 2010;11:245–252. doi: 10.1177/1099800409352237. [DOI] [PubMed] [Google Scholar]
- 153.Huppert FA. Solomou W. O'Connor S. Morgan K. Sussams P. Brayne C. Aging and lymphocyte subpopulations: whole-blood analysis of immune markers in a large population sample of healthy elderly individuals. Exp Gerontol. 1998;33:593–600. doi: 10.1016/s0531-5565(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 154.Husom AD. Peters EA. Kolling EA. Fugere NA. Thompson LV. Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 155.Hwang JS. Hwang JS. Chang I. Kim S. Age-associated decrease in proteasome content and activities in human dermal fibroblasts: restoration of normal level of proteasome subunits reduces aging markers in fibroblasts from elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62:490–499. doi: 10.1093/gerona/62.5.490. [DOI] [PubMed] [Google Scholar]
- 156.Hwang KA. Kim HR. Kang I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev. 2009;130:509–517. doi: 10.1016/j.mad.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ito K. Hirao A. Arai F. Takubo K. Matsuoka S. Miyamoto K. Ohmura M. Naka K. Hosokawa K. Ikeda Y. Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 158.Ito Y. Ponnappan U. Lipschitz DA. Excess formation of lysophosphatidic acid with age inhibits myristic acid-induced superoxide anion generation in intact human neutrophils. FEBS Lett. 1996;394:149–152. doi: 10.1016/0014-5793(96)00937-4. [DOI] [PubMed] [Google Scholar]
- 159.Jackson SP. Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jamieson BD. Douek DC. Killian S. Hultin LE. Scripture-Adams DD. Giorgi JV. Marelli D. Koup RA. Zack JA. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 161.Janssen-Heininger YM. Poynter ME. Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 162.Jenq RR. King CG. Volk C. Suh D. Smith OM. Rao UK. Yim NL. Holland AM. Lu SX. Zakrzewski JL. Goldberg GL. Diab A. Alpdogan O. Penack O. Na IK. Kappel LW. Wolchok JD. Houghton AN. Perales MA. van den Brink MR. Keratinocyte growth factor enhances DNA plasmid tumor vaccine responses after murine allogeneic bone marrow transplantation. Blood. 2009;113:1574–1580. doi: 10.1182/blood-2008-05-155697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jing Y. Gravenstein S. Chaganty NR. Chen N. Lyerly KH. Joyce S. Deng Y. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol. 2007;42:719–732. doi: 10.1016/j.exger.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 164.Johnson JA. Johnson DA. Kraft AD. Calkins MJ. Jakel RJ. Vargas MR. Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jung KJ. Lee EK. Kim JY. Zou Y. Sung B. Heo HS. Kim MK. Lee J. Kim ND. Yu BP. Chung HY. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 2009;58:143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- 166.Jung T. Catalgol B. Grune T. The proteasomal system. Mol Aspects Med. 2009;30:191–296. doi: 10.1016/j.mam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 167.Kashino G. Kodama S. Nakayama Y. Suzuki K. Fukase K. Goto M. Watanabe M. Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radic Biol Med. 2003;35:438–443. doi: 10.1016/s0891-5849(03)00326-5. [DOI] [PubMed] [Google Scholar]
- 168.Kato M. Slack FJ. microRNAs: small molecules with big roles—C. elegans to human cancer. Biol Cell. 2008;100:71–81. doi: 10.1042/BC20070078. [DOI] [PubMed] [Google Scholar]
- 169.Kawahara TL. Michishita E. Adler AS. Damian M. Berber E. Lin M. McCord RA. Ongaigui KC. Boxer LD. Chang HY. Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kawakami K. Nakamura A. Ishigami A. Goto S. Takahashi R. Age-related difference of site-specific histone modifications in rat liver. Biogerontology. 2009;10:415–421. doi: 10.1007/s10522-008-9176-0. [DOI] [PubMed] [Google Scholar]
- 171.Khan N. Shariff N. Cobbold M. Bruton R. Ainsworth JA. Sinclair AJ. Nayak L. Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 172.Khazaeli AA. Tatar M. Pletcher SD. Curtsinger JW. Heat-induced longevity extension in Drosophila. I. Heat treatment, mortality, and thermotolerance. J Gerontol A Biol Sci Med Sci. 1997;52:B48–B52. doi: 10.1093/gerona/52a.1.b48. [DOI] [PubMed] [Google Scholar]
- 173.Kiffin R. Kaushik S. Zeng M. Bandyopadhyay U. Zhang C. Massey AC. Martinez-Vicente M. Cuervo AM. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci. 2007;120:782–791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]
- 174.Kim HJ. Barajas B. Wang M. Nel AE. Nrf2 activation by sulforaphane restores the age-related decrease of T(H)1 immunity: role of dendritic cells. J Allergy Clin Immunol. 2008;121:1255–1261. doi: 10.1016/j.jaci.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kline GH. Hayden TA. Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol. 1999;162:3342–3349. [PubMed] [Google Scholar]
- 176.Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kohler S. Wagner U. Pierer M. Kimmig S. Oppmann B. Mowes B. Julke K. Romagnani C. Thiel A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35:1987–1994. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- 178.Kojima M. Hosoda H. Date Y. Nakazato M. Matsuo H. Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 179.Kong F. Chen CH. Cooper MD. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- 180.Kovacs EJ. Palmer JL. Fortin CF. Fulop T., Jr. Goldstein DR. Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30:319–324. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Krishnaraj R. Immunosenescence of human NK cells: effects on tumor target recognition, lethal hit and interferon sensitivity. Immunol Lett. 1992;34:79–84. doi: 10.1016/0165-2478(92)90030-r. [DOI] [PubMed] [Google Scholar]
- 182.Krishnaraj R. Bhooma T. Cytokine sensitivity of human NK cells during immunosenescence. 2. IL2-induced interferon gamma secretion. Immunol Lett. 1996;50:59–63. doi: 10.1016/0165-2478(96)02519-9. [DOI] [PubMed] [Google Scholar]
- 183.Kubo C. Day NK. Good RA. Influence of early or late dietary restriction on life span and immunological parameters in MRL/Mp-lpr/lpr mice. Proc Natl Acad Sci U S A. 1984;81:5831–5835. doi: 10.1073/pnas.81.18.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kubota H. Quality control against misfolded proteins in the cytosol: a network for cell survival. J Biochem. 2009;146:609–616. doi: 10.1093/jb/mvp139. [DOI] [PubMed] [Google Scholar]
- 185.Kumar R. Avagyan S. Snoeck HW. A quantitative trait locus on chr.4 regulates thymic involution. J Gerontol A Biol Sci Med Sci. 2010;65:620–625. doi: 10.1093/gerona/glq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kwak MK. Cho JM. Huang B. Shin S. Kensler TW. Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic Biol Med. 2007;43:809–817. doi: 10.1016/j.freeradbiomed.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 187.Kwak MK. Wakabayashi N. Greenlaw JL. Yamamoto M. Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kwon J. Devadas S. Williams MS. T cell receptor-stimulated generation of hydrogen peroxide inhibits MEK-ERK activation and lck serine phosphorylation. Free Radic Biol Med. 2003;35:406–417. doi: 10.1016/s0891-5849(03)00318-6. [DOI] [PubMed] [Google Scholar]
- 189.Labrie JE., III Sah AP. Allman DM. Cancro MP. Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lafferty-Whyte K. Cairney CJ. Jamieson NB. Oien KA. Keith WN. Pathway analysis of senescence-associated miRNA targets reveals common processes to different senescence induction mechanisms. Biochim Biophys Acta. 2009;1792:341–352. doi: 10.1016/j.bbadis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 191.Lages CS. Suffia I. Velilla PA. Huang B. Warshaw G. Hildeman DA. Belkaid Y. Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lamark T. Johansen T. Autophagy: links with the proteasome. Curr Opin Cell Biol. 2009;22:192–198. doi: 10.1016/j.ceb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 193.Larbi A. Dupuis G. Douziech N. Khalil A. Fulop T., Jr. Low-grade inflammation with aging has consequences for T-lymphocyte signaling. Ann N Y Acad Sci. 2004;1030:125–133. doi: 10.1196/annals.1329.016. [DOI] [PubMed] [Google Scholar]
- 194.Larbi A. Franceschi C. Mazzatti D. Solana R. Wikby A. Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- 195.Larbi A. Kempf J. Pawelec G. Oxidative stress modulation and T cell activation. Exp Gerontol. 2007;42:852–858. doi: 10.1016/j.exger.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 196.Larbi A. Kempf J. Wistuba-Hamprecht K. Haug C. Pawelec G. The heat shock proteins in cellular aging: is zinc the missing link? Biogerontology. 2006;7:399–408. doi: 10.1007/s10522-006-9055-5. [DOI] [PubMed] [Google Scholar]
- 197.Lazuardi L. Herndler-Brandstetter D. Brunner S. Laschober GT. Lepperdinger G. Grubeck-Loebenstein B. Microarray analysis reveals similarity between CD8+ Biogerontology. 2009;10:191–202. doi: 10.1007/s10522-008-9167-1. [DOI] [PubMed] [Google Scholar]
- 198.Lee HK. Marzella L. Regulation of intracellular protein degradation with special reference to lysosomes: role in cell physiology and pathology. Int Rev Exp Pathol. 1994;35:39–147. [PubMed] [Google Scholar]
- 199.Leiser SF. Miller RA. Nrf2 signaling: a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Levine B. Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 201.Levy SM. Herberman RB. Lee J. Whiteside T. Beadle M. Heiden L. Simons A. Persistently low natural killer cell activity, age, and environmental stress as predictors of infectious morbidity. Nat Immun Cell Growth Regul. 1991;10:289–307. [PubMed] [Google Scholar]
- 202.Li C. Capan E. Zhao Y. Zhao J. Stolz D. Watkins SC. Jin S. Lu B. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 203.Li J. Stein TD. Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004;18:261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 204.Li Y. Wang LX. Yang G. Hao F. Urba WJ. Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68:6889–6895. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Licastro F. Candore G. Lio D. Porcellini E. Colonna-Romano G. Franceschi C. Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ligthart GJ. Corberand JX. Geertzen HG. Meinders AE. Knook DL. Hijmans W. Necessity of the assessment of health status in human immunogerontological studies: evaluation of the SENIEUR protocol. Mech Ageing Dev. 1990;55:89–105. doi: 10.1016/0047-6374(90)90108-r. [DOI] [PubMed] [Google Scholar]
- 207.Lin J. Epel E. Cheon J. Kroenke C. Sinclair E. Bigos M. Wolkowitz O. Mellon S. Blackburn E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Linton PJ. Haynes L. Tsui L. Zhang X. Swain S. From naive to effector—alterations with aging. Immunol Rev. 1997;160:9–18. doi: 10.1111/j.1600-065x.1997.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 209.Lithgow GJ. White TM. Melov S. Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu B. Wang J. Chan KM. Tjia WM. Deng W. Guan X. Huang JD. Li KM. Chau PY. Chen DJ. Pei D. Pendas AM. Cadinanos J. Lopez-Otin C. Tse HF. Hutchison C. Chen J. Cao Y. Cheah KS. Tryggvason K. Zhou Z. Genomic instability in laminopathy-based premature aging. Nat Med. 2005;11:780–785. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]
- 211.Liu L. Wylie RC. Andrews LG. Tollefsbol TO. Aging, cancer and nutrition: the DNA methylation connection. Mech Ageing Dev. 2003;124:989–998. doi: 10.1016/j.mad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 212.Lombard DB. Chua KF. Mostoslavsky R. Franco S. Gostissa M. Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 213.Looney RJ. Falsey A. Campbell D. Torres A. Kolassa J. Brower C. McCann R. Menegus M. McCormick K. Frampton M. Hall W. Abraham GN. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 214.Lopatina N. Haskell JF. Andrews LG. Poole JC. Saldanha S. Tollefsbol T. Differential maintenance and de novo methylating activity by three DNA methyltransferases in aging and immortalized fibroblasts. J Cell Biochem. 2002;84:324–334. doi: 10.1002/jcb.10015. [DOI] [PubMed] [Google Scholar]
- 215.Lord JM. Butcher S. Killampali V. Lascelles D. Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122:1521–1535. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 216.Lord JM. Phillips AC. Arlt W. Synergistic effects of ageing and stress on neutrophil function. In: Fulop T, editor; Franceschi C, editor; Hirokawa K, editor; Pawelec G, editor. Handbook of Immunosenescence. Dordrecht, Netherlands: Springer; 2009. pp. 475–498. [Google Scholar]
- 217.Los M. Schenk H. Hexel K. Baeuerle PA. Droge W. Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995;14:3731–3740. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lung TL. Saurwein-Teissl M. Parson W. Schonitzer D. Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18:1606–1612. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 219.Lynch HE. Goldberg GL. Chidgey A. Van den Brink MRM. Boyd R. Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Maini MK. Casorati G. Dellabona P. Wack A. Beverley PC. T-cell clonality in immune responses. Immunol Today. 1999;20:262–266. doi: 10.1016/s0167-5699(99)01472-3. [DOI] [PubMed] [Google Scholar]
- 221.Mantovani A. Sozzani S. Locati M. Allavena P. Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 222.Mariani E. Mariani AR. Meneghetti A. Tarozzi A. Cocco L. Facchini A. Age-dependent decreases of NK cell phosphoinositide turnover during spontaneous but not Fc-mediated cytolytic activity. Int Immunol. 1998;10:981–989. doi: 10.1093/intimm/10.7.981. [DOI] [PubMed] [Google Scholar]
- 223.Mariani E. Meneghetti A. Neri S. Ravaglia G. Forti P. Cattini L. Facchini A. Chemokine production by natural killer cells from nonagenarians. Eur J Immunol. 2002;32:1524–1529. doi: 10.1002/1521-4141(200206)32:6<1524::AID-IMMU1524>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 224.Mariani E. Roda P. Mariani AR. Vitale M. Degrassi A. Papa S. Facchini A. Age-associated changes in CD8+ and CD16+ cell reactivity: clonal analysis. Clin Exp Immunol. 1990;81:479–484. doi: 10.1111/j.1365-2249.1990.tb05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Marone G. Poto S. di ML. Condorelli M. Human basophil releasability. I. Age-related changes in basophil releasability. J Allergy Clin Immunol. 1986;77:377–383. doi: 10.1016/s0091-6749(86)80121-x. [DOI] [PubMed] [Google Scholar]
- 226.Martinez-Vicente M. Sovak G. Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 227.Massie HR. Aiello VR. Williams TR. Inhibition of iron absorption prolongs the life span of Drosophila. Mech Ageing Dev. 1993;67:227–237. doi: 10.1016/0047-6374(93)90001-8. [DOI] [PubMed] [Google Scholar]
- 228.Mathur SK. Schwantes EA. Jarjour NN. Busse WW. Age-related changes in eosinophil function in human subjects. Chest. 2008;133:412–419. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Matthias P. Yoshida M. Khochbin S. HDAC6 a new cellular stress surveillance factor. Cell Cycle. 2008;7:7–10. doi: 10.4161/cc.7.1.5186. [DOI] [PubMed] [Google Scholar]
- 230.Maue AC. Yager EJ. Swain SL. Woodland DL. Blackman MA. Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30:301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mays-Hoopes L. Chao W. Butcher HC. Huang RC. Decreased methylation of the major mouse long interspersed repeated DNA during aging and in myeloma cells. Dev Genet. 1986;7:65–73. doi: 10.1002/dvg.1020070202. [DOI] [PubMed] [Google Scholar]
- 232.McElhaney JE. Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Messaoudi I. Warner J. Fischer M. Park B. Hill B. Mattison J. Lane MA. Roth GS. Ingram DK. Picker LJ. Douek DC. Mori M. Nikolich-Zugich J. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Michie CA. McLean A. Alcock C. Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 235.Min D. Panoskaltsis-Mortari A. Kuro O. Hollander GA. Blazar BR. Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Miyaji C. Watanabe H. Toma H. Akisaka M. Tomiyama K. Sato Y. Abo T. Functional alteration of granulocytes, NK cells, and natural killer T cells in centenarians. Hum Immunol. 2000;61:908–916. doi: 10.1016/s0198-8859(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 237.Miyamoto K. Araki KY. Naka K. Arai F. Takubo K. Yamazaki S. Matsuoka S. Miyamoto T. Ito K. Ohmura M. Chen C. Hosokawa K. Nakauchi H. Nakayama K. Nakayama KI. Harada M. Motoyama N. Suda T. Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 238.Mizushima N. Levine B. Cuervo AM. Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Morgan TE. Wong AM. Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- 240.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Morley JF. Brignull HR. Weyers JJ. Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Motohashi H. Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 243.Muljo SA. Ansel KM. Kanellopoulou C. Livingston DM. Rao A. Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Munz C. Enhancing immunity through autophagy. Annu Rev Immunol. 2009;27:423–449. doi: 10.1146/annurev.immunol.021908.132537. [DOI] [PubMed] [Google Scholar]
- 245.Murata S. Takahama Y. Tanaka K. Thymoproteasome: probable role in generating positively selecting peptides. Curr Opin Immunol. 2008;20:192–196. doi: 10.1016/j.coi.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 246.Murciano C. Yanez A. O'Connor JE. Gozalbo D. Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol Med Microbiol. 2008;53:214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 247.Nagel JE. Chopra RK. Chrest FJ. McCoy MT. Schneider EL. Holbrook NJ. Adler WH. Decreased proliferation, interleukin 2 synthesis, and interleukin 2 receptor expression are accompanied by decreased mRNA expression in phytohemagglutinin-stimulated cells from elderly donors. J Clin Invest. 1988;81:1096–1102. doi: 10.1172/JCI113422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nagel JE. Pyle RS. Chrest FJ. Adler WH. Oxidative metabolism and bactericidal capacity of polymorphonuclear leukocytes from normal young and aged adults. J Gerontol. 1982;37:529–534. doi: 10.1093/geronj/37.5.529. [DOI] [PubMed] [Google Scholar]
- 249.Napolitano LA. Lo JC. Gotway MB. Mulligan K. Barbour JD. Schmidt D. Grant RM. Halvorsen RA. Schambelan M. McCune JM. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS. 2002;16:1103–1111. doi: 10.1097/00002030-200205240-00003. [DOI] [PubMed] [Google Scholar]
- 250.Nardai G. Vegh EM. Prohaszka Z. Csermely P. Chaperone-related immune dysfunction: an emergent property of distorted chaperone networks. Trends Immunol. 2006;27:74–79. doi: 10.1016/j.it.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 251.Navon A. Ciechanover A. The 26 S proteasome: from basic mechanisms to drug targeting. J Biol Chem. 2009;284:33713–33718. doi: 10.1074/jbc.R109.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Naylor K. Li G. Vallejo AN. Lee WW. Koetz K. Bryl E. Witkowski J. Fulbright J. Weyand CM. Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 253.Nguyen T. Nioi P. Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nguyen T. Sherratt PJ. Huang HC. Yang CS. Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 255.Nguyen T. Sherratt PJ. Nioi P. Yang CS. Pickett CB. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2005;280:32485–32492. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- 256.Nguyen T. Sherratt PJ. Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 257.Niedernhofer LJ. Garinis GA. Raams A. Lalai AS. Robinson AR. Appeldoorn E. Odijk H. Oostendorp R. Ahmad A. van LW. Theil AF. Vermeulen W. van der Horst GT. Meinecke P. Kleijer WJ. Vijg J. Jaspers NG. Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 258.Nijnik A. Woodbine L. Marchetti C. Dawson S. Lambe T. Liu C. Rodrigues NP. Crockford TL. Cabuy E. Vindigni A. Enver T. Bell JI. Slijepcevic P. Goodnow CC. Jeggo PA. Cornall RJ. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 259.Nikolich-Zugich J. Non-human primate models of T-cell reconstitution. Semin Immunol. 2007;19:310–317. doi: 10.1016/j.smim.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nikolich-Zugich J. Messaoudi I. Mice and flies and monkeys too: caloric restriction rejuvenates the aging immune system of non-human primates. Exp Gerontol. 2005;40:884–893. doi: 10.1016/j.exger.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 262.Nishioka T. Shimizu J. Iida R. Yamazaki S. Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 263.Njemini R. Demanet C. Mets T. Aging-related differences in basal heat shock protein 70 levels in lymphocytes are linked to altered frequencies of lymphocyte subsets. Aging Cell. 2008;7:498–505. doi: 10.1111/j.1474-9726.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 264.Nomellini V. Gomez CR. Kovacs EJ. Aging and impairment of innate immunity. Contrib Microbiol. 2008;15:188–205. doi: 10.1159/000136358. [DOI] [PubMed] [Google Scholar]
- 265.O'Connell RM. Taganov KD. Boldin MP. Cheng G. Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.O'Mahony L. Holland J. Jackson J. Feighery C. Hennessy TP. Mealy K. Quantitative intracellular cytokine measurement: age-related changes in proinflammatory cytokine production. Clin Exp Immunol. 1998;113:213–219. doi: 10.1046/j.1365-2249.1998.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ogawa K. Suzuki K. Okutsu M. Yamazaki K. Shinkai S. The association of elevated reactive oxygen species levels from neutrophils with low-grade inflammation in the elderly. Immun Ageing. 2008;5:13. doi: 10.1186/1742-4933-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Oikawa S. Kawanishi S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999;453:365–368. doi: 10.1016/s0014-5793(99)00748-6. [DOI] [PubMed] [Google Scholar]
- 269.Osburn WO. Yates MS. Dolan PD. Chen S. Liby KT. Sporn MB. Taguchi K. Yamamoto M. Kensler TW. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ospelt C. Gay S. TLRs and chronic inflammation. Int J Biochem Cell Biol. 2010;42:495–505. doi: 10.1016/j.biocel.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 271.Otsuji M. Kimura Y. Aoe T. Okamoto Y. Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci U S A. 1996;93:13119–13124. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ouyang Q. Wagner WM. Voehringer D. Wikby A. Klatt T. Walter S. Muller CA. Pircher H. Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003;38:911–920. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 273.Ouyang Q. Wagner WM. Walter S. Muller CA. Wikby A. Aubert G. Klatt T. Stevanovic S. Dodi T. Pawelec G. An age-related increase in the number of CD8+ T cells carrying receptors for an immunodominant Epstein-Barr virus (EBV) epitope is counteracted by a decreased frequency of their antigen-specific responsiveness. Mech Ageing Dev. 2003;124:477–485. doi: 10.1016/s0047-6374(03)00026-5. [DOI] [PubMed] [Google Scholar]
- 274.Paganelli R. Scala E. Rosso R. Cossarizza A. Bertollo L. Barbieri D. Fabrizi A. Lusi EA. Fagiolo U. Franceschi C. A shift to Th0 cytokine production by CD4+ cells in human longevity: studies on two healthy centenarians. Eur J Immunol. 1996;26:2030–2034. doi: 10.1002/eji.1830260910. [DOI] [PubMed] [Google Scholar]
- 275.Palombella VJ. Conner EM. Fuseler JW. Destree A. Davis JM. Laroux FS. Wolf RE. Huang J. Brand S. Elliott PJ. Lazarus D. McCormack T. Parent L. Stein R. Adams J. Grisham MB. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–15676. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Panda A. Arjona A. Sapey E. Bai F. Fikrig E. Montgomery RR. Lord JM. Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pang KC. Sanders MT. Monaco JJ. Doherty PC. Turner SJ. Chen W. Immunoproteasome subunit deficiencies impact differentially on two immunodominant influenza virus-specific CD8+ T cell responses. J Immunol. 2006;177:7680–7688. doi: 10.4049/jimmunol.177.11.7680. [DOI] [PubMed] [Google Scholar]
- 278.Pani G. Colavitti R. Borrello S. Galeotti T. Redox regulation of lymphocyte signaling. IUBMB Life. 2000;49:381–389. doi: 10.1080/152165400410227. [DOI] [PubMed] [Google Scholar]
- 279.Paulsen CE. Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Pearson KJ. Lewis KN. Price NL. Chang JW. Perez E. Cascajo MV. Tamashiro KL. Poosala S. Csiszar A. Ungvari Z. Kensler TW. Yamamoto M. Egan JM. Longo DL. Ingram DK. Navas P. de CR. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pegoraro G. Kubben N. Wickert U. Gohler H. Hoffmann K. Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol. 2009;11:1261–1267. doi: 10.1038/ncb1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Perez VI. Buffenstein R. Masamsetti V. Leonard S. Salmon AB. Mele J. Andziak B. Yang T. Edrey Y. Friguet B. Ward W. Richardson A. Chaudhuri A. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Peters T. Weiss JM. Sindrilaru A. Wang H. Oreshkova T. Wlaschek M. Maity P. Reimann J. Scharffetter-Kochanek K. Reactive oxygen intermediate-induced pathomechanisms contribute to immunosenescence, chronic inflammation and autoimmunity. Mech Ageing Dev. 2009;130:564–587. doi: 10.1016/j.mad.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 284.Petersen S. Saretzki G. Von ZT. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res. 1998;239:152–160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- 285.Piatelli MJ. Doughty C. Chiles TC. Requirement for a hsp90 chaperone-dependent MEK1/2-ERK pathway for B cell antigen receptor-induced cyclin D2 expression in mature B lymphocytes. J Biol Chem. 2002;277:12144–12150. doi: 10.1074/jbc.M200102200. [DOI] [PubMed] [Google Scholar]
- 286.Piazzolla G. Tortorella C. Serrone M. Jirillo E. Antonaci S. Modulation of cytoskeleton assembly capacity and oxidative response in aged neutrophils. Immunopharmacol Immunotoxicol. 1998;20:251–266. doi: 10.3109/08923979809038543. [DOI] [PubMed] [Google Scholar]
- 287.Plowden J. Renshaw-Hoelscher M. Engleman C. Katz J. Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 288.Plunkett FJ. Franzese O. Belaramani LL. Fletcher JM. Gilmour KC. Sharifi R. Khan N. Hislop AD. Cara A. Salmon M. Gaspar HB. Rustin MH. Webster D. Akbar AN. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech Ageing Dev. 2005;126:855–865. doi: 10.1016/j.mad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 289.Plunkett FJ. Franzese O. Finney HM. Fletcher JM. Belaramani LL. Salmon M. Dokal I. Webster D. Lawson AD. Akbar AN. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 290.Ponnappan U. Ubiquitin-proteasome pathway is compromised in CD45RO+ and CD45RA+ T lymphocyte subsets during aging. Exp Gerontol. 2002;37:359–367. doi: 10.1016/s0531-5565(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 291.Ponnappan S. Ovaa H. Ponnappan U. Lower expression of catalytic and structural subunits of the proteasome contributes to decreased proteolysis in peripheral blood T lymphocytes during aging. Int J Biochem Cell Biol. 2007;39:799–809. doi: 10.1016/j.biocel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 292.Ponnappan S. Ponnappan U. Ubiquitin-proteasome pathway in immune-senescence. In: Pandalai S, editor. Recent Research Developments in Biochemistry Vol. 4 Part II. Trivandrum, India: Research Signpost; 2003. pp. 779–785. [Google Scholar]
- 293.Ponnappan U. Trebilcock GU. Zheng MZ. Studies into the effect of tyrosine phosphatase inhibitor phenylarsine oxide on NFkappaB activation in T lymphocytes during aging: evidence for altered IkappaB-alpha phosphorylation and degradation. Exp Gerontol. 1999;34:95–107. doi: 10.1016/s0531-5565(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 294.Ponnappan U. Zhong M. Trebilcock GU. Decreased proteasome-mediated degradation in T cells from the elderly: a role in immune senescence. Cell Immunol. 1999;192:167–174. doi: 10.1006/cimm.1998.1418. [DOI] [PubMed] [Google Scholar]
- 295.Pua HH. Dzhagalov I. Chuck M. Mizushima N. He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pua HH. He YW. Maintaining T lymphocyte homeostasis: another duty of autophagy. Autophagy. 2007;3:266–267. doi: 10.4161/auto.3908. [DOI] [PubMed] [Google Scholar]
- 297.Rabatic S. Sabioncello A. Dekaris D. Kardum I. Age-related changes in functions of peripheral blood phagocytes. Mech Ageing Dev. 1988;45:223–229. doi: 10.1016/0047-6374(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 298.Rajawat YS. Hilioti Z. Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 299.Reddy NM. Kleeberger SR. Bream JH. Fallon PG. Kensler TW. Yamamoto M. Reddy SP. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene. 2008;27:5821–5832. doi: 10.1038/onc.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Reddy NM. Kleeberger SR. Cho HY. Yamamoto M. Kensler TW. Biswal S. Reddy SP. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am J Respir Cell Mol Biol. 2007;37:3–8. doi: 10.1165/rcmb.2007-0004RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Reyes BM. Danese S. Sans M. Fiocchi C. Levine AD. Redox equilibrium in mucosal T cells tunes the intestinal TCR signaling threshold. J Immunol. 2005;175:2158–2166. doi: 10.4049/jimmunol.175.4.2158. [DOI] [PubMed] [Google Scholar]
- 302.Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;2:245–261. doi: 10.1016/s1568-1637(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 303.Rider DA. Sinclair AJ. Young SP. Oxidative inactivation of CD45 protein tyrosine phosphatase may contribute to T lymphocyte dysfunction in the elderly. Mech Ageing Dev. 2003;124:191–198. doi: 10.1016/s0047-6374(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 304.Rink L. Cakman I. Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 305.Ritz BW. Gardner EM. Malnutrition and energy restriction differentially affect viral immunity. J Nutr. 2006;136:1141–1144. doi: 10.1093/jn/136.5.1141. [DOI] [PubMed] [Google Scholar]
- 306.Robert MF. Morin S. Beaulieu N. Gauthier F. Chute IC. Barsalou A. MacLeod AR. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet. 2003;33:61–65. doi: 10.1038/ng1068. [DOI] [PubMed] [Google Scholar]
- 307.Rock KL. Goldberg AL. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–779. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 308.Rogerson BJ. Harris DP. Swain SL. Burgess DO. Germinal center B cells in Peyer's patches of aged mice exhibit a normal activation phenotype and highly mutated IgM genes. Mech Ageing Dev. 2003;124:155–165. doi: 10.1016/s0047-6374(02)00115-x. [DOI] [PubMed] [Google Scholar]
- 309.Romagnoli P. Strahan D. Pelosi M. Cantagrel A. van Meerwijk JP. A potential role for protein tyrosine kinase p56(lck) in rheumatoid arthritis synovial fluid T lymphocyte hyporesponsiveness. Int Immunol. 2001;13:305–312. doi: 10.1093/intimm/13.3.305. [DOI] [PubMed] [Google Scholar]
- 310.Rossi DJ. Bryder D. Seita J. Nussenzweig A. Hoeijmakers J. Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 311.Rossi SW. Jeker LT. Ueno T. Kuse S. Keller MP. Zuklys S. Gudkov AV. Takahama Y. Krenger W. Blazar BR. Hollander GA. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood. 2007;109:3803–3811. doi: 10.1182/blood-2006-10-049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rushmore TH. Morton MR. Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 313.Saccani S. Marazzi I. Beg AA. Natoli G. Degradation of promoter-bound p65/RelA is essential for the prompt termination of the nuclear factor kappaB response. J Exp Med. 2004;200:107–113. doi: 10.1084/jem.20040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Salminen A. Kaarniranta K. Genetics vs. entropy: longevity factors suppress the NF-kappaB-driven entropic aging process. Ageing Res Rev. 2009;9:298–314. doi: 10.1016/j.arr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 315.Salminen A. Kaarniranta K. NF-kappaB signaling in the aging process. J Clin Immunol. 2009;29:397–405. doi: 10.1007/s10875-009-9296-6. [DOI] [PubMed] [Google Scholar]
- 316.Salminen A. Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 317.Salminen A. Kauppinen A. Suuronen T. Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: regulation of aging via NF-kappaB acetylation? Bioessays. 2008;30:939–942. doi: 10.1002/bies.20799. [DOI] [PubMed] [Google Scholar]
- 318.Salminen A. Ojala J. Huuskonen J. Kauppinen A. Suuronen T. Kaarniranta K. Interaction of aging-associated signaling cascades: inhibition of NF-kappaB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci. 2008;65:1049–1058. doi: 10.1007/s00018-008-7461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Salminen A. Paimela T. Suuronen T. Kaarniranta K. Innate immunity meets with cellular stress at the IKK complex: regulation of the IKK complex by HSP70 and HSP90. Immunol Lett. 2008;117:9–15. doi: 10.1016/j.imlet.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 320.Salomons FA. Menendez-Benito V. Bottcher C. McCray BA. Taylor JP. Dantuma NP. Selective accumulation of aggregation-prone proteasome substrates in response to proteotoxic stress. Mol Cell Biol. 2009;29:1774–1785. doi: 10.1128/MCB.01485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Samorajski T. Delaney C. Durham L. Ordy JM. Johnson JA. Dunlap WP. Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiol Aging. 1985;6:17–24. doi: 10.1016/0197-4580(85)90066-1. [DOI] [PubMed] [Google Scholar]
- 322.Sansoni P. Vescovini R. Fagnoni F. Biasini C. Zanni F. Zanlari L. Telera A. Lucchini G. Passeri G. Monti D. Franceschi C. Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 323.Sarg B. Koutzamani E. Helliger W. Rundquist I. Lindner HH. Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J Biol Chem. 2002;277:39195–39201. doi: 10.1074/jbc.M205166200. [DOI] [PubMed] [Google Scholar]
- 324.Sarkar D. Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 325.Sauce D. Larsen M. Fastenackels S. Duperrier A. Keller M. Grubeck-Loebenstein B. Ferrand C. Debre P. Sidi D. Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sauer S. Bruno L. Hertweck A. Finlay D. Leleu M. Spivakov M. Knight ZA. Cobb BS. Cantrell D. O'Connor E. Shokat KM. Fisher AG. Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Saurwein-Teissl M. Lung TL. Marx F. Gschosser C. Asch E. Blasko I. Parson W. Bock G. Schonitzer D. Trannoy E. Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 328.Savino W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2006;2:e62. doi: 10.1371/journal.ppat.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Schlissel MS. Kaffer CR. Curry JD. Leukemia and lymphoma: a cost of doing business for adaptive immunity. Genes Dev. 2006;20:1539–1544. doi: 10.1101/gad.1446506. [DOI] [PubMed] [Google Scholar]
- 330.Schmid D. Pypaert M. Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schnaider T. Somogyi J. Csermely P. Szamel M. The Hsp90-specific inhibitor geldanamycin selectively disrupts kinase-mediated signaling events of T-lymphocyte activation. Cell Stress Chaperones. 2000;5:52–61. doi: 10.1043/1355-8145(2000)005<0052:THSIGS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Schreurs MW. Hermsen MA. Geltink RI. Scholten KB. Brink AA. Kueter EW. Tijssen M. Meijer CJ. Ylstra B. Meijer GA. Hooijberg E. Genomic stability and functional activity may be lost in telomerase-transduced human CD8+ T lymphocytes. Blood. 2005;106:2663–2670. doi: 10.1182/blood-2004-09-3742. [DOI] [PubMed] [Google Scholar]
- 333.Schroder AK. Rink L. Neutrophil immunity of the elderly. Mech Ageing Dev. 2003;124:419–425. doi: 10.1016/s0047-6374(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 334.Schwarzenbach HR. Nakagawa T. Conroy MC. de Weck AL. Skin reactivity, basophil degranulation and IgE levels in ageing. Clin Allergy. 1982;12:465–473. doi: 10.1111/j.1365-2222.1982.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 335.Sebastian C. Espia M. Serra M. Celada A. Lloberas J. MacrophAging: a cellular and molecular review. Immunobiology. 2005;210:121–126. doi: 10.1016/j.imbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 336.Sedelnikova OA. Horikawa I. Zimonjic DB. Popescu NC. Bonner WM. Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 337.Seggewiss R. Lore K. Guenaga FJ. Pittaluga S. Mattapallil J. Chow CK. Koup RA. Camphausen K. Nason MC. Meier-Schellersheim M. Donahue RE. Blazar BR. Dunbar CE. Douek DC. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood. 2007;110:441–449. doi: 10.1182/blood-2006-12-065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sempowski GD. Gooding ME. Liao HX. Le PT. Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 339.Sempowski GD. Hale LP. Sundy JS. Massey JM. Koup RA. Douek DC. Patel DD. Haynes BF. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164:2180–2187. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- 340.Seres I. Csongor J. Mohacsi A. Leovey A. Fulop T. Age-dependent alterations of human recombinant GM-CSF effects on human granulocytes. Mech Ageing Dev. 1993;71:143–154. doi: 10.1016/0047-6374(93)90042-p. [DOI] [PubMed] [Google Scholar]
- 341.Sheng WY. Chen YR. Wang TC. A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes. FEBS Lett. 2006;580:6819–6824. doi: 10.1016/j.febslet.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 342.Shodell M. Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 343.Simons K. Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 344.Sinclair DA. Oberdoerffer P. The ageing epigenome: damaged beyond repair? Ageing Res Rev. 2009;8:189–198. doi: 10.1016/j.arr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sinclair LV. Finlay D. Feijoo C. Cornish GH. Gray A. Ager A. Okkenhaug K. Hagenbeek TJ. Spits H. Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Skaug B. Jiang X. Chen ZJ. The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 347.Slagboom PE. Droog S. Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 348.Smith RG. Jiang H. Sun Y. Developments in ghrelin biology and potential clinical relevance. Trends Endocrinol Metab. 2005;16:436–442. doi: 10.1016/j.tem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 349.Sokol CL. Chu NQ. Yu S. Nish SA. Laufer TM. Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Solana R. Pawelec G. Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 351.Son NH. Joyce B. Hieatt A. Chrest FJ. Yanovski J. Weng NP. Stable telomere length and telomerase expression from naive to memory B-lymphocyte differentiation. Mech Ageing Dev. 2003;124:427–432. doi: 10.1016/s0047-6374(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 352.Son NH. Murray S. Yanovski J. Hodes RJ. Weng N. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with age. J Immunol. 2000;165:1191–1196. doi: 10.4049/jimmunol.165.3.1191. [DOI] [PubMed] [Google Scholar]
- 353.Soti C. Csermely P. Aging and molecular chaperones. Exp Gerontol. 2003;38:1037–1040. doi: 10.1016/s0531-5565(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 354.Souvannavong V. Lemaire C. Andreau K. Brown S. Adam A. Age-associated modulation of apoptosis and activation in murine B lymphocytes. Mech Ageing Dev. 1998;103:285–299. doi: 10.1016/s0047-6374(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 355.Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010;9:324–353. doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 356.Steger MM. Maczek C. Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin Exp Immunol. 1996;105:544–550. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Steinmann GG. Klaus B. Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 358.Stephan RP. Sanders VM. Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- 359.Stewart D. Killeen E. Naquin R. Alam S. Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 360.Stulnig T. Maczek C. Bock G. Majdic O. Wick G. Reference intervals for human peripheral blood lymphocyte subpopulations from “healthy” young and aged subjects. Int Arch Allergy Immunol. 1995;108:205–210. doi: 10.1159/000237155. [DOI] [PubMed] [Google Scholar]
- 361.Suh JH. Shenvi SV. Dixon BM. Liu H. Jaiswal AK. Liu RM. Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Sunil VR. Laumbach RJ. Patel KJ. Turpin BJ. Lim HJ. Kipen HM. Laskin JD. Laskin DL. Pulmonary effects of inhaled limonene ozone reaction products in elderly rats. Toxicol Appl Pharmacol. 2007;222:211–220. doi: 10.1016/j.taap.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Swift ME. Burns AL. Gray KL. DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 364.Sykiotis GP. Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Taganov KD. Boldin MP. Chang KJ. Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Tal MC. Sasai M. Lee HK. Yordy B. Shadel GS. Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Tan PH. Sagoo P. Chan C. Yates JB. Campbell J. Beutelspacher SC. Foxwell BM. Lombardi G. George AJ. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J Immunol. 2005;174:7633–7644. doi: 10.4049/jimmunol.174.12.7633. [DOI] [PubMed] [Google Scholar]
- 368.Tebbs RS. Flannery ML. Meneses JJ. Hartmann A. Tucker JD. Thompson LH. Cleaver JE. Pedersen RA. Requirement for the Xrcc1 DNA base excision repair gene during early mouse development. Dev Biol. 1999;208:513–529. doi: 10.1006/dbio.1999.9232. [DOI] [PubMed] [Google Scholar]
- 369.Terman A. Gustafsson B. Brunk UT. Autophagy, organelles and ageing. J Pathol. 2007;211:134–143. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- 370.Thoman ML. Weigle WO. Lymphokines and aging: interleukin-2 production and activity in aged animals. J Immunol. 1981;127:2102–2106. [PubMed] [Google Scholar]
- 371.Tomaru U. Ishizu A. Murata S. Miyatake Y. Suzuki S. Takahashi S. Kazamaki T. Ohara J. Baba T. Iwasaki S. Fugo K. Otsuka N. Tanaka K. Kasahara M. Exclusive expression of proteasome subunit {beta}5t in the human thymic cortex. Blood. 2009;113:5186–5191. doi: 10.1182/blood-2008-11-187633. [DOI] [PubMed] [Google Scholar]
- 372.Tonoki A. Kuranaga E. Tomioka T. Hamazaki J. Murata S. Tanaka K. Miura M. Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol Cell Biol. 2009;29:1095–1106. doi: 10.1128/MCB.01227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Tortorella C. Simone O. Piazzolla G. Stella I. Antonaci S. Age-related impairment of GM-CSF-induced signalling in neutrophils: role of SHP-1 and SOCS proteins. Ageing Res Rev. 2007;6:81–93. doi: 10.1016/j.arr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 374.Tothova Z. Kollipara R. Huntly BJ. Lee BH. Castrillon DH. Cullen DE. McDowell EP. Lazo-Kallanian S. Williams IR. Sears C. Armstrong SA. Passegue E. DePinho RA. Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 375.Tran QT. Xu L. Phan V. Goodwin SB. Rahman M. Jin VX. Sutter CH. Roebuck BD. Kensler TW. George EO. Sutter TR. Chemical genomics of cancer chemopreventive dithiolethiones. Carcinogenesis. 2009;30:480–486. doi: 10.1093/carcin/bgn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Trebilcock GU. Ponnappan U. Evidence for lowered induction of nuclear factor kappa B in activated human T lymphocytes during aging. Gerontology. 1996;42:137–146. doi: 10.1159/000213785. [DOI] [PubMed] [Google Scholar]
- 377.Tsitsiou E. Lindsay MA. microRNAs and the immune response. Curr Opin Pharmacol. 2009;9:514–520. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tsukamoto H. Clise-Dwyer K. Huston GE. Duso DK. Buck AL. Johnson LL. Haynes L. Swain SL. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Tullet JM. Hertweck M. An JH. Baker J. Hwang JY. Liu S. Oliveira RP. Baumeister R. Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Ugarte N. Petropoulos I. Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal. 2010;13:539–549. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- 381.Vallejo AN. Immune remodeling: lessons from repertoire alterations during chronological aging and in immune-mediated disease. Trends Mol Med. 2007;13:94–102. doi: 10.1016/j.molmed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 382.van DD. Mohanty S. Thomas V. Ginter S. Montgomery RR. Fikrig E. Allore HG. Medzhitov R. Shaw AC. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 383.van DD. Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc. 2007;55:1438–1444. doi: 10.1111/j.1532-5415.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- 384.van den Biggelaar AH. Huizinga TW. de Craen AJ. Gussekloo J. Heijmans BT. Frolich M. Westendorp RG. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp Gerontol. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 385.Vellai T. Autophagy genes and ageing. Cell Death Differ. 2009;16:94–102. doi: 10.1038/cdd.2008.126. [DOI] [PubMed] [Google Scholar]
- 386.Venugopal R. Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Verbeke P. Clark BF. Rattan SI. Reduced levels of oxidized and glycoxidized proteins in human fibroblasts exposed to repeated mild heat shock during serial passaging in vitro. Free Radic Biol Med. 2001;31:1593–1602. doi: 10.1016/s0891-5849(01)00752-3. [DOI] [PubMed] [Google Scholar]
- 388.Vernace VA. Arnaud L. Schmidt-Glenewinkel T. Figueiredo-Pereira ME. Aging perturbs 26S proteasome assembly in Drosophila melanogaster. FASEB J. 2007;21:2672–2682. doi: 10.1096/fj.06-6751com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Vijg J. Impact of genome instability on transcription regulation of aging and senescence. Mech Ageing Dev. 2004;125:747–753. doi: 10.1016/j.mad.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 390.Vittorini S. Paradiso C. Donati A. Cavallini G. Masini M. Gori Z. Pollera M. Bergamini E. The age-related accumulation of protein carbonyl in rat liver correlates with the age-related decline in liver proteolytic activities. J Gerontol A Biol Sci Med Sci. 1999;54:B318–B323. doi: 10.1093/gerona/54.8.b318. [DOI] [PubMed] [Google Scholar]
- 391.Wakabayashi N. Itoh K. Wakabayashi J. Motohashi H. Noda S. Takahashi S. Imakado S. Kotsuji T. Otsuka F. Roop DR. Harada T. Engel JD. Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 392.Walford RL. Mock D. Verdery R. MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–B224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- 393.Wan F. Lenardo MJ. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20:24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Weinberger B. Herndler-Brandstetter D. Schwanninger A. Weiskopf D. Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- 395.Weindruch RH. Kristie JA. Cheney KE. Walford RL. Influence of controlled dietary restriction on immunologic function and aging. Fed Proc. 1979;38:2007–2016. [PubMed] [Google Scholar]
- 396.Weiskopf D. Weinberger B. Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 397.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Weng NP. Telomere and adaptive immunity. Mech Ageing Dev. 2008;129:60–66. doi: 10.1016/j.mad.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Weng NP. Levine BL. June CH. Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Weyand CM. Brandes JC. Schmidt D. Fulbright JW. Goronzy JJ. Functional properties of CD4+ Mech Ageing Dev. 1998;102:131–147. doi: 10.1016/s0047-6374(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 401.Whisler RL. Chen M. Liu B. Newhouse YG. Age-related impairments in TCR/CD3 activation of ZAP-70 are associated with reduced tyrosine phosphorylations of zeta-chains and p59fyn/p56lck in human T cells. Mech Ageing Dev. 1999;111:49–66. doi: 10.1016/s0047-6374(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 402.Whisler RL. Grants IS. Age-related alterations in the activation and expression of phosphotyrosine kinases and protein kinase C (PKC) among human B cells. Mech Ageing Dev. 1993;71:31–46. doi: 10.1016/0047-6374(93)90033-n. [DOI] [PubMed] [Google Scholar]
- 403.Williams MS. Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 404.Wilson VL. Smith RA. Ma S. Cutler RG. Genomic 5-methyldeoxycytidine decreases with age. J Biol Chem. 1987;262:9948–9951. [PubMed] [Google Scholar]
- 405.Wright CA. Kozik P. Zacharias M. Springer S. Tapasin and other chaperones: models of the MHC class I loading complex. Biol Chem. 2004;385:763–778. doi: 10.1515/BC.2004.100. [DOI] [PubMed] [Google Scholar]
- 406.Wu D. Marko M. Claycombe K. Paulson KE. Meydani SN. Ceramide-induced and age-associated increase in macrophage COX-2 expression is mediated through up-regulation of NF-kappa B activity. J Biol Chem. 2003;278:10983–10992. doi: 10.1074/jbc.M207470200. [DOI] [PubMed] [Google Scholar]
- 407.Wu D. Meydani SN. Age-associated changes in immune and inflammatory responses: impact of vitamin E intervention. J Leukoc Biol. 2008;84:900–914. doi: 10.1189/jlb.0108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Wullschleger S. Loewith R. Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 409.Xiao C. Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 410.Yan Z. Banerjee R. Redox remodeling as an immunoregulatory strategy. Biochemistry. 2010;49:1059–1066. doi: 10.1021/bi902022n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Yan Z. Garg SK. Kipnis J. Banerjee R. Extracellular redox modulation by regulatory T cells. Nat Chem Biol. 2009;5:721–723. doi: 10.1038/nchembio.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Yang H. Youm YH. Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol. 2009;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Yeung F. Hoberg JE. Ramsey CS. Keller MD. Jones DR. Frye RA. Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Zea AH. Curti BD. Longo DL. Alvord WG. Strobl SL. Mizoguchi H. Creekmore SP. O'Shea JJ. Powers GC. Urba WJ. Alterations in T cell receptor and signal transduction molecules in melanoma patients. Clin Cancer Res. 1995;1:1327–1335. [PubMed] [Google Scholar]
- 415.Zhang C. Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Zhao Y. Sun H. Lu J. Li X. Chen X. Tao D. Huang W. Huang B. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol. 2005;208:697–705. doi: 10.1242/jeb.01439. [DOI] [PubMed] [Google Scholar]
- 417.Zheng H. Li Z. Cutting edge: cross-presentation of cell-associated antigens to MHC class I molecule is regulated by a major transcription factor for heat shock proteins. J Immunol. 2004;173:5929–5933. doi: 10.4049/jimmunol.173.10.5929. [DOI] [PubMed] [Google Scholar]