Abstract
Excessive nitrosative and oxidative stress is thought to trigger cellular signaling pathways leading to neurodegenerative conditions. Such redox dysregulation can result from many cellular events, including hyperactivation of the N-methyl-d-aspartate-type glutamate receptor, mitochondrial dysfunction, and cellular aging. Recently, we and our colleagues have shown that excessive generation of free radicals and related molecules, in particular nitric oxide species (NO), can trigger pathological production of misfolded proteins, abnormal mitochondrial dynamics (comprised of mitochondrial fission and fusion events), and apoptotic pathways in neuronal cells. Emerging evidence suggests that excessive NO production can contribute to these pathological processes, specifically by S-nitrosylation of specific target proteins. Here, we highlight examples of S-nitrosylated proteins that regulate misfolded protein accumulation and mitochondrial dynamics. For instance, in models of Parkinson's disease, these S-nitrosylation targets include parkin, a ubiquitin E3 ligase and neuroprotective molecule, and protein-disulfide isomerase, a chaperone enzyme for nascent protein folding. S-Nitrosylation of protein-disulfide isomerase may also be associated with mutant Cu/Zn superoxide dismutase toxicity in amyotrophic lateral sclerosis. Additionally, in models of Alzheimer's disease, excessive NO generation leads to the formation of S-nitrosylated dynamin-related protein 1 (forming SNO-Drp1), which contributes to abnormal mitochondrial fragmentation and resultant synaptic damage. Antioxid. Redox Signal. 14, 1479–1492.
Introduction
Many neurodegenerative diseases manifest excessive generation of reactive oxygen species (ROS), such as superoxide anion (O2•−), and reactive nitrogen species (RNS), including nitric oxide (NO•), which contribute to neuronal cell injury and death (10, 79, 84). N-methyl-d-aspartate (NMDA)–type glutamate receptors have been linked to ROS and RNS production in the nervous system. Overactivation of NMDA receptors causes excessive influx of Ca2+ ions, which generates ROS and activates neuronal NO synthase (nNOS) (48). Reaction of the NO group with critical cysteine thiols of target proteins results in the formation of S-nitrosoproteins and can thus regulate protein function (81). Lipton and Stamler initially discovered and characterized this biochemical process on the NMDA receptor itself (NO inhibits excessive NMDA receptor activity via S-nitrosylation) (81), and the activity of potentially hundreds or even thousands of other proteins may well be modulated in this fashion. Analogous to phosphorylation, they coined the term “S-nitrosylation,” indicating a biological effect of the chemical reaction of S-nitrosation. S-Nitrosylation can mediate either protective or neurotoxic effects depending on the action of the target protein. Additionally, NADPH oxidase as well as mitochondrial respiration produce free radicals, principally superoxide anion (O2•−), upon NMDA receptor activation (19). Superoxide anion thus generated from both mitochondrial and nonmitochondrial (e.g., NADPH oxidase) sources reacts rapidly with free radical NO to form the very toxic product peroxynitrite (ONOO−) (81) (Figs. 1 and 2).
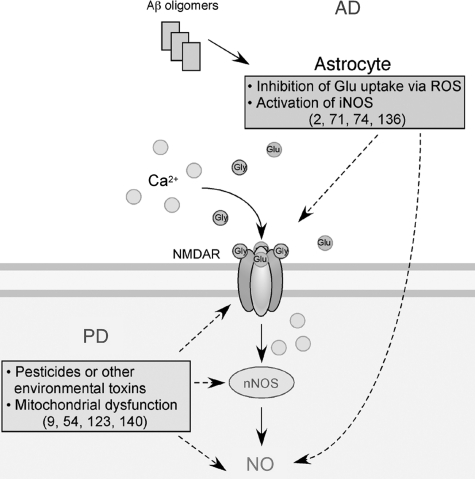
FIG. 1.
Possible mechanisms whereby Ca2+ signaling contributes to NO generation in neurodegenerative conditions. Hyperactivation of N-methyl-d-aspartate receptors (NMDARs) by glutamate (Glu) and glycine (Gly) induces excessive Ca2+ influx and activation of neuronal nitric oxide synthase (nNOS). nNOS produces NO from l-arginine. In Alzheimer's disease (AD), soluble oligomers of amyloid-β (Aβ) peptide, thought to be a key mediator in AD pathogenesis, can facilitate neuronal NO production in both NMDAR-dependent and NMDAR-independent manners (2, 71, 74, 136). In Parkinson's disease (PD), mitochondrial dysfunction caused by pesticides, herbicides, or other environmental toxins can trigger NO production, possibly via mitochondrial pathways and the NMDAR/Ca2+ cascade (9, 54, 123, 140). Note that in addition to reactive nitrogen species, reactive oxygen species (ROS) are also produced in response to Aβ and pesticides.
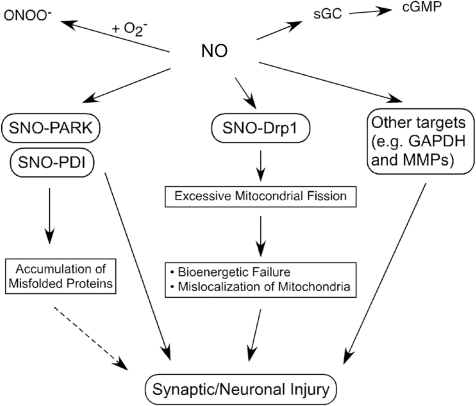
FIG. 2.
Overproduction of NO triggers formation of S-nitrosylated (SNO) proteins. NO produced by nNOS reacts with sulfhydryl groups to form SNO proteins. Physiological levels of NO mediate neuroprotective effects, at least in part, by S-nitrosylating the NMDAR and caspases, thus inhibiting their activity. In contrast, we postulate that overproduction of NO can be neurotoxic via S-nitrosylation of Parkin (forming SNO-PARK), protein-disulfide isomerase (PDI) (forming SNO-PDI), GAPDH, MMP-2/9, PrxII, and COX-2. S-Nitrosylated parkin and PDI contribute to neuronal cell injury by triggering accumulation of misfolded proteins. S-Nitrosylation of dynamin-related protein 1 (Drp1) (forming SNO-Drp1) causes excessive mitochondrial fragmentation in neurodegenerative conditions. NO also activates soluble guanylate cyclase (sGC) to produce cyclic guanosine-3′,5′-monophosphate (cGMP), and cGMP can activate cGMP-dependent protein kinase. Peroxynitrite (ONOO−), derived from reaction of NO and superoxide anion (O2•−), can oxidize vicinal sulfhydryl groups to disulfide bonds and can also nitrate tyrosine residues to form 3-nitrotyrosine.
In recent work, we have shown that S-nitrosylation and further oxidation of critical cysteine residues can lead to protein misfolding. Misfolded proteins form aggregates in many neurodegenerative diseases, and soluble oligomers of these aberrantly folded proteins are thought to adversely affect cell function by interfering with normal cellular processes or initiating cell death signaling pathways (91). As examples, α-synuclein and synphilin-1 in Parkinson's disease (PD), and amyloid-β (Aβ) and tau in Alzheimer's disease (AD) form toxic oligomers of non-native secondary structures. The formation of larger aggregates may be an attempt of the cell to wall off these toxic proteins. Protein aggregation is also a signature of Huntington's disease (a polyQ disorder), amyotrophic lateral sclerosis (ALS), and prion disease (32).
Sporadic forms of neurodegenerative diseases, rather than single gene mutation, constitute the vast majority of cases, and pathologic protein misfolding in these diseases may be the result of posttranslational changes to the protein engendered by nitrosative and/or oxidative stress, which can thus mimic the more rare genetic variants of the disease (145). Here we focus on specific examples that show the critical roles of S-nitrosylation of ubiquitin E3 ligases, for example, parkin, and endoplasmic reticulum (ER) chaperones, such as protein-disulfide isomerase (PDI), in accumulation of misfolded proteins in neurodegenerative diseases (31, 123, 140) (Fig. 1). We also review recent findings on S-nitrosylation of dynamin-related protein 1 (Drp1), which can contribute to the pathological fragmentation of mitochondria (Fig. 1).
Generation of ROS/RNS by Ca2+ Influx Through NMDA Receptor Channels in Response to Glutamatergic Signaling
The amino acid glutamate functions as the major excitatory neurotransmitter and is present at millimolar concentrations in the adult central nervous system. Ca2+ stimulates release of glutamate from the presynaptic nerve terminal into the synaptic cleft, where it diffuses to postsynaptic receptors on an adjacent neuron. Normal excitatory neurotransmission is essential for synaptic development and plasticity as well as learning and memory. In contrast, excessive glutamate excitation plays a role in a variety of neurological disorders ranging from acute hypoxic-ischemic brain injury to chronic neurodegenerative diseases. Survival pathways appear to be mediated via NMDA receptor synaptic activity, whereas neuronal damage may be mediated by excessive extrasynaptic activity (see below for further discussion of synaptic versus extrasynaptic NMDA receptor activity) (95, 100). Severe overstimulation of excitatory receptors can cause necrotic cell death, whereas less fulminant or chronic overstimulation can cause apoptotic or other forms of cell death (3, 12, 21).
Glutamate receptors in the nervous system are divided into two groups, ionotropic (representing ligand-gated ion channels) and metabotropic (coupled to G-proteins). Ionotropic glutamate receptors are represented by three separate classes, NMDA, α-amino–3-hydroxy-5 methyl-4-isoxazole propionic acid, and kainate; each receptor type is named for the synthetic ligands that selectively activate them. Functional NMDA receptors are likely to be heterotetramers in vivo, containing NR1, NR2 (with NR2A-NR2D subtypes), and, in some cases, NR3 subunits (26). In neurons, both synaptic and extrasynaptic sites express functional NMDA receptors. Synaptic NMDA receptor activity generally signals to molecular pathways promoting neuronal survival, for example, by enhancing the expression of antioxidative enzymes (100). Conversely, excessive activation of extrasynaptic NMDA receptors mediates molecular pathways that trigger neurotoxicity associated with accumulation of misfolded proteins (95). Evidence suggests that the NR2A subtype is predominantly located at synaptic sites, whereas NR2B is enriched at extrasynaptic sites but this differential localization is not exclusive; the subcellular localization of NR2 subtypes at best can only explain in part the differential effects of synaptic and extrasynaptic NMDA receptor activity on neuronal survival (79). Via postsynaptic density (PSD)-95 protein complexes, synaptic NMDA receptors are known to couple to multiple intracellular signaling molecules, including nNOS, located just beneath the postsynaptic membrane (68, 115). Recently, extrasynaptic NMDA receptors were also reported to be associated with PSD-95 (103), suggesting that nNOS/NO toxicity can play a role in cell death pathways triggered by extrasynaptic NMDA receptors.
NMDA receptors, unlike most other glutamate receptors, are highly permeable to Ca2+. Depolarization relieves blockade of NMDA receptor-coupled ion channels by Mg2+ (89). Ca2+ influx promotes many normal intracellular signaling pathways, but excessive influx promotes pathological signaling, contributing to cell injury and death via production of free radicals and related molecules such as ROS and NO, as well as other enzymatic processes (12, 21, 40, 81, 84) (Figs. 1 and 2). Excitotoxicity is defined as neuronal damage caused by excessive activation of glutamate receptors (96) and is at least partly mediated by excessive Ca2+ influx through NMDA receptor-associated ion channels (26, 79, 84). Increased levels of neuronal Ca2+, in conjunction with the Ca2+-binding protein, calmodulin, trigger the activation of nNOS and subsequent generation of NO from the amino acid l-arginine (1, 18, 115) (Figs. 1 and 2). Three subtypes of NOS have been identified; the two constitutive forms of NOS—nNOS and endothelial NOS—take their names from the cell type in which they were first found. The name of the third subtype—inducible NOS (iNOS)—indicates that expression of the enzyme is induced by acute inflammatory stimuli. For example, activated glial cells may produce neurotoxic amounts of NO via iNOS expression in various neurodegenerative diseases. All three isoforms are widely distributed in the brain. Each NOS isoform contains an oxidase domain at its amino terminal and a reductase domain at its carboxy terminal, separated by a Ca2+/CaM binding site (1, 16, 18, 46, 52). Constitutive and inducible NOS are also further distinguished by CaM binding; nNOS and endothelial NOS bind CaM in a reversible Ca2+-dependent manner and are thus activated by Ca2+. In contrast, iNOS binds CaM so tightly at resting intracellular Ca2+ concentrations that its activity does not appear to be affected by transient variations in Ca2+ concentration. To terminate iNOS-mediated NO production, microglia may redistribute iNOS to the aggresome for inactivation (70).
A connection between RNS and mitochondrial dysfunction in neurodegenerative diseases, especially PD, has recently been postulated (10, 11). Pesticides and other environmental toxins specifically inhibit mitochondrial complex I, generating excessive ROS/RNS, thus contributing to aberrant protein accumulation (31, 123, 140). In animal models, administration of complex I inhibitors, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, 6-hydroxydopamine, rotenone, and paraquat, recapitulates many features of sporadic PD, including degeneration of dopaminergic neurons, overproduction and aggregation of α-synuclein, accumulation of Lewy body-like intraneuronal inclusions, and impairment of behavioral functions (10, 11). Studies such as these strongly suggest a relationship between RNS and protein misfolding. Each may be the pathogenic trigger for the other in neurodegenerative diseases, but the mechanism has not yet been determined.
Alternatively, accumulation of ROS can trigger caspase activation resulting in synaptic damage and apoptosis (1). This process can be exacerbated by the action of endogenous neurotoxic electrophiles such as the prostaglandin derivative 15d-PGJ2 and catecholamine metabolites (including dopamine) (1). Such electrophiles compromise the reductive capacity of the cell by binding reduced cysteine residues, such as in glutathione, through a reaction called S-alkylation.
Nitrosative Stress, Protein Misfolding, Synaptic Injury, and Neuronal Cell Death
NO participates in cellular signaling pathways that regulate broad aspects of brain function, including synaptic plasticity, normal development, and neuronal cell death (40). These effects were thought to be largely achieved by activation of guanylate cyclase to form cyclic guanosine-3′,5′-monophosphate, but emerging evidence suggests that a more prominent reaction of NO is S-nitros(yl)ation of regulatory protein thiol groups (48, 62, 81). S-Nitrosylation is the covalent addition of an NO group to a cysteine thiol/sulfhydryl (RSH or, more properly, thiolate anion, RS−) to form an S-nitrosothiol derivative (R-SNO). Such regulatory modifications are broadly found in mammalian, plant, and microbial proteins. We and our colleagues have found that a consensus motif of nucleophilic residues (generally an acid and a base) surround a critical cysteine, increasing the susceptibility of the sulfhydryl to S-nitrosylation (56). This process is counterbalanced by denitrosylation by means of thioredoxin/thioredoxin reductase, class III alcohol dehydrogenase, PDI, intracellular glutathione, and other mechanisms. We first identified the physiological relevance of the redox-based mechanisms by which NO and related RNS exert seemingly paradoxical effects in the central nervous system (81). NO is neuroprotective through S-nitrosylation of NMDA receptors and caspases, yet is neurodestructive through formation of peroxynitrite or S-nitrosylation of matrix metalloproteinase-9, GAPDH, and other targets, as discussed below (53, 55, 81) (Fig. 2).
Accumulating evidence suggests that S-nitrosylation is analogous to phosphorylation in regulating the biological activity of many proteins (31, 53, 55, 56, 81, 118, 123, 140). However, the chemistry of NO is much more complex. NO is often a good “leaving group,” resulting in further oxidation of the thiol to a disulfide bond between neighboring (vicinal) cysteine residues. Alternatively, as NO leaves because of another reaction partner, the thiol group can react with ROS to yield sulfenic (-SOH), sulfinic (-SO2H), or sulfonic (-SO3H) acid derivatives on the cysteine residue of the protein (53, 123, 140). S-Nitrosylation may also possibly produce a nitroxyl disulfide, in which the NO group is shared by proximate cysteine thiols (60).
At low (physiological) levels, NO can mediate neuroprotective functions. For example, our group first identified the physiological relevance of S-nitrosylation by showing that NO reacts with the NMDA receptor to downregulate its excessive activity (79–81). Specifically, we found that five different cysteine residues on extracellular domains of the NMDA receptor could react with NO. One of these, located at cysteine residue #399 (Cys399) on the NR2A subunit of the NMDA receptor, mediates ≥90% of the effect of NO under our experimental conditions (30). From crystal structure models and electrophysiological experiments, we further found that NO binding to the NMDA receptor at Cys399 may induce a conformational change in the receptor protein that makes glutamate and Zn2+ bind more tightly to the receptor. The enhanced binding of glutamate and Zn2+ in turn causes the receptor to desensitize and, consequently, the ion channel to close (82). As opposed to ambient air with an oxygen tension of 150 torr, a pO2 of 10–20 torr is found in normal brain, and even lower levels occur under hypoxic/ischemic conditions. We recently found that as the oxygen tension is lowered the NMDA receptor becomes more sensitive to inhibition by S-nitrosylation (121). In sum, NO inhibits hyperactivation of the NMDA receptor through S-nitrosylation, providing neuroprotective affects under excitotoxic conditions.
In contrast, we and other colleagues have also studied the consequence of excessive (pathophysiological) generation of oxidative/nitrosative reaction. For example, recent evidence suggests that the presence of excessive NO-related species may play a significant role in the process of protein misfolding. Increased nitrosative and oxidative stress are associated with chaperone and proteasomal dysfunction, resulting in accumulation of misfolded aggregates (62, 145). However, until recently little was known regarding the molecular and pathogenic mechanisms underlying contributions of NO to the formation of aggregates such as amyloid plaques in AD or Lewy bodies in PD. We and others recently presented physiological and chemical evidence that S-nitrosylation modulates the ubiquitin E3 ligase activity of parkin (31, 83, 140). Additionally, we found that S-nitrosylation regulates the chaperone and isomerase activities of PDI (123), contributing to protein misfolding and neurotoxicity in models of neurodegenerative disorders.
Another reaction of NO, in this case with superoxide anion, to form peroxynitrite can contribute to nitration of tyrosine residues, which may also potentially contribute to dysfunctional protein folding and neuronal cell injury. For instance, nitration of α-synuclein and tau affects oligomer formation in vitro. Further, nitrated α-synuclein and tau selectively accumulate in inclusion bodies in PD and neurofibrillary tangles in AD brains (50, 112, 113, 124). Collectively, these findings support the proposition that S-nitrosylation cysteine residues and possibly nitration or tyrosine residues can influence protein aggregation and neurotoxicity.
NO has also been reported to regulate mitochondrial function. Under physiological conditions, the NO-cyclic guanosine-3′,5′-monophosphate pathway induces mitochondrial biogenesis through peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) (94). In contrast, increased nitrosative stress can result in defects in mitochondrial function. For example, NO/S-nitrosylation affects mitochondrial respiration by reversibly inhibiting complexes I and IV (14, 22, 33, 34, 39), and the formation of SNO complex I may be implicated in PD (27, 28, 61). Mitochondria thus compromised will release ROS, and this in turn could contribute to brain aging and/or pathological conditions associated with neurodegenerative diseases. Additionally, increased nitrosative stress can elicit dysfunction of mitochondrial dynamics (fission and fusion events) (8, 15, 144). Although the exact mechanism whereby NO contributes to excessive fragmentation of mitochondria remains enigmatic, our recent findings have shed light on the molecular events underlying this relationship, particularly in AD. Specifically, we have recently discovered (patho)physiological and chemical evidence that S-nitrosylation modulates the GTPase activity of the mitochondrial fission protein, Drp1 (dynamin related protein 1), thus contributing to mitochondrial fragmentation. We found that excessive mitochondrial fragmentation results in bioenergetic impairment, synaptic damage, and eventually frank neuronal loss in models of AD (29).
Protein Misfolding and Aggregation in Neurodegenerative Diseases
Healthy neurons generally show no accumulation of protein aggregates, indicating that the appearance of such structures is a response to pathological stresses. Considerable evidence suggests that misfolded or otherwise abnormal proteins are produced even in healthy cells. The difference can largely be accounted for by cellular mechanisms for quality control, such as molecular chaperones, the ubiquitin-proteasome system (UPS), and autophagy/lysosomal degradation. A reduction in molecular chaperone or proteasome activities under pathological conditions can result in deposition and accumulation of aberrant proteins either within or outside of cells in the brain. Several mutations in molecular chaperones or UPS-associated enzymes are known to contribute to neurodegeneration (36, 91). For example, a reduction in proteasome activity was found in the substantia nigra of PD patients (90), and overexpression of the molecular chaperone HSP70 prevented neurodegeneration in vivo in models of PD (6). Aggregated proteins were first considered to be pathogenic. However, recent evidence suggests that macroscopic aggregates are an attempt by the cell to sequester aberrant proteins, whereas soluble (micro-) oligomers of such proteins are the most toxic forms (18).
S-Nitrosylation of Parkin and the UPS
Studies of rare mutations have revealed key components of the mechanism for protein aggregation and pathology in PD, including sporadic forms of the disease. Such studies revealed that mutated α-synuclein is a major constituent of Lewy bodies in PD patient brains, and that mutant forms of the ubiquitin E3 ligase parkin or the ubiquitin carboxy-terminal hydrolase UCH-L1 (a deubiquinating enzyme) may result in UPS dysfunction and also result in hereditary forms of PD. Formation of polyubiquitin chains on a peptide constitutes the signal for proteasomal degradation. The cascade of activation (E1), conjugation (E2), and ubiquitin-ligase (E3)-type enzymes catalyzes the conjugation of the ubiquitin chain to the proteins marked for degradation. Individual E3 ubiquitin ligases play a key role in the recognition of specific peptide substrates (114).
Parkin is a member of a large family of E3 ubiquitin ligases. Parkin contains a total of 35 cysteine residues, many of which coordinate structurally important zinc atoms, which are often involved in catalysis (88). Parkin recruits substrate proteins as well as an E2 enzyme (e.g., UbcH7, UbcH8, or UbcH13). Mutations in the gene encoding parkin have been associated with autosomal recessive juvenile PD. In this case, mutations underlying this disorder usually do not produce Lewy bodies. However, other mutations in parkin resulting in adult onset PD have been associated with Lewy body formation. Mutations in both alleles of the parkin gene will cause dysfunction in its activity, although not all mutations result in loss of parkin E3 ligase activity (36). Additionally, wild-type parkin can mediate the formation of nonclassical and nondegradative lysine 63-linked polyubiquitin chains (76, 77). Parkin can also monoubiquitinate Eps15, HSP70, and itself, possibly at multiple sites. These activities may explain why some parkin mutations result in the formation of Lewy bodies, whereas others do not. Synphilin-1 (α-synuclein interacting protein) is a well-characterized substrate for parkin ubiquitination, and is found in Lewy body-like inclusions in cultured cells when coexpressed with α-synuclein. Accumulation of these proteins portends a poor prognosis for the survival of dopaminergic neurons in familial PD and possibly also in sporadic PD.
PD is the second most prevalent neurodegenerative disease and is characterized by the progressive loss of dopamine neurons in the substantia nigra pars compacta. Aberrant protein accumulation is observed in patients with genetically encoded mutant proteins, and recent evidence from our and other laboratories suggests that nitrosative/oxidative stress acts as a potential causal factor for protein misfolding in the much more common sporadic form of PD. Nitrosative/oxidative stress can mimic hereditary PD by promoting protein misfolding in the absence of a genetic mutation (31, 83, 140). In fact, S-nitrosylation and further oxidation of parkin result in a dysfunctional enzyme and disruption of UPS function (31, 140). We found that nitrosative stress produces S-nitrosylation of parkin (forming SNO-parkin) in rodent models of PD and in brains of human patients with PD and the related α-synucleinopathy, diffuse Lewy body disease. Initially, S-nitrosylation of parkin stimulates its ubiquitin E3 ligase activity, which may contribute to Lewy body formation. Subsequently, with time we found that the E3 ligase activity of SNO-parkin decreases, resulting in UPS dysfunction (83, 140) (Fig. 3). Importantly, S-nitrosylation of parkin on critical cysteine residues also compromises its neuroprotective activity (31). It is likely that S-nitrosylation influences the enzymatic functions of similar ubiquitin E3 ligases, suggesting that this process may be involved in a number of degenerative disorders.
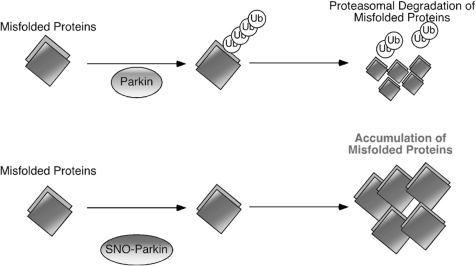
FIG. 3.
Possible mechanism whereby S-nitrosylated parkin contributes to the accumulation of aberrant proteins and neuronal damage. The ubiquitin E3 ligase parkin is believed to be involved in ubiquitin-proteasome-dependent degradation of misfolded proteins (top). S-Nitrosylation of parkin alters its E3 ligase activity, thus inducing ubiquitin-proteasome system dysfunction (bottom). Thus, S-nitrosylation of parkin (forming SNO-Parkin) can contribute to neuronal cell injury in part by triggering accumulation of misfolded proteins.
In addition to parkin, recent studies on rare genetic forms of PD have found that mutations in the genes encoding PINK1 (PARK6), α-synuclein (PARK1/4), DJ-1 (PARK7), leucine-rich repeat kinase-2 (LRRK2) (PARK8), or ATP13A2 (PARK9) are also associated with PD pathology (13, 69, 72, 97, 105, 110, 125, 147). NO can bind to cysteine residue(s) of DJ-1 to form an S-nitrosothiol and may affect its dimerization (63). However, the exact mechanism of SNO-DJ1 contributing to PD pathogenesis remains to be elucidated. It remains possible that S-nitrosylation may regulate the activity of additional PD-related gene products, such as PINK1, LRRK2, and ATP13A2 (but not α-synuclein because it lacks cysteine residues), but further studies will be needed to investigate this possibility.
S-Nitrosylation of PDI Mediates Protein Misfolding and Neurotoxicity in Cell Models of PD and AD
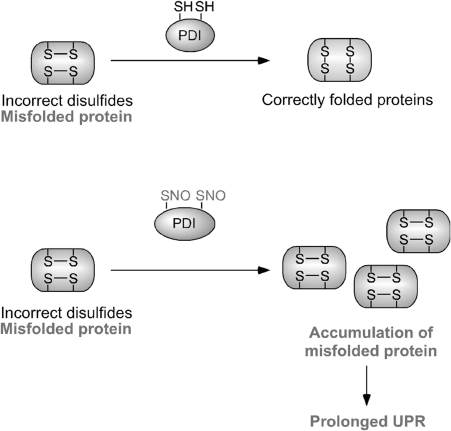
In the ER, PDI facilitates proper protein folding by introducing disulfide bonds into proteins (oxidation), breaking disulfide bonds (reduction), and catalyzing thiol/disulfide exchange (isomerization), thus facilitating disulfide bond formation, rearrangement reactions, and structural stability (87). During oxidation of a target protein, oxidized PDI catalyzes disulfide formation in the substrate protein, resulting in the reduction of PDI. In contrast, the reduced form of the active-site cysteines can initiate isomerization by attacking the disulfide of a substrate protein and forming a transient intermolecular disulfide bond. As a consequence, an intramolecular disulfide rearrangement occurs within the substrate itself, resulting in the generation of reduced PDI (Fig. 4). Several mammalian PDI homologs, such as ERp57 and PDIp, also localize to the ER and may manifest similar functions (35). Increased expression of PDIp in neuronal cells under conditions mimicking PD suggests the possible contribution of PDIp to neuronal survival (35). In many neurodegenerative disorders and cerebral ischemia, the accumulation of immature and denatured proteins results in ER dysfunction (35), but upregulation of PDI represents an adaptive response promoting protein refolding and may offer neuronal cell protection (35). We recently reported that S-nitrosylation of PDI (to form SNO-PDI) disrupts normal protein folding and this neuroprotective role (123).
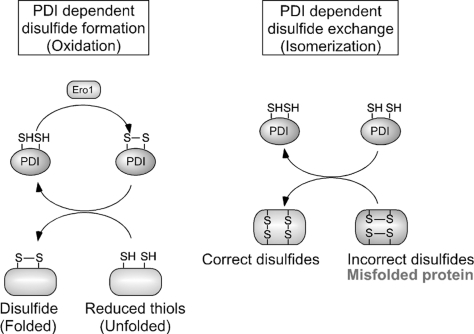
FIG. 4.
Molecular mechanisms of PDI-dependent oxidative protein folding in the endoplasmic reticulum (ER). Left: Oxidized PDI (top), which contains a disulfide bond at its active site, catalyzes the formation of a disulfide bond in a substrate protein (bottom), resulting in the reduction of PDI. Conversely, the ER oxidoreduction protein, Ero1, can reoxidize and regenerate the PDI active site. By repeating this cycle, PDI can continuously insert disulfide bonds into different substrate proteins. Right: In the early process of protein folding in the ER, cysteine residues often form inaccurate disulfide bonds (resulting in a misfolded protein). The isomerase activity of PDI (top) converts these incorrect disulfide bonds to their correct native form. This reaction occurs through breakage of substrate disulfide, formation of intramolecular disulfide, and reformation of intermolecular disulfide bonds with different thiols in the target substrate protein (bottom). For simplicity, the redox state of only one PDI active site is shown.
The ER normally manifests a relatively positive redox potential in contrast to the highly reducing environment of the cytosol and mitochondria. This redox environment in the ER can influence the stability of protein S-nitrosylation and oxidation reactions (45). Excessive NO is known to create ER stress by disruption of Ca2+ homeostasis. One possible mechanism is the increased activity of the ER Ca2+ channel-ryanodine receptor through S-nitrosylation (137). We have recently reported that excessive NO, as well as rotenone exposure, which is known to lead to PD, can lead to S-nitrosylation of the active-site thiols of PDI, inhibiting its isomerase and chaperone activities (123). S-Nitrosylation of PDI prevented its attenuation of neuronal cell death triggered by ER stress, misfolded proteins, or proteasome inhibition. Also, PDI was S-nitrosylated in the brains of virtually all cases we examined of sporadic AD and PD. These results suggest that SNO-PDI can mediate protein misfolding and consequent neuronal cell death or injury (Fig. 5).
FIG. 5.
Possible mechanism whereby S-nitrosylated PDI contributes to the accumulation of aberrant proteins and neuronal damage. PDI is known to associate with nascent or misfolded proteins and to refold them correctly (top). Under conditions of nitrosative stress, the decreased chaperone and isomerase activity of PDI due to S-nitrosylation enhances accumulation of misfolded proteins, thus activating a prolonged unfolded protein response (UPR) and neuronal cell death (bottom). In this manner, S-nitrosylation of PDI (forming SNO-PDI) can contribute to neuronal cell injury, in part by triggering accumulation of misfolded proteins.
The activity of the UPS and molecular chaperones normally decline with age (102). Since we have not found detectable levels of SNO-parkin and SNO-PDI in normal aged brain but only in disease states (31, 123, 140), it is likely that S-nitrosylation of these and similar proteins is a key mechanism contributing to neurodegenerative conditions.
PDI activity in ALS and prion disease
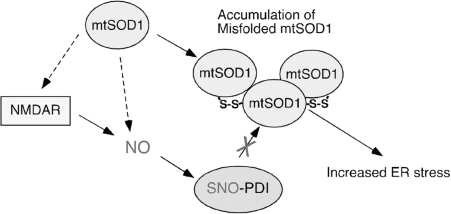
Recently, PDI has also been implicated in the pathophysiology of familial ALS (5). Mutations in Cu/Zn superoxide dismutase (SOD1) are known to be involved in motor neuron death in some forms of familial ALS. SOD1 is an intracellular homodimeric metalloprotein that forms a stable intrasubunit disulfide bond. Biochemical evidence suggests that the disulfide-reduced monomer of mutant SOD1 (mtSOD1) forms inclusion bodies (4, 43, 47, 108, 122), and aggregates of misfolded mtSOD1 are commonly associated with the disease, as seen at postmortem examination. In addition, although wild-type SOD1 is found predominantly in the cytoplasm, mtSOD1 forms monomers or insoluble high molecular weight multimers within the ER (67). Intriguingly, PDI has been found to colocalize and bind to intracellular aggregates of mtSOD1. Also, upregulation of the unfolded protein response in the ER is observed in mtSOD1 mice. Recent studies have shown that inhibition of PDI activity with bacitracin can increase aggregation of mtSOD1 in neuronal cells, and that regulation of endogenous PDI activity by reticulons protects against neurodegeneration (5, 139). In contrast, overexpression of PDI decreases mtSOD aggregation and mtSOD1-induced neuronal cell death. Taken together, these findings suggest that ER stress may contribute to the pathophysiology of familial ALS, and that increased PDI activity may reduce mtSOD1 aggregation and promote neuronal survival (5, 127, 139). Moreover, S-nitrosothiol levels were also found to be abnormal in the spinal cords of mtSOD1 transgenic mice (116), including elevated SNO-PDI (127) (Fig. 6). Sporadic ALS patients were also reported to manifest increased SNO-PDI levels, suggesting that S-nitrosylation of PDI may contribute to the pathogenesis of sporadic ALS (127). It will be important to determine whether SNO-PDI is involved in protein aggregation and motoneuron injury in ALS in the absence of SOD1 mutations.
FIG. 6.
Possible mechanism whereby S-nitrosylated PDI contributes to the accumulation of aberrant mutant Cu,Zn superoxide dismutase (mtSOD1) and neuronal damage in familial amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis-linked mtSOD1 is prone to form high-molecular-weight complexes (or aggregates) that contain non-native disulfide bonds. Accumulation of mtSOD1 is known to trigger ER stress. Although most mtSOD1 is present in the cytoplasm, recent lines of evidence suggest that mtSOD1 may be secreted via the ER-Golgi pathway. Further, the ER resident protein PDI binds to both wild-type SOD1 and mtSOD1, thereby reducing mtSOD1 aggregation and toxicity (5, 127). Since S-nitrosylation inhibits the enzymatic activity of PDI, overproduction of NO may contribute to mtSOD1 toxicity via the formation of SNO-PDI. Excessive NMDAR activity may contribute to mtSOD1 toxicity via production of NO to facilitate SNO-PDI formation (130).
Additionally, transmissible spongiform encephalopathies, also known as prion diseases, are transmissible neurodegenerative disorders and include Creutzfeldt-Jacob disease, bovine spongiform encephalopathy, and scrapie. Cerebral accumulation of misfolded prion protein (PrP) and extensive neuronal apoptosis represent pathological hallmarks of these prion diseases. Recent reports have suggested that a prolonged unfolded protein response due to PrP misfolding in the ER may contribute to neuronal dysfunction (57, 58, 142). This ER stress response is mainly associated with upregulation of GRP58, an ER chaperone with PDI-like activity, suggesting that this chaperone may play an important role in the cellular response to prion infection (58). In fact, in vitro studies on GRP58, either overexpressing (via transfection) or downregulating (via RNAi), demonstrated that this ER chaperone protects cells against PrP misfolding and toxicity. Collectively, these studies raise the possibility that SNO-PDI and S-nitrosylation of other chaperone molecules may represent potential therapeutic targets to prevent protein aggregation in several neurodegenerative diseases.
Mitochondrial Fission/Fusion Machinery in Nerve Cells
Neurons are particularly vulnerable to mitochondrial defects because they require high levels of energy for their survival and specialized function. Mitochondrial biogenesis, an event that produces new mitochondria, is required in regions that demand high concentrations of ATP, especially the synapse. The distribution of mitochondria at the nerve terminal can control synaptic transmission and structure (25, 73, 75).
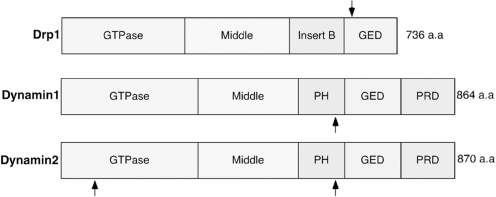
In healthy neurons, the fission/fusion machinery proteins maintain mitochondrial integrity and insure their presence at critical locations. These proteins includes Drp1 and Fis1, acting as fission proteins, and mitofusin (Mfn) and Opa1, operating as fusion proteins (143). In both familial and sporadic neurodegenerative conditions, abnormal mitochondria regularly appear in the brain as a result of dysfunction in the fission/fusion machinery. Genetic mutations in Mfn2 can cause Charcot-Marie-Tooth disease, a hereditary peripheral neuropathy that affects both motor and sensory neurons (66, 149). Mutations in Opa1 cause autosomal dominant optic atrophy, characterized by the loss of retinal ganglion cells and the optic nerve, representing their axons (41). Recently, Waterham et al. described a heterozygous, dominant-negative mutation of Drp1 in a patient whose symptoms were broadly similar to those of Charcot-Marie-Tooth neuropathy and autosomal dominant optic atrophy (135). Drp1 includes four distinct structural domains: an N-terminal GTPase domain, a dynamin-like middle domain, an insert B domain, and a C-terminal GTPase effector domain (Fig. 7). The mutation (Ala 395 to Asp) was found in the middle domain of Drp1. This case study further suggested that a defect in mitochondrial fission may have more severe consequences than those of fusion defects, since the Drp1 mutation caused a much earlier onset (prenatal) and fatal outcome. Additionally, it is apparent that the balance between fission and fusion is critical for normal function of mitochondria and determination of phenotype in disease. These fission/fusion proteins are widely expressed in human tissues, clearly supporting the notion that neurons are particularly sensitive to mitochondrial dysfunction.
FIG. 7.
Schematic structure of the domains of Drp1 and Dynamin1/2. The length and topology of amino acids for each region are shown. Each protein has a GTPase domain, a middle domain, and a GTPase effector domain (GED). Dynamin1/2 also contains a pleckstrin homology (PH) domain and a highly basic C-terminal proline-rich domain (PRD). Arrows indicate S-nitrosylated cysteine residues (Cys644 of Drp1, Cys 607 of Dynamin1, and Cys 60 and 607 of Dynamin2). S-Nitrosylation of these proteins upregulates their GTPase activity (29, 64, 128).
Dysregulation of mitochondrial dynamics in AD
An estimated 26 million people globally have AD, which is thought to be the most common form of dementia. In AD brains, neuronal loss in the hippocampus and cerebral cortex mainly accounts for the cognitive decline. Degenerating AD brains contain aberrant accumulations of misfolded, aggregated proteins—Aβ and tau—which can adversely affect neuronal connectivity and plasticity, and trigger cell death signaling pathways. These aggregates are recognized as either intracellular neurofibrillary tangles, which contain hyperphosphorylated tau, or extracellular plaques, which contain Aβ. β-Secretase and γ-secretase proteolytically cleave amyloid precursor protein in its transmembrane region to generate Aβ. It is currently thought that soluble oligomers of misfolded protein are pathogenic and that the large aggregates may actually be an attempt by the cell to wall off the aberrant proteins (although such aggregates could potentially be toxic by location or if not contained by the proper chaperones). A recent study showed that an N-terminal fragment of amyloid precursor protein may also contribute to neurodegeneration in AD models (93).
Emerging evidence suggests that mitochondrial dysfunction plays a prominent role in the pathogenesis of AD (133). Analyses of autopsy and biopsy samples revealed that mitochondria isolated from AD brains exhibit diminished respiratory capacity (101), and that AD neurons contain a number of mitochondria with fractured cristae (59). Additionally, electron-microscopic studies have described an increase in mitochondrial fragmentation in human AD brains (7, 131). In cell-based experiments, Aβ production resulted in the appearance of fragmented and abnormally distributed mitochondria (8, 132), suggesting that Aβ (possibly in the form of soluble oligomers) may trigger excessive mitochondrial fission in AD patients. Pathological forms of tau may also contribute to mitochondrial fragmentation in AD brains since expression of caspase-cleaved tau induced mitochondrial fission in a calcineurin-dependent manner (107).
Similarly, dysfunction in mitochondrial integrity is associated with PD (126). For instance, the Parkinsonian neurotoxins, rotenone and l-methyl-4-phenyl-pyridinium ion, which inhibit complex I of the mitochondrial electron transport chain, can induce excessive mitochondrial fragmentation and cell death (8). Additionally, multiple groups recently observed that a deficiency in familial PD-related proteins, such as parkin and PINK1, led to the appearance of mitochondrial pathology (44, 51, 86, 106). Exogenous expression of mitochondrial fusion proteins, Mfn2 and OPA1, or dominant negative Drp1 rescued the altered mitochondrial morphology, suggesting that parkin or PINK1 deficiency promoted mitochondrial fragmentation (86). Additionally, Drp1 seems to activate autophagy/mitophagy pathways for morphologic remodeling of mitochondria in PINK1-deficient neuroblastoma cells (38). Further, PINK1 escorts parkin to damaged mitochondria, where parkin ubiquitinates the voltage-dependent anion channel 1 and promotes autophagic clearance of damaged mitochondria (mitophagy) (49). In contrast, the PINK1/Parkin pathway also appears to promote mitochondrial fission and has also been reported to inhibit mitochondrial fusion in Drosophila (42, 106, 138). For example, the Drosophila PINK1/parkin pathway induces Mfn ubiquitination, which results in lower Mfn levels and thus the appearance of small-sized mitochondria (148). Thus, although further studies will be needed to understand the significance of these findings for the pathogenesis of PD, it is clear that PD-associated genes influence mitochondrial dynamics.
S-Nitrosylation of Drp1 Mediates Mitochondrial Fission and Neurotoxicity in Cell Models of AD
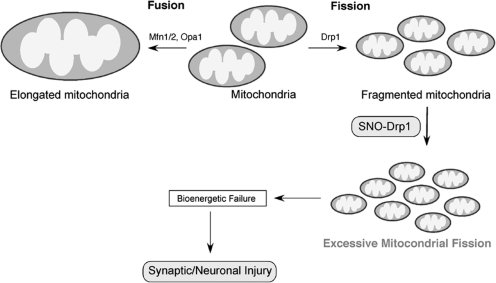
In addition to rare hereditary mutations in the genes encoding mitochondrial fission and fusion proteins, recent studies have demonstrated that posttranslational modification of these proteins can contribute to altered mitochondria dynamics. For example, phosphorylation, ubiquitination, sumoylation, and proteolytic cleavage of Drp1 regulate mitochondrial fission by affecting Drp1 activity, at least in cell culture systems (17, 23, 37, 65, 92, 120, 134, 141). Excessive activation of mitochondrial fission or fusion proteins by posttranslational modification was posited to contribute to neurodegeneration by compromising mitochondrial function. Along these lines, we recently reported that excessive NO can lead to S-nitrosylation of Drp1 at Cys644 (29) (Fig. 8). Cys644 resides within the GTPase effector domain of Drp1, which influences both GTPase activity and oligomer formation of Drp1 (85, 104, 109, 146). S-Nitrosylation of Drp1 (forming SNO-Drp1) induces formation of Drp1 dimers, which function as building blocks for tetramers and higher order structures of Drp1, and activates Drp1 GTPase activity; however, substitution of Cys644 to Ala (C644A) abrogated these effects of NO.
FIG. 8.
Possible mechanism whereby S-nitrosylated Drp1 contributes to abnormal mitochondrial function and neuronal damage. In healthy neurons, mitochondria continuously undergo fission and fusion to maintain their integrity and insure their presence at critical locations. Mitochondrial fission is driven by fission proteins such as Drp1 and fis1. Components involved in mitochondrial membrane fusion include Opa1 and mitofusin (Mfn)1/2. The NO group reacts with a critical cysteine thiol on Drp1 by S-nitrosylation to form SNO-Drp1. SNO-Drp1 contributes to synaptic and neuronal injury by leading to excessive mitochondrial fission/fragmentation and bioenergetic impairment.
We further demonstrated that exposure to oligomeric Aβ peptide results in formation of SNO-Drp1 in cell culture models. Moreover, we and our colleagues have observed that Drp1 is S-nitrosylated in the brains of virtually all cases of sporadic AD (29, 131). To determine the consequences of S-nitrosylated Drp1 in neurons, we exposed cultured cerebrocortical neurons to the physiological NO donor, S-nitrosocysteine, or to Aβ oligomers and found that both induced SNO-Drp1 formation and led to the accumulation of excessively fragmented mitochondria. Moreover, mutation of a specific cysteine residue in Drp1 (C644A) prevented these effects of S-nitrosocysteine or Aβ on mitochondrial fragmentation, consistent with the notion that SNO-Drp1 triggered excessive mitochondria fission or fragmentation. Finally, in response to Aβ, SNO-Drp1-induced mitochondrial fragmentation caused synaptic damage, an early characteristic feature of AD, and eventually apoptotic neuronal cell death. Importantly, blockade of Drp1 nitrosylation [using the Drp1(C644A) mutant] prevented Aβ-mediated synaptic loss and neuronal cell death, suggesting that SNO-Drp1 may represent a potential therapeutic target to protect neurons and their synapses in AD. Thus, the posttranslational modification of S-nitrosylation can mimic the effect of rare genetic mutations in contributing to the AD phenotype.
Unlike AD and PD, HD is a purely genetic disease caused by mutations that result in CAG expansion in the first exon of the Huntingtin (Htt) gene, with more than ∼35 CAGs being pathogenic and resulting in polyglutamine expression. Mitochondrial dysfunction is also associated with pathogenesis of HD (111). Respiratory electron transport chain activity and ATP levels are decreased in mitochondria from HD patients and Htt transgenic mice (98, 117, 119). Additionally, the complex II inhibitor, 3-nitropropionic acid (3-NP), causes a movement disorder similar in many respects to HD (20). Moreover, mutant Htt directly impairs mitochondrial membrane potential, calcium homeostasis, and mitochondrial axonal trafficking (24, 99). Recently, Wang et al. demonstrated that expression of mutant Htt sensitizes cells to oxidative stress-induced mitochondrial fission and reduces ATP levels by inhibiting mitochondrial fusion (129). Overexpression of the Drp1 dominant-negative K38A or the fusion protein Mfn2 reduces mutant Htt-induced mitochondrial fragmentation as well ATP loss and cell death. Importantly, RNAi against Drp1 also reduces the motility defect in a worm model of HD. In addition, 3-NP exposure induces increased mitochondrial fragmentation in an NMDA receptor-dependent manner in cerebrocortical neurons (78). Remarkably, we have also observed S-nitrosylation of Drp1 in HD brains similar to that seen in AD brains, raising the possibility that this redox event may play a pathogenic role in HD in addition to AD. Collectively, these studies suggest that alterations in mitochondrial dynamics may be involved in the pathogenesis of HD.
Conclusions
Sporadic forms of neurodegenerative diseases can be caused by excessive NMDA receptor activation and/or mitochondrial dysfunction that results in excessive nitrosative and oxidative stress. These pathological processes can result in malfunction of the UPS and/or molecular chaperones and contribute to abnormal protein accumulation and neuronal damage. Here we have described a mechanistic link between free radical production, protein misfolding, abnormal mitochondrial dynamics, and neuronal cell injury in neurodegenerative disorders such as PD and AD. The elucidation of NO-mediated S-nitrosylation of parkin, PDI, and Drp1 in neurodegenerative disease may promote development of new drugs to prevent aberrant protein misfolding or excessive mitochondrial fission by targeted disruption of this process.
Abbreviations Used
- Aβ
amyloid-β
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- cGMP
cyclic guanosine-3′,5′-monophosphate
- Drp1
dynamin-related protein 1
- ER
endoplasmic reticulum
- GED
GTPase effector domain
- Htt
Huntingtin
- iNOS
inducible nitric oxide synthase
- Mfn
mitofusin
- mtSOD1
mutant Cu,Zn superoxide dismutase
- NMDA
N-methyl-d-aspartate
- nNOS
neuronal NOS
- NO
nitric oxide
- PD
Parkinson's disease
- PDI
protein disulfide isomerase
- PrP
prion protein
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SNO
S-nitrosylated
- UPR
unfolded protein response
- UPS
ubiquitin-proteasome system
Acknowledgments
This work was supported in part by NIH grants P01 ES016738, P01 HD29587, R01 EY05477, and R01 EY09024; the American Parkinson's Disease Association, San Diego Chapter; and an Ellison Senior Scholars Award in Aging (to S.A.L.).
References
- 1.Abu-Soud HM. Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A. 1993;90:10769–10772. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akama KT. Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- 3.Ankarcrona M. Dypbukt JM. Bonfoco E. Zhivotovsky B. Orrenius S. Lipton SA. Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 4.Arnesano F. Banci L. Bertini I. Martinelli M. Furukawa Y. O'Halloran TV. The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279:47998–48003. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 5.Atkin JD. Farg MA. Turner BJ. Tomas D. Lysaght JA. Nunan J. Rembach A. Nagley P. Beart PM. Cheema SS. Horne MK. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J Biol Chem. 2006;281:30152–30165. doi: 10.1074/jbc.M603393200. [DOI] [PubMed] [Google Scholar]
- 6.Auluck PK. Chan HY. Trojanowski JQ. Lee VM. Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 7.Baloyannis SJ. Mitochondrial alterations in Alzheimer's disease. J Alzheimers Dis. 2006;9:119–126. doi: 10.3233/jad-2006-9204. [DOI] [PubMed] [Google Scholar]
- 8.Barsoum MJ. Yuan H. Gerencser AA. Liot G. Kushnareva Y. Graber S. Kovacs I. Lee WD. Waggoner J. Cui J. White AD. Bossy B. Martinou JC. Youle RJ. Lipton SA. Ellisman MH. Perkins GA. Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol. 1998;44:S110–S114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 10.Beal MF. Experimental models of Parkinson's disease. Nat Rev Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 11.Betarbet R. Sherer TB. MacKenzie G. Garcia-Osuna M. Panov AV. Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 12.Bonfoco E. Krainc D. Ankarcrona M. Nicotera P. Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonifati V. Rizzu P. van Baren MJ. Schaap O. Breedveld GJ. Krieger E. Dekker MC. Squitieri F. Ibanez P. Joosse M. van Dongen JW. Vanacore N. van Swieten JC. Brice A. Meco G. van Duijn CM. Oostra BA. Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 14.Borutaite V. Brown GC. S-nitrosothiol inhibition of mitochondrial complex I causes a reversible increase in mitochondrial hydrogen peroxide production. Biochim Biophys Acta. 2006;1757:562–566. doi: 10.1016/j.bbabio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Bossy-Wetzel E. Lipton SA. Nitric oxide signaling regulates mitochondrial number and function. Cell Death Differ. 2003;10:757–760. doi: 10.1038/sj.cdd.4401244. [DOI] [PubMed] [Google Scholar]
- 16.Boucher JL. Moali C. Tenu JP. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol Life Sci. 1999;55:1015–1028. doi: 10.1007/s000180050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breckenridge DG. Kang BH. Kokel D. Mitani S. Staehelin LA. Xue D. Caenorhabditis elegans drp-1 and fis-2 regulate distinct cell-death execution pathways downstream of ced-3 and independent of ced-9. Mol Cell. 2008;31:586–597. doi: 10.1016/j.molcel.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredt DS. Hwang PM. Glatt CE. Lowenstein C. Reed RR. Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 19.Brennan AM. Suh SW. Won SJ. Narasimhan P. Kauppinen TM. Lee H. Edling Y. Chan PH. Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brouillet E. Hantraye P. Ferrante RJ. Dolan R. Leroy-Willig A. Kowall NW. Beal MF. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc Natl Acad Sci U S A. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budd SL. Tenneti L. Lishnak T. Lipton SA. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc Natl Acad Sci U S A. 2000;97:6161–6166. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burwell LS. Nadtochiy SM. Tompkins AJ. Young S. Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CR. Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 24.Chang DT. Rintoul GL. Pandipati S. Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Chen H. Chan DC. Critical dependence of neurons on mitochondrial dynamics. Curr Opin Cell Biol. 2006;18:453–459. doi: 10.1016/j.ceb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Chen HS. Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 27.Chinta SJ. Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson's disease. Free Radic Biol Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Chinta SJ. Kumar MJ. Hsu M. Rajagopalan S. Kaur D. Rane A. Nicholls DG. Choi J. Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho DH. Nakamura T. Fang J. Cieplak P. Godzik A. Gu Z. Lipton SA. S-Nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YB. Tenneti L. Le DA. Ortiz J. Bai G. Chen HS. Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- 31.Chung KK. Thomas B. Li X. Pletnikova O. Troncoso JC. Marsh L. Dawson VL. Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 32.Ciechanover A. Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 33.Cleeter MW. Cooper JM. Darley-Usmar VM. Moncada S. Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 34.Clementi E. Brown GC. Feelisch M. Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conn KJ. Gao W. McKee A. Lan MS. Ullman MD. Eisenhauer PB. Fine RE. Wells JM. Identification of the protein disulfide isomerase family member PDIp in experimental Parkinson's disease and Lewy body pathology. Brain Res. 2004;1022:164–172. doi: 10.1016/j.brainres.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 37.Cribbs JT. Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagda RK. Cherra SJ., 3rd Kulich SM. Tandon A. Park D. Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahm CC. Moore K. Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J Biol Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 40.Dawson VL. Dawson TM. London ED. Bredt DS. Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delettre C. Lenaers G. Griffoin JM. Gigarel N. Lorenzo C. Belenguer P. Pelloquin L. Grosgeorge J. Turc-Carel C. Perret E. Astarie-Dequeker C. Lasquellec L. Arnaud B. Ducommun B. Kaplan J. Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 42.Deng H. Dodson MW. Huang H. Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doucette PA. Whitson LJ. Cao X. Schirf V. Demeler B. Valentine JS. Hansen JC. Hart PJ. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J Biol Chem. 2004;279:54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 44.Exner N. Treske B. Paquet D. Holmstrom K. Schiesling C. Gispert S. Carballo-Carbajal I. Berg D. Hoepken HH. Gasser T. Kruger R. Winklhofer KF. Vogel F. Reichert AS. Auburger G. Kahle PJ. Schmid B. Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forrester MT. Benhar M. Stamler JS. Nitrosative stress in the ER: a new role for S-nitrosylation in neurodegenerative diseases. ACS Chem Biol. 2006;1:355–358. doi: 10.1021/cb600244c. [DOI] [PubMed] [Google Scholar]
- 46.Forstermann U. Boissel JP. Kleinert H. Expressional control of the “constitutive” isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- 47.Furukawa Y. O'Halloran TV. Amyotrophic lateral sclerosis mutations have the greatest destabilizing effect on the apo- and reduced form of SOD1, leading to unfolding and oxidative aggregation. J Biol Chem. 2005;280:17266–17274. doi: 10.1074/jbc.M500482200. [DOI] [PubMed] [Google Scholar]
- 48.Garthwaite J. Charles SL. Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 49.Geisler S. Holmstrom KM. Skujat D. Fiesel FC. Rothfuss OC. Kahle PJ. Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 50.Giasson BI. Duda JE. Murray IV. Chen Q. Souza JM. Hurtig HI. Ischiropoulos H. Trojanowski JQ. Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 51.Greene JC. Whitworth AJ. Kuo I. Andrews LA. Feany MB. Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groves JT. Wang CC. Nitric oxide synthase: models and mechanisms. Curr Opin Chem Biol. 2000;4:687–695. doi: 10.1016/s1367-5931(00)00146-0. [DOI] [PubMed] [Google Scholar]
- 53.Gu Z. Kaul M. Yan B. Kridel SJ. Cui J. Strongin A. Smith JW. Liddington RC. Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 54.Gupta SP. Patel S. Yadav S. Singh AK. Singh S. Singh MP. Involvement of nitric oxide in maneb- and paraquat-induced Parkinson's disease phenotype in mouse: is there any link with lipid peroxidation? Neurochem Res. 2010;35:1206–1213. doi: 10.1007/s11064-010-0176-5. [DOI] [PubMed] [Google Scholar]
- 55.Hara MR. Agrawal N. Kim SF. Cascio MB. Fujimuro M. Ozeki Y. Takahashi M. Cheah JH. Tankou SK. Hester LD. Ferris CD. Hayward SD. Snyder SH. Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 56.Hess DT. Matsumoto A. Kim SO. Marshall HE. Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 57.Hetz C. Russelakis-Carneiro M. Maundrell K. Castilla J. Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hetz C. Russelakis-Carneiro M. Walchli S. Carboni S. Vial-Knecht E. Maundrell K. Castilla J. Soto C. The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J Neurosci. 2005;25:2793–2802. doi: 10.1523/JNEUROSCI.4090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirai K. Aliev G. Nunomura A. Fujioka H. Russell RL. Atwood CS. Johnson AB. Kress Y. Vinters HV. Tabaton M. Shimohama S. Cash AD. Siedlak SL. Harris PL. Jones PK. Petersen RB. Perry G. Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Houk KN. Hietbrink BN. Bartberger MD. McCarren PR. Choi BY. Voyksner RD. Stamler JS. Toone EJ. Nitroxyl disulfides, novel intermediates in transnitrosation reactions. J Am Chem Soc. 2003;125:6972–6976. doi: 10.1021/ja029655l. [DOI] [PubMed] [Google Scholar]
- 61.Hsu M. Srinivas B. Kumar J. Subramanian R. Andersen J. Glutathione depletion resulting in selective mitochondrial complex I inhibition in dopaminergic cells is via an NO-mediated pathway not involving peroxynitrite: implications for Parkinson's disease. J Neurochem. 2005;92:1091–1103. doi: 10.1111/j.1471-4159.2004.02929.x. [DOI] [PubMed] [Google Scholar]
- 62.Isaacs AM. Senn DB. Yuan M. Shine JP. Yankner BA. Acceleration of amyloid beta-peptide aggregation by physiological concentrations of calcium. J Biol Chem. 2006;281:27916–27923. doi: 10.1074/jbc.M602061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito G. Ariga H. Nakagawa Y. Iwatsubo T. Roles of distinct cysteine residues in S-nitrosylation and dimerization of DJ-1. Biochem Biophys Res Commun. 2006;339:667–672. doi: 10.1016/j.bbrc.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 64.Kang-Decker N. Cao S. Chatterjee S. Yao J. Egan LJ. Semela D. Mukhopadhyay D. Shah V. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J Cell Sci. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- 65.Karbowski M. Neutzner A. Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kijima K. Numakura C. Izumino H. Umetsu K. Nezu A. Shiiki T. Ogawa M. Ishizaki Y. Kitamura T. Shozawa Y. Hayasaka K. Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2A. Hum Genet. 2005;116:23–27. doi: 10.1007/s00439-004-1199-2. [DOI] [PubMed] [Google Scholar]
- 67.Kikuchi H. Almer G. Yamashita S. Guegan C. Nagai M. Xu Z. Sosunov AA. McKhann GM., 2nd Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci U S A. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim E. Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 69.Kitada T. Asakawa S. Hattori N. Matsumine H. Yamamura Y. Minoshima S. Yokochi M. Mizuno Y. Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 70.Kolodziejska KE. Burns AR. Moore RH. Stenoien DL. Eissa NT. Regulation of inducible nitric oxide synthase by aggresome formation. Proc Natl Acad Sci U S A. 2005;102:4854–4859. doi: 10.1073/pnas.0500485102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lauderback CM. Harris-White ME. Wang Y. Pedigo NW., Jr. Carney JM. Butterfield DA. Amyloid beta-peptide inhibits Na+-dependent glutamate uptake. Life Sci. 1999;65:1977–1981. doi: 10.1016/s0024-3205(99)00459-2. [DOI] [PubMed] [Google Scholar]
- 72.Leroy E. Boyer R. Auburger G. Leube B. Ulm G. Mezey E. Harta G. Brownstein MJ. Jonnalagada S. Chernova T. Dehejia A. Lavedan C. Gasser T. Steinbach PJ. Wilkinson KD. Polymeropoulos MH. The ubiquitin pathway in Parkinson's disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 73.Li H. Chen Y. Jones AF. Sanger RH. Collis LP. Flannery R. McNay EC. Yu T. Schwarzenbacher R. Bossy B. Bossy-Wetzel E. Bennett MV. Pypaert M. Hickman JA. Smith PJ. Hardwick JM. Jonas EA. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:2169–2174. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S. Hong S. Shepardson NE. Walsh DM. Shankar GM. Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z. Okamoto K. Hayashi Y. Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Lim KL. Chew KC. Tan JM. Wang C. Chung KK. Zhang Y. Tanaka Y. Smith W. Engelender S. Ross CA. Dawson VL. Dawson TM. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci. 2005;25:2002–2009. doi: 10.1523/JNEUROSCI.4474-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim KL. Dawson VL. Dawson TM. Parkin-mediated lysine 63-linked polyubiquitination: a link to protein inclusions formation in Parkinson's and other conformational diseases? Neurobiol Aging. 2006;27:524–529. doi: 10.1016/j.neurobiolaging.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 78.Liot G. Bossy B. Lubitz S. Kushnareva Y. Sejbuk N. Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 80.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 81.Lipton SA. Choi YB. Pan ZH. Lei SZ. Chen HS. Sucher NJ. Loscalzo J. Singel DJ. Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 82.Lipton SA. Choi YB. Takahashi H. Zhang D. Li W. Godzik A. Bankston LA. Cysteine regulation of protein function—as exemplified by NMDA-receptor modulation. Trends Neurosci. 2002;25:474–480. doi: 10.1016/s0166-2236(02)02245-2. [DOI] [PubMed] [Google Scholar]
- 83.Lipton SA. Nakamura T. Yao D. Shi ZQ. Uehara T. Gu Z. Comment on “S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function.”. Science. 2005;308:1870. doi: 10.1126/science.1110353. author reply 1870. [DOI] [PubMed] [Google Scholar]
- 84.Lipton SA. Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 85.Low HH. Lowe J. A bacterial dynamin-like protein. Nature. 2006;444:766–769. doi: 10.1038/nature05312. [DOI] [PubMed] [Google Scholar]
- 86.Lutz AK. Exner N. Fett ME. Schlehe JS. Kloos K. Laemmermann K. Brunner B. Kurz-Drexler A. Vogel F. Reichert AS. Bouman L. Vogt-Weisenhorn D. Wurst W. Tatzelt J. Haass C. Winklhofer KF. Loss of parkin or PINK1 function increases DRP1-dependent mitochondrial fragmentation. J Biol Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lyles MM. Gilbert HF. Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: dependence of the rate on the composition of the redox buffer. Biochemistry. 1991;30:613–619. doi: 10.1021/bi00217a004. [DOI] [PubMed] [Google Scholar]
- 88.Marin I. Ferrus A. Comparative genomics of the RBR family, including the Parkinson's disease-related gene parkin and the genes of the ariadne subfamily. Mol Biol Evol. 2002;19:2039–2050. doi: 10.1093/oxfordjournals.molbev.a004029. [DOI] [PubMed] [Google Scholar]
- 89.Mayer ML. Westbrook GL. Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 90.McNaught KS. Perl DP. Brownell AL. Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol. 2004;56:149–162. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- 91.Muchowski PJ. Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura N. Kimura Y. Tokuda M. Honda S. Hirose S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nikolaev A. McLaughlin T. O'Leary DD. Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Nisoli E. Clementi E. Paolucci C. Cozzi V. Tonello C. Sciorati C. Bracale R. Valerio A. Francolini M. Moncada S. Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 95.Okamoto SI. Pouladi MA. Talantova M. Yao D. Xia P. Ehrnhoefer DE. Zaidi R. Clemente A. Kaul M. Graham RK. Zhang D. Vincent Chen HS. Tong G. Hayden MR. Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 97.Paisan-Ruiz C. Jain S. Evans EW. Gilks WP. Simon J. van der Brug M. Lopez de Munain A. Aparicio S. Gil AM. Khan N. Johnson J. Martinez JR. Nicholl D. Carrera IM. Pena AS. de Silva R. Lees A. Marti-Masso JF. Perez-Tur J. Wood NW. Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 98.Pandey M. Varghese M. Sindhu KM. Sreetama S. Navneet AK. Mohanakumar KP. Usha R. Mitochondrial NAD+-linked State 3 respiration and complex-I activity are compromised in the cerebral cortex of 3-nitropropionic acid-induced rat model of Huntington's disease. J Neurochem. 2008;104:420–434. doi: 10.1111/j.1471-4159.2007.04996.x. [DOI] [PubMed] [Google Scholar]
- 99.Panov AV. Gutekunst CA. Leavitt BR. Hayden MR. Burke JR. Strittmatter WJ. Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 100.Papadia S. Soriano FX. Leveille F. Martel MA. Dakin KA. Hansen HH. Kaindl A. Sifringer M. Fowler J. Stefovska V. McKenzie G. Craigon M. Corriveau R. Ghazal P. Horsburgh K. Yankner BA. Wyllie DJ. Ikonomidou C. Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parker WD., Jr. Parks J. Filley CM. Kleinschmidt-DeMasters BK. Electron transport chain defects in Alzheimer's disease brain. Neurology. 1994;44:1090–1096. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- 102.Paz Gavilan M. Vela J. Castano A. Ramos B. del Rio JC. Vitorica J. Ruano D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging. 2006;27:973–982. doi: 10.1016/j.neurobiolaging.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 103.Petralia RS. Wang YX. Hua F. Yi Z. Zhou A. Ge L. Stephenson FA. Wenthold RJ. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167:68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pitts KR. McNiven MA. Yoon Y. Mitochondria-specific function of the dynamin family protein DLP1 is mediated by its C-terminal domains. J Biol Chem. 2004;279:50286–50294. doi: 10.1074/jbc.M405531200. [DOI] [PubMed] [Google Scholar]
- 105.Polymeropoulos MH. Lavedan C. Leroy E. Ide SE. Dehejia A. Dutra A. Pike B. Root H. Rubenstein J. Boyer R. Stenroos ES. Chandrasekharappa S. Athanassiadou A. Papapetropoulos T. Johnson WG. Lazzarini AM. Duvoisin RC. Di Iorio G. Golbe LI. Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 106.Poole AC. Thomas RE. Andrews LA. McBride HM. Whitworth AJ. Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quintanilla RA. Matthews-Roberson TA. Dolan PJ. Johnson GV. Caspase-cleaved tau expression results in mitochondrial dysfunction in cortical neurons. Implications for the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284:18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rakhit R. Crow JP. Lepock JR. Kondejewski LH. Cashman NR. Chakrabartty A. Monomeric Cu,Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J Biol Chem. 2004;279:15499–15504. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- 109.Ramachandran R. Surka M. Chappie JS. Fowler DM. Foss TR. Song BD. Schmid SL. The dynamin middle domain is critical for tetramerization and higher-order self-assembly. EMBO J. 2007;26:559–566. doi: 10.1038/sj.emboj.7601491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramirez A. Heimbach A. Grundemann J. Stiller B. Hampshire D. Cid LP. Goebel I. Mubaidin AF. Wriekat AL. Roeper J. Al-Din A. Hillmer AM. Karsak M. Liss B. Woods CG. Behrens MI. Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 111.Reddy PH. Mao P. Manczak M. Mitochondrial structural and functional dynamics in Huntington's disease. Brain Res Rev. 2009;61:33–48. doi: 10.1016/j.brainresrev.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reynolds MR. Berry RW. Binder LI. Nitration in neurodegeneration: deciphering the “Hows” “nYs.”. Biochemistry. 2007;46:7325–7336. doi: 10.1021/bi700430y. [DOI] [PubMed] [Google Scholar]
- 113.Reynolds MR. Reyes JF. Fu Y. Bigio EH. Guillozet-Bongaarts AL. Berry RW. Binder LI. Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer's disease and other tauopathies. J Neurosci. 2006;26:10636–10645. doi: 10.1523/JNEUROSCI.2143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ross CA. Pickart CM. The ubiquitin-proteasome pathway in Parkinson's disease and other neurodegenerative diseases. Trends Cell Biol. 2004;14:703–711. doi: 10.1016/j.tcb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 115.Sattler R. Xiong Z. Lu WY. Hafner M. MacDonald JF. Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 116.Schonhoff CM. Matsuoka M. Tummala H. Johnson MA. Estevez AG. Wu R. Kamaid A. Ricart KC. Hashimoto Y. Gaston B. Macdonald TL. Xu Z. Mannick JB. S-nitrosothiol depletion in amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:2404–2409. doi: 10.1073/pnas.0507243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seong IS. Ivanova E. Lee JM. Choo YS. Fossale E. Anderson M. Gusella JF. Laramie JM. Myers RH. Lesort M. MacDonald ME. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet. 2005;14:2871–2880. doi: 10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- 118.Stamler JS. Lamas S. Fang FC. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 119.Tabrizi SJ. Cleeter MW. Xuereb J. Taanman JW. Cooper JM. Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington's disease brain. Ann Neurol. 1999;45:25–32. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 120.Taguchi N. Ishihara N. Jofuku A. Oka T. Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi H. Shin Y. Cho SJ. Zago WM. Nakamura T. Gu Z. Ma Y. Furukawa H. Liddington R. Zhang D. Tong G. Chen HS. Lipton SA. Hypoxia enhances S-nitrosylation-mediated NMDA receptor inhibition via a thiol oxygen sensor motif. Neuron. 2007;53:53–64. doi: 10.1016/j.neuron.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tiwari A. Hayward LJ. Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction. J Biol Chem. 2003;278:5984–5992. doi: 10.1074/jbc.M210419200. [DOI] [PubMed] [Google Scholar]
- 123.Uehara T. Nakamura T. Yao D. Shi ZQ. Gu Z. Ma Y. Masliah E. Nomura Y. Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 124.Uversky VN. Yamin G. Munishkina LA. Karymov MA. Millett IS. Doniach S. Lyubchenko YL. Fink AL. Effects of nitration on the structure and aggregation of alpha-synuclein. Brain Res Mol Brain Res. 2005;134:84–102. doi: 10.1016/j.molbrainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 125.Valente EM. Abou-Sleiman PM. Caputo V. Muqit MM. Harvey K. Gispert S. Ali Z. Del Turco D. Bentivoglio AR. Healy DG. Albanese A. Nussbaum R. Gonzalez-Maldonado R. Deller T. Salvi S. Cortelli P. Gilks WP. Latchman DS. Harvey RJ. Dallapiccola B. Auburger G. Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 126.Van Laar VS. Berman SB. Mitochondrial dynamics in Parkinson's disease. Exp Neurol. 2009;218:247–256. doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walker AK. Farg MA. Bye CR. McLean CA. Horne MK. Atkin JD. Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain. 2009;133(Pt 1):105–116. doi: 10.1093/brain/awp267. [DOI] [PubMed] [Google Scholar]
- 128.Wang G. Moniri NH. Ozawa K. Stamler JS. Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci U S A. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang H. Lim PJ. Karbowski M. Monteiro MJ. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet. 2009;18:737–752. doi: 10.1093/hmg/ddn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang R. Zhang D. Memantine prolongs survival in an amyotrophic lateral sclerosis mouse model. Eur J Neurosci. 2005;22:2376–2380. doi: 10.1111/j.1460-9568.2005.04431.x. [DOI] [PubMed] [Google Scholar]
- 131.Wang X. Su B. Lee HG. Li X. Perry G. Smith MA. Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang X. Su B. Siedlak SL. Moreira PI. Fujioka H. Wang Y. Casadesus G. Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X. Su B. Zheng L. Perry G. Smith MA. Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Neurochem. 2009;109(Suppl 1):153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wasiak S. Zunino R. McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Waterham HR. Koster J. van Roermund CW. Mooyer PA. Wanders RJ. Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 136.Weldon DT. Rogers SD. Ghilardi JR. Finke MP. Cleary JP. O'Hare E. Esler WP. Maggio JE. Mantyh PW. Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J Neurosci. 1998;18:2161–2173. doi: 10.1523/JNEUROSCI.18-06-02161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu L. Eu JP. Meissner G. Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 138.Yang Y. Gehrke S. Imai Y. Huang Z. Ouyang Y. Wang JW. Yang L. Beal MF. Vogel H. Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang YS. Harel NY. Strittmatter SM. Reticulon-4A (Nogo-A) redistributes protein disulfide isomerase to protect mice from SOD1-dependent amyotrophic lateral sclerosis. J Neurosci. 2009;29:13850–13859. doi: 10.1523/JNEUROSCI.2312-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yao D. Gu Z. Nakamura T. Shi ZQ. Ma Y. Gaston B. Palmer LA. Rockenstein EM. Zhang Z. Masliah E. Uehara T. Lipton SA. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yonashiro R. Ishido S. Kyo S. Fukuda T. Goto E. Matsuki Y. Ohmura-Hoshino M. Sada K. Hotta H. Yamamura H. Inatome R. Yanagi S. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 2006;25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoo BC. Krapfenbauer K. Cairns N. Belay G. Bajo M. Lubec G. Overexpressed protein disulfide isomerase in brains of patients with sporadic Creutzfeldt-Jakob disease. Neurosci Lett. 2002;334:196–200. doi: 10.1016/s0304-3940(02)01071-6. [DOI] [PubMed] [Google Scholar]
- 143.Youle RJ. Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 144.Yuan H. Gerencser AA. Liot G. Lipton SA. Ellisman M. Perkins GA. Bossy-Wetzel E. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death Differ. 2007;14:462–471. doi: 10.1038/sj.cdd.4402046. [DOI] [PubMed] [Google Scholar]
- 145.Zhang K. Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 146.Zhu PP. Patterson A. Stadler J. Seeburg DP. Sheng M. Blackstone C. Intra- and intermolecular domain interactions of the C-terminal GTPase effector domain of the multimeric dynamin-like GTPase Drp1. J Biol Chem. 2004;279:35967–35974. doi: 10.1074/jbc.M404105200. [DOI] [PubMed] [Google Scholar]
- 147.Zimprich A. Biskup S. Leitner P. Lichtner P. Farrer M. Lincoln S. Kachergus J. Hulihan M. Uitti RJ. Calne DB. Stoessl AJ. Pfeiffer RF. Patenge N. Carbajal IC. Vieregge P. Asmus F. Muller-Myhsok B. Dickson DW. Meitinger T. Strom TM. Wszolek ZK. Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 148.Ziviani E. Tao RN. Whitworth AJ. Drosophila Parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A. 2010;107:5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zuchner S. Mersiyanova IV. Muglia M. Bissar-Tadmouri N. Rochelle J. Dadali EL. Zappia M. Nelis E. Patitucci A. Senderek J. Parman Y. Evgrafov O. Jonghe PD. Takahashi Y. Tsuji S. Pericak-Vance MA. Quattrone A. Battaloglu E. Polyakov AV. Timmerman V. Schroder JM. Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]