Abstract
Purpose
To determine the in-field and out-of-field cell survival of cells irradiated with either primary field or scattered radiation in the presence and absence of intercellular communication.
Methods and Materials
Cell survival was determined by clonogenic assay in human prostate cancer (DU145) and primary fibroblast (AGO1552) cells following exposure to different field configurations delivered using a 6-MV photon beam produced with a Varian linear accelerator.
Results
Nonuniform dose distributions were delivered using a multileaf collimator (MLC) in which half of the cell population was shielded. Clonogenic survival in the shielded region was significantly lower than that predicted from the linear quadratic model. In both cell lines, the out-of-field responses appeared to saturate at 40%–50% survival at a scattered dose of 0.70 Gy in DU-145 cells and 0.24 Gy in AGO1522 cells. There was an approximately eightfold difference in the initial slopes of the out-of-field response compared with the α-component of the uniform field response. In contrast, cells in the exposed part of the field showed increased survival. These observations were abrogated by direct physical inhibition of cellular communication and by the addition of the inducible nitric oxide synthase inhibitor aminoguanidine known to inhibit intercellular bystander effects. Additional studies showed the proportion of cells irradiated and dose delivered to the shielded and exposed regions of the field to impact on response.
Conclusions
These data demonstrate out-of-field effects as important determinants of cell survival following exposure to modulated irradiation fields with cellular communication between differentially irradiated cell populations playing an important role. Validation of these observations in additional cell models may facilitate the refinement of existing radiobiological models and the observations considered important determinants of cell survival.
Keywords: Out-of-field, Modulated fields, Cell survival, Bystander
INTRODUCTION
The delivery of clinical radiotherapy is assumed to result in a radiobiological response within the target tumor volume that is proportional to the dose delivered (1). In advanced megavoltage radiotherapy techniques such as intensity-modulated radiation therapy (IMRT), sequential delivery of highly modulated beam profiles are used, resulting in a high degree of dose conformity to the target volume and reducing the dose and risk of complication to normal tissue. Even in the most conformal of treatments, regions of significant dose can accumulate out-of-field because of scattered photons, which may have an impact on cellular response in these regions. With more advanced radiotherapy delivery techniques in clinical use, a more comprehensive understanding of beam quality and its effect on biological responses in and out of the primary treatment field is necessary.
Differences in beam quality outside the primary treatment field for 6-MV photons have been reported in several Monte Carlo studies (2, 3). Kirby et al. (2) demonstrated a significant increase in the low energy component of the fluence spectra outside of the primary field corresponding to increased linear energy transfer (LET). Similarly, Liu and Verhaegen (3) showed a 20% variation in beam quality comparing penumbra and central axis. In contrast, Moiseenko et al. (4) showed no significant difference in beam quality between central axis and penumbra regions of a tomotherapy fan beam out to a distance of 0.6 cm. Recent in vitro experimental evidence has shown significant enhancement of DNA damage out-of-field in normal human fibroblasts irradiated with a 6-MV beam (5).
In addition to differences in quality of the beam, communication between irradiated and nonirradiated cell populations through the radiation induced bystander effect (RIBE) may affect biological response (6). Because IMRT beams are by definition spatially modulated, cell communication between differentially irradiated cells populations within the target tumor volume may also have an important role.
Several in vitro studies have attempted to address this question (7-10). Using a wedge to create a nonuniform field, Suchowerska et al. (7) observed differences in survival response between cell populations in which intercellular communication was either intact or physically inhibited (7). Differences in cell survival were also shown by Claridge Makonis et al. (8) by comparing delivery of a uniform field with delivery to 25% of the cell population as a single region or as three parallel stripes within the same flask. Moiseenko et al. (9) reported reduced cell kill for IMRT treatment plans compared with acute irradiation for head and neck treatment plans delivered in vitro.
Recent evidence from our laboratory showed no significant difference in the survival response following exposure to modulated and nonmodulated 6-MV fields under conditions in which modulation was delivered as a series of step functions across the cell population (10). However, it is difficult to draw conclusions from these reports because of differences in the spatial and temporal components of modulated beam delivery, with protracted delivery times associated with reduced cell kill (11-13).
In this study, we determined the survival responses in field and out of field for a modulated 6-MV photon beam in a human normal and tumor cell line using a multileaf collimator (MLC) to define the in-field exposed area. The effect or intercellular communication between the in and out-of-field cell populations was investigated. Additional studies were performed varying the proportion of cells in and out of the primary field.
METHODS AND MATERIALS
Cell culture
Experiments were conducted using two cell lines, the human prostate cancer cell line, DU-145, and the human fibroblast cell line, AGO-1552. Cell lines were obtained from Cancer Research UK and selected as malignant and transformed models with different radiosensitivity. DU-145 cells were grown in RPMI-1640 with L-glutamine (Lonza, Cambridge, United Kingdom) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Gibco, Paisley, United Kingdom). AGO-1522 cells were grown in Eagle's minimum essential medium with deoxyribonucleosides and deoxyribonucleotides (Lonza) supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin. All cell lines were maintained at 37°C in a humidified atmosphere of 5% CO2.
Clonogenic assay
Cell survival was determined by clonogenic assay as previously reported (10). Cells were plated and allowed to adhere overnight. Culture flasks were filled with serum-free medium and sealed immediately before irradiation. Cells were irradiated at room temperature (25 ± 2 °C). Following irradiation, serum-free medium was removed and replaced with complete culture medium. Cultures were incubated for 10–14 days before staining with 0.5% crystal violet in 50% methanol. DU-145 colonies were scored using a Colcount (Oxford Optronix, United Kingdom) automated counter which optimized for the cell line. AGO-1522 colonies were scored manually applying a 50-cell exclusion rule. For each experiment, unexposed controls were prepared and treated as sham exposures. Experiments were conducted under standard culture conditions or in the presence of the inducible nitric oxide synthase (iNOS) inhibitor aminoguanidine (AG) at a concentration of 100 μM. AG was diluted in phosphate-buffered saline to the desired final concentration and added to culture medium 2 hours before irradiation. AG remained present in the culture medium for the duration of the assay.
Irradiation setup and validation of experimental design
Cells were irradiated in either T75 or T25 culture flasks (Nunc, Loughborough, United Kingdom) with a 6-MV photon beam produced by a Varian 600CD medical linear accelerator with 120-leaf millennium MLC (Varian Medical Systems, Palo Alto, CA) calibrated according to the UK Code of Practice (Institute of Physical Sciences in Medicine, 1990).
The same experimental setup was used as previously described (10). Full scatter conditions were achieved by filling the flask with culture medium before irradiation and submersing in a water phantom on top of a 30 × 30 cm, 5-cm-deep block of solid water that was placed on the treatment couch. The gantry was placed at 180°, and the couch was placed so that the source to surface distance to the couch top was 100 cm. All calculations for set monitor units included a factor to account for the attenuation of the couch. The setup was CT scanned using a Siemens Emotion 6 (Siemens, Erlangen, Germany), and a plan was created using Nucletron Oncentra (Nucletron, Veenendal, the Netherlands) to ensure uniform irradiation of the culture flask using a 20 × 20 cm field gave the number of monitor units required to give the prescribed doses to cells in the flasks of (0–6.28 Gy) for AGO and (0–12.40 Gy) for DU145 cells. Plans were also created with MLCs shielding 50% of the 20 × 20 cm field, and monitor units were calculated to give equivalent doses to the exposed area as was given for the open field.
MLC leaf transmission and leakage is known to be higher than for standard collimators. To confirm that this was not affecting the results, the secondary collimators were used to shield half of the 20 × 20 cm field in place of the MLC leaves. Using the secondary collimators to deliver a nonuniform field resulted in a lower scattered dose of 0.39 ± 0.08 Gy being delivered compared with 0.47 ± 0.09 Gy when using the MLC (due to transmission through the MLC). However, both the in-field and out-of-field survival responses when using the secondary collimators showed no significant difference in response than when using the MLC as shielding. Figure 1 shows that the out-of-field region corresponded to the neck of the T75 flask. To confirm that this was not adversely affecting the data, the collimator was rotated by 180°, and the 8 Gy was delivered in-field to the neck end. The delivery of the same dose to the flask in the opposite orientation had no significant impact on the relative survival responses in field and out of field.
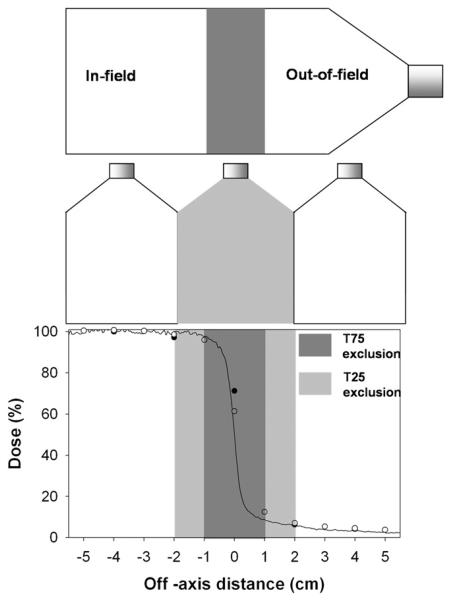
Fig. 1.
Field orientations and dose profiles for nonuniform radiation fields. Cells were irradiated in a single T75 flask or as two separate T25 flasks. Dose profiles were measured using Gafchromic film (solid line), an ionization chamber array (○), and a farmer ionization chamber (●). Penumbra regions were excluded from analysis.
A number of measurements were performed to ensure that the T75 flask was receiving the correct absolute dose using Gafchromic EBT film (ISP, Wayne, NJ). Gafchromic film was cut into the shape of the T75 flasks and placed at the bottom of the water tank. Each of the fields was delivered to the flask to ensure a dose of 2 Gy on the central axis at the plane of the film (coincident with the plane of the cells). Figure 1 shows line profiles along the central axis.
A 2-cm “exclusion zone” was set where cells were not analyzed to allow for uncertainties in setup and to avoid analysis at any steep dose gradients. For the physically inhibited communication experiment, a T25 flask was used as the exclusion area. The dose to the out-of-field area was taken as the average dose to the area. Confirmatory measurements were performed using Gafchromic film, a farmer ionization chamber placed at 2 cm intervals along the central axis and an ionization chamber array (IBA MatriXX Evolution) in solid water at the radiological equivalent depth of 6.6 cm. Due to increased uncertainty at low dose for Gafchromic EBT (ISP, Wayne, NJ), out-of-field doses were calculated by averaging the coinciding 2D array ionization chamber readings. Out-of-field doses to the T25 flasks were lower than those for the T75 flasks as the area analyzed was further from the central axis. Dose uncertainties were calculated as the standard deviation of the average dose measured within the shielded area (19.5%).
Data analysis
For each of the irradiation conditions, surviving fractions (SF) were calculated as the ratio of the number of colonies in the exposed flask to the number of seeded cells corrected for the plating efficiency of sham irradiated control cells. Survival curves were fitted to the form SF = exp [− (αD + βD2)] using Origin Pro version 8. Statistical errors on fit values were calculated as the standard error. All experiments were performed in triplicate at least three times with the data presented as ± standard error in all cases. Statistical analysis comparing the survival values for nonmodulated and modulated field configurations was carried out using the Mann-Whitney U test. Statistical significant differences were assumed at the level of p < 0.05. Calculations were performed using Statistical Package for Social Sciences version 15.0.1.1 (SPSS, Chicago, IL).
RESULTS
Cell survival data in field and out of field following nonuniform exposure was obtained for human fibroblast (AGO-1522) and prostate cancer cells (DU-145). Nonuniform fields were delivered using the MLC to shield a percentage of the field area. Cells were irradiated in either T25 or T75 culture flask with the penumbra region omitted from analysis. The nonuniform dose profiles for each configuration are shown in Fig. 1. Cell survival following exposure to a uniform field compared with the in-field and out-of-field responses following nonuniform exposure is shown in Fig. 2.
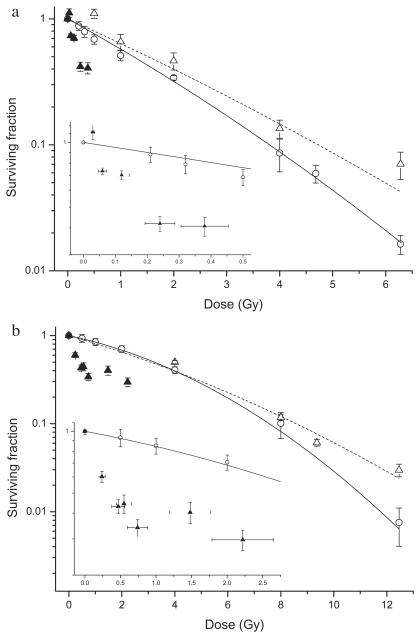
Fig. 2.
Cell survival following exposure to a uniform radiation field (○) compared with in-field (△) and out-of-field (▲) survival following exposure to a nonuniform field using the MLC to shield 50% of the area of the flask. Data are shown for (a) AGO-1522 cells and (b) DU-145 cells. In-field survival data was fitted to the LQ model. Out-of-field survival data are inset for clarity. Y error bars indicate ± standard error of the mean; X error bars indicate uncertainty in the low-dose region.
In AGO-1522 cells (Fig. 2a), the in-field dose response curve (α = 0.53 ± 0.05, β = 0.02 ± 0.01) appeared to diverge from uniform field response (α = 0.44 ± 0.10, β = 0.01 ± 0.02) as a function of dose; however, a statistically significant difference in survival was only detected at 6.28 Gy (p < 0.001). Out-of-field cell survival is dramatically lower than predicted from the linear quadratic (LQ) model and suggests a level of saturation at 40%–50% survival for scattered doses >0.24 Gy. Similar responses were observed in DU-145 cells (Fig. 2b) with the in-field dose response curve (α = 0.15 ± 0.03, β = 0.02 ± 0.01) diverging from the uniform field curve (α = 0.20 ± 0.03, β = 0.01 ± 0.01) with significantly different survival at 12.4 Gy (p < 0.001). Out-of-field cell survival is dramatically lower than predicted from the LQ model and again suggests a level of saturation between 40% and 50% survival at scattered radiation doses >0.74 Gy.
In both cell lines, the out-of-field survival responses in the initial part of the curve were fitted to the form SF = exp (−αD) (AGO – 1522, a = 1.64 ± 0.07; DU-145, a = 3.65 ± 0.18). The α-component of the uniform field response values were found to be 8.29 and 8.20 times greater for AGO-1522 and DU-145 cells, respectively.
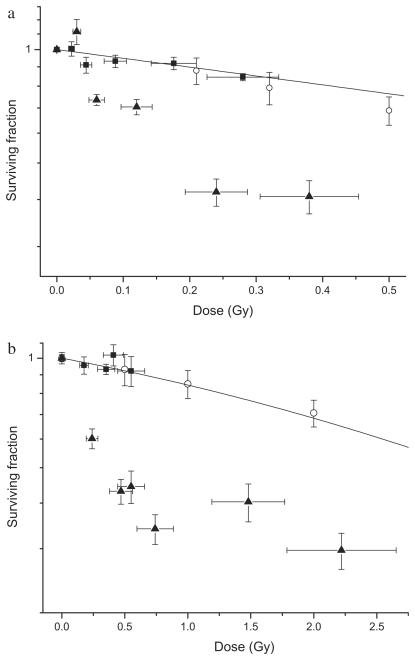
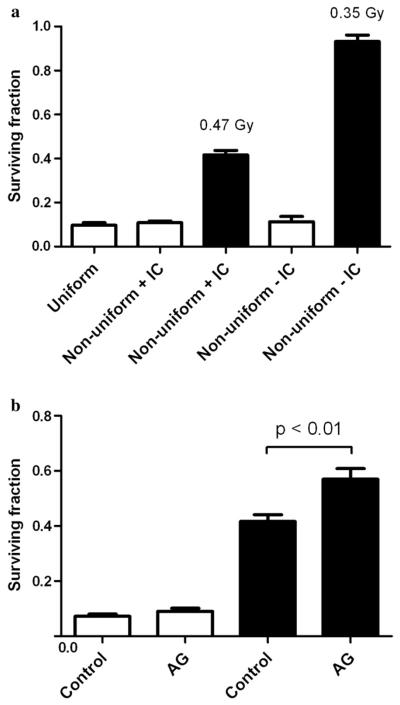
The effect of direct physical inhibition of intracellular communication on the out-of-field responses was demonstrated using two separate T25 culture flasks placed in and out of field (Fig. 1). These responses compared with those observed when intercellular communication is intact are shown Fig. 3. Inboth AGO-1522 (Fig. 3a) and DU-145 cells (Fig. 3b), inhibition of direct intercellular communication was shown to abrogate the observed out-of-field responses when differentially irradiated cell populations are free to communicate, with cell survival being the same as predicted from the LQ model. These responses were further investigated at a single dose of 8 Gy and compared with the response for a uniform field (Fig. 4a). The effect of biological inhibition of intercellular communication using the iNOS inhibitor AG is shown in Fig. 4b. Pretreatment with AG had no significant effect on in-field survival response compared with the uniform field (p = 0.12). However, a significant increase of around 15% was observed for the out-of-field AG pretreated cells compared with untreated controls (p < 0.01).
Fig. 3.
Cell survival following exposure to a uniform radiation field (○) compared with out-of-field survival for nonuniform fields in which intracellular communication is intact (▲) or physically inhibited (■). Data are shown for (a) AGO-1522 cells and (b) DU-145 cells. Yerror bars indicate ± standard error of the mean; X error bars indicate uncertainty in the low-dose region.
Fig. 4.
Comparison of in- and out-of-field cell survival for DU-145 cells irradiated with different field configurations. A dose of 8 Gy was delivered to in-field regions shown as open columns; doses delivered to out-of-field regions are stated and shown as solid columns. (a) Comparison of cell survival to a nonuniform field when irradiated with intact (+IC) and inhibited (−IC) intracellular communication. (b) Biological modulation of in-field and out-of-field survival responses by pretreatment with aminoguanidine (AG). Error bars indicate ± standard error of the mean.
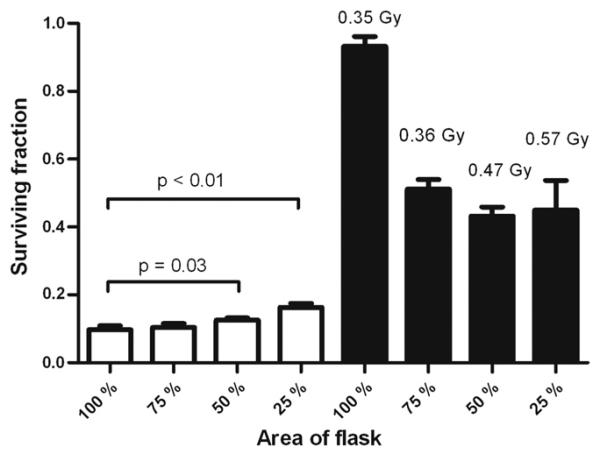
The effect of the irradiated area in and out of field as defined using the MLC is shown in Fig. 5. Increased survival was observed as the in-field area decreased, with significant differences being shown at 50% (p = 0.03) and 25% (p < 0.01) compared with a uniform field. Variation of the out-of-field area showed a trend toward increased survival with increased area out of field; however, variation in the scattered dose delivered prevented statistical comparison of the data.
Fig. 5.
Cell survival for DU-145 cells irradiated with varying areas and in- and out-of-field. A dose of 8 Gy was delivered to the in-field regions shown as open columns; doses delivered to out-of-field regions are stated and shown as solid columns. Error bars indicate ± standard error of the mean.
DISCUSSION
This study demonstrates differences between the in- and out-of-field survival responses of cells exposed to intensity-modulated radiation beams, which are shown to be dependent on intercellular communication. In-field cell survival shows a trend toward increased survival that diverged from the uniform field dose response curve as a function of dose, although these differences were shown to be significant only at high doses. Similar effects have been reported by Suchowerska et al. (7) following exposure to dose gradients and by Claridge Makonis et al. (8) in MLC-shielded regions from modulated field exposures using a 6-MV photon beam. The data presented in this study also showed the in-field response to be dependent on the relative proportion of cells in and out of field.
Out-of-field cell survival is dramatically less than would be predicted solely due to scattered dose. Physical inhibition of intercellular communication abrogated the level of response showing intercellular communication between the in-field and out-of-field cell populations to be a crucial determinant of cellular response, observations again reported by Suchowerska et al. (7) using the same method to inhibit cellular communication between populations exposed to a dose gradient. Our data have also shown a mechanistic role for nitric oxide in mediating the out-of-field response because pretreatment of cells with AG significantly increased cell survival. Taken together, these results are consistent with the involvement of the RIBE in out-of-field responses.
The RIBE describes the response of nonirradiated cells to signals produced by neighboring cells that have been directly traversed by the radiation field (6). Out-of-field responses share several characteristics manifested by the RIBE. The data in this study shows the out-of-field responses occurring as a low-dose phenomenon, and above a certain dose, there is less additional effect (0.24 Gy in AGO-1522 cells; 0.74 Gy in DU-145 cells). It is well established that the RIBE is a low-dose biological response that saturates at doses <1 Gy (14-20). An important observation is that the level of cell survival appears to be limited to a maximum of 40% to 50% in both cell lines, but the dose at which the response begins to plateau is around 3 times greater in the more radioresistant DU-145 cells. These data show the magnitude of out-of-field response to be constant and suggest the dose at which the response appears to reach a plateau is inversely related to the radiosensitivity of the cell line. Furthermore, comparison of the initial slopes of the outof-field response compared with the α-component of the uniform field showed an eightfold difference in both cell lines.
Decreased clonogenic survival in the region of 10%–30% has been demonstrated by the transfer of irradiated cell–conditioned media onto unexposed cells (15, 21, 22). Although the level of out-of-field response is greater than those previously reported, the experiments conducted in this study differ in that the cells were exposed to a highly modulated field, share the same cell density in and out of field, and are free to communicate from the time of irradiation and for the duration of the clonogenic assay.
The RIBE is mediated through gap junction intercellular communication or the secretion of factors into the culture medium. These experiments were performed using sparsely seeded cell cultures; therefore, the RIBE can occur only through the release of soluble factors from irradiated cells. Although the exact biological mechanism responsible for the RIBE remains to be identified, several molecular pathways have been shown to be involved (6). Consistent with other reports (22-25), our data provide evidence for the involvement of NO in the RIBE as inhibition of iNOS significantly increased out-of-field cell survival. It is likely that decreased levels of NO will reduce levels of other downstream signaling molecules such as TGF-β1, which has been shown to be a product of radiation-induced NO (23, 24) and merits further mechanistic investigation.
Although the data presented in this study suggest the RIBE as a possible mechanism driving out-of-field response, it is likely that a local dose component may also affect out-offield response because dose delivered out-of-field occurs primarily as a result of scattered photons and charged particles that have a larger low energy component. Because LET is inversely related to electron energy, out-of-field dose could potentially have different biological effectiveness compared with that within the primary field. Significant increases in the low energy component of the fluence spectra out of field for a 6-MV photon beam have been reported using Monte Carlo modeling (2). In addition, variation in beam quality of up to 20% has been reported between penumbra and central axis due to changes in the energy spectrum of the photon fields (3). This would suggest increased biological effectiveness per unit dose delivered out of field compared with in field, which has been supported by Syme et al. (5) who observed 27% enhancement in net mean nuclear γ-H2AX fluorescence intensity at 2 Gy and 48% enhancement at 4 Gy in normal human fibroblast irradiated with 6-MV photons. Our data show no difference in response between uniform fields and the out-of-field response when intercellular communication was inhibited, suggesting the RIBE as the predominant factor.
Considering the experimental challenges and debate concerning survival data in response to modulated fields (8, 26, 27) extensive dosimetric measurements were performed and penumbra regions excluded from the analysis. The experimental design was verified by rotation of the MLC through 180° and by using the secondary collimators to produce the modulated beam, with both responses showing no significant difference from the original modulated exposure setup shown in Fig. 1.
Our observations show significant effects outside the primary treatment field that has relevance to intensity-modulated radiation treatments. Further work is required to extrapolate to the in vivo scenario in which tumor cells and surrounding normal tissue are free to communicate not only through secreted factors but also gap junction intercellular communication. This study provides further evidence for the importance of the RIBE, even when cells receive differential doses by the delivery of a dose gradient and may inform the refinement of traditional radiobiological models to facilitate the optimization of advanced radiotherapy treatment plans.
Acknowledgments
This work was supported by Cancer Research UK (Grant no. C1513/A7047) to KMP. CKM is supported by a Health and Social Care Research and Development of the Public Health Agency Training Fellowship Award.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 6th ed. Lippincott William & Wilkins; Philadelphia: 2006. [Google Scholar]
- 2.Kirkby C, Field C, MacKenzie M, et al. A Monte Carlo study of the variation of electron fluence in water from a 6 MV photon beam outside of the field. Phys Med Biol. 2007;52:3563–3578. doi: 10.1088/0031-9155/52/12/015. [DOI] [PubMed] [Google Scholar]
- 3.Liu HH, Verhaegen F. An investigation of energy spectrum and lineal energy variations in mega-voltage photon beams used for radiotherapy. Radiat Prot Dosimetry. 2002;99:425–427. doi: 10.1093/oxfordjournals.rpd.a006824. [DOI] [PubMed] [Google Scholar]
- 4.Moiseenko V, Mulligan M, Kron T. Radiation quality of a tomotherapy photon fan beam. Health Phys. 2004;87:166–170. doi: 10.1097/00004032-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Syme A, Kirkby C, Mirzayans R, et al. Relative biological damage and electron fluence in and out of a 6 MV photon field. Phys Med Biol. 2009;54:6623–6633. doi: 10.1088/0031-9155/54/21/012. [DOI] [PubMed] [Google Scholar]
- 6.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suchowerska N, Ebert MA, Zhang M, Jackson M. In vitro response of tumour cells to non-uniform irradiation. Phys Med Biol. 2005;50:3041–3051. doi: 10.1088/0031-9155/50/13/005. [DOI] [PubMed] [Google Scholar]
- 8.Claridge Mackonis EC, Suchowerska N, Zhang M, et al. Cellular response to modulated radiation fields. Phys Med Biol. 2007;52:5469–5482. doi: 10.1088/0031-9155/52/18/001. [DOI] [PubMed] [Google Scholar]
- 9.Moiseenko V, Duzenli C, Durand RE. In vitro study of cell survival following dynamic MLC intensity-modulated radiation therapy dose delivery. Med Phys. 2007;34:1514–1520. doi: 10.1118/1.2712044. [DOI] [PubMed] [Google Scholar]
- 10.Butterworth KT, McGarry CK, O'Sullivan JM, et al. A study of the biological effects of modulated 6 MV radiation fields. Phys Med Biol. 2010;55:1607–1618. doi: 10.1088/0031-9155/55/6/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bewes JM, Suchowerska N, Jackson M, et al. The radiobiological effect of intra-fraction dose-rate modulation in intensity modulated radiation therapy (IMRT) Phys Med Biol. 2008;53:3567–3578. doi: 10.1088/0031-9155/53/13/012. [DOI] [PubMed] [Google Scholar]
- 12.Ogino H, Shibamoto Y, Sugie C, Ito M. Biological effects of intermittent radiation in cultured tumor cells: influence of fraction number and dose per fraction. J Radiat Res (Tokyo) 2005;46:401–406. doi: 10.1269/jrr.46.401. [DOI] [PubMed] [Google Scholar]
- 13.Shibamoto Y, Ito M, Sugie C, et al. Recovery from sublethal damage during intermittent exposures in cultured tumor cells: implications for dose modification in radiosurgery and IMRT. Int J Radiat Oncol Biol Phys. 2004;59:1484–1490. doi: 10.1016/j.ijrobp.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 14.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 15.Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 16.Lehnert BE, Goodwin EH, Deshpande A. Extracellular factor (s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997;57:2164–2171. [PubMed] [Google Scholar]
- 17.Belyakov OV, Malcolmson AM, Folkard M, et al. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br J Cancer. 2001;84:674–679. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prise KM, Folkard M, Michael BD. A review of the bystander effect and its implications for low-dose exposure. Radiat Prot Dosimetry. 2003;104:347–355. doi: 10.1093/oxfordjournals.rpd.a006198. [DOI] [PubMed] [Google Scholar]
- 19.Schettino G, Folkard M, Prise KM, et al. Low-dose studies of bystander cell killing with targeted soft X rays. Radiat Res. 2003;160:505–511. doi: 10.1667/rr3060. [DOI] [PubMed] [Google Scholar]
- 20.Ryan LA, Smith RW, Seymour CB, Mothersill CE. Dilution of irradiated cell conditioned medium and the bystander effect. Radiat Res. 2008;169:188–196. doi: 10.1667/RR1141.1. [DOI] [PubMed] [Google Scholar]
- 21.Balduzzi M, Sapora O, Matteucci A, Paradisi S. Modulation of the bystander effects induced by soluble factors in HaCaT cells by different exposure strategies. Radiat Res. 2010;173:779–788. doi: 10.1667/RR1835.1. [DOI] [PubMed] [Google Scholar]
- 22.Shao C, Stewart V, Folkard M, et al. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 2003;63:8437–8442. [PubMed] [Google Scholar]
- 23.Shao C, Folkard M, Prise KM. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 2008;27:434–440. doi: 10.1038/sj.onc.1210653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao C, Prise KM, Folkard M. Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts. Mutat Res. 2008;638:139–145. doi: 10.1016/j.mrfmmm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Tomita M, Maeda M, Maezawa H, et al. Bystander cell killing in normal human fibroblasts is induced by synchrotron X-ray microbeams. Rad Res. 2010;173:380–385. doi: 10.1667/RR1995.1. [DOI] [PubMed] [Google Scholar]
- 26.Claridge Mackonis E, Suchowerska N, McKenzie DR, et al. Reply to Comments on “Cellular response to modulated radiation fields”. Phys Med Biol. 2009;54:L15–L20. doi: 10.1088/0031-9155/54/5/L02. [DOI] [PubMed] [Google Scholar]
- 27.Ross CK, Klassen NV. Comments on “Cellular response to modulated radiation fields.”. Phys Med Biol. 2009;54:L11–L13. doi: 10.1088/0031-9155/54/5/L02. [DOI] [PubMed] [Google Scholar]