Abstract
Amphetamine, which is known to cause sensitization, potentiates the hormonal and neurobiological signatures of stress and may also increase sensitivity to stress-inducing stimuli in limbic areas. Trimethylthiazoline (5 μL TMT) is a chemical constituent of fox feces that evokes innate fear and activates the neuronal and hormonal signatures of stress in rats. We used blood oxygen level dependent (BOLD) MRI to test whether amphetamine sensitization (1 mg/kg, i.p. X 3 days) in female rats has a lasting effect on the neural response to a stress-evoking stimulus, the scent of a predator, during the postpartum period. The subiculum and dopamine-enriched midbrain VTA/SN of amphetamine-sensitized, but not control mothers showed a greater BOLD signal response to predator odor than a control putrid scent. The greater responsiveness of these two brain regions following stimulant sensitization might impact neural processing in response to stressors in the maternal brain.
Keywords: Amphetamine, Sensitization, Maternal rats, Withdrawal, Gestation, Stress, Fear, Predator Odor, Fox Feces, Trimethylthiazoline, TMT, Maternal Stress, Butyrate, Cortex, Limbic System, Freezing Behavior, Female rat
1. INTRODUCTION
Although the rates of illicit drug use among females 12 and older has declined, they still remain high according to the 2008 National Survey on Drug Use and Health (SAMHSA, 2009). This is worrisome in light of that fact that drug use and abuse, especially of stimulant drugs, can exert long lasting effects on neurobiology and behavior that can lead to addiction during critical reproductive epochs such as lactation (Febo and Ferris, 2007; Grimm et al., 2003; Hyman et al., 2006). Our previous work shows that pre-pregnancy cocaine sensitization can have lasting effects on maternal behavior during the postpartum period and on maternal prefrontal cortical responses to suckling stimulation from pups (Febo and Ferris, 2007; Nephew and Febo). Addiction during early motherhood has been associated with problems with the ability to cope with stressors (Arevalo et al., 2008; Harmer et al., 1999). Amphetamine sensitization is associated with heightening of stress responses through cross-sensitization with hypothalamic-pituitary-adrenal (HPA) axis function (Antelman et al., 1980; Barr et al., 2002). Amphetamine-sensitized rats show alterations in glucorticoid receptor levels and elevated plasma levels of adrenocorticotropic hormone (ACTH) and corticosterone (CORT) in response to a variety widely used experimental stressors in rodents (Schmidt et al., 2001; Shilling et al., 1996). Even a single injection of amphetamine can increase CORT and ACTH responses to stressors for as long as 22 but not 46 days (Schmidt et al., 2001). Heightened sensitivity to stressful stimuli itself is a vulnerability factor for reinstatement of stimulant self-administration (Ahmed and Koob, 1997). Based on these data, it is possible that development of amphetamine sensitization alters the neural processing of stimuli that evoke stress and anxiety in the maternal brain. Modeling this phenomenon in rodents is especially difficult since one needs to model the day-to-day stressors for humans using day-to-day stressors that a maternal rodent might encounter in the wild. One such stressor in rodents is that of a predator. The chemical 2,4,5,-trimethylthiazoline (or TMT) is a synthetic analog of the chemical constituent of fox feces that is widely known to evoke unconditioned fear responses to rodents (Blanchard et al., 2003; Endres and Fendt, 2008; Morrow et al., 2000; Rosen et al., 2005; Staples et al., 2008; Wallace and Rosen, 2001). TMT emits a putrid smell that has been shown to elevate plasma CORT levels, autonomic activation, defensive postures and prefrontal DA release in rats much like other stressors (Hamamura and Fibiger, 1993; Morrow et al., 2000; Staples et al., 2008). Therefore, TMT may be viewed as an ecologically valid model as a stressor that may also cause strong distress in rodents. Here, we test whether amphetamine sensitization before pregnancy alters how the maternal rat brain processes a stressful stimulus such as the scent of the predator.
2. RESULTS
2.1 Amphetamine Sensitization
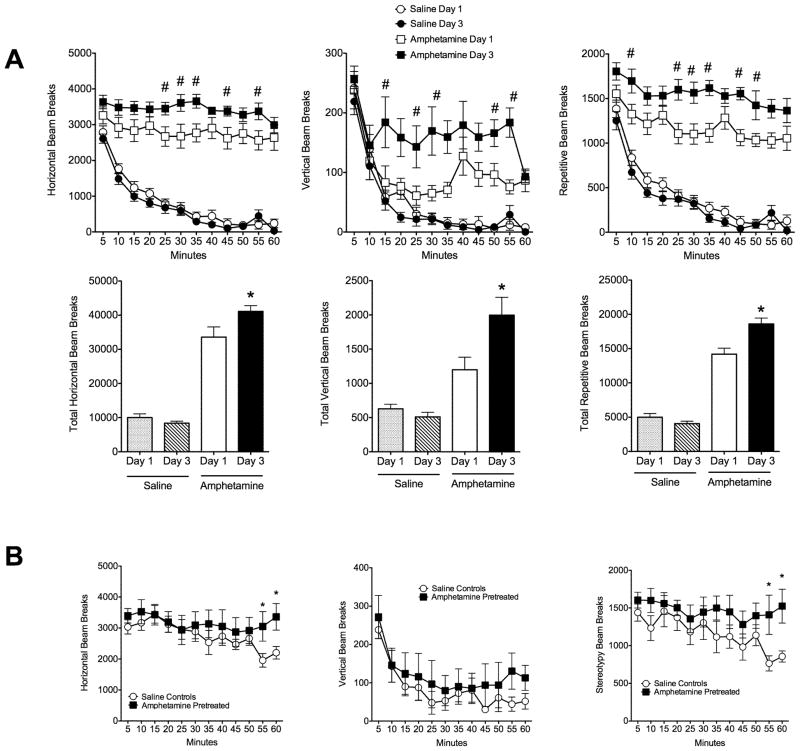
An interaction between drug treatment (saline X amphetamine) and day of treatment was observed for horizontal activity (F1,17 = 14.8, p = 0.001), stereotyped activity (F1,17 = 19.4, p = 0.0004), and vertical activity (F1,17 = 11.6, p = 0.003). Administration of 1 mg/kg amphetamine on the 3rd day of injections resulted in a greater motor reactivity in comparison to first exposure (Figure 1A). Females showed a greater horizontal, vertical and repetitive infrared beam breaks (p < 0.01 Bonferroni multiple comparisons test). Females showed evidence of sensitization after about 35 days of withdrawal, with saline controls showing a faster decline in the motor response to amphetamine challenge (Figure 1B).
Figure 1.
Development of amphetamine sensitization before pregnancy and its expression after drug challenge in the postpartum period. A) Time courses for horizontal, vertical and stereotypic locomotor activity on days 1 and 3 following saline (n = 10) and 1 mg/kg, i.p. amphetamine (n = 9) administration to virgin rats. Total horizontal, vertical and stereotypic activity counts are summarized in the column graphs below the time course plots. Symbols (# and *) denote significant differences between corresponding time points (Bonferroni’s multiple comparison test, p <0.05). B) Time courses for horizontal, vertical and stereotypic locomotor activity on the day of amphetamine challenge in the postpartum period. A group of saline (n = 5) and amphetamine pretreated animals (n = 5) were challenged with 1 mg/kg i.p. amphetamine. Asterisks denote significant differences between corresponding time points (Mann-Whitney U test for specific time points, p <0.05). All data are expressed as mean ± standard error.
2.2 Light-dark box results
There was no effect of amphetamine pretreatment on time spent in the dark chamber of a light-dark box. Furthermore, no difference in baseline anxiety level was observed before pregnancy or the postpartum period.
2.3 BOLD signal response to TMT vs SB
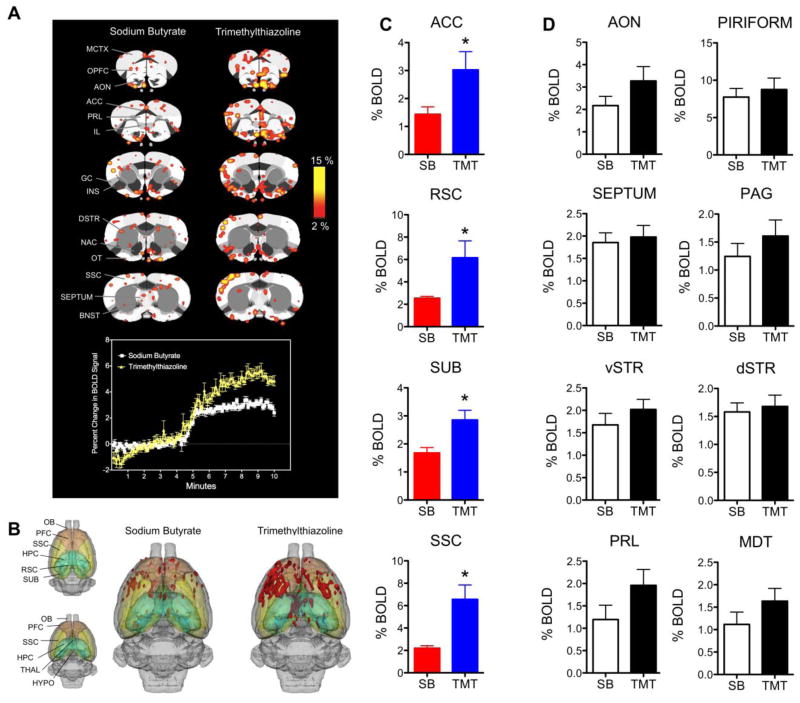
Postpartum day 2–4 rats were presented with SB and TMT during MR scanning. Fifty-eight ROI were segmented and analyzed. We first analyzed the effects of TMT vs SB in 12 rats collapsed across saline (n = 5) and amphetamine (n = 7) groups (Figure 2A-2B). The composite maps show that TMT evokes BOLD activity across limbic prefrontal regions such as the medial-to-infralimbic prefrontal cortex, the anterior cingulate, the lateral orbital regions and the insular cortex (Figure 2A). TMT produced almost twice the magnitude BOLD signal response of SB in several areas (Figure 2A time course below 2D maps). Significant differences were also observed in the number of activated voxels (Figure 2B). Statistically significant differences in BOLD signal changes between SB and TMT were observed in the anterior cingulate cortex (p = 0.03, two-tailed Mann- Whitney U test for independent samples), retrosplenial cortex (p = 0.001), subiculum (p = 0.02), somatosensory cortex (p = 0.02), lateral amygdala (p = 0.02) and motor cortex (p = 0.02) (Figure 2C). Figure 2D shows additional ROI previously associated with the neural response to TMT or the expression of anxiety-related behavior that did not show significant differences between SB and TMT in the present study.
Figure 2.
Blood oxygen level dependent (BOLD) signal response to the scent of sodium butyrate (SB) and 2,4,5-trimethylthiazoline (TMT). A) Composite maps of SB and TMT induced BOLD responses. Each odor stimulus condition SB and TMT consists of 12 rats collapsed from 5 saline and 7 amphetamine animals. Scale bar hue indicates percent change in BOLD (2% - 15%, minimum p value for maps is 0.05 corrected for multiple comparisons). Below the 2D maps is an average time course for the cortical BOLD signal response to SB (white) and TMT (yellow). Scents were presented just before the 5-minute mark (4:40–45 minutes). B) Threedimensional composite maps of SB and TMT induced BOLD activity. Red areas are indicative of signal increases. Maps are segmented to highlight areas showing significantly more BOLD activation to TMT than SB (in C). C) Summary of regions showing significantly greater BOLD signal changes in response to TMT (blue) than SB (red). Data are presented as mean ± standard error. Asterisks denote significant differences (Mann-Whitney U test, p < 0.05). D) Regions previously identified as being involved in conditioned and unconditioned fear behavior and emotion expression that do not show significant differences here. Abbreviations: ACC, anterior cingulate cortex; AON, anterior olfactory nucleus; BNST, bed nucleus of stria terminalis; DSTR, dorsal striatum; GC, gustatory cortex; HYPO, hypothalamus; HPC, hippocampus; IL, infralimbic cortex; INS, insular cortex; MCTX, motor cortex; NAC, nucleus accumbens; OB, olfactory bulb; OPFC, orbital prefrontal cortex; OT, olfactory tubercle; PAG, periaqueductal grey; PRL, prelimbic cortex; PFC, prefrontal cortex; RSC, retrosplenial cortex; SEPTUM, septum; SSC, somatosensory cortex; dSTR, dorsal striatum; vSTR, ventral striatum; SUB, subiculum; SN, substantia nigra; THAL, thalamus; VTA, ventral tegmental area.
2.4 Effect of amphetamine sensitization on the BOLD signal response to TMT
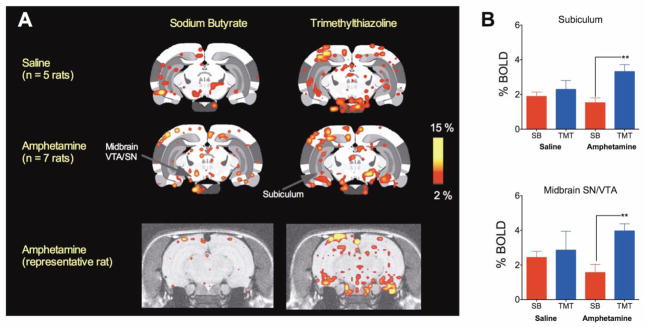
We next analyzed the interaction between odor presentation (TMT vs SB) and drug treatment (saline vs amphetamine pretreatment) and observed that the subiculum and the midbrain showed a significant interaction between odor and drug (Kruskall-Wallis ANOVA p < 0.05). Amphetamine, but not saline pretreated animals showed a greater BOLD activation in response to TMT than SB in the subiculum (t11 = 3.7, p = 0.0032, unpaired two-tailed t-test for homoscedastic variances) and midbrain (t11 = 3.8, p = 0.0029) (Figure 3A-C).
Figure 3.
BOLD signal response to SB and TMT in the subiculum and the midbrain. A) Composite 2D BOLD activation maps for saline pretreated (n = 5) and amphetamine-sensitized (n = 7) maternal rats presented with SB and TMT are shown above a representative brain maps of an amphetamine treated animal. Scale bar hue indicates percent change in BOLD (2% - 15%). Arrows indicate regions of interest under study. Abbreviations are as in Figure 1. B) The average BOLD signal response to SB and TMT in saline treated and amphetamine sensitized maternal rats. Asterisks indicate significant differences between SB and TMT in amphetamine-sensitized animals (t11 > 3.6, p < 0.005, two tailed t-test). Data presented as mean ± standard error.
2.5 Physiological alterations with TMT
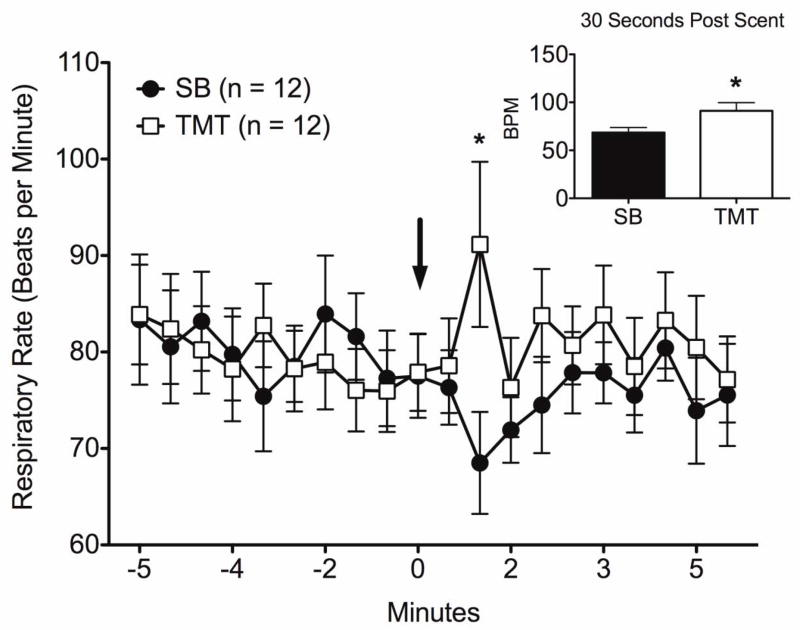
No differences in baseline respiratory rates were observed between SB and TMT. However, TMT elicited a slight, but significant increase in respiratory rate above baseline and above that observed for SB (Figure 4). No significant differences were observed between saline and amphetamine treatment groups.
Figure 4.
Changes in respiratory rates measured non-invasively during presentation of SB and TMT in maternal rats. Data are shown for five minutes before and after the time of scent presentation during scanning. Asterisk represents significant difference for the specific time point using t-test (p = 0.03). Inset shows significant time point 30 seconds post scent presentation. All data are expressed as mean ± standard error.
2.6 TMT-induced freezing in saline and amphetamine treated dam’s
The behavioral effects of the TMT and SB were confirmed post-imaging (Figure 5), with greater levels of freezing induced by TMT (p < 0.0001 two-tailed Mann-Whitney U test for independent samples). However, no differences were observed between saline and amphetamine treatment groups.
Figure 5.
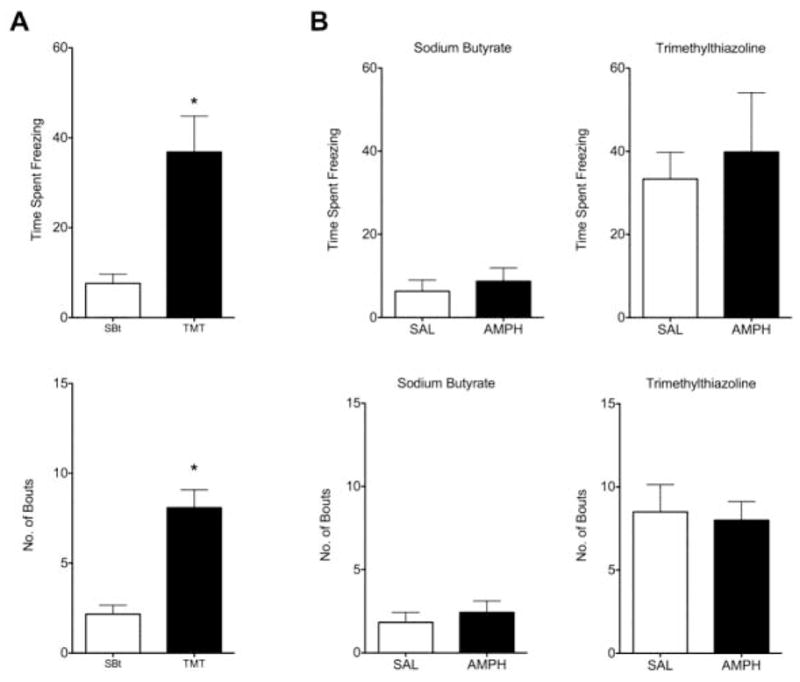
Expression of freezing postures during a 10-minute post-imaging test in the rats home cage. A) TMT induced significantly greater levels of freezing behavior and spent more time freezing than SB (n = 12; *p < 0.005). B) Freezing in the presence of TMT and SB in groups subdivided for pretreatment with saline (n = 5) or amphetamine (n = 7). No effect of amphetamine treatment on TMT-induced freezing was observed.
3. DISCUSSION
Presenting the scent of a predator to maternal rats that were sensitized to amphetamine before pregnancy increased BOLD activation in the ventral hippocampus and midbrain. TMT activated the subiculum, the VTA and SN to a greater magnitude than SB in amphetamine but not saline pretreated dams. It is possible that these two brain regions represent sites of long-term neuroadaptations to repeat amphetamine treatment that result in greater sensitivity stimuli that evoke stress or anxiety in dams. Activity between these two sites is related both anatomically and physiologically and their co-activation may play key roles in reward seeking, anxiety states and in mental diseases such addiction and schizophrenia. Studies by Floresco and Grace (2001) have shown that the ventral hippocampus modulates DA neuron firing through a polysynaptic pathway involving the NAC, ventral pallidum and VTA (Floresco et al., 2001). Using a rat prenatal drug exposure model of psychosis, Lodge and Grace show that excessive spontaneous firing of midbrain VTA DA neurons was suppressed by inactivation of the ventral hippocampus (Lodge and Grace, 2006; Lodge and Grace, 2007). Activity of the ventral hippocampus at specific burst frequencies seems to be key in reinstatement of drug seeking behavior (Vorel et al., 2001). The ventral hippocampus has also been studied for its role in the expression of fear and anxiety. Lesions to the ventral hippocampus but not the dorsal hippocampus result in anxiolytic traits such as reduced freezing behavior, increasing social interactions, and reduced fear of novel environments (Bannerman et al., 2004). Lesions to this site also increase open arm transitions in an elevated plus maze and reduce CORT levels in response to high illumination in a confined space (Kjelstrup et al., 2002). Neuroadaptations, such as long-term accumulation of brain derived neurotrophic factor (Grimm et al., 2003) and ΔFosB (Perrotti et al., 2005), have also been described in the VTA. Here, stress plays a critical role in addiction and reinstatement since changes in synaptic plasticity have been described in response to both stress and cocaine (Niehaus et al.). Glucocorticoid receptors on DA neurons within the VTA increase cocaine self-administration (Ambroggi et al., 2009) and CRF enhances NMDA dependent postsynaptic currents (Hahn et al., 2009).
We used SB as a control odor for TMT. Physiological differences between TMT and SB have been well documented. Heale et al (1994) presented a host of chemical olfactory stimuli to the anesthetized rats while registering 15–30 Hz spikes in the dentate gyrus and observed greater sensitivity of this region to TMT and weasel gland secretions than to SB (Heale et al., 1994). In a follow up study, this group shows that rats will show differential food preferences with chemicals such as weasel gland extracts or cadaverine, but are indifferent to foods sprayed with butyrate (Heale et al., 1996). This makes SB an adequate choice as a ‘neutral’ scent. Indeed, SB has been shown to not be aversive in rats (Panhuber, 1982). At a systems level, SB may have some unusual effects and therefore is not entirely neutral. Although, unlike TMT, SB does not induce a large increase in plasma corticosterone in rats (Morrow et al., 2000), these effects are dependent on length of exposure and reexposure(Dias Soares et al., 2003).
TMT evoked greater activity across several areas that were not previously described to be part of the neural circuitry responsive to this synthetic analog of a predator scent (Rosen et al., 2005; Staples et al., 2008). These regions included the anterior cingulate cortex, retrosplenial cortex, the ventral hippocampus, lateral amygdala and regions of the somatosensory cortex. We have reported activation of the anterior cingulate in a previous fMRI study in the rat (Febo et al., 2009); however, the present study used a comparison scent that allowed a clearer discernment of brain activation in response to TMT. The results from the lateral amygdala are perplexing since it has been previously shown that it is involved in conditioned and not unconditioned fear (Wallace and Rosen, 2001). It is possible that the greater BOLD activation in the lateral amygdala may be due to the higher amount of TMT used in the present study (Blanchard et al., 2003). We have observed previously that presentation of a male intruder rat in the presence of pups, which may elicit aggression in mothers and arguably anxiety as well, activates the somatosensory cortex (Caffrey et al.; Nephew et al., 2009). As indicated before, corticopetal projections, especially to the somatosensory cortex might regulate unconditioned fear specifically through cholinergic neurons from the nucleus basalis magnocellularis(Knox and Berntson, 2006). The retrosplenial cortex, specifically the caudal portions seen here to become activated by TMT, show specificity in their projections to the caudo-ventral portions of the subiculum (Wyss and Van Groen, 1992) and also show increased c-fos like immunoreactivity following conditioned fear stimuli (Beck and Fibiger, 1995). We did not find significant differences between the scent of a predator and a putrid smell produced by butyrate in other areas that have been previously identified using cellular markers such as c-fos or zif268-like immunoreactivity (Rosen et al., 2005; Staples et al., 2008). These included the PAG and septum, among others. It is possible that these sites become active gradually over time with prolonged exposure to TMT. Here, the TMT evoked activity within the first 1–2 minutes and thus corresponds precisely to the reaction of the animal at the moment when the odor is encountered. Alternatively, the mismatch in findings can also be due to the restraint position of the animals inside the MRI scanner. This would perhaps occur with structures such as the PAG that are thought to mediate the behavioral inhibition during behavioral reactions to fear and anxiogenic stimuli (McNaughton and Corr, 2004). On the other hand, increased BOLD activation with TMT in the ACC, RSC, SSC and SUB might reflect instead higher level processing of the anxiogenic stimulus (McNaughton and Corr, 2004). The septum shares connectivity with the ventral hippocampus and this latter structure can also be attributed to behavioral inhibition and fear responses in other experimental settings(Degroot and Treit, 2004). Tetrodotoxin-induced inactivation of the septum and ventral hippocampus result in similar anxiolytic effects(Degroot and Treit, 2004). Therefore, the disconnect between the present fMRI results and the freezing behavior observed, which is known to involve the septum, PAG and BNST, might be due to the restraint conditions of the procedure used for imaging. Activation of higher centers could be associated with upper level cognitive and emotion-related processing in the absence of overt behavioral changes during fMRI (‘emotion’ here is used to denote top-down autonomic modulation that would result in increased physiological activity).
We would like to point to several shortcomings of the study that need further attention in future experiments employing fMRI studies in rodents to assess the effects of stress. First, given that the animals used in the study were screened on multiple tests for freezing responses, light-dark tests, locomotor activity, it would not be improbable that the neural response to the TMT and other stressors might vary from entirely naïve animals that were only imaged. This might be further compounded by acclimation to restraint for MRI procedures and acclimation to test boxes. A strength of the present study design, however, is that fMRI results were accompanied by behavioral tests that aided in the interpretations of the data. For instance, we observed that TMT evoked greater levels of overall freezing responses than SB and this helped confirm that the animals presented with the scent during imaging were in fact sensitive to the fear-related behavioral effects. The other point is in regards to the longevity of the habituated state in animals. As previously reported, rats are acclimated to restraint and MR sounds before imaging methods. It is unknown (1) whether all rats or just a portion of these acclimate to restraint conditions and (2) whether the habituated state lasts over an extended period of time as required in the present study (ca. 34 days after acclimation rats were imaged). Given that animals were lost due to motion, it might be that either one of the above scenarios incurs in a bias towards losing awake animals in which the acclimation never develops well or does not last. We are currently conducting studies to better understand the effects of acclimation. It is important to note however that past research has shown that that rats habituate to repeated daily 1–2 hour restraint for 4–9 days (Dhabhar et al., 1997; Melia et al., 1994). Rats show normal patterns of food intake and heart rate following habituation to restraint (Haleem, 1996; Stamp and Herbert, 2001). Importantly, habituation to repeated daily sessions of restraint is not indicative of impaired HPA axis function, since rats acclimated to restraint stress still show increased c-fos activation and corticosterone levels to a novel stressor (Melia et al., 1994). Finally, comparison virgin rat controls were not included in the present study. As mentioned in the methods section, the amphetamine sensitization paradigm used here has been reported to produce long-term sensitization in female rats (Afonso et al., 2009). It also known that virgin rats a more fearful and less resilient to stress than lactating rats and therefore the present results might vary, perhaps be of greater magnitude, in virgin versus lactating rats (Slattery and Neumann, 2008).
Overall, the present findings show a pattern of brain activity corresponding to the lactating rats response to a distressful stimulus, such as the scent of a predator. Further, it appears that the ventral hippocampus and midbrain are possible sites of long-term sensitization to stressors in maternal rats that have been sensitized to amphetamine before pregnancy and that remain drug-free during gestation.
4. METHODS
4.1 Subjects
Long-Evans female rats (175–225 g) were purchased from Charles River Laboratories (Wilmington, MA). Females were housed in pairs in a temperature and humidity controlled room and maintained on a 12L: 12D light-dark cycle (lights off at 1900 hr). Following amphetamine administration and mating, females were single housed during their pregnancy and the postpartum period. All experiments are carried out with primiparous rats. Home cages consisted of hanging plastic microisolater cages of standard dimensions with woodchip bedding. Water and Purina rat chow were provided ad libitum. Rats were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 85-23, Revised 1985) and adhere to the National Institutes of Health and the American Association for Laboratory Animal Science guidelines. The Institutional Animal Care and Use Committee at Northeastern University approved the protocols used for this study.
4.2 Acclimatization for awake magnetic resonance imaging procedures
Rats were acclimated for 5 days to MRI restraint procedures before drug treatment and breeding. On each day, they were anesthetized with 4% isoflurane gas anesthesia and placed into a replica of the restraint unit used for functional magnetic resonance imaging (fMRI) studies. A topical anesthetic of 4 % lidocaine cream was applied to the skin around the ear canals and over the bridge of the nose before the animal is placed inside the restraint unit. This minimizes discomfort on pressure points around the head. Most of the restraint is around the head and above the shoulders; therefore, animals are in a hunched posture with limbs free and unrestrained. The head holder has a bite bar over which the incisors are placed and held by lowering and locking-in a plastic nose bar. Plastic headgear has built-in blunted ear bars that are locked in place by lateral screws, thereby preventing lateral movement. A pair of shoulder bars was lowered behind the animal’s neck, thereby further restricting vertical movement. Once setup is complete, the rat is placed into an opaque environment simulating the spectrometer bore. They are then exposed to pre-recorded MR pulse sequence sound. The acclimatization procedures are repeated over 5 days, during which the time spent under restraint is increased from 20 minutes the 1st day to a maximum 60 minutes on the last.
4.3 Development and expression of amphetamine sensitization
Two days following the final 5th day of acclimatization to restraint, animals were injected with either saline or amphetamine in order to test for sensitization of its locomotor stimulant effects. D-Amphetamine HCl was purchased from Sigma-Aldrich (St. Louis, MO) and given intraperitoneally (i.p.) at a dose of 1 mg/kg/mL. Control animals received an i.p. injection of 1ml/kg of 0.9% sterile saline solution. Rats were provided with amphetamine injections every other day for 3 days (3 days of injection). This schedule of amphetamine injections has previously been shown to produce sensitization in Long Evans female rats (Afonso et al., 2009) that result in long lasting behavioral effects (ca. 22 days of withdrawal before subsequent testing). Locomotor activity was recorded in automated activity cages (Accuscan Instruments, Columbus, OH), as previously reported (Febo et al., 2003). The day before testing, rats were habituated to the cage environment for 60 minutes. Over the three amphetamine treatment days, animals were given a saline or amphetamine injection and locomotor activity recorded for 60 minutes. The activity data were collected on a Windows XP PC as the number of total vertical, horizontal and repetitive infrared beam breaks in 60 minutes or changes in activity over 5 minute bins. Activity data in response to saline and amphetamine injections were statistically evaluated for drug × day interactions using a two-factor analysis of variance with drug and day of treatment as independent variables (significance at p < 0.05). Bonferroni’s multiple comparison test was used for post-hoc determination of significant differences between day 1 and day 3 for amphetamine treatments.
4.4 Light-dark box testing for baseline anxiety levels
Two days after the last amphetamine injection, rats were screened for the time spent in the dark side of a light-dark shuttle box. The cage used for testing was a modified Shuttleflex system (Accuscan Instruments), in which one side of the cage was completely concealed from external light. The cage has a dividing wall with a center hole in which the animal is free to move from either the light or dark sides. The testing was done in 5 minutes. A 2nd 5-minute test was performed during the postpartum period following fMRI studies. Time spent in the dark side was statistically compared between saline and amphetamine animals using two-tailed t-test for independent samples (α< 0.05). The day after testing, rats were housed with a male breeder for up to 5 days, or until pregnancy was confirmed by light microscopic examination for the presence of sperm in the vaginal canal. Rats were drug free during gestation.
4.5 Imaging the response to sodium butyrate and synthetic 2,4,5-trimethylthiazoline (‘fox scent’)
The BOLD signal response to TMT was measured on the early postpartum period (days 2–4), over 34 days since the last amphetamine injection. Imaging per rat was done on a single session. Synthetic TMT or fox scent was obtained from CONTECH (Victoria, British Columbia, CA) and sodium butyrate (SB) from Sigma-Aldrich (St. Louis, MO). Both chemicals emit strong odors that are detected by rodents and humans. TMT, but not SB, has been reported to elevate plasma levels of the stress hormone, corticosterone, in rats (Morrow et al., 2000) and is thought to evoke stress in addition to modulating unconditioned fear and anxiety in rats (Wallace and Rosen, 2001). Odor delivery for scanning was achieved by pumping air through rubber tubing connected at one end to an aquarium pump and at the other end placed near the rats nose inside the bore of the magnet. Just outside of the magnet, an easily removable tube connector attached the clean tubing to the outlet tubing towards the rats nose. During odor delivery the connector was switched with a connector containing the odor on a strip of absorbent paper with 5 μL of SB or TMT (pure undiluted) (Morrow et al., 2000). The concentrated volume is slightly lower than our prior study (Febo et al., 2009), however, we determined through pilot work that it is sufficient to elicit freezing behavior in the Long Evans rat and is sufficient for odor delivery during fMRI studies. The volume of TMT used here is 100 times the minimum volume used to produce freezing in rats (0.05 μL in Blanchard et al., 2003), but only 4–7 times less than the amount used previously to determine the neural circuitry and neurochemistry of unconditioned fear (Morrow et al., 2000; Fendt et al., 2003; Febo et al., 2009). The bore of the magnet was thoroughly cleansed after each experiment using ethanol (95%). Fifteen milligrams of SB was dissolved in 1 mL of water before use. Clean and unused tubing was used for each study. Odor presentation was not counterbalanced, with SB always presented before TMT. A third pilot scan was used before the presentation of the SB, in which rats were presented with a novel citric scent (data not shown). This additional (control) run with the delivery of an alternate odor was done to prepare the animals for the actual experimental odors (SB vs TMT). This was done in all animals and it helped control for the novelty of the odor presentation. Each scan lasted 10 minutes with odors presented at 5 minutes into the scan. Pups were not present during scanning.
4.6 Functional MRI methods
Before MR scanning, dams were anesthetized with 4 % isoflurane. A topical anesthetic of 4 % lidocaine cream was applied to the skin and soft tissue around the ear canals and over the bridge of the nose before the animal is placed inside the restraint unit. This procedure took 5–6 min, after which gaseous anesthesia flow was turned off and the entire unit was placed through the bore of the magnet for imaging. After the entire unit was placed in the magnet, scanning preparations controlled by Paravision 4.0 typically took 10–15 minutes and thereafter the entire imaging session including 1 anatomical scan (ca. 6 minutes) and 3 10-minute functional scans lasted about 36 minutes. Thus, the entire experiment per animal in an unanaesthetized state lasted 50–60 minutes. A pneumatic respiratory pillow was placed underneath each animal’s chest during scanning to non-invasively measure breaths per minute using a Control/Gating Module (SA Instruments Inc, Stony Brook, NY). This allowed us to determine whether there were differences in baseline physiology among the rats that were imaged and to also determine whether TMT altered a physiological measure during its presentation.
Experiments were conducted in a 300 Mhz Bruker USR 7T/20 cm horizontal magnet (Bruker, Germany) equipped with a Paravision 4.0 console (Bruker, Billerica, MA U.S.A). Studies were performed with a quadrature (transmit/receive) radiofrequency (Rf) coil, with easy access head and body restrainer for rats (Ekam Imaging, Shrewsbury, MA). The Rf coil produces a homogenous B1 field across the entire brain, allowing very high SNR’s. Radiofrequency signals are sent and received with electronics built into the animal restrainer (Ludwig et al., 2004). Functional imaging was performed using a multi-segmented T2-weighted fast spin echo pulse sequence with the following parameters: repetition time TR = 1562 msec, echo time TE = 7.5, effective echo time TEeff= 45 msec and an echo train length ETL = 16. Geometry was setup as follows: 12 slices, field of view of 28 mm, 1.0 mm thick slices with no gaps, data matrix of 642 for functional scans and 2562 for anatomical scans (Thus the in plane 2D pixel resolution was 438 μm2 for functional and 117 μm2 for anatomical scans). A full set of 12 coronal slices across the brain was collected at each effective repetition time and was completed every 6 seconds.
4.7 Statistical analysis
Full details for the MRI data analysis using in-house software has been previously reported (Ferris et al., 2008). Scans were pre-screened for motion and drift using previously described criteria (Ferris et al., 2008). Out of 19 rats initially included in the study (10 saline and 9 amphetamine), 2 were excluded due to excessive motion or signal drift that could not be corrected and 5 were excluded because of not achieving pregnancy after a single 5-day mating session (n = 4) or infanticide (n = 1). A second round of mating was not used in order to maintain comparable drug free periods before scanning (see Figure 1A-1B). The final group sizes for fMRI studies were 5 saline and 7 amphetamine rats. Group sizes for some of the behavior measures or collapsed imaging data were higher and are indicated in graph legends.
Each subject was registered to a fully segmented electronic rat brain atlas (Paxinos & Watson, 1997; Swanson, 1999). Statistical t tests are performed on each subject within the original coordinate system. The baseline period used was 48 repetitions immediately preceding odor and the stimulation window was 48 repetitions. Statistical t tests used a 95 % confidence level, two-tailed distribution, and heteroscedastic variance assumptions. In order to provide a conservative estimate of significance, a false-positive detection-controlling algorithm is introduced into the analysis (Genovese et al., 2002). This ensures that the false-positive detection rate is below our confidence level of 5 % (Ferris et al., 2005). Statistically significant pixels were assigned their percentage change values (stimulus mean minus control mean). Activated voxel numbers and percent signal changes were exported to SPSS for statistical comparisons between groups. Details for the MRI data analysis using in-house software has been previously reported (Ferris et al., 2008; Nephew et al., 2009). The number of voxels per region of interest (ROI) and their corresponding average percent change values were statistically evaluated between 4 scan groups (Saline-SB, Saline-TMT, Amphetamine-SB, Amphetamine-TMT) using Kruskall-Wallis analysis of variance (ANOVA p < 0.05). Posthoc testing for specific differences between treatment groups was done using Mann-Whitney U test (p < 0.05). Experimental conditions related to drug treatments and litter size and health were uniform across saline and amphetamine groups. No significant differences were observed between the drug treatment groups. Dams were imaged at about the same postpartum day (saline: 3 ± 0.6 and amphetamine 3.3 ± 1.5), thus the average withdrawal length was around 32–34 days (saline: 34 ± 5.8 and amphetamine 32 ± 4.4). Litter sizes were 12 ± 1.4 for saline and 13 ± 3.5 for amphetamine rats (average weights were saline = 6.8 ± 0.5 and amphetamine 7.9 ± 0.6).
4. 8 Test for innate fear: TMT-induced Freezing Behavior
Thirty minutes after fMRI studies, rats were tested in their home cage for freezing in response to TMT or SB. Pups were in the cage during testing. Cages were placed inside a fume hood to avoid spread of odors. Behavior measurements were taken during a 10-minute test by a trained observer blind to the drug treatment condition (saline and amphetamine). For testing, 5 uL of SB or TMT was applied to a strip of absorbent paper and into a 1 mL Eppendorf tube that was attached to the upper inner side of the cage. As during scanning, SB was always tested before TMT. Both time spent freezing and number of times the animal froze was registered. During freezing the animal arched or stretched its back, slightly lifted its head upward and stopped all movement for several seconds(Blanchard et al., 2003). Freezing behavior was statistically compared between SB and TMT or saline and amphetamine animals using two-tailed independent samples t-test (p < 0.05).
Acknowledgments
Support: funding provided by NIH grant DA019946 and Northeastern University seed funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso VM, Mueller D, Stewart J, Pfaus JG. Amphetamine pretreatment facilitates appetitive sexual behaviors in the female rat. Psychopharmacology (Berl) 2009;205:35–43. doi: 10.1007/s00213-009-1511-x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132:289–95. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schutz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–9. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–31. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Arevalo S, Prado G, Amaro H. Spirituality, sense of coherence, and coping responses in women receiving treatment for alcohol and drug addiction. Eval Program Plann. 2008;31:113–23. doi: 10.1016/j.evalprogplan.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacology. 2002;26:286–94. doi: 10.1016/S0893-133X(01)00308-6. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–20. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117:360–8. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- Caffrey MK, Nephew BC, Febo M. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology. 58:107–16. doi: 10.1016/j.neuropharm.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Res. 2004;1001:60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–8. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Dias Soares D, Fernandez F, Aguerre S, Foury A, Mormede P, Chaouloff F. Fox odour affects corticosterone release but not hippocampal serotonin reuptake and open field behaviour in rats. Brain Res. 2003;961:166–70. doi: 10.1016/s0006-8993(02)03944-6. [DOI] [PubMed] [Google Scholar]
- Endres T, Fendt M. Inactivation of the lateral septum blocks fox odor-induced fear behavior. Neuroreport. 2008;19:667–70. doi: 10.1097/WNR.0b013e3282fb78d9. [DOI] [PubMed] [Google Scholar]
- Febo M, Gonzalez-Rodriguez LA, Capo-Ramos DE, Gonzalez-Segarra NY, Segarra AC. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J Neurochem. 2003;86:405–12. doi: 10.1046/j.1471-4159.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Febo M, Ferris CF. Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience. 2007;148:400–12. doi: 10.1016/j.neuroscience.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Shields J, Ferris CF, King JA. Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Res. 2009;1302:183–93. doi: 10.1016/j.brainres.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–8. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and threedimensional computational analysis. J Neurosci. 2005;25:149–56. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–22. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–7. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29:6535–44. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleem DJ. Adaptation to repeated restraint stress in rats: failure of ethanol-treated rats to adapt in the stress schedule. Alcohol Alcohol. 1996;31:471–7. doi: 10.1093/oxfordjournals.alcalc.a008181. [DOI] [PubMed] [Google Scholar]
- Hamamura T, Fibiger HC. Enhanced stress-induced dopamine release in the prefrontal cortex of amphetamine-sensitized rats. Eur J Pharmacol. 1993;237:65–71. doi: 10.1016/0014-2999(93)90094-x. [DOI] [PubMed] [Google Scholar]
- Harmer AL, Sanderson J, Mertin P. Influence of negative childhood experiences on psychological functioning, social support, and parenting for mothers recovering from addiction. Child Abuse Negl. 1999;23:421–33. doi: 10.1016/s0145-2134(99)00020-4. [DOI] [PubMed] [Google Scholar]
- Heale VR, Vanderwolf CH, Kavaliers M. Components of weasel and fox odors elicit fast wave bursts in the dentate gyrus of rats. Behav Brain Res. 1994;63:159–65. doi: 10.1016/0166-4328(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Heale VR, Petersen K, Vanderwolf CH. Effect of colchicine-induced cell loss in the dentate gyrus and Ammon’s horn on the olfactory control of feeding in rats. Brain Res. 1996;712:213–20. doi: 10.1016/0006-8993(95)01416-0. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–30. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Berntson GG. Effect of nucleus basalis magnocellularis cholinergic lesions on fear-like and anxiety-like behavior. Behav Neurosci. 2006;120:307–12. doi: 10.1037/0735-7044.120.2.307. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–61. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–30. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R, Bodgdanov G, King J, Allard A, Ferris CF. A dual RF resonator system for high-field functional magnetic resonance imaging of small animals. J Neurosci Methods. 2004;132:125–35. doi: 10.1016/j.jneumeth.2003.08.017. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–38. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000;864:146–51. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Febo M. Effect of cocaine sensitization prior to pregnancy on maternal care and aggression in the rat. Psychopharmacology (Berl) 2010;209:127–35. doi: 10.1007/s00213-010-1777-z. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Caffrey MK, Felix-Ortiz AC, Ferris CF, Febo M. Blood oxygen level-dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing intruder threat to pups. Eur J Neurosci. 2009;30:934–45. doi: 10.1111/j.1460-9568.2009.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci. 32:108–17. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhuber H. Effect of odor quality and intensity on conditioned odor aversion learning in the rat. Physiol Behav. 1982;28:149–54. doi: 10.1016/0031-9384(82)90116-0. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–24. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Adamec RE, Thompson BL. Expression of egr-1 (zif268) mRNA in select fear-related brain regions following exposure to a predator. Behav Brain Res. 2005;162:279–88. doi: 10.1016/j.bbr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Schoffelmeer AN, De Vries TJ, Wardeh G, Dogterom G, Bol JG, Binnekade R, Tilders FJ. A single administration of interleukin-1 or amphetamine induces long-lasting increases in evoked noradrenaline release in the hypothalamus and sensitization of ACTH and corticosterone responses in rats. Eur J Neurosci. 2001;13:1923–30. doi: 10.1046/j.0953-816x.2001.01569.x. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Kelsoe JR, Segal DS. Hippocampal glucocorticoid receptor mRNA is up-regulated by acute and down-regulated by chronic amphetamine treatment. Brain Res Mol Brain Res. 1996;38:156–60. doi: 10.1016/0169-328x(96)00009-5. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–85. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp J, Herbert J. Corticosterone modulates autonomic responses and adaptation of central immediate-early gene expression to repeated restraint stress. Neuroscience. 2001;107:465–79. doi: 10.1016/s0306-4522(01)00364-5. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–47. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–8. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci. 2001;21:3619–27. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus. 1992;2:1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]