Abstract
Background and study aims
Most natural orifice transluminal endoscopic surgery (NOTES) procedures have been performed in animal models through the anterior stomach wall, but this approach does not provide efficient access to all anatomic areas of interest. Moreover, injury of the adjacent structures has been reported when using a blind access. The aim of the current study was to assess the utility of a CT-based (CT: computed tomography) image registered navigation system in identifying safe gastrointestinal access sites for NOTES and identifying intraperitoneal structures.
Methods
A total of 30 access procedures were performed in 30 pigs: anterior gastric wall (n = 10), posterior gastric wall (n = 10), and anterior rectal wall (n = 10). Of these, 15 procedures used image registered guidance (IR-NOTES) and 15 procedures used a blind access (NOTES only). Timed abdominal exploration was performed with identification of 11 organs. The location of the endoscopic tip was tracked using an electromagnetic tracking system and was recorded for each case. Necropsy was performed immediately after the procedure. The primary outcome was the rate of complications; secondary outcome variables were number of organs identified and kinematic measurements.
Results
A total of 30 animals weighting a mean (± SD) of 30.2 ± 6.8 kg were included in the study. The incision point was correctly placed in 11 out of 15 animals in each group (73.3 %). The mean peritoneoscopy time and the number of properly identified organs were equivalent in the two groups. There were eight minor complications (26.7 %), two (13.3 %) in the IR-NOTES group and six (40.0 %) in the NOTES only group (P = n. s.). Characteristics of the endoscope tip path showed a statistically significant improvement in trajectory smoothness of motion for all organs in the IR-NOTES group.
Conclusion
The image registered system appears to be feasible in NOTES procedures and results from this study suggest that image registered guidance might be useful for supporting navigation with an increased smoothness of motion.
Introduction
Natural orifice transluminal endoscopic surgery (NOTES) has changed the approach to the peritoneum in the past few years [1 – 7]. This novel technique permits access to the peritoneal organs through the mouth, rectosigmoid or vagina, with diagnostic and therapeutic purposes. Numerous hybrid NOTES procedures (combining NOTES with laparoscopy) have been described during the past five years [8–10]. As highlighted in the White Paper from the American Society for Gastrointestinal Endoscopy (ASGE)/Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) [11], one of the issues to be addressed before translating NOTES to human practice is providing the physician with adequate visual feedback and clear indicators of instrument location and orientation, thereby improving instrument navigation and facilitating the recognition of anatomic structures. In addition, most NOTES procedures have been performed in animal models through the anterior stomach wall but this approach does not provide efficient access to all anatomic areas of interest. Moreover, injury of the adjacent structures has been reported when using a blind access. To overcome these complexities, some authors have used endoscopic ultrasound (EUS) guidance either to identify pre-assessed target organs [12] or to have more secure access to the peritoneal cavity [13–16]. However, there are no reports on the feasibility and usefulness of other image-guided systems.
Over the past two decades, many investigators have sought to show image and instrument position in 3D models. Vosburgh et al. [17, 18] have explored the use of real-time systems that track instruments and display relevant data in the context of registered 3D models of the patient extracted from preoperative computed tomography (CT). This work is based on the recent development of tiny but very accurate position sensors and very fast interface and software systems that permit complex visualization with no discernible lag. These “image registered” techniques have been used to improve task performance in laparoscopy and endoscopy, and have been explored for guiding transgastric access as a first step in developing image registered NOTES techniques [19, 20].
The aims of the present study were to demonstrate the feasibility of an image registered system for guiding NOTES procedures in a porcine model, and to compare an image registered-guided approach with conventional endoscopic NOTES alone.
Materials and methods
Animals
Experiments were performed in 30 female Yorkshire pigs weighting 27 – 35 kg. The studies were approved by the Animal Research Committee at the University of Barcelona. Animals underwent a 2-day quarantine and acclimation period during which they were individually caged, fed the same diet, and had unlimited access to water. All procedures were performed under general anesthesia using desflurane and tracheal intubation.
Study protocol
A total of 30 NOTES peritoneoscopies were performed through three different access sites: anterior gastric wall (n = 10), posterior gastric wall (n = 10), and anterior rectal wall (n = 10). The animals were divided into two groups: NOTES with image registered guidance (IR-NOTES, n = 15) and conventional NOTES without image registered guidance (NOTES, n = 15). Following the procedure, necropsy was immediately performed and incision sites and the peritoneal cavity were examined for evidence of bleeding, perforation or other complications.
The primary outcome of the study was the rate of complications; secondary outcome variables were the number of organs identified and kinematic measurements.
Image registered system
The image registered system is a real-time guidance system with one synthetic display driven by the position of the endoscope. The display shows a 3D anatomic model of the anatomy derived from a volumetric CT data set, and the tip of the tracked scope provides contextual information of its position with respect to overall anatomy. The image registered system uses established techniques for the visualization of the probe position and image registration but implements them in real time by using an electromagnetic tracker (3D Guidance trakSTAR; Ascension Technology Corp., Burlington, Vermont, USA) with a mid-range transmitter and a miniaturized sensor (2-mm outer diameter). The tracker system has been tested to meet International Electrotechnical Commission (IEC) 60601-01 standards. The electromagnetic sensor was tightly attached to the tip of the endoscope using shrink wrap.
For these studies, the volumetric data were collected by using a Siemens Sensation 64 slice CT system (Siemens Medical Systems, Erlangen, Germany). A contrast-enhanced arterial phase image was acquired using a 70-mL bolus triggered at 2.5 mL/second when the density in the aorta reached 150 HU. Images were acquired using 120 kVp and 210mAs with an image field of view of 380 mm. The images were reconstructed in a 512 × 512 matrix with a B20f reconstruction kernel and 1-mm slice thickness. The synthetic 3D models of reference anatomy were then created using a semiautomatic approach [19] using the open-source image analysis software 3D slicer (www.slicer.org). Anatomic models were made for the rib cage and spine, lungs, gallbladder, aortic trunk, celiac and superior mesenteric artery branches, kidneys, and bladder.
The image registered system display was based on the display previously reported for laparoscopic and endoscopic image registered ultrasound [19, 20]. For this study, the display (Figs. 1 and 2) was composed of two views: an external view of the anatomy and a virtual endoscopic view.
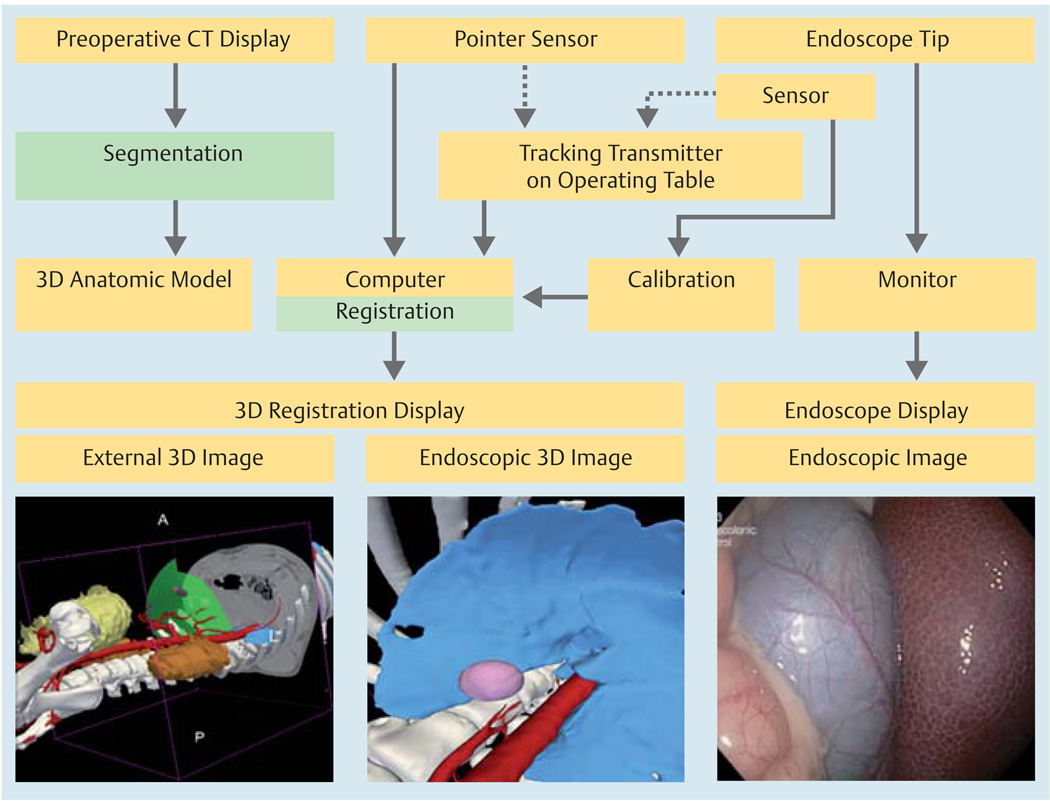
Fig. 1.
Construction of the 3D display from pre-operative computed tomography (CT) and the integration of the tracking system components with different computational elements of the image registered-guided natural orifice transluminal endoscopic surgery (IR-NOTES) system.
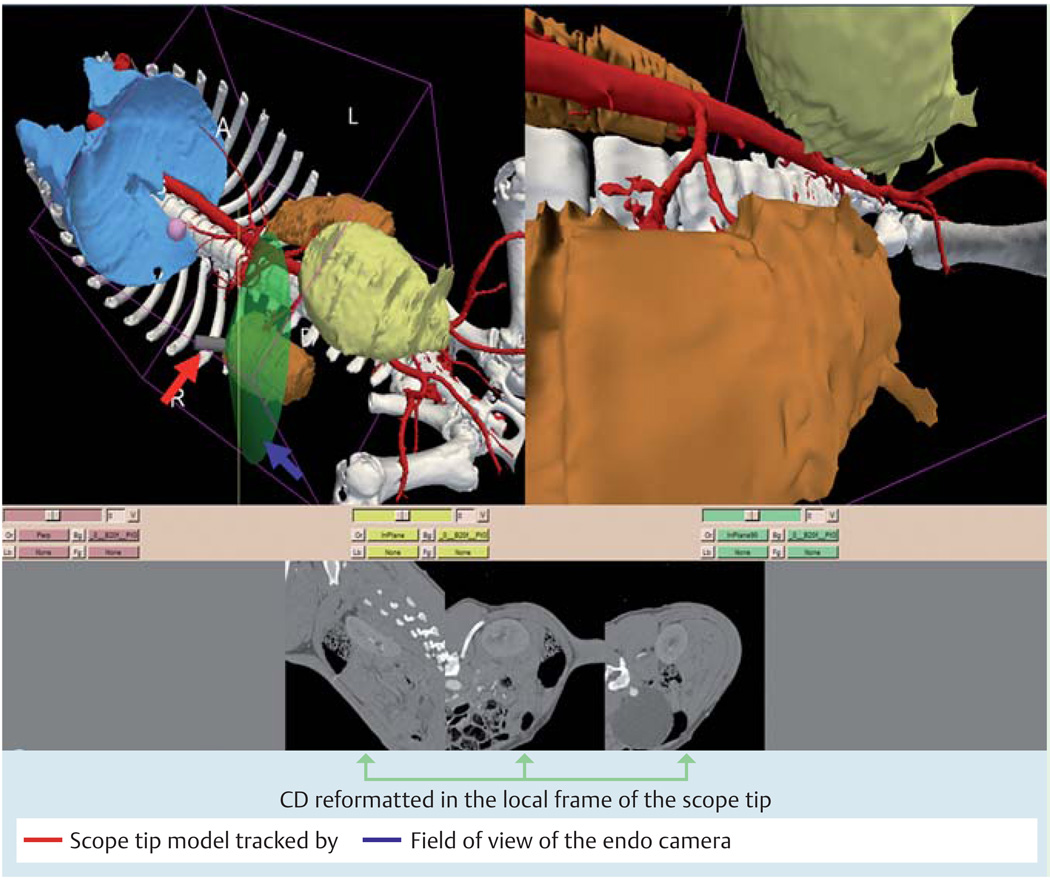
Fig. 2.
Annotated screen capture of the image registered-guided natural orifice transluminal endoscopic surgery (IR-NOTES) that was displayed during the guided procedures. The upper left part of the screen shows a 3D display of the general anatomy and the location of scope tip with respect to the rendered organs. The upper right part of the screen shows the virtual endoscopic image that is generated from the structures extracted from preoperative computed tomography (CT). The lower part of the screens shows the CT re-formatted in the local frame with respect to the scope tip.
The external view shows the tracked tip of the scope with respect to the displayed anatomy. The virtual endoscopic view shows the 3D anatomy from the view point of the endoscope camera as a complement to the real endoscopic view.
The preoperative images were registered to the coordinate system defined by the electromagnetic transmitter using a continuous set of points acquired from the skin surface using a separate electromagnetic sensor. Those points were aligned with the model of the skin using the iterative closest point (ICP) algorithm [21] to yield a rigid body transformation.
NOTES peritoneoscopy
A standard flexible gastroduodenoscope (GIF Q140; Olympus Europe, Hamburg, Germany) was used. Reusable endoscopic tools used throughout the procedure included conventional needle- knives, Jagwire guide wires (Boston Scientific, Natick, Massachusetts, USA), and 18-mm controlled radial expansion (CRE) balloon dilators.
For transgastric NOTES procedures, indentation of the gastric wall produced by palpating the ventral abdomen was used to identify the anterior and posterior wall. After the location for the incision had been chosen, a needle-knife was used to make a 5 – 10-mm incision, which was then dilated with an 18-mm CRE balloon.
For transrectal NOTES procedures, the colonic incision site was carefully chosen between 15 and 20 cm from the anal verge after identifying anterior positioning with external palpation. A needle- knife was used to make an 8 – 10 mm linear incision and, using the needle-knife as a guide, the endoscope was advanced through the colonic wall into the peritoneal cavity.
Peritoneoscopy consisted of timed abdominal exploration with identification of 11 pre-determined organs in the following order: liver, gallbladder, spleen, large intestine, small intestine, bladder, fallopian tubes, stomach, right and left kidneys, and pancreas.
The position series of the endoscopic tip was continuously recorded using the electromagnetic tracking system to perform a kinematic analysis [22]. As well as the linear metrics described in angular kinematics, measurements were also computed to characterize the motions in the angular directions (endoscope tip roll, elevation, and azimuth). Kinematic variables are described in Table 1.
Table 1.
Description of kinematic variables.
| Elapsed time (seconds) | Time employed to accomplish the task |
| Path length (mm) | Length of the trajectory followed by the probe to complete the task |
| Linear velocity (mm/second) | Velocity in the linear component |
| Linear acceleration (mm/second2) | Acceleration in the linear component |
| Linear jerk (mm/second3) | Total amount of jerk for the linear component. Jerk tracks changes in acceleration that are associated with smoothness of motion |
| Depth perception | Perception of depth in the distal direction of the scope |
| Rotation1 (radians) | Total amount of rotation around the axis of the scope |
| Rotation2 (radians) | Total amount of rotation in the transversal plane of the scope tip (elevation and azimuth) |
| Angular velocity1 (radians/second) | Angular velocity around the scope axis (roll) |
| Angular velocity2 (radians/second) | Angular velocity in the elevation and azimuth orientations |
| Angular acceleration1 (radians/second2) | Angular acceleration in roll orientation |
| Angular acceleration2 (radians/second2) | Angular acceleration in elevation and azimuth |
| Angular Jerk1 (radians/second3) | Smoothness of rotational motions of the scope tip in the roll orientation |
| Angular Jerk2 (radians/second3) | Smoothness of rotation motions of the scope tip in the elevation and azimuth orientations |
Statistical analysis
Categorical data were evaluated by Fisher exact test, and continuous data were compared by the Wilcoxon test. Multiple comparisons were performed with the analysis of variance and the Newman-Keuls test for continuous data and Fisher exact test for categorical data.
The sample size was calculated to achieve 80% power to detect a difference in the incidence of complications of 12% with a significance level of 0.05.
All continuous variables were expressed as the median plus range. A P value of less than 0.05 was considered statistically significant. All calculations were made using the SPSS statistical package (SPSS Inc., Chicago, Illinois, USA).
Results
All 30 animals provided good data for analysis. A total of 20 animals underwent transgastric peritoneoscopy (10 through anterior gastric wall access and 10 via the posterior gastric wall) and 10 underwent transcolonic peritoneoscopy. The incision point was correctly placed in the pre-assigned site in 22 (73.3 %) cases. A correctly placed incision was achieved in all of the transrectal attempts but only 12 out of the 20 (60 %) transgastric procedures (P = 0.02). The anterior transgastric access was the most difficult, with five out of 10 (50 %) being located in the posterior wall. By contrast, when the posterior wall was attempted, the incision was correctly placed in seven out of 10 cases (70 %) (P = n. s.). The procedures are described in detail in Table 2.
Table 2.
Characteristics of natural orifice transluminal endoscopic surgery procedures performed using three different access routes.
| Overall n = 30 |
Transgastric anterior n = 10 |
Transgastric posterior n = 10 |
Transrectal n = 10 |
P | |
|---|---|---|---|---|---|
| Weight, median (range), kg | 30 (27 – 44) | 31 (29 – 34) | 30 (27 – 44) | 30 (28 – 34) | n. s. |
| Incision time, median (range), seconds | 443 (36 – 1180) | 540 (241 – 910) | 532 (173 – 1180) | 175 (36 – 240) | n. s. |
| Visualized structures, median (range) | 10 (6 – 10) | 10 (7 – 10)* | 9 (6 – 10)* | 10 (7 – 10) | n. s. |
| Peritoneoscopy time, median (range), seconds | 1145 (685 – 1980) | 1034 (815 – 1740) | 1149 (685 – 1980) | 1253 (900 – 1584) | n. s. |
| Total procedure time, median (range), seconds | 1632 (1073 – 3344) | 1656 (1355 – 2770) | 2235 (1123 – 3344) | 1417 (1073 – 3240) | n. s. |
| Correct incision point, n | 22 | 5 | 7 | 10 | n. s. |
P = 0.04
n. s., not significant.
According to the study protocol, 15 animals underwent the IR-NOTES procedure. Details of peritoneoscopies are shown in Table 3.
Table 3.
Characteristics of the procedures performed with image registered-guided natural orifice transluminal endoscopic surgery (IR-NOTES) and blind NOTES.
| IR-NOTES n = 15 |
NOTES n = 15 |
P | |
|---|---|---|---|
| Weight, median (range), kg | 31 (28– 44) | 30 (27 – 35) | n. s. |
| Incision time, median (range), seconds | 360 (36 – 910) | 540 (38 – 1180) | n. s. |
| Visualized structures, median (range) | 10 (6 – 10) | 10 (7 – 10) | n. s. |
| Peritoneoscopy time, median (range), seconds | 1145 (840 – 1980) | 1080 (685 – 1740) | n. s. |
| Total procedure time, median (range), seconds | 1612 (1223 – 3344) | 1693 (1073 – 3240) | n. s. |
| Correct incision point, n | 11 | 11 | n. s. |
n. s., not significant.
The incision point was correctly placed in 11 out of 15 animals in each group (73.3 %), and time for performing the incision and reaching the peritoneal cavity was slightly shorter with IR-NOTES than with NOTES alone (360 seconds [range 36 – 910] vs. 540 seconds [range 38 – 1180], respectively; P = n. s.). When considering the three different access sites, incision time was also slightly shorter in all the IR-NOTES procedures (transgastric anterior: 448 seconds [range 241 – 910] vs. 660 seconds [range 530 – 780]; P = n. s.; transgastric posterior: 460 seconds [range 173 – 840] vs. 612 seconds [range 438 – 1180]; P = n. s.; transrectal: 171 seconds [range 36 – 189] vs. 180 seconds [range 38 – 240]; P = n. s.). However, these figures were statistically significant when the 20 cases with a transgastric access were evaluated together (454 seconds [range 173 – 910] and 636 seconds [range 438 – 1180]; P = 0.04). We did not find any differences regarding either the number of visualized structures or the duration of peritoneoscopy. Large intestine, small intestine, liver, spleen, and bladder were always visualized whereas pancreas and fallopian tubes were seen in only 24 (80.0 %) and 25 (83.3 %) cases, respectively. Gallbladder and kidneys were missed in two cases each.
No major complications occurred but minor complications were observed in eight cases (26.6 %) (Table 4). The number of complications was lower in the IR-NOTES group (13.3% vs. 40.0 %) but this was not statistically significant.
Table 4.
Minor complications observed at the end of procedures.
| Overall n = 30 |
IR-NOTES n = 15 |
NOTES n = 15 |
P | |
|---|---|---|---|---|
| Transgastric anterior | 4 | Hemorrhage (n = 1) | Hemorrhage (n = 2) Liver laceration (n = 1) | |
| Transgastric posterior | 1 | 0 | Liver laceration (n = 1) | |
| Transrectal | 3 | Abdominal wall tear (n = 1) | Abdominal wall tear (n = 2) | |
| 8 | 2 (13.3%) | 6 (40.0%) | n. s. |
IR-NOTES, image registered-guided natural orifice transluminal endoscopic surgery; n. s., not significant.
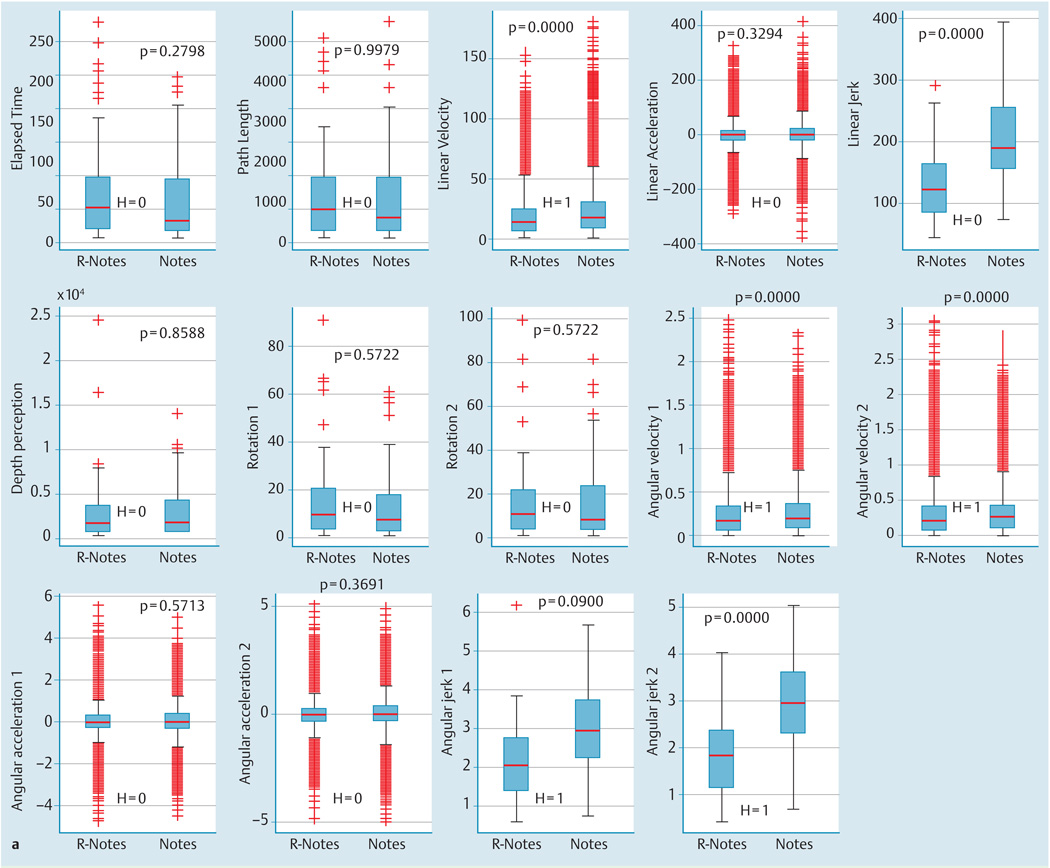
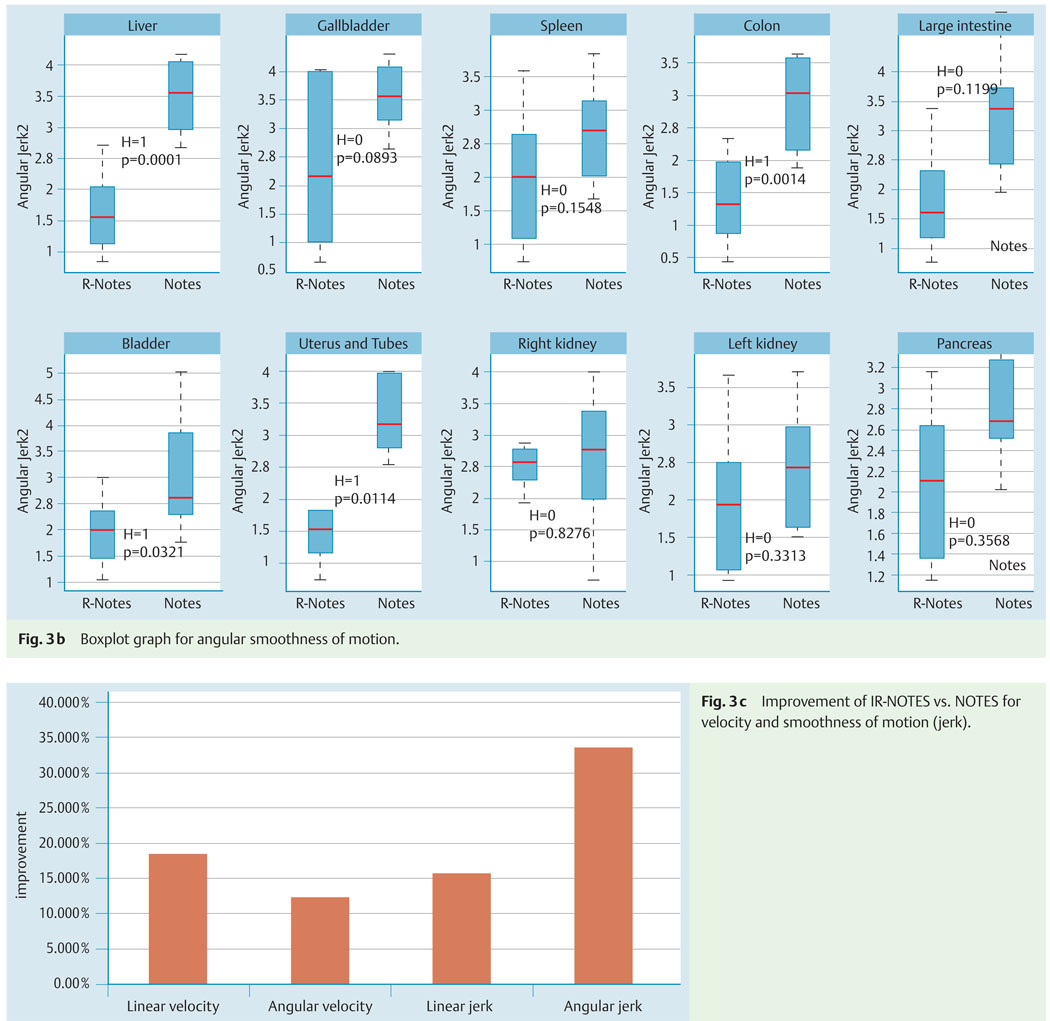
The kinematic evaluation for IR-NOTES vs. NOTES alone showed improved smoothness of motion and velocity with a statistically significant difference (P < 0.01) between the groups when all organs were considered (Fig. 3a) both for linear and angular measurements. When each organ was independently analyzed, velocity was statistically different for all the organs, whereas jerk (both angular and linear) was statistically different (P < 0.05) for liver, colon, large intestine, uterus and Fallopian tubes, and bladder (Fig. 3b).
Fig. 3.
Kinematic analysis comparison for image registered-guided natural orifice transluminal endoscopic surgery (IR-NOTES) and NOTES alone.
a Boxplot graph for each kinematic measurement for all organs. Results of the Wilcoxon test are shown in each plot.
The range of smoothness of motion was 1.63 – 2.50 radians/second3 and 98.68 – 156.97 mm/second3 for the angular and linear component, respectively. The improvement in velocity of IR-NOTES vs. NOTES was 18.4% and 8.7% for the linear and angular components, respectively. Meanwhile, the improvement in smoothness of motion for IR-NOTES vs. NOTES was 35.76% and 30.04% for the linear and angular components, respectively (Fig. 3c).
Discussion
Image registered techniques have been used to improve task performance in laparoscopy [23] and endoscopy [18] and have been explored for guiding transgastric access [24] as a first step in developing image registered NOTES techniques. In these studies, both timed task performance and analysis of the kinematics data on probe motion were used to quantify the benefits of image registered technology. The overall conclusion was that such augmented reality techniques as image registration significantly improve the utility of intracorporeal ultrasound; this was supported by structured survey analyses of the users, in which they reported much less frustration and need to concentrate when using the image registered-augmented systems, while also scoring much higher on the task metrics.
This is the first study that has used an image registered system to guide the navigation of the endoscope inside the peritoneal cavity. The issue of endoscope orientation outside the gastrointestinal lumen once transluminal access to the peritoneal space has been created and an endoscope has been passed through was previously addressed in a study performed by Fritscher-Ravens et al. [15]. These authors explored the usefulness of EUS to guide the scope not only inside the peritoneal space but also in the mediastinum. All the complications they observed occurred in the group in which NOTES procedures were performed without guidance. They concluded that the most obvious benefit in reducing complications was in the narrow space of the mediastinum but they identified other potential utility such as access to structures difficult to reach by the direct endoscopic route (e. g. adrenal glands).
Our study protocol involved only a timed peritoneoscopy. We did not perform any complex surgery and we did not try to target difficult structures because this would have required a change in position of the animal in order to reach the structures; this movement would have affected the calibration of the magnetic sensors. This lack of complexity could explain the fact that the use of image registered guidance did not shorten the duration of the procedure. To date, transgastric peritoneoscopy with current flexible endoscopic technology has been demonstrated to be inferior to laparoscopy [25], and the introduction of image registered systems for targeting difficult structures might improve the efficacy of this procedure. Another possible factor could be the expertise of the operator, who had experience with NOTES and was familiar with pig anatomy. In any case, the introduction of sophisticated technology in the laboratory did not have a negative impact on efficiency. Because this system has already been used for guidance of EUS and laparoscopy with very encouraging results, we think that this technology warrants further study (maybe in the mediastinum).
Until now, the majority of studies reported in the literature concerning diagnostic NOTES peritoneoscopy have used the anterior transgastric approach [1, 4, 7, 12, 16, 26, 27]. The alternate access sites evaluated in this study may have important future NOTES applications. The rectum has already been reported to be an efficient point of entry for cholecystectomy [6]. Additional potential applications of transrectal access include all the interventions in the superior abdomen. Posterior gastric access may provide an important platform for procedures that involve retroperitoneal structures such as the pancreas or could complement anterior gastric access for diagnostic applications, such as cancer staging. In our study, the posterior gastric access was the most difficult to achieve, with fewer structures identified and longer median procedure time, but it was also the most secure with only one minor complication.
One of the main problems when using an anterior gastric access in pigs is that, because the porcine stomach is not attached to other structures, it turns up easily with insufflation. Therefore, although the incision point appears to be anterior, it is located in the posterior wall, close to the great curvature and the omentum. This makes the entry to the peritoneal space more difficult and time-consuming. Because navigation with our image registered system is based on a 3D reconstruction of pre-operative CT and is not a fully real-time system, changes in the anatomy during endoscopic navigation due to breathing or endoscopic insufflation were not represented in the display in a real-time manner and the use of image registered guidance did not improve the accuracy of the selection of the right incision point in transgastric NOTES procedures. This characteristic could be a limitation when performing more complex surgeries that includes organ removal. However, a new method of registration that uses non-optimized algorithm implementations has recently been developed and has demonstrated that local translation displacements can be easily recovered so that full real operations appears likely [28].
Image registered-guided access substantially reduced but did not completely eliminate the risk of complications. Complications (all minor) were more frequent in unguided NOTES procedures and could be attributed to the blinded access to the peritoneal space through the gastrointestinal wall and the lack of orientation. Bleeding is one of the most serious complications and may occur at any time during surgery, but it is more likely to occur when access is obtained with the endoscopic needle-knife incision of the gut wall. We experienced three episodes of bleeding in the anterior gastric access (one in the IR-NOTES group), and hemostasis was achieved using the coagulation settings of the needle-knife. By contrast, all the complications described in the study by Elmunzer et al. [16] in the group with EUS-guided access procedures were observed in transrectal access. In our study, the use of an image registered system for guidance could not prevent the incision of a small blood vessel because the 3D display did not include the vasculature anatomy of the stomach. However, it is possible to delineate arterial vessels and other structures outside the gastric wall with an image registered system [20] (Fig. 4).
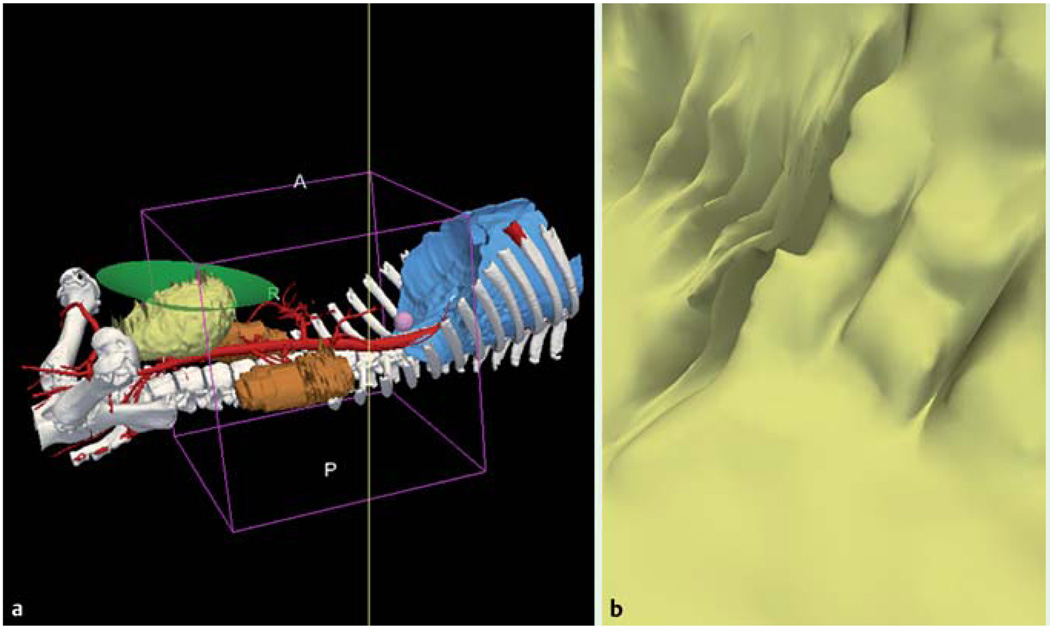
Fig. 4.
Augmented reality effects gained by the virtual endoscopic display provided by image registered- guided natural orifice transluminal endoscopic surgery. In this case, the display shows a view of the bladder while the scope is in the colon. b Endoscopic view of the anatomy shown in a. The bladder can be visualized beyond the visual feedback provided by the endoscopic camera to avoid any injuries during the access to the peritoneum. A, anterior; P, posterior; R, right.
The solution for this is not as simple as just making a pre-procedure CT and then tracking the instruments, because the insufflation of the stomach to gain a better working space certainly distorts the position of the vessels attached to the outer stomach wall. Further evaluation will be necessary to determine the limitations of the image registration technique in this context.
Characteristics of the endoscope tip path (the shape of which correlates with operator expertise) showed a statistically significant improvement in trajectory smoothness of motion for all organs. Previous studies [18, 22] indicate that such differences in performance kinematics are correlated with operator expertise and reduced operator stress, implying that the image registered system assists operators in performing the procedures. The observation that the smoothness of movements was greater when using the image registered guidance was not perceived by the operator. In this case, the operator did not find any differences driving the scope but felt more confident due to the capability of the image registered system to enable gross orientation and navigation. This improvement in smoothness could be more substantial when performing more complex surgeries and might have a potential benefit on the inflammatory response and surgical trauma associated with NOTES procedures [29, 30].
Several technical and design limitations must be addressed. These experiments were conducted in an animal model, with the animal breathing. However, the image registered system capability was more than sufficient for the guidance task because most targets of interest are large. Moreover, porcine anatomy differs quite substantially from that of human beings, making the pig a suboptimal model for determining immediate clinical applicability. Another limitation of the study is the lack of randomization for determining which system was used, and it had an unavoidable learning curve. This study, however, established the feasibility of the system and we plan to address randomization issues in future comparative trials. Finally, the major limitation of the study is that it was not conducted on difficult structures, the inclusion of which would have provided more information about targeting during NOTES and could have better demonstrated the actual utility of an image registered system for improving navigation.
In conclusion, the image registered system appears feasible in NOTES and may be superior to conventional NOTES in efficiency and accuracy of probe positioning. Moreover, image registered-guided access might reduce the risk of complications. When considering these results, the image registered system could find utilization in many NOTES procedures and may lead to the development of additional indications for NOTES.
Acknowledgments
This research project was granted by euroNOTES foundation in 2008. G. Fernández-Esparrach thanks the Generalitat de Catalunya (AGAUR, BE-100022) and the Societat Catalana de Digestologia for supporting her training and research in NOTES.
R. San José Estepar was supported by NIH U41 RR019703. K. G. Vosburgh was supported by the Center for Integration of Medicine and Innovative Technology (CIMIT).
The authors thank Olympus Medical Europe (Hamburg, Germany) for providing the equipment for this study free of charge.
Footnotes
Competing interests: C. C. Thompson is consultant for Boston Scientific and Olympus and a member of the Advisory Board of Covidien.
References
- 1.Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114–117. doi: 10.1016/s0016-5107(04)01309-4. [DOI] [PubMed] [Google Scholar]
- 2.Kantsevoy SV, Jagannath SB, Niiyama H, et al. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc. 2005;62:287–292. doi: 10.1016/s0016-5107(05)01565-8. [DOI] [PubMed] [Google Scholar]
- 3.Park PO, Bergstrom M, Ikeda K, et al. Experimental studies of transgastric gallbladder surgery: cholecystectomy and cholecystogastric anastomosis. Gastrointest Endosc. 2005;61:601–606. doi: 10.1016/s0016-5107(04)02774-9. [DOI] [PubMed] [Google Scholar]
- 4.Wagh MS, Merrifield BF, Thompson CC. Endoscopic transgastric abdominal exploration and organ resection: initial experience in a porcine model. Clin Gastroenterol Hepatol. 2005;3:892–896. doi: 10.1016/s1542-3565(05)00296-x. [DOI] [PubMed] [Google Scholar]
- 5.Wagh MS, Merrifield BF, Thompson CC. Survival studies after endoscopic transgastric oophorectomy and tubectomy in a porcine model. Gastrointest Endosc. 2006;63:473–478. doi: 10.1016/j.gie.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 6.Pai RD, Fong DG, Bundga ME, et al. Transcolonic endoscopic cholecystectomy: a NOTES survival study in a porcine model (with video) Gastrointest Endosc. 2006;64:428–434. doi: 10.1016/j.gie.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 7.Fong DG, Pai RD, Thompson CC. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65:312–318. doi: 10.1016/j.gie.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Lacy AM, Delgado S, Rojas OA, et al. Hybrid vaginal MA-NOS sleeve gastrectomy: technical note on the procedure in a patient. Surg Endosc. 2009;23:1130–1137. doi: 10.1007/s00464-008-0292-3. [DOI] [PubMed] [Google Scholar]
- 9.Lacy AM, Delgado S, Rojas OA, et al. MA-NOS radical sigmoidectomy: report of a transvaginal resection in the human. Surg Endosc. 2008;22:1717–1723. doi: 10.1007/s00464-008-9956-2. [DOI] [PubMed] [Google Scholar]
- 10.Dallemagne B, Perretta S, Allemann P, et al. Transgastric hybrid cholecystectomy. Br J Surg. 2009;96:1162–1166. doi: 10.1002/bjs.6704. [DOI] [PubMed] [Google Scholar]
- 11.Rattner D, Kalloo A ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. White Paper October 2005. Surg Endosc. 2006;20:329–333. doi: 10.1007/s00464-005-3006-0. [DOI] [PubMed] [Google Scholar]
- 12.Voermans RP, van Berge Henegouwen MI, Bemelman WA, et al. Feasibility of transgastric and transcolonic natural orifice transluminal endoscopic surgery peritoneoscopy combined with intraperitoneal EUS. Gastrointest Endosc. 2009;69:e61–e67. doi: 10.1016/j.gie.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm D, Meining A, von Delius S, et al. An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy. 2007;39:401–406. doi: 10.1055/s-2007-966438. [DOI] [PubMed] [Google Scholar]
- 14.Fritscher-Ravens A, Patel K, Ghanbari A, et al. Natural orifice transluminal endoscopic surgery (NOTES) in the mediastinum: long-term survival animal experiments in transesophageal access, including minor surgical procedures. Endoscopy. 2007;39:870–875. doi: 10.1055/s-2007-966907. [DOI] [PubMed] [Google Scholar]
- 15.Fritscher-Ravens A, Ghanbari A, Cuming T, et al. Comparative study of NOTES alone vs. EUS-guided NOTES procedures. Endoscopy. 2008;40:925–930. doi: 10.1055/s-2008-1077732. [DOI] [PubMed] [Google Scholar]
- 16.Elmunzer BJ, Schomisch SJ, Trunzo JA, et al. EUS in localizing safe alternate access sites for natural orifice transluminal endoscopic surgery: initial experience in a porcine model. Gastrointest Endosc. 2009;69:108–114. doi: 10.1016/j.gie.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Vosburgh KG, Stylopoulos N, Thompson CC, et al. Novel real time tracking interface improves the use of laparoscopic and endoscopic ultrasound in the abdomen. Computer Assisted Radiology and Surgery (CARS); Presented at: CARS/ISCAS; June 26–July 3, 2006; Osaka, Japan. [Google Scholar]
- 18.Vosburgh KG, Stylopoulos N, San José Estépar R, et al. EUS with CT improves efficiency and structure identification over conventional EUS. Gastrointest Endosc. 2007;65:866–870. doi: 10.1016/j.gie.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 19.San José Estépar R, Stylopoulos N, Ellis RE, et al. Towards scarless surgery: an endoscopic-ultrasound navigation system for transgastric access procedures. Comput Aided Surg. 2007;12:311–324. doi: 10.3109/10929080701746892. [DOI] [PubMed] [Google Scholar]
- 20.Vosburgh KG, San José Estépar R. Natural orifice transluminal endoscopic surgery (NOTES): an opportunity for augmented reality guidance. Stud Health Technol Inform. 2007;125:485–490. [PubMed] [Google Scholar]
- 21.Besl PJ, McKay HD. A method for registration of 3-D shapes. IEEE Transactions on pattern analysis and machine intelligence. 1992;12:239–256. [Google Scholar]
- 22.Stylopoulos N, Vosburgh KG. Assessing technical skill in surgery and endoscopy: a set of metrics and an algorithm (C-PASS) to assess skills in surgical and endoscopic procedures. Surg Innov. 2007;14:113–121. doi: 10.1177/1553350607302330. [DOI] [PubMed] [Google Scholar]
- 23.Ellsmere J, Stoll J, Wells W, 3rd, et al. A new visualization technique for laparoscopic ultrasonography. Surgery. 2004;136:84–92. doi: 10.1016/j.surg.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 24.San Jose Estepar R, Stylopoulos N, Ellis RE, et al. Towards scarless surgery: an endoscopic ultrasound navigation system for transgastric access procedures. Med Image Comput Assist Interv. 2006;9(Pt 1):445–453. doi: 10.1007/11866565_55. [DOI] [PubMed] [Google Scholar]
- 25.Voermans RP, Sheppard B, van Berge Henegouwen MI, et al. Comparison of transgastric NOTES and laparoscopic peritoneoscopy for detection of peritoneal metastases. Ann Surg. 2009;250:255–259. doi: 10.1097/SLA.0b013e3181ae6d9d. [DOI] [PubMed] [Google Scholar]
- 26.von Delius S, Feussner H, Wilhelm D, et al. Transgastric in vivo histology in the peritoneal cavity using miniprobe-based confocal fluorescence microscopy in an acute porcine model. Endoscopy. 2007;39:407–411. doi: 10.1055/s-2007-966439. [DOI] [PubMed] [Google Scholar]
- 27.Feretis C, Kalantzopoulos D, Koulouris P, et al. Endoscopic transgastric procedures in anesthetized pigs: technical challenges, complications, and survival. Endoscopy. 2007;39:394–400. doi: 10.1055/s-2007-966430. [DOI] [PubMed] [Google Scholar]
- 28.San José Estépar R, Westin CF, Vosburgh KG. Towards real time 2D to 3D registration for ultrasound-guided endoscopic and laparoscopic procedures. Int J Comput Assist Radiol Surg. 2009;4:549–560. doi: 10.1007/s11548-009-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGee MF, Schomisch SJ, Marks JM, et al. Late phase TNF-alpha depression in natural orifice translumenal endoscopic surgery (NOTES) peritoneoscopy. Surgery. 2008;143:318–328. doi: 10.1016/j.surg.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Delgado S, Lacy AM, Filella X, et al. Acute phase response in laparoscopic and open colectomy in colon cancer: randomized study. Dis Colon Rectum. 2001;44:638–646. doi: 10.1007/BF02234558. [DOI] [PubMed] [Google Scholar]