Abstract
Introduction
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II-receptor blockers (ARBs) are prototypes of “upstream” therapy for the management of atrial fibrillation (AF). Ectopic activity arising from the PV sleeves plays a prominent role in the development of AF.
Methods
Transmembrane action potentials were recorded from canine superfused left superior or inferior PV sleeves using standard microelectrode techniques. Acetylcholine (ACh, 1 µM), isoproterenol (1 µM), high calcium ([Ca2+]o=5.4mM) or a combination was used to induce early or delayed afterdepolarizations (EADs or DADs) and triggered activity.
Results
The ARB losartan (1 µM, n=5) and the ACE inhibitor enalapril (10 µM, n=5) produced no significant change in action potential duration, maximum rate of rise of action potential upstroke (Vmax), action potential amplitude or take-off potential at basic cycle lengths of 200 to 2000 ms. Losartan (1 µM) and enalapril (10–20 µM) markedly attenuated or suppressed EADs and DAD–induced triggered activity elicited by exposure of the PV sleeves to ACh, isoproterenol or high calcium following rapid pacing in 6 of 6 (losartan) and 4 of 5 (enalapril) PV sleeve preparations. Neither losartan or enalapril altered Ca2+ or K+ channel currents in enzymatically-dissociated atrial myocytes at these concentrations.
Conclusions
Our data suggest that in addition to their “upstream” effects to reduce atrial structural remodeling, ACE inhibitors and ARBs exert a “direct” antiarrhythmic effect by suppressing triggers responsible for the genesis of AF and other atrial arrhythmias.
Keywords: atrial fibrillation, antiarrhythmic drugs, sodium channel blocker, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia encountered in the clinic.1 In addition to the traditional approaches for the treatment of AF involving ion channel modulation are new strategies that target fibrosis, inflammation and oxidative injury.2–4 These approaches are termed “upstream therapy”. Angiotensin II -receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors are prototypes of “upstream” therapy for the management of AF, in addition to their use as anti-hypertensives. The renin-angiotensin-aldosterone (RAS) system, including angiotensin-II, has been shown to exert proarrhythmic effects.5 In animal experimental models, ARBs as well as ACE inhibitors have been shown to suppress or reduce apoptosis, fibrosis, and AF maintenance in dogs with ventricular tachypacing–induced congestive heart failure.3 Thus, ACE inhibitors and ARBs are able to reverse electrical remodeling induced by rapid activation of the atria as well as modify the substrate for the maintenance of AF. In addition, a number of retrospective and recent prospective clinical studies suggest a role for ACE inhibitors and ARBs in the prevention of AF.2, 6, 7
Ectopic activity arising from the pulmonary veins (PV) plays a prominent role in the development of AF.8 Delayed afterdepolarizations (DADs) and late-phase 3 early afterdepolarizations (EADs), originating from PV sleeves following parasympathetic and /or sympathetic stimulation, have been proposed as potential triggers in initiation of AF.9–15
The present study was designed to evaluate the electrophysiologic and possible antiarrhythmic effects of the ARB losartan and the ACE-inhibitor enalapril in superfused PV sleeve preparations isolated from canine hearts. Preliminary data have been presented in abstract form.16
METHODS
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No 85-23, Revised 1996) and was approved by the ACUC of the Masonic Medical Research Laboratory.
Adult mongrel dogs weighing 20–35 kg were anticoagulated with heparin (180 IU/kg) and anesthetized with sodium pentobarbital (35 mg/kg, IV). The chest was opened via a left-thoracotomy and the heart excised and placed in a cold cardioplegic solution ([K+]0 = 8 mmol/L, 4°C).
Superfused pulmonary vein sleeve preparation
PV sleeve preparations (approximately 2.0 × 1.5 cm) were isolated from left canine atria. The thickness of the preparation was approximately 2 mm. Left superior pulmonary veins were used preferentially in most experiments. The preparations were placed in a small tissue bath and superfused with Tyrode's solution of the following composition (mM): 129 NaCl, 4 KCl, 0.9 NaH2PO4, 20 NaHCO3, 1.8 CaCl2, 0.5 MgSO4, 5.5 glucose, buffered with 95% O2/5% CO2 (35 ± 0.5°C). PV preparations were stimulated at a basic cycle length (BCL) of 1000 ms during the equilibration period (1h) using electrical stimulation (1–3 ms duration, 2.5 times diastolic threshold intensity) delivered through silver bipolar electrodes insulated except at the tips. Transmembrane potentials were recorded using glass microelectrodes filled with 3 M KCl (10–20 MΩ DC resistance) connected to a high input-impedance amplification system (World Precision Instruments, model KS-700, New Haven, CT). The following parameters were measured: take-off potential (TOP), action potential amplitude (APA), action potential duration at 85% repolarization (APD85) and maximum rate of rise of action potential upstroke (Vmax). Transmembrane action potentials were recorded at a sampling rate of 41 kHz. Take-off potential measurement was used instead of resting membrane potential because of the slow phase 3 of the action potential of the PV sleeve preparation that fails to return to the resting potential at the fastest rates.
Isolation of adult canine cardiomyocytes
Myocytes were prepared from canine atria as described previously. Briefly, the atrium were dissected as described above and initially perfused via the osteum with nominally Ca2+-free solution (mM): NaCl 129, KCl 5.4, MgSO4 2.0, NaH2PO4 0.9, glucose 5.5, NaHCO3 20, bubbled with 95% O2/5% CO2 containing 0.1% BSA for a period of about 5 minutes. The preparation was then subjected to enzyme digestion with the nominally Ca2+-free solution supplemented with 0.5 mg/ml collagenase (Type II, Worthington Biochemical Corp., Lakewood, NJ), 0.1 mg/ml protease (Type XIV, Sigma-Aldrich Co., St. Louis, MO), 2,3-butanedione monoxime 20, and 1 mg/ml BSA for 30 minutes. The pectinate muscle and appendage were then placed in separate beakers, minced and incubated in fresh buffer containing 0.5 mg/ml collagenase, 1 mg/ml BSA and agitated. The supernatant was filtered, centrifuged at 200 rpm for 2 minutes and the myocyte containing pellet was stored in 0.5 mM Ca2+ HEPES buffer at room temperature.
Voltage Clamp Recordings
Cells were placed in a temperature controlled chamber (PDMI-2, Medical Systems Corp., Greenvale, NY) mounted on the stage of an inverted microscope (Nikon TE300). Voltage-clamp recordings were made using a MultiClamp 700A amplifier and MultiClamp Commander (Axon Instruments Molecular Devices, Inc., Sunnyvale, CA). Patch pipettes were fabricated from borosilicate glass capillaries (1.5 mm O.D., Fisher Scientific, Pittsburgh, PA). The pipettes were pulled using a gravity puller (Model PP-830, Narishige, Tokyo, Japan) and the pipette resistance ranged from 1–3 MΩ when filled with the internal solution (see below). After a whole cell patch was established, cell capacitance was measured by applying −5 mV voltage steps. Electronic compensation of series resistance to 60–70% was applied to minimize voltage errors. All current signals were acquired at 10–50 kHz, filtered at 4–6 kHz, digitized with a Digidata 1322 converter (Axon Instruments Molecular Devices, Inc., Sunnyvale, CA) and stored using pClamp9 software.
Cardiomyocytes were superfused with a HEPES buffer of the following composition (mM): NaCl 126, KCl 5.4, MgCl2 1.0, CaCl2 2.0, HEPES 10, and glucose 11. pH adjusted to 7.4 with NaOH. For ICa recordings, the patch pipette solution had the following composition (mM): CsCl 120, MgCl2 1.0, EGTA 10, MgATP 5, HEPES 10, CaCl2 5, pH = 7.2 with CsOH. For Ito and IK recordings, the pipette solution had the following composition (mM): K-aspartate 90, KCl 30, glucose 5.5, MgCl2 1.0, EGTA 5, MgATP 5, HEPES 10, NaCl 10. pH = 7.2 with KOH17. In addition, 300 µM CdCl2 was present to block L-type Ca2+ channels. All experiments were performed at 37° C.
Drugs
Losartan (Sigma-Aldrich Corp., St. Louis, Missouri) and enalapril (VWR, West Chester, PA) were used at final concentrations of 1 µM and 10–20 µM, respectively. Isoproterenol (Sigma-Aldrich Corp., St. Louis, MO) was dissolved in distilled water to form a stock solution of 1 mM and used at final concentrations of 1 µM. Acetylcholine (ACh) was dissolved in distilled water to form a stock solution of 10 mM and used at a concentration of 1 µM.
Statistics
Statistical analysis was performed using one way repeated measures analysis of variance (ANOVA) followed by Bonferroni’s test. Mean values were considered to be different at p < 0.05.
RESULTS
Effects of losartan on pulmonary vein (PV) sleeve preparations
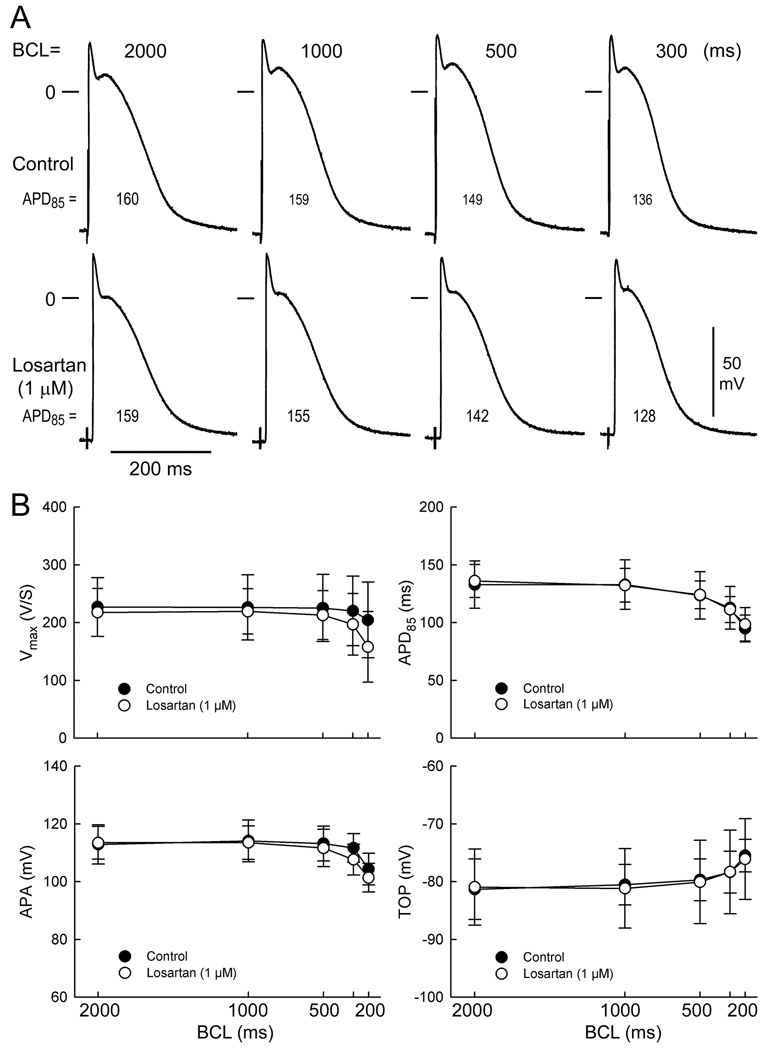
Figure 1A illustrates a representative example of the effects of the ARB losartan on the action potential of a PV sleeve preparation. Shown are control action potentials recorded at basic cycle lengths (BCLs) of 2000, 1000, 500 and 300 ms. Losartan (1 µM) induced no change in action potential duration (APD) at any basic cycle length (BCL) and a small decrease in the plateau phase of the action potential. Similar findings were observed in five experiments.
Figure 1.
Effect of losartan (1 µM) on pulmonary vein (PV) sleeve preparations. (A) Control action potentials recorded at basic cycle lengths (BCLs) of 2000, 1000, 500 and 300ms. Effects of losartan. (B). Composite data (n=5) of the rate-dependent effects of losartan (1 µM) on maximum rate of rise of action potential upstroke (Vmax), APD measured at 85% repolarization (APD85), action potential amplitude (APA) and take off potential (TOP) in PV sleeve preparations. Losartan produced no significant changes in any of these parameters. BCL: basic cycle length.
Composite data (n=5) of the rate-dependent effects of losartan (`1 µM) on Vmax, APD85, APA and TOP are graphically illustrated in Figure 1B. Losartan induced no significant change in any of these parameters at any cycle length.
Effects of losartan on DAD- and EAD-induced triggered activity
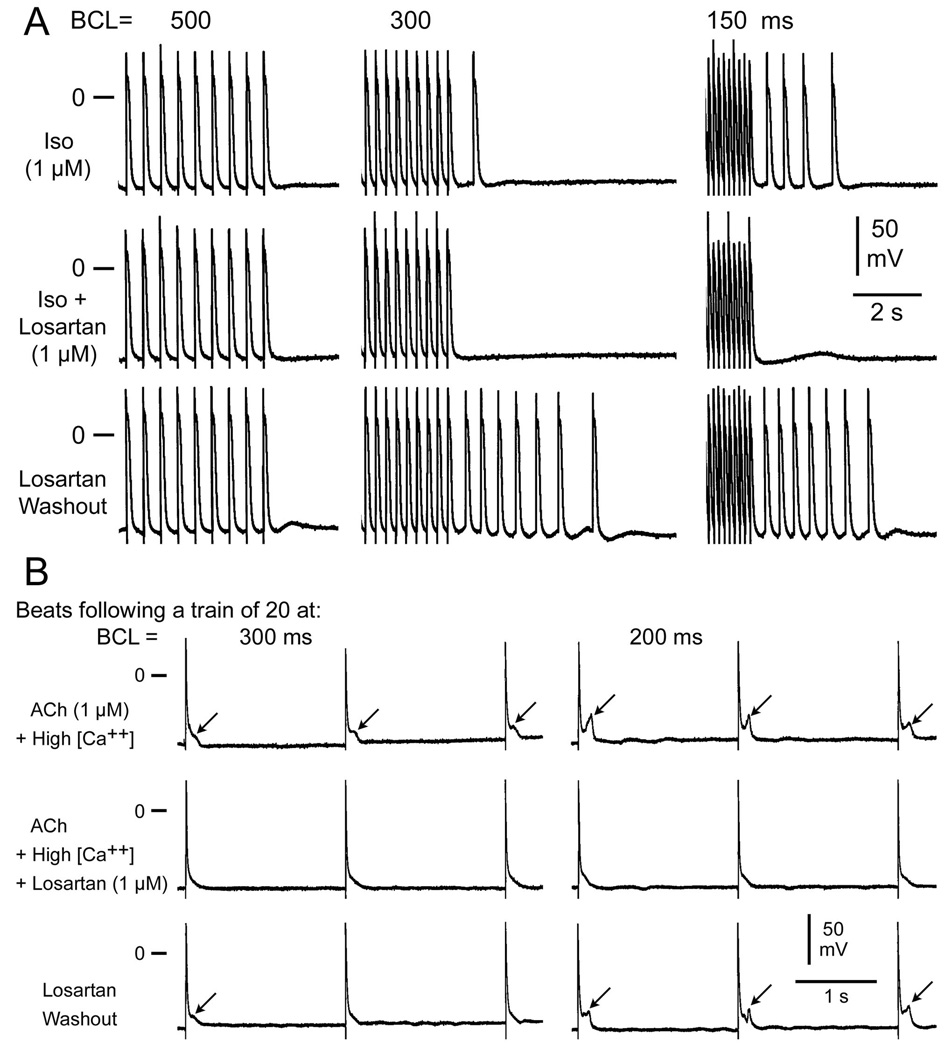
In another series of experiments, we determined the effects of losartan on delayed afterdepolarizations, late-phase 3 early afterdepolarizations and triggered activity elicited by exposure of PV sleeve preparations to acetylcholine (ACh), isoproterenol, or high calcium and rapid pacing. “Time control” studies in PV sleeve preparations exposed to isoproterenol, high calcium, ACh or their combination were performed over a 30–60 minute period and indicate no change in the development of EADs, DADs and triggered activity over this time period. Figure 2A shows a representative experiment of the effects of losartan on isoproterenol-induced DADs and triggered activity in a PV sleeve preparation. Each panel shows a train of 10 beats at a BCL of 500, 300 and 150 ms followed by a pause. Isoproterenol (Iso) induced prominent rate-dependent DADs and triggered activity following the pause. Addition of losartan (1 µM) suppressed the triggered beats and a small DAD persisted at a BCL 150ms. Washout of losartan (isoproterenol alone) restored DAD activity and triggered beats. Figure 2B shows an example of the effects of losartan on late-phase 3 EADs induced in PV sleeve preparations. Each panel shows the first three beats following a train of 20 at BCLs of 300 and 200 ms. ACh and high calcium induced rate-dependent late phase 3 EADs. Losartan eliminated EAD activity, which returned upon washout.
Figure 2.
Effects of losartan on isoproterenol-induced DADs and triggered activity in a PV sleeve preparation. (A) Each panel depicts a train of 10 beats at a basic cycle length (BCL) of 500, 300 and 150 ms followed by a pause. Isoproterenol (Iso, 1 µM) induces prominent rate-dependent DADs and triggered activity during the pause. Addition of losartan (1 µM) suppresses the triggered beats and a small DAD persists at BCL 150ms Washout of losartan restores DADs and triggered beats. (B) Effects of losartan on acetylcholine (ACh)/high calcium-induced late-phase 3 EADs in a PV sleeve preparation. Each panel shows the first 3 beats following a train of 20 beats at basic cycle length (BCL) of 300 and 200 ms. (ACh (1µM) and high calcium (5.4mM) induce rate-dependent late-phase 3 EADs. Losartan (1uM) eliminates EAD activity. Washout of losartan restores EADs.
Losartan eliminated or reduced ACh, isoproterenol or high calcium-induced DADs, late phase 3 EADs and triggered activity in 6 of 6 PV sleeve preparations. In addition, losartan (1 µM) prevented the development of EADs, DADs and triggered activity in 4 of 4 PV preparations (data not shown).
Effects of angiotensin II on pulmonary vein sleeve preparations
In another series of experiments, we evaluated the effects of angiotensin-II in PV sleeves preparations. Angiotensin–II (10–100 nM) did not induce EADs or DADs activity (n=4) in any of the PV sleeve preparations studied indicating that the effect of losartan on EADs and DADS was not mediated by angiotensin-II blocking activity.
Effects of enalapril on pulmonary vein sleeve preparations
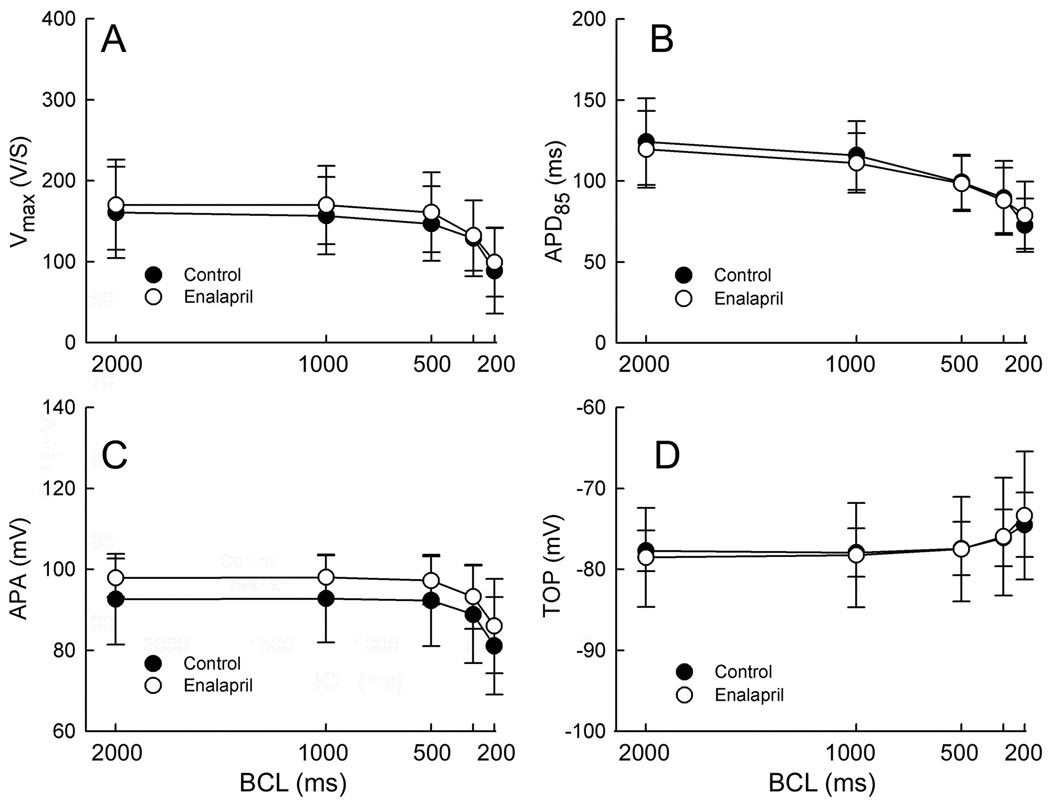
In another set of experiments, we evaluated the effects of the ACE inhibitor enalapril in PV sleeve preparations. Composite data (n=5) of the rate-dependent effects of enalapril (`10 µM) on Vmax, APD85, APA and TOP are graphically illustrated in Figure 3. Enalapril produced no significant changes in any of the parameters evaluated at any cycle length.
Figure 3.
Composite data (n=5) of the rate-dependent effects of enalapril (10 µM) on Vmax, APD85, APA and TOP in PV sleeve preparations. Enalapril produced no significant change in any of these parameters. BCL: basic cycle length.
Effects of enalapril on EAD- and DAD- induced triggered activity in PV sleeve preparations
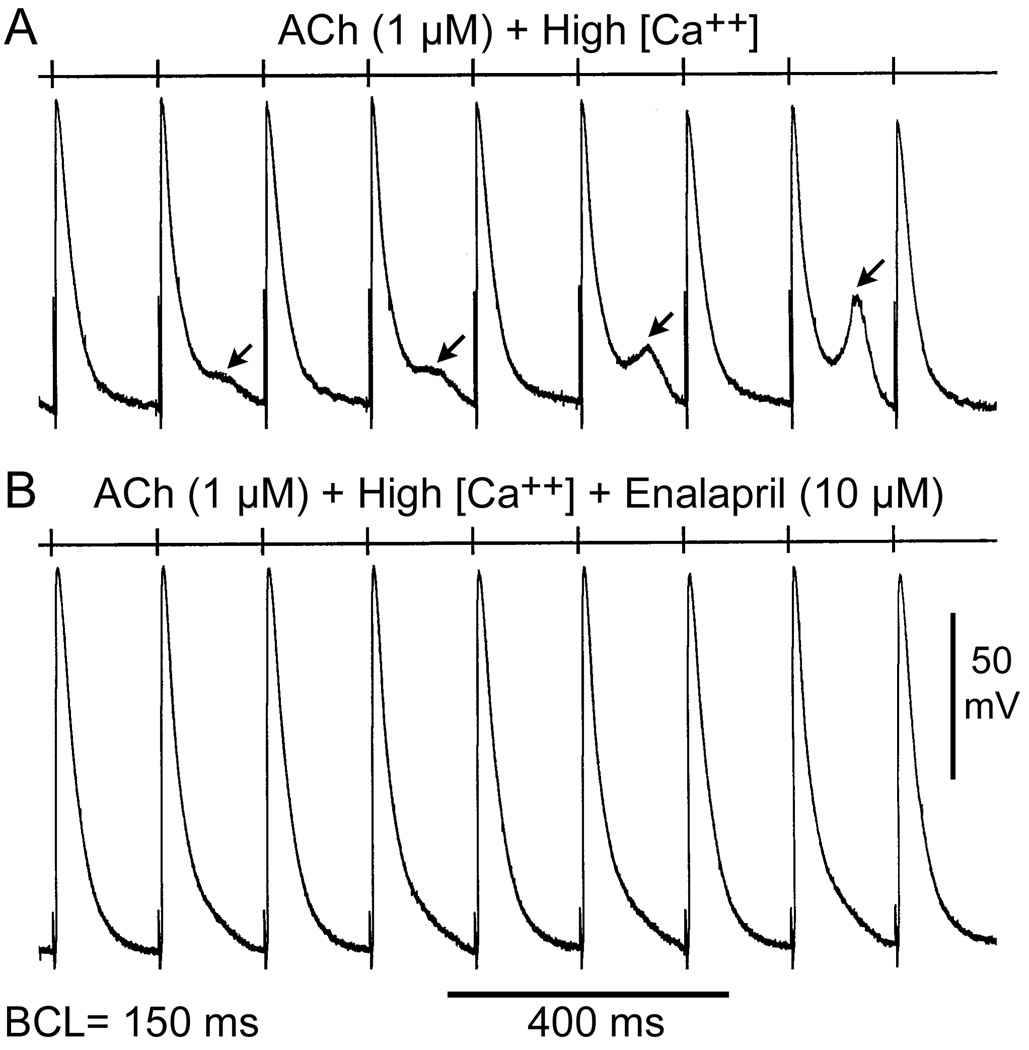
Figure 4 shows the effects of enalapril on ACh/high calcium-induced late-phase 3 EADs at rapid pacing rates. At a BCL of 150 ms, ACh and high calcium induced late-phase 3 EADs in alternate beats. Addition of enalapril (10 µM) suppressed all EAD activity. Figure 5 illustrates the effects of enalapril to eliminate DAD-induced triggered activity and reduce DAD.
Figure 4.
Effects of enalapril on acetylcholine (ACh)/high calcium induced early afterdepolarizations (EADs) at fast pacing rates. (A) ACh (1 µM) and high calcium (5.4 mM) induce late-phase 3 EADs in alternating beats at a basic cycle length (BCL) of 150 ms. (B) Addition of enalapril (10 µM) suppresses EAD activity.
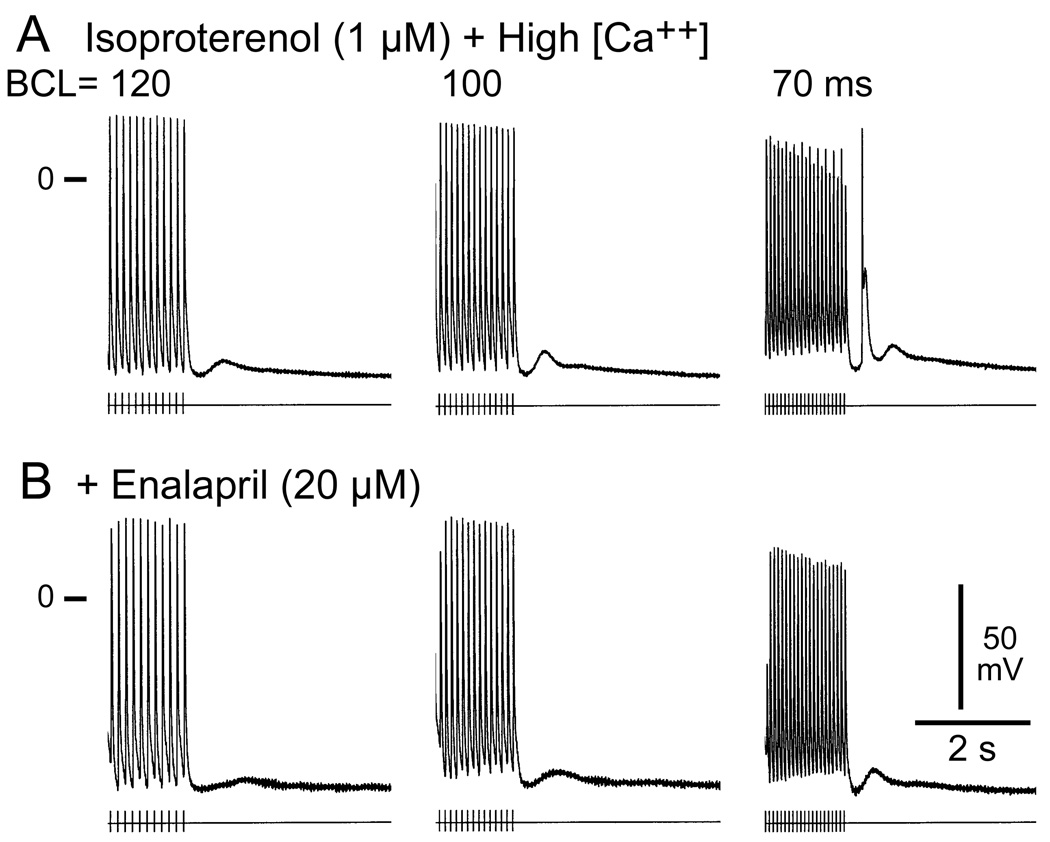
Figure 5.
Effects of enalapril on isoproterenol and high calcium-induced delayed afterdepolarizations (DADs) and triggered activity in a PV sleeve preparation. Each panel shows a train of 10 beats at basic cycle lengths (BCL) of 120, 100 and 70 ms followed by a pause. (A) Isoproterenol (Iso, 1 µM) and high calcium (5.4 mM) induced prominent DADs and a DAD-mediated triggered beat during the pause following pacing at 70 ms. (B) Addition of enalapril (10 µM) suppresses the triggered beat and reduces the amplitude of the DADs.
Enalapril (10–20 µM) eliminated or reduced late-phase 3 EADs and DADs in 4 of 5 PV sleeve preparations. In addition, enalapril (10 µM) prevented the development of EADs, DADs and triggered activity in 3 of 4 PV preparations (data not shown).
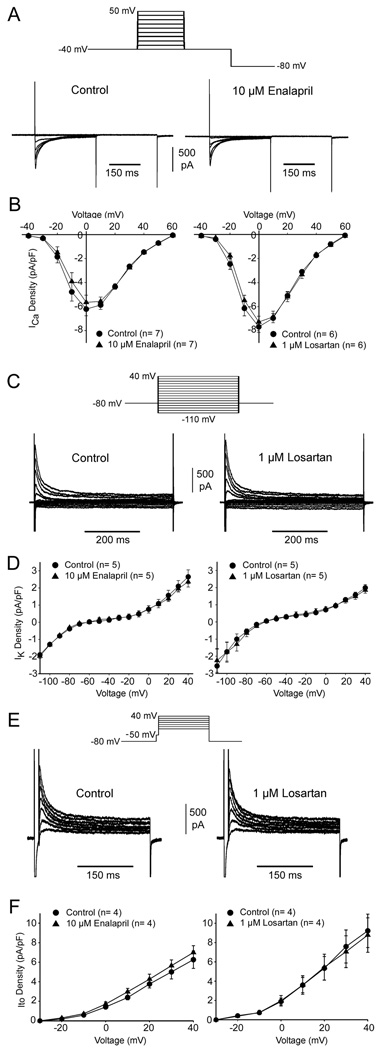
Both losartan and enalapril reduced DADs suggesting these drugs reduced intracellular Ca2+ overload. In addition, a small effect on the plateau of the PV action potential was observed suggesting that ICa may be affected. In another series of experiments we measured ICa in atrial myocytes in the absence and presence of the two compounds. Following a series of five pre-pulse (not shown), ICa was measured at voltages between −40 and +60 mV (Figure 6A). The current-voltage relations for ICa were not significantly different in the presence of either losartan or enalapril (Figure 6B). These results demonstrate that reduction of DADs by either drug was not due to blockade of ICa. We next evaluated whether either drug affected K+ currents. Representative total ionic currents elicited by step increments of 10 mV between −110 and +40 mV from a holding potential of −80 mV are illustrated in Figure 6C. In response to hyperpolarizing voltage the inward rectifier current (IK1) could be observed. At positive potentials, a prominent transient outward current (Ito) and sustained component (IKur) was observed. We compared the differences in K+ currents in the absence and presence of both drugs by measuring the current at the end of the 500 msec voltage clamp pulse (Figure 6D). We found that K+ channel current at the end of the 500msec pulse (IK1 and IKur) was unaffected by application of either drug. We next examined the magnitude of Ito in the absence and presence of each drug. Following a brief pre-pulse to −50 mV to discharge Na+ channels, Ito was elicited by step increments of 10 mV between −30 and +40 mV (Figure 6E). Neither losartan nor enalapril affected Ito at the concentration tested (Figure 6F).
Figure 6.
(A) Representative whole cell ICa recordings from an atrial cell under control conditions and after addition of 10 µM enalapril. Five pre-pulses were applied (not shown) and currents were obtained at test potentials between −40 and +60 mV incremented in 10 mV steps from a holding potential of −80 mV. (B) I-V relation for ICa in the absence and presence of either 10 µM enalapril or 1 µM losartan. (C) Representative IK recordings from an atrial cell in the absence and presence of 1 µM losartan. K+ currents were measured at the end of the 500msec pulse using the protocol shown in the inset. (D) I-V relation for IK in the absence and presence of either 10 µM enalapril or 1 µM losartan. (E) Representative Ito recordings from an atrial cell in the absence and presence of 1 µM losartan. Ito was measured following a brief pre-pulse to −50 mV to discharge Na+ current (see inset). (F) I-V relation for Ito in the absence and presence of either 10 µM enalapril or 1 µM losartan.
DISCUSSION
AF is the most common arrhythmia requiring medical attention. Ectopic activity arising from the PV sleeves is thought to be an important source of triggers and in some cases substrate for the development of AF.18–20 The results of the present study indicate that concentrations of losartan and enalapril within the therapeutic range induce little change in action potential characteristics of canine pulmonary vein (PV) sleeve superfused preparations. The dose of enalapril (10–20 uM) correlates with plasma concentrations of an oral therapeutic dose of 10 mg21 and the dose of losartan (1 uM) with an oral therapeutic dose of 50 mg. Both losartan and enalapril eliminated or reduced ACh, isoproterenol, and high calcium-induced DADs, late phase 3 EADs and triggered activity in 6 of 6 (losartan) and 4 of 5 (enalapril) PV preparations and prevented the development of EADs, DADs and triggered activity in 4 of 4 (losartan) and 3 of 4 (enalapril) PV preparations.
These data suggest a “direct” antiarrhythmic effect of losartan and enalapril in PV sleeve preparations, in addition to the previously described effects of ARBs and ACE-inhibitors to diminish atrial electrical remodeling.3
Losartan and enalapril eliminate DADs and late-phase 3 EADs in PV sleeves
The present study shows that late phase 3 EADs and DADs can be easily induced in PV sleeve preparations following the addition of isoproterenol, ACh, high calcium alone or in combination. Similar findings were previously reported by Chen and co-workers in canine and rabbit isolated single PV myocytes,22, 23 by Patterson and co-workers, Sicouri and co-workers in isolated canine pulmonary vein sleeve preparations,9, 10, 12, 13, 24 and Burashnikov and co-workers in coronary-perfused right atrial preparations.11, 25 In isolated PV myocytes, isoproterenol was shown to increase automaticity and induce spontaneous activity as well as EAD or DAD-induced triggered activity.22, 23 Late-phase 3 EADs11, 25 are due to abbreviated repolarization that permits normal sarcoplasmic reticulum calcium release and associated sodium-calcium exchange inward current to induce a late-phase 3 EADs. Conditions permitting intracellular calcium loading facilitate the development of late phase 3 EADs. Patterson et al. showed that autonomic stimulation could give rise to late phase 3 EADs in canine PV sleeves, which may result in a run of triggered responses.12, 13 Our data show that losartan and enalapril in concentrations within the therapeutic range are capable of eliminating late-phase 3 EADs and DADs as well as EAD- and DAD–induced triggered activity originating in PV sleeves by these two distinct mechanisms.
Ionic mechanisms
Our data show that the action potential of PV sleeve preparations is little affected by either losartan or enalapril. A small decrease in the voltage of the action potential plateau is observed in PV action potentials exposed to losartan. Losartan has previously been shown to increase the action potential notch and the transient outward current in canine myocytes exposed to 1 µM losartan for 2 to 24 hrs26. This effect of losartan was not observed in the present study or in canine wedge preparations exposed acutely to losartan (Sicouri and Antzelevitch, unpublished observation), suggesting differences in ionic mechanism between acute and chronic exposure to losartan. Previous studies have shown that angiotensin-II increases the L-type calcium current as well as the sodium-calcium exchange current.5 Our data show that losartan as well as enalapril do not affect calcium channel current (Ica) density (Figure 6). A lack of effect of losartan on ICa has been reported in isolated rabbit PV cells5 and guinea pig ventricular myocytes 27. Losartan has also been shown to decrease PV spontaneous activity, contractile force, myocytes, transient inward current (Iti) and sodium/calcium exchange current.5 Suppression of DAD and late-phase 3 EAD activity by the ARB losartan and the ACE-inhibitor enalapril maybe explained by their actions to reduce the transient inward current (Iti) as well as to reduce intracellular calcium loading secondary to their effects to block the sodium/calcium exchange current.5, 28 The effects of losartan on EADs and DADs do not appear to depend on an effect to block angiotensin-II receptors, since angiotensin-II did not induce DAD or EAD activity in any of the PV sleeve preparations studied. In a cellular model of simulated ischemia and reperfusion, losartan did not affect APD or ICa yet improved recovery of contraction and inhibited Iti during reperfusion, suggesting losartan reduces signs of intracellular Ca2+ overload27 Consistent with that study, we found no effect of losartan on either ICa or IK but a dramatic suppression of DADs induced by high Ca2+.
Clinical implications: antiarrhythmic effects of losartan and enalapril
Angiotensin II -receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors are the most common anti-hypertensive drugs used in the clinics. In addition to their use as anti-hypertensive agents, ACE inhibitors and ARBs have been shown to prevent the development of AF in clinical trials (LIFE, CHARM, VALUE).2, 6, 7 However, in a recent large clinical study involving the ARB valsartan (GISSI-AF),29 the authors concluded that, at one year, valsartan was not associated with a reduction of incidence of AF. Difference in patient selection, duration of the study and endpoints (primary prevention or no) may account for differences encountered in the studies.
The data presented in the paper suggest that ARB and ACE-inhibitor may play a role in the treatment of AF, alone or associated with other agents, perhaps allowing for the use of lower doses of antiarrhythmic agents.
Limitations of the study
Transient inward current (Iti), NCX or internal calcium were not measured in the PV sleeve preparations. It should be noted that the concentration of isoproterenol and calcium used are out of the physiological range. The whole perfused left atrium was used for cell isolation. Pulmonary vein myocytes were not isolated in this study because the chunk method of dissociation that would be required provides a relatively poor yield and condition of single myocytes.
Conclusion
The data suggest that, in addition to their “upstream” antiarrhythmic action (effect on atrial structural remodeling), ACE inhibitors and angiotensin II -receptor blockers have a “direct” antiarrhythmic effect by suppressing triggers responsible for the genesis of AF and other atrial arrhythmias
Acknowledgments
Supported by grant HL47678 from NHLBI (CA) and NYS and Florida Grand Lodges F. & A.M.
Footnotes
No disclosures.
References
- 1.Fuster V. Atrial fibrillation: an epidemiologic, scientific and clinical challenge. Nat Clin Pract Cardiovasc Med. 2005;2:225. doi: 10.1038/ncpcardio0199. [DOI] [PubMed] [Google Scholar]
- 2.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 4.Gramley F, Lorenzen J, Plisiene J, Rakauskas M, Benetis R, Schmid M, Autschbach R, Knackstedt C, Schimpf T, Mischke K, Gressner A, Hanrath P, Kelm M, Schauerte P. Decreased plasminogen activator inhibitor and tissue metalloproteinase inhibitor expression may promote increased metalloproteinase activity with increasing duration of human atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1076–1082. doi: 10.1111/j.1540-8167.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen YJ, Chen YC, Tai CT, Yeh HI, Lin CI, Chen SA. Angiotensin II and angiotensin II receptor blocker modulate the arrhythmogenic activity of pulmonary veins. Br J Pharmacol. 2006;147:12–22. doi: 10.1038/sj.bjp.0706445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salehian O, Healey J, Stambler B, Alnemer K, Almerri K, Grover J, Bata I, Mann J, Matthew J, Pogue J, Yusuf S, Dagenais G, Lonn E. Impact of ramipril on the incidence of atrial fibrillation: results of the Heart Outcomes Prevention Evaluation study. Am Heart J. 2007;154:448–453. doi: 10.1016/j.ahj.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 7.Yin Y, Dalal D, Liu Z, Wu J, Liu D, Lan X, Dai Y, Su L, Ling Z, She Q, Luo K, Woo K, Dong J. Prospective randomized study comparing amiodarone vs. amiodarone plus losartan vs. amiodarone plus perindopril for the prevention of atrial fibrillation recurrence in patients with lone paroxysmal atrial fibrillation. Eur Heart J. 2006;27:1841–1846. doi: 10.1093/eurheartj/ehl135. [DOI] [PubMed] [Google Scholar]
- 8.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 9.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sicouri S, Belardinelli L, Carlsson L, Antzelevitch C. Potent antiarrhythmic effects of chronic amiodarone in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2009;20:803–810. doi: 10.1111/j.1540-8167.2009.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE. 2006;29:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Wongcharoen W, Chen YC, Chen YJ, Chen SY, Yeh HI, Lin CI, Chen SA. Aging increases pulmonary veins arrhythmogenesis and susceptibility to calcium regulation agents. Heart Rhythm. 2007;4:1338–1349. doi: 10.1016/j.hrthm.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Lo LW, Chen YC, Chen YJ, Wongcharoen W, Lin CI, Chen SA. Calmodulin kinase II inhibition prevents arrhythmic activity induced by alpha and beta adrenergic agonists in rabbit pulmonary veins. Eur J Pharmacol. 2007;571:197–208. doi: 10.1016/j.ejphar.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 16.Sicouri S, Talarico M, Antzelevitch C. Antiarrhythmic effects of losartan and enalapril in canine pulmonary vein sleeves. Heart Rhythm. 2009;6:S91. doi: 10.1111/j.1540-8167.2010.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumaine R, Cordeiro JM. Comparison of K+ currents in cardiac Purkinje cells isolated from rabbit and dog. J Mol Cell Cardiol. 2007;42:378–389. doi: 10.1016/j.yjmcc.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 19.Nattel S, Allessie MA, Haissaguerre M. Spotlight on atrial fibrillation-the 'complete arrhythmia'. Cardiovasc Res. 2002;54:197–203. doi: 10.1016/s0008-6363(02)00324-3. [DOI] [PubMed] [Google Scholar]
- 20.Nattel S. Combined parasympathetic-sympathetic nerve discharge and pulmonary vein afterdepolarizations: A new unifying concept with basic and clinical relevance. Heart Rhythm. 2005;2:632–633. doi: 10.1016/j.hrthm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura M, Imanishi M, Matsushima Y, Akabane S, Kuramochi M, Ito K, Omae T. A comparison of lisinopril with enalapril by monitoring plasma angiotensin II levels in humans. Jpn J Pharmacol. 1990;54:143–149. doi: 10.1254/jjp.54.143. [DOI] [PubMed] [Google Scholar]
- 22.Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen YJ, Chen SA. Electrophysiology of pulmonary veins. J Cardiovasc Electrophysiol. 2006;17:220–224. doi: 10.1111/j.1540-8167.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 24.Sicouri S, Glass A, Carlsson L, Antzelevitch C. Electophysiologic and antiarrythmic effects of AZD1305 in canine pulmonary vein sleeves. Heart Rhythm. 2008;5S:S163. doi: 10.1124/jpet.110.166702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, Rosen MR, Cohen IS. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 27.Louch WE, Ferrier GR, Howlett SE. Losartan improves recovery of contraction and inhibits transient inward current in a cellular model of cardiac ischemia and reperfusion. J Pharmacol Exp Ther. 2000;295:697–704. [PubMed] [Google Scholar]
- 28.Sipido KR, Volders PG, Vos MA, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc Res. 2002;53:782–805. doi: 10.1016/s0008-6363(01)00470-9. [DOI] [PubMed] [Google Scholar]
- 29.Disertori M, Latini R, Barlera S, Franzosi MG, Staszewsky L, Maggioni AP, Lucci D, Di PG, Tognoni G. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–1617. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]