Abstract
In this manuscript, we characterize for the first time an animal model of non-myeloablative bone marrow transplantation (BMT) using the purine analog pentostatin [P]. Other cohorts of mice received a distinct purine analog, fludarabine [F], which we and others have previously evaluated in non-myeloablative murine models. In this project, we have characterized pentostatin for its ability to: (1) operate synergistically with cyclophosphamide [C] to induce host T cell depletion; (2) induce host T cell suppression, as defined by modulation of cytokine secretion in vitro and abrogation of host-versus-graft reactivity (HVGR) in vivo; (3) constrain host T cell recovery post-chemotherapy; and (4) prevent the rejection of T-cell depleted (TCD), fully MHC mismatched bone marrow allografts. Relative to single-agent regimens, combination PC or FC regimens worked synergistically to deplete host CD4+ and CD8+ T cells; PC and FC regimens were developed that yielded similar levels of host T cell and myeloid cell depletion. In the setting of these generally comparable states of host T and myeloid cell depletion, the PC regimen was found to be highly immune suppressive, as evidenced by reduced host T cell capacity to: (1) secrete IL-2 and IFN-γ in vitro; (2) mediate HVGR in vivo; and (3) recover numerically and functionally during a two-week observation period post-chemotherapy. Finally, using B6 hosts treated with the 14-day chemotherapy regimens, the PC regimen more consistently prevented the rejection of BALB/c TCD-allografts than the FC regimen (rate of alloengraftment, 14/15 [93%] of PC-treated recipients vs. 8/14 [57%] of FC-treated recipients; p<0.05); similar results were observed using an 8-day conditioning regimen. These data suggest that host T cell suppression, distinct from T cell depletion, may therefore represent a critical determinant of engraftment after purine analog-based regimens and may also be preferentially attained by use of pentostatin.
INTRODUCTION
Reduced-intensity conditioning regimens prior to allogeneic bone marrow transplantation spare severe bone marrow toxicity associated with myeloablative regimens but increase the barrier of host immunity to donor engraftment. (1, 2) Fludarabine has been employed to inhibit host immunity prior to transplant and belongs to a class of purine nucleoside analogs that mediate cytotoxicity by incorporation into DNA and blockade of elongation by DNA polymerase. (3) Initial non-myeloablative regimens utilized fludarabine in combination with idarubicin or total body irradiation (TBI) (4, 5), and current regimens also utilize fludarabine in combination with the alkylating agent cyclophosphamide. (6, 7) Other purine analogs, such as pentostatin, have been less frequently evaluated for host preparation prior to transplant.
Pentostatin is a purine analog with a unique mechanism of action relative to fludarabine, as it inhibits adenosine deaminase (ADA) and thereby results in lymphocyte toxicity due to the accumulation of deoxyadenosine-triphosphate. (8, 9) Indeed, an inherited deficiency of ADA is one cause of congenital severe combined immunodeficiency, or SCID, a disease in which both B and T cells fail to mature. (10) In both the setting of hairy cell leukemia (11) or GVHD therapy (12), pentostatin results in profound reduction in host immunity, which has primarily been characterized in numerical terms, specifically a reduction of absolute T cell counts. In spite of the compelling potential of pentostatin for modulation of host immunity, only a limited number of clinical trials have evaluated this drug for non-myeloablative transplantation. (13, 14) Furthermore, to our knowledge, no direct comparative data exist with respect to the relative immune modulation effects of fludarabine and pentostatin in the clinic or in animal models. As such, it is unclear whether distinct purine analogs might result in differential efficacy in host preparation for alloengraftment.
Previously, we found that fludarabine and cyclophosphamide acted synergistically to induce a depth of immune depletion sufficient for facilitation of MHC-mismatched, T-cell depleted murine alloengraftment. (15) The fludarabine-based model we used was stringent in terms of studying host-versus-graft rejection (HVGR) because of the major MHC-mismatch utilized and the use of T-cell depleted allografts. Because no reports exist in the literature pertaining to the ability of pentostatin to facilitate alloengraftment after experimental murine BMT, we set out to develop murine models focusing on host conditioning with pentostatin. Because of our previous finding that fludarabine and cyclophosphamide acted in synergy, we evaluated pentostatin either alone or in combination with this DNA alkylator. Although host NK cells can mediate rejection (16), we focused our studies on purine analog modulation of host CD4+ and CD8+ T cells, which are key mediators of graft rejection (17) and known targets of purine analog therapy.
In this project, we pursued three specific objectives. First, we characterized the ability of pentostatin to cause immune depletion when utilized in combination with cyclophosphamide and characterized immune recovery following such depletion. Secondly, we examined the immune suppressive effect of pentostatin containing regimens; that is, we tested whether T cells that survived host conditioning could mediate effector function, including generation of an in vivo HVGR response upon adoptive cell transfer to a secondary host. Lastly, we evaluated the ability of pentostatin- containing regimens to facilitate stable, long-term engraftment of MHC-mismatched, T-cell depleted marrow allografts.
MATERIALS AND METHODS
Mice
Adult female C57BL/6 (B6, H-2Kb) and BALB/c (H-2Kd) mice 8- to 10- weeks old were obtained from Frederick Cancer Research Facility. Mice were maintained in a specific pathogen-free facility, and treated according to an approved animal protocol. Drinking water was supplemented with ciprofloxacin from day −7 to day +14 after chemotherapy treatment [concentration of 100 mg/L; volume of water intake not monitored].
Preparative Regimen
BALB/c or B6 mice were treated by intra-peritoneal (i.p.) injection with either pentostatin or fludarabine; these agents were administered either alone or in combination with cyclophosphamide. Control cohorts received cyclophosphamide alone, vehicle (HBSS) or lethal total-body irradiation (BALB/c 950 cGy, B6 1100 cGy; 137Cs gamma radiation, gamma Cell 40, Atomic Energy of Canada). The drug schedules for each experiment vary and are described in the figure legends.
Bone Marrow Transplant
Bone marrow (BM) cells were harvested and T-cell depleted (T-depleted; anti-CD90 [Thy1.2] microbeads; Miltenyi®); the marrow product contained <1% CD3+ cells by flow cytometry. Host T cells for post-irradiation add-back were obtained from the spleen and were purified to >99% purity (pan-T-cell selection kit; Miltenyi®). After completion of the preparative regimen, the T-depleted BM was injected intravenously (i.v.) into hosts at a dose of 2 × 107 cells per recipient.
Determination of Cytokine Phenotype
Recipient spleen cells were harvested, red cells were lysed (ACK buffer, Quality Biologicals) and adjusted to 0.5 × 106 cells/ml in 24-well plates. Anti-CD3, anti-CD28 coated beads (CD3/CD28 beads) were produced as previously detailed (18); spleen T cells were co-stimulated (3:1 ratio of beads to T cells) for 24 h in complete medium (CM) consisting of RPMI 1640 (Mediatech), 10% FCS (Gemini Bio-Products), pen-strep-glut, nonessential amino acids (Invitrogen), and 2-ME (5 × 10−5 M; Invitrogen). In some experiments, T cells were stimulated with B6 or BALB/c dendritic cells (DC) (spleen cell to DC ratio, 10:1) for 24 h. Resultant culture supernatants were evaluated for cytokine content by multiplex bead array (Bio-Rad).
Secondary Transfer Experiments
BALB/c mice were treated for 3 days with combination pentostatin plus cyclophosphamide or with combination fludarabine plus cyclophosphamide; control hosts received TBI. Spleen cells were harvested the day after the end of treatment and T cells were purified. Such T cells were then injected (i.v.) into BALB/c hosts that were lethally irradiated 24 h prior to injection. On the day after host T cell infusion, mice were challenged with fully allogeneic, TCD BM cells (B6 donors; 10 × 106 cells; i.v. injection). Subsequently, at day 5 after bone marrow infusion, HVGR was quantified.
Quantification of HVGR
Post-transplant splenic single cells were adjusted to 0.5 × 106 cells/ml and stimulated with B6 or BALB/c DC. Such DC were generated by culturing marrow cells for 6 days in rmGM-CSF and rmIL-4 (each at 1000 IU/ml; PeproTech); bacterial LPS (1 μg/ml; Calbiochem) was added to the final 24 h of DC culture. Expanded DC were washed and used at a spleen cell to DC ratio of 10:1. After 24 h, supernatants were collected for cytokine analysis, and cells were evaluated by cytokine capture flow cytometry using IFN-γ catch reagent (Miltenyi®) followed by 45 min of incubation (RPMI 1640 with 10% FCS) at 37°C (slow rotation). Cells were washed with cold buffer, labeled with IFN-γ detection Ab (APC) and previously described surface Abs, washed, and analyzed. Flow cytometric frequency data was multiplied by splenic cell yields to obtain the absolute number of cytokine secreting cells per spleen; allospecific values were calculated by subtracting values obtained after syngeneic stimulation.
Determination of Chimerism
Spleens and bone marrow were harvested at different times after the end of treatment, and single cell suspensions were labeled with anti-CD3, -CD4, -CD8, -NK1.1 or -Gr1, conjugated with FITC, PE, APC (PharMingen). For chimerism analysis, post-transplant blood, spleen, and bone marrow cells were labeled with anti-H-2Kb and anti-H-2Kd to differentiate donor vs. host, and with anti-B220 or anti-CD19, -CD3 and –Gr1 to identify cell lineage. Four-color flow cytometry was performed on a FACSCalibur instrument (BD); five-color flow cytometry was performed on an LSR II instrument using FACSDiva software (BD). Live events (10,000–20,000) were acquired, with 7-AAD used to exclude dead cells. Percentage donor chimerism was calculated according to the following equation: % donor chimerism (donor cells/[host cells + donor cells]) × 100.
Statistics
Survival analysis was performed according to the Kaplan-Maier method and survival curves were compared using log-rank testing. Flow and cytokine data were analyzed using Student’s 2-tailed t tests. Values of p<0.05 were considered statistically significant.
RESULTS
Pentostatin combined with cyclophosphamide synergistically induces host immune depletion and immune suppression
To evaluate immune depletion and immune suppression caused by pentostatin (P) or fludarabine (F), we chose a dose of fludarabine (100 mg/kg/day) that was found to be effective for the treatment of leukemia and was associated with a defined incidence of neurotoxicity (19); of note, this was the same dose of fludarabine that we previously evaluated in combination with cyclophosphamide. (15) By comparison, pentostatin has immune suppressive activity at a dose of approximately 1 mg/kg/day (20), with increasing toxicity at doses higher than 1 mg/kg/day (21); based on these data, we initiated our experiments using pentostatin at a dose of 1 mg/kg/day. In previous work, (15) we found that fludarabine caused minimal immune depleting effects unless combined with cyclophosphamide (C or Cy); this observation is consistent with the characterized synergistic relationship between purine analogs and alkylating agents. (22) Therefore, in our initial experiments, which were designed to detect synergy between the different purine analogs and the DNA alkylator, we utilized an intermediate dose of cyclophosphamide that we previously evaluated. (15)
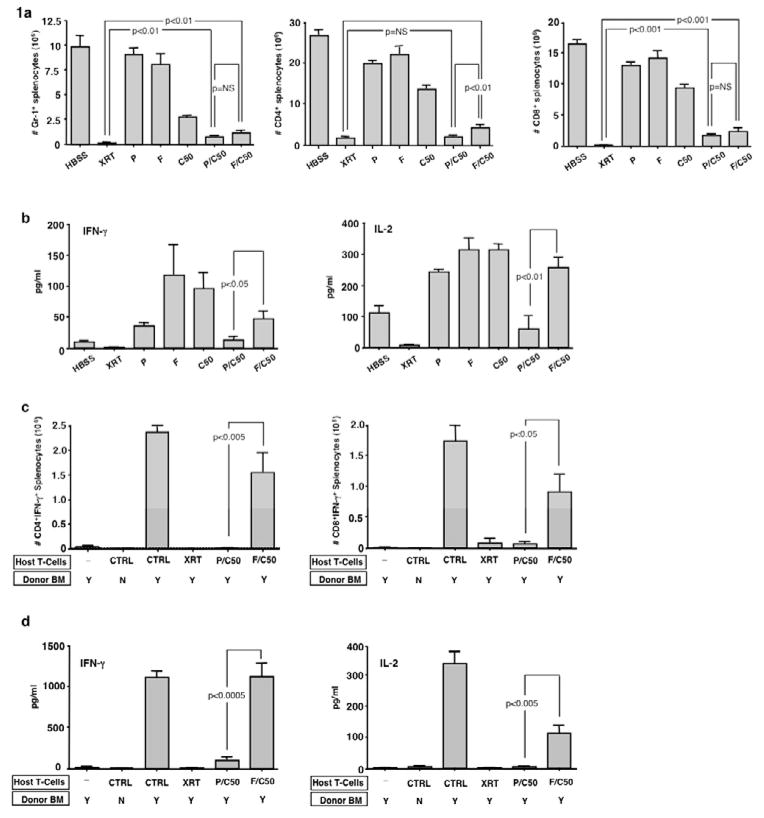
First, we evaluated a 3-day regimen of P or F either alone or in combination with Cy. Either purine analog alone had minimal effect on host myeloid cell depletion; however, addition of Cy significantly and similarly reduced host myeloid cells to a level that did not approach myeloablative TBI levels (Fig 1a, left panel). Because these PC and FC regimens yielded similar myeloid cell depletion, we were able to assess whether the regimens mediated differential immune suppressive and immune depletive effects when controlled for myeloid cell toxicity. Recipients of either P or F alone had minimally decreased numbers of CD4+ T cells (mean reduction of 25% and 17%, respectively); CD4+ T cells decreased by 48% in recipients of single agent Cy (Fig 1a, middle panel). In contrast, recipients of the combination regimens had marked CD4+ T cell depletion to levels lower than would be expected if the purine analog and alkylator had an additive effect (PC: 92% CD4+ depletion [actual] vs. 74% depletion [calculated value if additive]; p <0.05) (FC: 84% CD4+ depletion [actual] vs. 66% depletion [calculated value if additive]; p <0.05). In this experiment, the PC regimen caused slightly more severe CD4+ T cell depletion than the FC regimen and was comparable to a lethal TBI regimen in terms of CD4+ depletion. Similar synergy between the purine analog and DNA alkylator was seen in terms of CD8+ T cell depletion (PC: 90% CD8+ depletion [actual] vs. 64% depletion [calculated value if additive]; p <0.05) (FC: 85% CD8+ depletion [actual] vs. 57% depletion [calculated value if additive]; p <0.05) (Fig 1a, right panel). The PC and FC regimens were comparable in the severity of CD8+ T-cell depletion and both regimens were less CD8-depleting relative to the TBI regimen.
Figure 1. Pentostatin and cyclophosphamide synergistically induce host immune depletion and suppression.
BALB/c hosts were injected i.p. once daily for three days with either: HBSS (vehicle), pentostatin (P; 1mg/kg/d), fludarabine (F; 100mg/kg/d), cyclophosphamide (C50; 50 mg/kg/d), pentostatin plus cyclophosphamide (P/C50), fludarabine plus cyclophosphamide (F/C50) or treated with lethal total body irradiation (XRT; 950 cGy). (a) Three days post-therapy spleen cells were isolated, counted and analyzed by flow cytometry to determine the levels of depletion of myeloid cells (Gr-1+) and CD4+ and CD8+ T cells. (b) Spleen cells were co-stimulated and the resultant 24-hr supernatant was tested for IFN-γ and IL-2 content. (c) In a separate experiment, BALB/c hosts were lethally irradiated and then injected with syngeneic BALB/c host T cells (0.1 × 106) obtained from mice treated with either HBSS (control), lethal irradiation (XRT; 1050 cGy), combination pentostatin plus cyclophosphamide (P/C50), or combination fludarabine plus cyclophosphamide (F/C50). One day following injection of host T cells, the hosts were transplanted with fully MHC-mismatched T-cell depleted bone marrow from B6 mice (10 × 106 cells). On day 8 post-BMT, spleen cells were isolated and stimulated with syngeneic or allogeneic dendritic cells for 24 hours. The number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by cytokine capture flow cytometry. (d) Resultant culture supernatants were also tested for cytokine content (allogeneic stimulation condition is shown). All results are shown as mean ± SEM of n=5–10 per cohort.
Next, we evaluated pentostatin- and fludarabine-containing regimens for their immune suppressive effect, as defined by a reduced capacity of post-regimen host splenocytes to secrete cytokines. Post-regimen splenocytes were normalized to equal cell concentrations and cytokine secretion potential was tested. As anticipated, post-TBI splenocytes were immune suppressed relative to control splenocytes in terms of IFN-γ secretion (Fig 1b: median values, pg/ml: 13 vs. 2; p <0.01) and IL-2 secretion (median values, pg/ml: 134 vs. 8; p <0.001). In marked contrast, post-regimen splenocytes from single-agent P, F, or Cy recipients unexpectedly had increased IFN-γ and IL-2 secretion. Such “rebound” cytokine production was ameliorated by the combination PC regimen and, to a lesser extent, by the combination FC regimen.
We also evaluated whether T cells harvested from PC-and FC-treated hosts were immune suppressed as defined by a reduced capacity to mount an in vivo alloreactive host-versus-graft-reaction (HVGR). Host-type T cells harvested from FC-treated mice induced a similar magnitude of HVGR as control host T cells (as defined by allospecific IFN-γ secreting CD4+ and CD8+ T cells; Fig 1c, Miltenyi flow cytometry cytokine capture assay). In contrast, host-type T cells harvested from PC- or lethal TBI-treated mice mediated greatly reduced CD4+ and CD8+-driven HVGR. HVGR was also greatly reduced in recipients of PC-treated host T cells when tested by IFN-γ and IL-2 supernatant analysis (Fig 1d).
Host immune recovery after PC or FC therapy: a potential barrier to engraftment
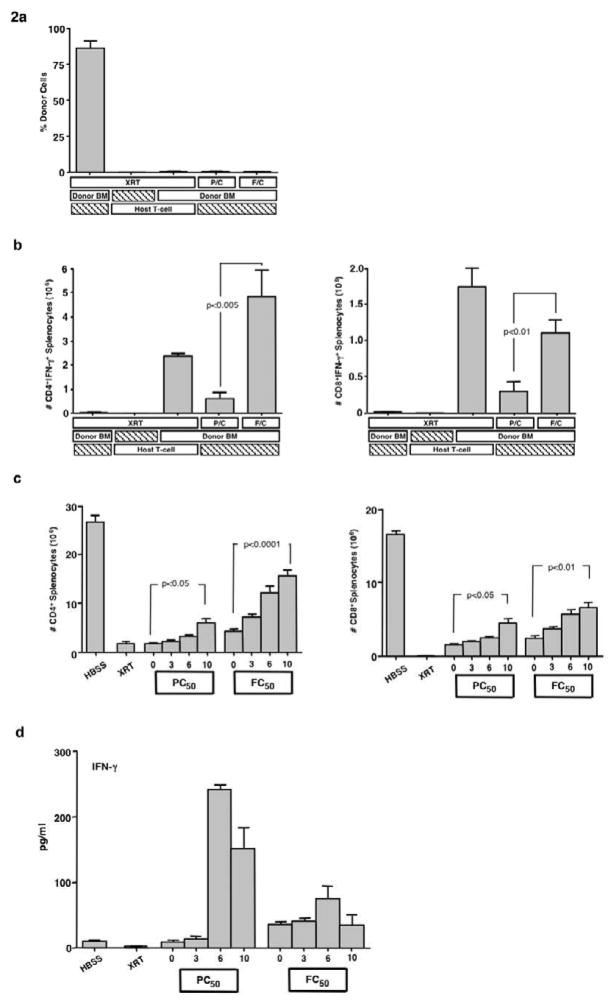
We next tested the combination PC and FC regimens in a fully MHC-mismatched BMT setting. In spite of the significant host immune suppression and immune depletion caused by these regimens, graft rejection was uniformly observed (Fig 2a). Of note, PC-treated hosts had reduced CD4+-and CD8+- mediated HVGR compared to FC-treated hosts; however, recipients of either regimen had increased HVGR relative to TBI recipients (Fig 2b).
Figure 2. Host immune recovery after PC or FC therapy.
(a) BALB/c hosts were transplanted with T-cell depleted B6 BM cells (10 × 106 cells) following conditioning with either lethal irradiation (XRT; 1050 cGy), combination pentostatin plus cyclophosphamide (P/C), or combination fludarabine plus cyclophosphamide (F/C). A graft rejection control group received lethal irradiation followed by an infusion of unmanipulated host T cells (0.1 × 106). Spleen cells were isolated on day 8 post-BMT and analyzed by flow cytometry for percent donor cells. (b) In addition, the total number of day 8 post-BMT host CD4+ and CD8+ T cells producing IFN-γ after 24h stimulation with syngeneic (BALB/c) or allogeneic (B6) DC was determined (results shown are with allogeneic stimulation). (c) In a separate experiment, BALB/c mice were injected i.p. once daily for three days with either: HBSS, pentostatin plus cyclophosphamide (P/C50), fludarabine plus cyclophosphamide (F/C50) or treated with lethal total body irradiation (XRT). Subsequently, spleen cells were obtained at days 3, 6 and 10 after therapy and analyzed by flow cytometry for number of CD4+ and CD8+ T-cells. (d) In addition, spleen cells at each time point were co-stimulated and the resultant supernatants tested for IFN-γ content. All results are shown as mean ± SEM of n=5 per cohort for each time.
Given the occurrence of HVGR and graft rejection in PC- or FC-treated hosts, we reasoned that the immune suppressive and immune depleting state induced by these 3-day regimens may have been too transient to allow for a clinically meaningful engraftment result. As such, we set out to characterize the period of immune suppression and immune depletion, and to develop alternative regimens with enhanced durability. The absolute number of CD4+ and CD8+ T cells progressively increased between days 3 and 10 after either the PC or FC regimens (Fig 2c). Strikingly, splenocytes harvested from mice at day 6 and 10 after the PC regimen had a marked increase in capacity for IFN-γ secretion relative to splenocytes harvested immediately after completion of the PC regimen (Fig. 2d). By comparison, FC treated spleen cells secreted moderately increased amounts of IFN-γ immediately post-regimen, with sustained elevation through the 10-day period.
Intensified PC and FC regimens: profound immune depletion
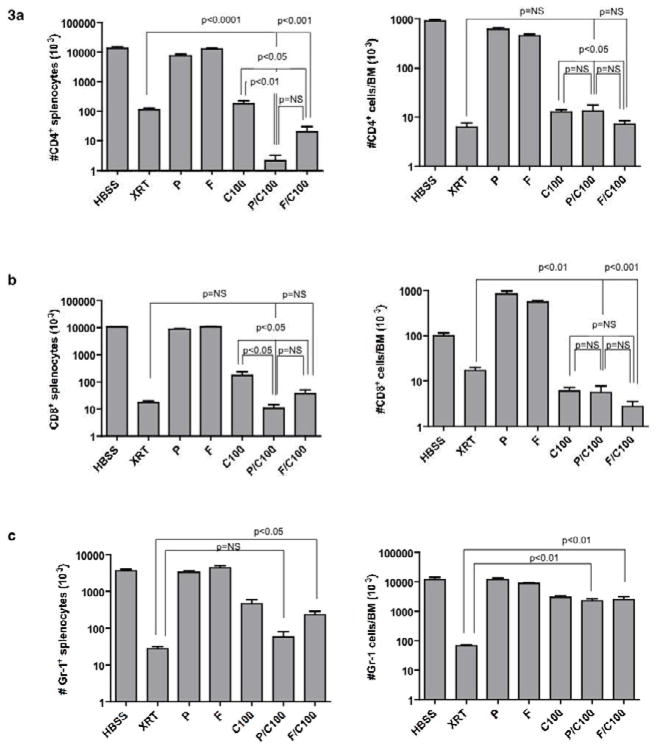
These data indicated that more severe host T cell depletion and impairment of immune recovery might be required to permit fully MHC-mismatched engraftment after PC or FC therapy. Therefore, we sought to develop regimens that would yield both CD4+ and CD8+ T cell depletion to levels comparable to lethal doses of TBI. For this purpose, we tested regimens that incorporated either pentostatin or fludarabine every third day in combination with daily cyclophosphamide for 14 days. This intermittent purine analog therapy, which was necessitated by increased toxicity with prolonged daily administration, theoretically takes advantage of the relatively long biologic half-life of purine analogs. (3) Similar to the results with the 3-day regimen, splenic CD4+ T cells in both the 14-day PC and FC combinations were greatly reduced compared to single drug administration (Fig 3a, left panel). Consistent with our objective, recipients of the 14-day PC or FC regimens had more profound CD4+ T cell depletion in the spleen than recipients of the lethal TBI regimen. Similar intensive immune depletion was observed upon evaluation of the bone marrow after PC and FC treatment. That is, CD4+ T cells harvested from PC or FC recipients were reduced to levels equivalent to lethal TBI-treated hosts (Fig 3a, right panel). Host mice that received combination therapy were also more severely depleted of splenic CD8+ T cells than recipients of P, F or Cy alone (Fig 3b, left panel) and such CD8+ T cell values were comparable to levels achieved after lethal TBI. Combination PC or FC regimens, as well as single-agent Cy alone, mediated profound depletion of CD8+ T cells in the bone marrow to values less than those obtained with the TBI regimen (Fig 3b, right panel). NK cells were equally depleted by PC or FC regimens (data not shown).
Figure 3. Intensified PC and FC regimens result in profound immune depletion.
(a) Host B6 mice were treated with a 14-day course of either pentostatin (P; 1mg/kg on days 1, 4, 8 and 12) or fludarabine (F; 100mg/kg on days 1, 4, 8. and 12) alone; daily cyclophosphamide alone (C100; 100 mg/kg/day); a combination of intermittent pentostatin or fludarabine with daily cyclophosphamide (P/C100 or F/C100); or treated with lethal total body irradiation (1150 cGy). Spleen cells were isolated and evaluated by flow cytometry for total number of CD4+ and CD8+ T cells and myeloid cells (Gr-1+). (b) Bone marrow cells were also analyzed for T cell composition and myeloid lineage. All results are shown as mean ± SEM of n=5 per cohort.
In addition to the induction of profound immune depletion, the 14-day PC or FC regimens also mediated substantial depletion of myeloid cells. That is, recipients of the PC regimen had similar reduction in number of splenic Gr-1+ cells as TBI recipients (Fig 3c, left panel); recipients of the FC regimen also had greatly reduced splenic Gr-1+ cells, although not to levels observed with lethal TBI. Myeloid cells in the bone marrow were preserved in PC and FC recipients: such recipients had approximately 2-log higher numbers of myeloid cells in the marrow compared to lethal TBI recipients (Fig 3c, right panel). Therefore, both the 14-day PC and FC regimens were non-myeloablative yet yielded similar depletion of host CD4+ and CD8+ T cells compared to lethal TBI recipients.
Intensified PC and FC regimens: kinetics of immune recovery
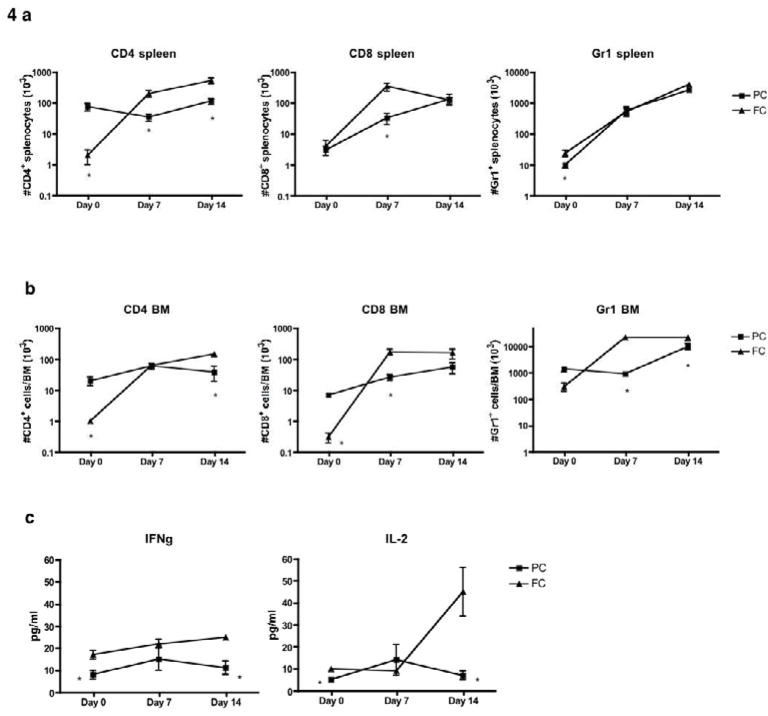
Next, we evaluated whether the 14-day regimens might limit the immune T cell recovery that was observed with the 3-day regimens. In this experiment, splenic CD4+ T cell depletion was more profound in FC recipients relative to PC recipients (Fig 4a and 4b, “Day 0” values); however, at days 7 and 14 post-chemotherapy, there was substantial recovery of splenic CD4+ T cells in FC recipients but not in PC recipients (Fig 4a, left panel). Indeed, splenic CD4+ T cells at days 7 and 14 post-chemotherapy were greater in FC recipients than in PC recipients. In addition, splenic CD8+ T cell numbers, which were depleted to similar values just after PC and FC chemotherapy, recovered to a greater extent in FC recipients than in PC recipients at day 7 post-chemotherapy (Fig 4a, middle panel). There was an approximate two-log increase in splenic Gr-1+ myeloid cells in both PC and FC recipients in the two-week post-chemotherapy period.
Figure 4. Intensified PC regimen effectively constrains host immune recovery.
(a) Host B6 mice received either pentostatin (1 mg/kg on days 1, 4, 8 and 12) or fludarabine (100 mg/kg on days 1, 4, 8 and 12), with each drug administered in combination with cyclophosphamide (100 mg/kg/d; days 1 through 14). After treatment with these PC or FC regimens, spleen cells were harvested on days 0, 7 and 14 post-regimen and evaluated by flow cytometry for total number of CD4+ and CD8+ T cells and myeloid cells (Gr-1+). (b) Bone marrow cells were also harvested on days 0, 7 and 14 and analyzed for T cell composition and myeloid lineage. (c) On days 0, 7 and 14 post-regimen, spleen cells were stimulated with anti-CD3/CD28 beads and 24-hr supernatant was tested for IFN-γ and IL-2 content. All results are shown as mean ± SEM of n=5 per cohort for each time point.
Relative to the PC regimen, the FC regimen also induced more profound CD4+ T cell depletion in the bone marrow (Fig 4b, left panel); such enhanced CD4+ depletion was relatively transient because levels of marrow CD4+ T cells were actually higher in FC recipients relative to PC recipients at day 14 post-chemotherapy. Similarly, relative to PC recipients, FC recipients had more profound CD8+ T cell depletion in the marrow immediately post-chemotherapy but had increased CD8+ T cell numbers at day 7 post-chemotherapy (Fig 4b, middle panel). In contrast to this severe host T cell depletion, myeloid cells in the marrow were relatively spared for both regimens, with further Gr-1+ myeloid cell recovery over two weeks (Fig 4b, right panel). Recovery of myeloid cells was somewhat constrained in PC recipients relative to FC recipients at days 7 and 14 post-chemotherapy. In sum, these data suggest that PC recipients had a reduced capacity for T cell recovery post-chemotherapy relative to FC recipients; that is, FC recipients in general had lower CD4+ and CD8+ T cell numbers in the spleen and marrow just post-chemotherapy but had more rapid recovery of CD8+ T cell numbers by day 7 post-therapy and higher CD4+ T cell numbers at day 14 post-therapy.
Given this evidence that immune T-cell recovery was constrained after the 14-day regimens, particularly the PC regimen, we also investigated whether the functional T cell rebound observed with the 3-day regimens might be similarly constrained. Indeed, splenocytes from PC recipients had minimal capacity to secrete IFN-γ and IL-2; remarkably, such T cell dysfunction persisted over the two-week post-chemotherapy period (Fig 4c). Relative to PC recipients, FC recipients had modestly increased splenocyte capacity for IFN-γ and increased recovery of IFN-γ and IL-2 secretion at day 14 post-chemotherapy.
Intensified PC and FC regimens: successful abrogation of graft rejection
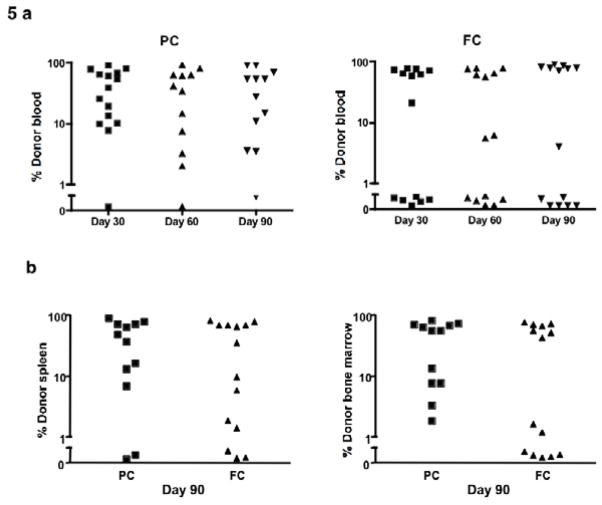
Having shown the ability of the intensified PC and FC regimens to induce profound host immune depletion and immune suppression with limited immune recovery, we hypothesized that such regimens would prevent graft rejection in a fully MHC-disparate transplantation model. Indeed, 14/15 recipients of PC conditioning and 8/14 recipients of FC conditioning had alloengraftment by peripheral blood analysis at day 30 post-BMT (Table 1; PC>FC, p <0.05). In contrast, recipients of single-agent Cy conditioning did not engraft (0/10 cases of alloengraftment). For both PC and FC recipients, alloengraftment was relatively stable at days 60 and 90 post-BMT (Fig 5a); of note, the PC regimen yielded several cases of stable mixed chimerism whereas engraftment in FC recipients was in general an all-or-none phenomenon. There were three deaths in recipients of the PC regimen between days 30 and 90 post-transplant; we observed no overt clinical signs of GVHD such as skin lesions or diarrhea. At day 90 post-BMT, chimerism analysis of harvested spleen and bone marrow confirmed the peripheral blood results: that is, there was an increased frequency of successful alloengraftment in PC recipients relative to FC recipients (Fig 5b) [cases of alloengraftment, day 90 spleen analysis: 10/12 (PC recipients) vs. 8/14 (FC recipients), p <0.05] [cases of alloengraftment, day 90 bone marrow analysis: 12/12 (PC recipients) vs. 9/14 (FC recipients), p <0.05].
Table 1.
Pentostatin facilitates fully MHC-disparate mini-transplant1
| Cohort | Engraftment2 | Statistics | |
|---|---|---|---|
| 1 | TBI | 5/5 | |
| 2 | Cyclophosphamide | 0/10 | |
| 3 | Pentostatin/Cyclophosphamide | 14/15 | PC>FC, p<0.05 |
| 4 | Fludarabine/Cyclophosphamide | 8/14 |
Peripheral blood analyzed at day 30 post-BMT
Chimerism values > 1% donor
Figure 5. Alloengraftment after intensified PC and FC regimens (14-day regimen).
(a) Host B6 mice received treatment with the 14-day PC or FC regimens and were subsequently transplanted with T-cell depleted BALB/c bone marrow (20 × 106 cells). The percentage of total donor cells in the blood was evaluated by flow cytometry at 30, 60 and 90 days after transplant. (b) The percentage of donor cells in the spleen and bone marrow was determined on day 90 post-BMT.
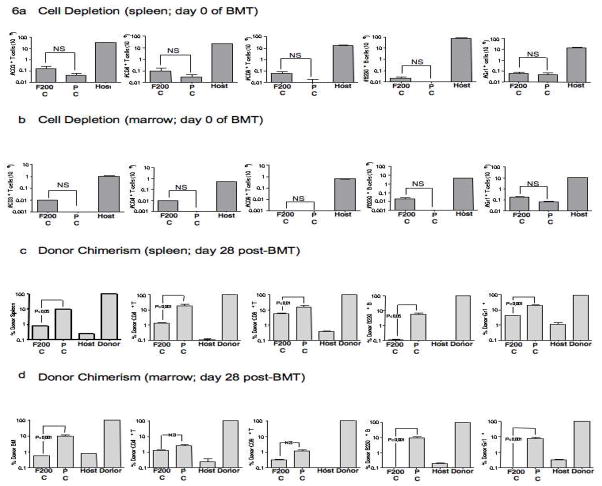
The PC and FC regimens we evaluated yielded similar levels of host immune T cell depletion, and as such, the differential alloengraftment results observed may have been due to some other effect of the conditioning, such as differential immune suppression or differential capacity for immune recovery. Alternatively, it is possible that higher doses of fludarabine might improve alloengraftment in FC recipients. As a step towards addressing this latter possibility, we performed a second BMT experiment using 8-day conditioning regimens comprised of PC (same pentostatin dose as previous experiments, 1 mg/kg) and an FC regimen containing a 100% increase in fludarabine (fludarabine dose increased from 100 mg/kg to 200 mg/kg). In this experiment, we found that the PC and FC regimens equally depleted myeloid cells and caused similar, marked depletion of total CD3+ T cells, CD4+ T cells, CD8+ T cells, and B cells (Fig 6a, splenic absolute cell numbers; Fig 6b, marrow absolute cell numbers; PC vs. FC, each comparison, p = NS). And, similar to the previous BMT experiment, recipients of the PC regimen had increased alloengraftment relative to recipients of the FC regimen (Fig 6c, percent donor chimerism in spleen cells; Fig 6d, percent donor chimerism in marrow cells).
Figure 6. Alloengraftment after intensified PC and FC regimens (8-day regimen; increased fludarabine dosing).
Host B6 mice received either pentostatin (1 mg/kg on days 1, 4, and 7) or fludarabine (200 mg/kg on days 1, 4, and 7); each drug was administered in combination with cyclophosphamide (100 mg/kg/d, days 1 through 8; “F200C” regimen, “PC” regimen). After treatment, cohorts of mice were euthanized (n=10 in each cohort); spleen and marrow were harvested and the absolute numbers of post-conditioning CD3+ T cells, CD4+ T cells, CD8+ T cells, B cells, and Gr-1+ myeloid cells were quantified by flow cytometry. “Host” indicates cell subset numbers in untreated host mice. Absolute cell numbers (mean values ± SEM) in the spleen are shown in (a) whereas absolute cell numbers in the marrow are shown in (b) (NS, not statistically significant). Other cohorts of mice treated with the F200C regimen (n=10) and the PC regimen (n=5) were subsequently transplanted with T-cell depleted BALB/c bone marrow (20 × 106 cells). The percentage of donor/host chimerism was then determined by flow cytometry at day 28 post-transplant for total cells, CD3+ T cells, CD4+ T cells, CD8+ T cells, B220+ B cells, and Gr-1+ myeloid cells in the spleen (c) and in the bone marrow (d). “Host” and “Donor” values represent chimerism control values obtained from untreated host and donor mice; results are mean values ± SEM.
DISCUSSION
In this study, we have characterized the immune-depleting and immune-suppressive properties of pentostatin combined with cyclophosphamide, and have for the first time identified pentostatin-based regimens that facilitate the engraftment of fully MHC-disparate, T cell-depleted murine bone marrow allografts. We found that both pentostatin and fludarabine profoundly influenced host immunity but also identified potentially important differential effects. Pentostatin and fludarabine were relatively comparable in their ability to operate synergistically with a secondary agent, the DNA alkylator cyclophosphamide, to achieve marked immune T cell depletion. However, in experimental settings that yielded comparable host T cell depletion, we consistently observed that the PC regimen mediated more severe immune functional deficits relative to the FC regimen. Indeed, recipients of the PC regimen were less likely to reject a T-cell depleted, fully MHC-disparate bone marrow allograft than recipients of the FC regimen.
Our finding that pentostatin worked synergistically with cyclophosphamide to achieve severe host T cell depletion extends our prior observation with respect to fludarabine and cyclophosphamide (15), thereby providing further in vivo evidence to support the previously described synergy between purine analogs and DNA alkylators. (23, 24) This synergy results from purine analog-induced inhibition of DNA synthesis and repair, thus enhancing T cell susceptibility to DNA-damaging agents. (3) The PC and FC regimens we evaluated were not only similar in terms of host CD4+ and CD8+ T cell depletion, but were each non-myeloablative and generally equivalent with respect to the extent of myeloid cell depletion. Given the similar immune T cell and myeloid cell depletion profiles of these regimens, an opportunity was created to compare pentostatin- and fludarabine-based regimens for their ability to mediate immune suppression, as defined by reduced T cell effector function in vitro and reduced capacity to mediate host-versus-graft reactivity (HVGR) in vivo. Although we evaluated regimens of PC and FC that were similarly immune-depleting across each of the several experiments performed, it is possible that the differential immune suppression, immune recovery, and alloengraftment results we observed with the PC and FC regimens would have been minimized if higher doses of fludarabine were utilized. However, in an experiment performed as a step towards evaluating this possibility, we found that host conditioning with an FC regimen containing a 100% increased dose of fludarabine (dose increased from 100 to 200 mg/kg) yielded less alloengraftment than the PC regimen. It is perhaps unlikely that further escalation of fludarabine in our model would be feasible because the LD10 for a single dose of fludarabine has been reported to be 234 mg/kg. (25) It is also important to note that significant species effects exist with respect to nucleoside analog metabolism (26), and as such, it is difficult to predict the degree to which the current experimental findings would translate into the clinic.
The current experimental results support the concept that chemotherapy-mediated host immune T cell suppression represents an important and separate parameter from immune T cell depletion. By comparison, our previous murine experiments that evaluated fludarabine and cyclophosphamide conditioning focused solely on immune cell depletion as a biomarker for host capacity to mediate graft rejection. (15) Initial experiments in our new efforts, which utilized 3-day treatment regimens, yielded two findings that support our conclusion that pentostatin mediated more profound host T cell suppression than fludarabine. First, we found that T cells remaining after purine analog therapy alone paradoxically had an increased capacity for cytokine secretion; we speculate that this “rebound” of function may relate to a relative resistance of cytokine-secreting, effector T cells to purine analog therapy. Importantly, addition of cyclophosphamide to the pentostatin therapy, but not to the fludarabine therapy, minimized this post-regimen increase in host T cell cytokine secretion. And second, in adoptive transfer experiments, we found that T cells harvested from recipients of the PC regimen had a reduced capacity to mediate HVGR relative to T cells harvested from recipients of the FC regimen.
Furthermore, experiments that evaluated post-regimen immune T cell recovery also point to the preferential immune suppressive effects of pentostatin. That is, after both the 3-day and intensified 14-day treatment regimens, recipients of the PC regimen had reduced recovery of T cell numbers and T cell cytokine secretion relative to recipients of the FC regimen. Remarkably, recipients of the 14-day PC regimen, but not the 14-day FC regimen, showed minimal evidence for numerical or functional T cell recovery over a two-week observation period. Further studies will be required to determine whether this differential effect might be the result of pentostatin inhibition of adenosine deaminase and resultant accumulation of adenosine, which potently inhibits T cell function (27, 28); of note, T cells of Th1/Tc1 phenotype that are known to mediate murine marrow graft rejection (29) are preferentially sensitive to the immune suppressive effects of adenosine (30). It is possible that this differential immune recovery capacity may have accounted in part for the more consistent ability of the PC regimen to prevent fully MHC-disparate marrow graft rejection than the FC regimen.
In summary, pentostatin, when combined with cyclophosphamide, caused severe host immune T cell depletion in a non-myeloablative manner and induced profound immune suppression in residual host T cells. Specifically, pentostatin effectively impaired host T cell recovery in both numerical and functional terms, with resultant abrogation of alloantigen-driven rejection responses. These data add to the limited body of knowledge regarding the immune suppressive effects of purine analogs and may facilitate further murine research pertaining to non-myeloablative transplantation. Finally, these results help provide a rationale to perform pilot clinical trials of non-myeloablative transplantation using pentostatin in combination with cyclophosphamide.
Footnotes
Financial Disclosure statement: the authors have no financial interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Georges GE, Storb R. Review of “minitransplantation”: nonmyeloablative allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2003;77:3–14. doi: 10.1007/BF02982597. [DOI] [PubMed] [Google Scholar]
- 2.Giralt S, Khouri I, Champlin R. Non myeloablative “mini transplants”. Cancer Treat Res. 1999;101:97–108. [PubMed] [Google Scholar]
- 3.Robak T, Lech-Maranda E, Korycka A, Robak E. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: mechanism of action and clinical activity. Curr Med Chem. 2006;13:3165–3189. doi: 10.2174/092986706778742918. [DOI] [PubMed] [Google Scholar]
- 4.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 5.Storb R, Yu C, Barnett T, et al. Stable mixed hematopoietic chimerism in dog leukocyte antigen-identical littermate dogs given lymph node irradiation before and pharmacologic immunosuppression after marrow transplantation. Blood. 1999;94:1131–1136. [PubMed] [Google Scholar]
- 6.Childs RW, Clave E, Tisdale J, Plante M, Hensel N, Barrett J. Successful treatment of metastatic renal cell carcinoma with a nonmyeloablative allogeneic peripheral-blood progenitor-cell transplant: evidence for a graft-versus-tumor effect. J Clin Oncol. 1999;17:2044–2049. doi: 10.1200/JCO.1999.17.7.2044. [DOI] [PubMed] [Google Scholar]
- 7.Kuwatani M, Ikarashi Y, Mineishi S, Asaka M, Wakasugi H. An irradiation-free nonmyeloablative bone marrow transplantation model: importance of the balance between donor T-cell number and the intensity of conditioning. Transplantation. 2005;80:1145–1152. doi: 10.1097/01.tp.0000183289.79693.3d. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell BS, Mejias E, Daddona PE, Kelley WN. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978;75:5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauter C, Lamanna N, Weiss MA. Pentostatin in chronic lymphocytic leukemia. Expert Opin Drug Metab Toxicol. 2008;4:1217–1222. doi: 10.1517/17425255.4.9.1217. [DOI] [PubMed] [Google Scholar]
- 10.Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2:1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- 11.Spiers AS, Parekh SJ, Bishop MB. Hairy-cell leukemia: induction of complete remission with pentostatin (2′-deoxycoformycin) J Clin Oncol. 1984;2:1336–1342. doi: 10.1200/JCO.1984.2.12.1336. [DOI] [PubMed] [Google Scholar]
- 12.Bolanos-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23:2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 13.Miller KB, Roberts TF, Chan G, et al. A novel reduced intensity regimen for allogeneic hematopoietic stem cell transplantation associated with a reduced incidence of graft-versus-host disease. Bone Marrow Transplant. 2004;33:881–889. doi: 10.1038/sj.bmt.1704454. [DOI] [PubMed] [Google Scholar]
- 14.Pavletic SZ, Bociek RG, Foran JM, et al. Lymphodepleting effects and safety of pentostatin for nonmyeloablative allogeneic stem-cell transplantation. Transplantation. 2003;76:877–881. doi: 10.1097/01.TP.0000084869.08639.A0. [DOI] [PubMed] [Google Scholar]
- 15.Petrus MJ, Williams JF, Eckhaus MA, Gress RE, Fowler DH. An immunoablative regimen of fludarabine and cyclophosphamide prevents fully MHC-mismatched murine marrow graft rejection independent of GVHD. Biol Blood Marrow Transplant. 2000;6:182–189. doi: 10.1016/s1083-8791(00)70041-3. [DOI] [PubMed] [Google Scholar]
- 16.Murphy WJ, Kumar V, Bennett M. Acute rejection of murine bone marrow allografts by natural killer cells and T cells. Differences in kinetics and target antigens recognized. J Exp Med. 1987;166:1499–1509. doi: 10.1084/jem.166.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallera DA, Taylor PA, Sprent J, Blazar BR. The role of host T cell subsets in bone marrow rejection directed to isolated major histocompatibility complex class I versus class II differences of bm1 and bm12 mutant mice. Transplantation. 1994;57:249–256. doi: 10.1097/00007890-199401001-00017. [DOI] [PubMed] [Google Scholar]
- 18.Jung U, Foley JE, Erdmann AA, Eckhaus MA, Fowler DH. CD3/CD28-costimulated T1 and T2 subsets: differential in vivo allosensitization generates distinct GVT and GVHD effects. Blood. 2003;102:3439–3446. doi: 10.1182/blood-2002-12-3936. [DOI] [PubMed] [Google Scholar]
- 19.Adjei AA, Dagnino L, Wong MM, Paterson AR. Protection against fludarabine neurotoxicity in leukemic mice by the nucleoside transport inhibitor nitrobenzylthioinosine. Cancer Chemother Pharmacol. 1992;31:71–75. doi: 10.1007/BF00695997. [DOI] [PubMed] [Google Scholar]
- 20.Tedde A, Balis ME, Ikehara S, Pahwa R, Good RA, Trotta PP. Animal model for immune dysfunction associated with adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1980;77:4899–4903. doi: 10.1073/pnas.77.8.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JB, Lee G, Grimm GR, Barrett TA. Therapeutic benefit of pentostatin in severe IL-10−/− colitis. Inflamm Bowel Dis. 2008;14:880–887. doi: 10.1002/ibd.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi T, Nowak BJ, Keating MJ, Plunkett W. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroperoxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin Cancer Res. 2001;7:3580–3589. [PubMed] [Google Scholar]
- 23.Johnston JB, Verburg L, Shore T, Williams M, Israels LG, Begleiter A. Combination therapy with nucleoside analogs and alkylating agents. Leukemia. 1994;8 (Suppl 1):S140–143. [PubMed] [Google Scholar]
- 24.Weiss MA, Maslak PG, Jurcic JG, et al. Pentostatin and cyclophosphamide: an effective new regimen in previously treated patients with chronic lymphocytic leukemia. J Clin Oncol. 2003;21:1278–1284. doi: 10.1200/JCO.2003.08.100. [DOI] [PubMed] [Google Scholar]
- 25.Avramis VI, Plunkett W. Metabolic and Therapeutic Efficacy of 9-β-D-Arabinofuranosyl 1–2-fluoradenine against murine leukemia P388. Cancer Res. 1982;42:2587–2591. [PubMed] [Google Scholar]
- 26.Sirotnak FM, Chello PL, Dorick DM, Montgomery JA. Specificity of systems mediating transport of adenosine, 9-β-D-Arabinofuranosyl 1–2-fluoradenine, and other purine analogues in L1210 cells. Cancer Res. 1983;43:104–109. [PubMed] [Google Scholar]
- 27.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 28.Trotta PP, Tedde A, Ikehara S, Pahwa R, Good RA, Balis ME. Specific immunosuppressive effects of constant infusion of 2′-deoxycoformycin. Cancer Res. 1981;41:2189–2196. [PubMed] [Google Scholar]
- 29.Mariotti J, Foley J, Ryan K, et al. Graft rejection as a Th1-type process amenable to regulation by donor Th2-type cells through an interleukin-4/STAT6 pathway. Blood. 2008;112:4765–4775. doi: 10.1182/blood-2008-05-154278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdmann AA, Gao ZG, Jung U, et al. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]