Abstract
Object
Heme toxicity may contribute to the pathogenesis of intracerebral hemorrhage. The primary defense against extracellular heme is provided by hemopexin, a serum and neuronal glycoprotein that binds it with very high affinity and mitigates its pro-oxidant effect. In the present study, we tested the hypothesis that hemopexin knockout mice would sustain more injury after experimental intracerebral hemorrhage than their wild-type counterparts.
Methods
Striatal intracerebral hemorrhage was induced by stereotactic injection of bacterial collagenase or autologous blood. Three days later, striatal protein oxidation was assessed by carbonyl assay. Cell viability was quantified at 8-9 days using the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay. Behavioral deficits were detected by high resolution digital analysis of six hour home cage video recordings and standard testing.
Results
Perihematomal protein oxidation was increased in wild-type collagenase-injected striata by approximately 2.1-fold compared with contralateral striata vs. 3-fold in knockouts. Striatal cell viability was reduced in wild-type mice to 52.9±6.5% of that in contralateral striata by collagenase injection, and to 31.1%±3.7% in knockouts; similar results were obtained after blood injection. Digital analysis of home cage video recordings demonstrated an activity deficit in both models that was significantly exacerbated at 8 days in knockouts. Striatal heme content 9 days after blood injection was increased approximately 2.7-fold by hemopexin knockout.
Conclusion
These results suggest that hemopexin has a protective effect against hemorrhagic CNS injuries. Hemopexin deficiency, which is often associated with sickle cell disease, may worsen outcome after intracerebral hemorrhage.
Keywords: Free Radical, Hemin, Iron, Oxidative Stress, Stroke
Introduction
A growing body of experimental evidence supports the hypothesis that hemoglobin may contribute to oxidative injury in tissue surrounding an intracerebral hemorrhage (ICH).44 The deleterious effect of extracellular hemoglobin does not appear to be due to the intact protein, but rather to release of its heme moieties, which is facilitated by their oxidation.2,12 Hemin, the oxidized form of heme, accumulates in intracranial hematomas,16 and may be directly cytotoxic by both oxidative and colloid-osmotic mechanisms.5,28,41 In addition, hemin breakdown by the heme oxygenase enzymes releases iron, which in some experimental models appears to be the principal oxidizing species.30,33 The first line of defense against extracellular hemin is provided by hemopexin, a ~60 kDa serum glycoprotein that binds heme or hemin with very high affinity (Kd < 1 pM), thereby mitigating its toxicity.12,37 The hemopexin-heme complex binds to a specific receptor, LDL receptor-related protein (LRP)-1, which is expressed primarily by macrophages and hepatocytes, and to a much lesser extent by a variety of cell types including neurons.13 Endocytosis of the receptor-ligand complex results in release and degradation of hemopexin-heme, following which the receptor is recycled to the cell membrane. In addition to heme scavenging, the heme-hemopexin complex activates signaling pathways and increases the expression of several proteins that may promote cell survival in an oxidative environment.39
Although it is a relatively abundant serum protein (reference range 0.4-1.5 g/l in humans7), hemopexin has not been intensively investigated in heme-mediated or other acute injury models, due at least in part to the high cost and limited availability of purified apo-hemopexin. Specific assessment of its effect has been greatly facilitated by the development of hemopexin knockout mice, which are fertile, have normal serum iron and blood hemoglobin levels, and are otherwise similar to wild-type littermates under physiologic conditions. Tolosano et al. reported that hemopexin knockouts sustained more renal injury after intravascular hemolysis than their wild-type counterparts, despite compensatory upregulation of the hemoglobin-binding protein haptoglobin.40 In the CNS, knockouts sustain larger infarct volumes and more pronounced neurological deficits after transient middle cerebral artery occlusion, suggesting that release of heme from cell hemoproteins may contribute to the pathogenesis of ischemic injury.17 However, despite the 10 mM heme content in blood, and the tendency of extracellular hemoglobin to oxidize and release its heme moieties,1 the effect of hemopexin gene knockout in models directly relevant to intracranial hemorrhage has not yet been reported. In the present study, we tested the hypothesis that perihematomal tissue injury would be increased in knockouts after striatal hemorrhage, increasing behavioral deficits.
Materials and Methods
Experimental Animals
Breeding and housing of mice were conducted exclusively at our animal care facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal care and treatments were in compliance with the standards described in Guide for Care and Use of Laboratory Animals, 1996, National Research Council, and were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee. Animals were provided with food and water ad libitum and a 12-hour light/dark cycle. Founding pairs of hemopexin knockout mice (Hex −/−) were provided by Dr. Frank Berger, University of South Carolina, USA, and were originally produced by Dr. Emanuela Tolosano and colleagues at the University of Turin, Italy.40 Mice were bred with wild-type B6;129 mice that are maintained in our colony. In order to minimize genetic differences between wild-type and knockout groups, heterozygotes were used for breeding. Genotype was determined by PCR of genomic DNA extracted from proteinase K-treated tail clippings, using the following three primers:
Hex wild-type and knockout forward: 5-TCC TGT GTG GCC TTT GCA GC-3
Hex wild-type reverse: 5-CAA CTT CGG CAA CTC TCC CG-3.
Hex knockout reverse: 5-GAT GCG GTG GGC TCT ATG GC-3
Knockout and wild-type reactions were run concomitantly in the same tube. Absence of hemopexin expression in mice designated knockouts by this procedure was confirmed by immunoblotting as previously described4, using a 1:2000 dilution of rabbit anti-hemopexin (Life Diagnostics, West Chester, PA, USA).
Striatal Blood or Collagenase Injections
Mice were used for experiments when 4-6 months old. Homozygous knockout and wild-type mice were anesthetized with 2% isoflurane in oxygen, and were secured into a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). Type VIIS collagenase (Sigma-Aldrich, St. Louis MO, USA) 0.028 U was then injected 3 mm below the skull surface through a burr hole drilled 2.5 mm lateral to bregma, using a glass syringe with 30-33 gauge needle (Hamilton, Reno, NV, USA) and a minipump (Harvard Apparatus, Holliston, MA). Alternatively, mice were injected with 20 μl autologous blood (1 μl /minute) which had been collected from a tail vein. In some experiments, mice were injected at the same coordinates with 6 μl of a 12 mg/ml hemin solution. Ten minutes later, the needle was gently removed and the wound was sutured. Surgical control mice were treated in the same manner, but were injected with an equal volume of artificial CSF (NaCl 148 mmol/L, KCl 3 mmol/L, CaCL2 1 mmol/L, MgCL2 0.8 mmol/L, Na2HPO4 0.8 mmol/L, NaH2PO4 0.2 mmol/L).
Protein Oxidation Assay
At 72 h after collagenase injection, injected and contralateral striata were rapidly removed and were homogenized in ice-cold cell lysis buffer (210 mmol/L mannitol, 70 mmol/L sucrose, 5 mmol/L HEPES, 1 mmol/L ethylenediaminetetraacetic acid, and 0.1% sodium dodecyl sulfate). After sonication and centrifugation, the lysate protein concentration was determined (Pierce/ Thermo-Fisher BCA assay, Rockford, IL). Proteins were denatured in 12 % sodium dodecyl sulfate (SDS), and carbonyl groups were derivatized by reaction with 2,4-dinitrophenylhydrazine, using the Oxyblot ™ Kit (Chemicon, Inc., Temecula, CA, USA) and following the manufacturer’s instructions. Proteins were then separated on a 12% polyacrylamide gel and were transferred to a polyvinylidene difluoride Imobilon-P transfer membrane filter (Millipore, Billerica, MA, USA) using a semidry transfer apparatus (Bio-Rad, Hercules, CA, USA). Carbonylated proteins were detected with rabbit anti-DNP (1:150, Chemicon) followed by goat anti-rabbit IgG (1:300). Immunoreactive proteins were visualized using Super Signal West Femto Reagent (Pierce, Rockford, IL, USA) and Kodak Gel Logic 2200.
Cell Viability Assay
Striatal cell viability was assessed two days after hemin injection, eight days after collagenase injection, and nine days after striatal blood injection, using the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay, modified for in vivo use. Prior studies have demonstrated that this method correlates well with quantification of injury via cell counts of histologic sections.25,26 Tetrazolium salts such as MTT and 2,3,5-triphenyl-tetrazolium chloride (TTC) are reduced by viable cells to formazan products that are easily extracted and quantified by their absorbance. In order to ensure that a uniform concentration of MTT was delivered to all cells, striatal tissue was first dissociated by gentle trituration, a method used extensively in this laboratory to prepare primary cell cultures.
Mice were anesthetized with isoflurane and were then euthanized by cervical dislocation. Brains were rapidly removed and placed into a 60 mm culture dish containing 3 ml dissecting medium (Hanks Balanced Salt Solution supplemented with 27.8 mM glucose, 20.5 mM sucrose, and 4.2 mM sodium bicarbonate31). Injected and contralateral striata were excised under a dissecting microscope, minced with forceps, and placed into separate centrifuge tubes in 1 ml dissecting medium. Tissue was then dissociated by trituration through a Pasteur pipette, followed by passage through another pipette with narrowed (flame-polished) tip. After addition of 1 ml of 0.25 mg/mL MTT in Dulbecco’s mimimal essential medium (DMEM), tubes were incubated in a water bath at 37°C for four minutes. The cell suspension was then collected (1380 x g, 1 minute), the supernatant was discarded, and the purple formazan was extracted in 2 ml isopropanol. After centrifugation to remove debris (1380 x g, 5 minutes), the absorbance of the supernatant was determined at 562 nm with a reference wavelength of 650 nm. The formazan signal in the injected striatum was expressed as a percentage of that in the contralateral striatum.
Haptoglobin Assay
Serum haptoglobin concentrations were quantified before collagenase injection and 24h and 72h later using an ELISA kit (Life Diagnostics, West Chester, PA, Cat. #2410-1) according to the manufacturer’s instructions. Serum samples were diluted 25,000-fold with diluent provided with the kit in order to be consistently within the range of the standard curve.
Tissue Heme Assay
Tissue heme was assayed following the method of Kuross et al.15 Injected and contralateral striata were removed and placed into separate vials containing 500 μl concentrated formic acid. After trituration and centrifugation, (18,188 x g, 10 min), the absorbance of the supernatant at 398 nm was then determined and was compared with that of a hemin in formic acid standard curve, using a microtiter plate reader (Molecular Devices, Sunnyvale, CA). The signal in the contralateral striatum was subtracted from that in the ipsilateral striatum to calculate the heme content specific for blood injection.
Behavioral Testing
Quantification of Locomotor Activity
At three and eight days after injection, each mouse was placed into a separate cage that was identical to its home cage, with fresh bedding, food and water. A video camera (Sony DCR-HC62) was positioned perpendicular to the long axis of the cage so that the field of view included the entire length of the cage. Mice were acclimated to their new environment for one hour, and then activity was recorded for six hours (4PM-10PM) under standard fluorescent room lighting. Mice were then returned to their home cages. The full length of each video was analyzed using HomeCageScan (Version 3.0, Clever Systems Inc., Reston, VA USA), specifically quantifying the following behaviors: walking, feeding, vertical hanging, rearing, and jumping, as previously described for quantification of mouse motor deficits.35 The time spent in each behavior was summed to yield an index of total activity.
Corner test
Two Styrofoam boards (30×20×1cm) were placed and attached at one end at a 30 degree angle, with a small opening between the boards to encourage entry. As the mouse entered the corner to explore, both sides of the vibrissae were stimulated, resulting in rearing upward and turning towards the open end. The percentage of right turns was recorded for six trials. Turning that was not associated with rearing was not scored. A mouse with a right striatal lesion will preferentially turn to the right.45
Elevated body swing test
Each mouse was habituated in an empty cage for 10 minutes. It was then held by the tester approximately 3 cm from the base of its tail, and lifted so that its head was 3 cm above the base of the cage. Left and right swings were recorded over 45 seconds, and the percentage left swings was calculated. A right striatal lesion produces a leftward swinging bias.19
Adhesive removal test
Adhesive dots (6mm diameter) were attached to the medial aspect of the left or right forepaw, with randomization of the placement order. The mouse was then returned to its home cage, and the interval until dot removal was recorded in two trials. Mean removal time from the right (ipsilateral to hemorrhage) forepaw was subtracted from that of the left to calculate an asymmetry score.20 All mice received four training sessions prior to formal testing.
Statistical analysis
Data were analyzed with one-way ANOVA in experiments containing three or more groups, followed by the Bonferroni multiple comparisons test; differences between two groups were assessed with Student’s t-test. Significance was assigned to a p-value < 0.05.
Results
Genotyping
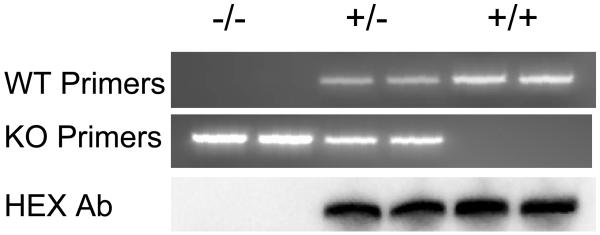
PCR genotyping demonstrated an approximately 220 bp product for the hemopexin knockout gene and a 190 bp product for the wild-type (Fig. 1). Immunoblotting of serum proteins confirmed the presence of hemopexin in mice identified as wild-type or heterozygotes by this method, and its absence in homozygous knockouts.
Fig. 1.
Top and middle: Representative 2% agarose gels showing PCR products from hemopexin knockout (KO, −/−), heterozygous(+/−), and wild-type (WT, +/+) mice. Lower: Immunoblot of cortical lysates, stained with anti- hemopexin (HPX).
Mortality
A total of 178 mice (87 wild-type and 91 homozygous hemopexin knockout) received collagenase injections in this study. 12 wild-type (13.8%) and 23 knockout mice (25.3%) died within 48 hours of injection (relative risk for death in knockouts = 1.83, two sided P value = 0.061). No animals died at later time points. One of 27 knockout mice (3.7%) receiving striatal injection of autologous blood died within 48 hours; all 26 wild-type mice survived.
Hemopexin knockout increases protein oxidation after experimental ICH
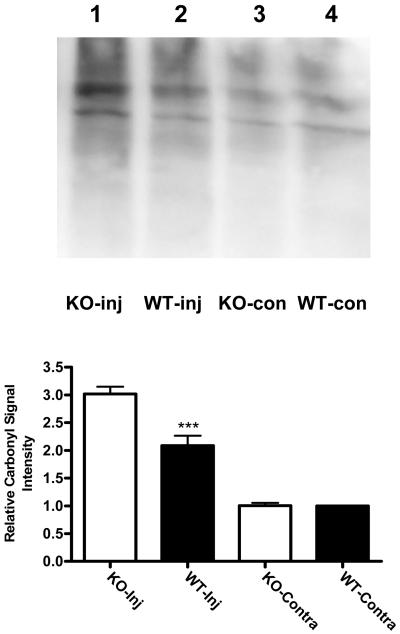
Protein carbonylation is a sensitive marker iron-mediated oxidative injury,34 and is increased in the mouse striatum 3-5 days after injection of autologous blood or hemoglobin.4,26 Consistent with these observations, the mean carbonyl signal intensity in the striata of wild-type mice three days after collagenase injection was 2.1-fold higher than that in contralateral striata (Fig. 2). In hemopexin knockout mice, protein carbonyls were increased threefold compared with contralateral values.
Fig. 2.
Increased protein oxidation after experimental ICH in hemopexin knockout mice. Immunoblot of lysates from collagenase-injected (Inj) and contralateral striata of hemopexin KO and WT mice, collected 72 hours after injection and stained with antibody to derivitized protein carbonyls. Bars represent mean lane densities (±S.E.M., 6/condition), normalized to the mean value in WT striata contralateral to collagenase injection (= 1.0). ***P< 0.001 compared with the mean signal in KO striata injected with collagenase, Bonferroni multiple comparisons test.
Hemopexin knockout reduces striatal cell viability after hemorrhage
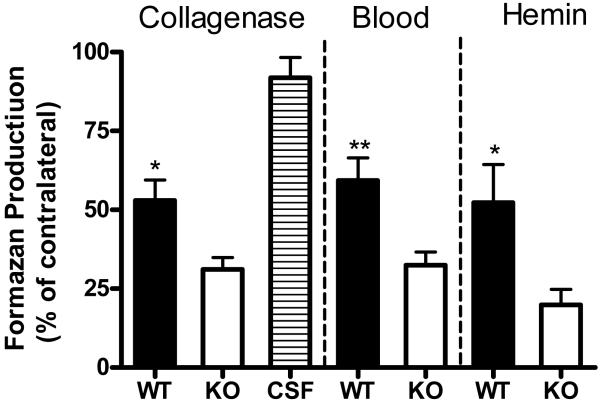
Striatal cell viability was assessed at eight days after collagenase injection or 9 days after blood injection; both time points are after hematoma resolution in mice.4 In wild-type mice, mean MTT reduction to formazan in the collagenase model was 52.9 ± 6.5% of that in contralateral striata (Fig. 3). In hemopexin knockouts, it was reduced to 31.2 ± 3.7% of contralateral (P < 0.05). Similar results were obtained in mice receiving striatal injections of autologous blood instead of collagenase. In order to more directly assess if the deleterious effect of hemopexin knockout was due to exacerbation of heme toxicity, additional wild-type and knockout mice were injected with freshly-prepared hemin (6 μl of a 12 mg/ml solution) rather than collagenase. Striatal cell viability three days later was significantly less in knockouts than in wild-type mice.
Fig. 3.
Bars represent mean striatal cell viability in hemopexin WT and KO mice, 8 days after striatal collagenase injection (mean ± S.E.M., n= 8/condition), 9 days after autologous blood injection (14/condition), or 2 days after striatal hemin injection (5/condition), as assessed by MTT reduction to formazan. Control mice were injected with artificial CSF solution only. MTT reduction is expressed as a percentage of that in the hemisphere contralateral to the injection site. The CSF group consisted of four KO and four WT mice, which had similar values. *P<0.05, **P < 0.01 versus ratio in KO mice, Bonferroni multiple comparisons test.
Effect of hemopexin knockout on serum haptoglobin levels
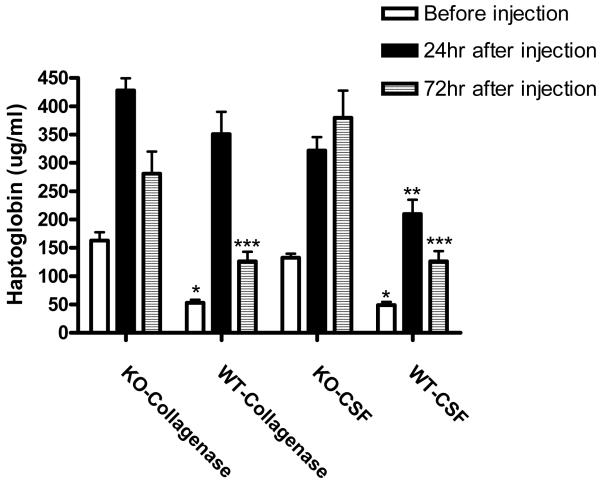
Hemopexin knockout mice upregulate expression of the hemoglobin-binding protein haptoglobin.38 Since the latter may protect against hemorrhagic CNS injuries,46 serum haptoglobin levels were quantified prior to induction of ICH, and 24 and 72 hours later. Hemopexin knockout increased pre-injection haptoglobin by approximately 2.9-fold compared with wild-type controls (Fig. 4). However, at 24 hours after collagenase injection serum levels were similar due to a 6.6-fold increase in wild-type mice but only a 2.6-fold increase in knockouts. Surprisingly, levels were also significantly increased at this time point in mice undergoing anesthesia and then striatal injection of artificial CSF rather than collagenase. By 72 hours, haptoglobin had declined in both collagenase-injected groups, but remained elevated in CSF-injected knockouts.
Fig. 4.
Serum haptoglobin is increased in hemopexin knockout mice. Haptoglobin levels were assayed before and 24 or 72 hr after striatal injection of collagenase or artificial CSF. N = 6/condition, *P<0.05, **P<0.01, ***P<0.001 compared with corresponding KO condition, Bonferroni multiple comparisons test.
Reduced locomotor activity in hemopexin knockouts after ICH
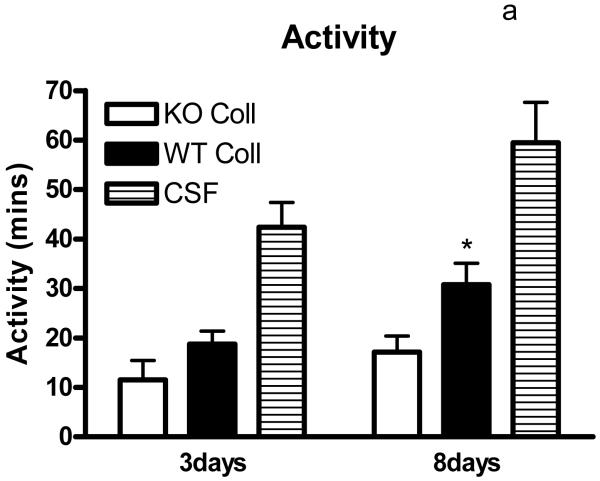
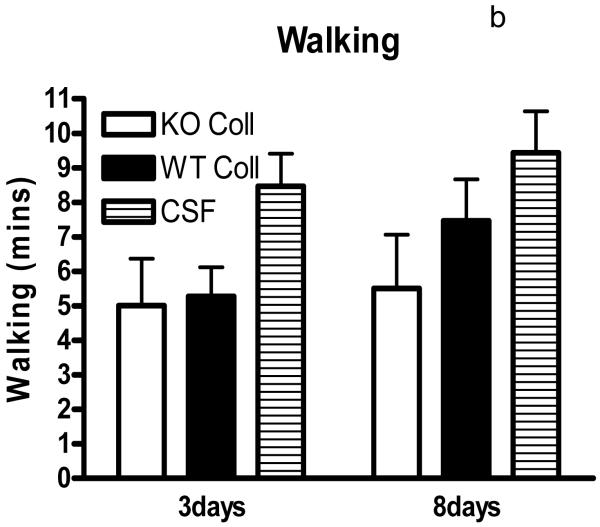
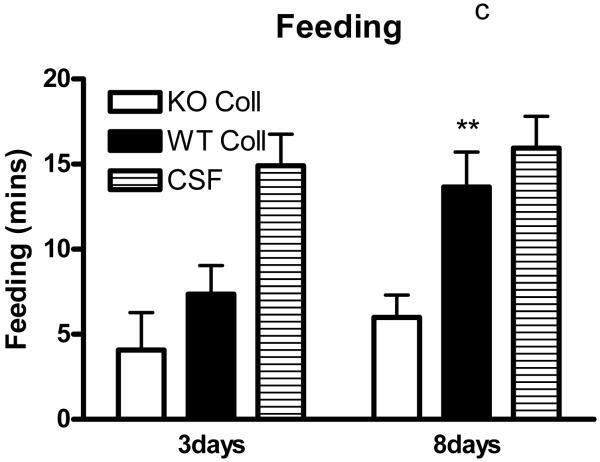
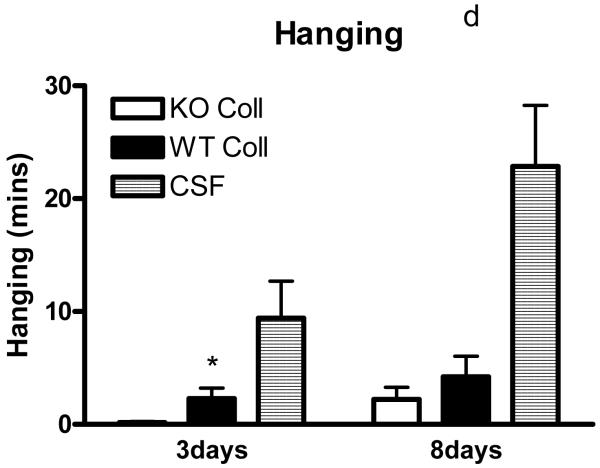
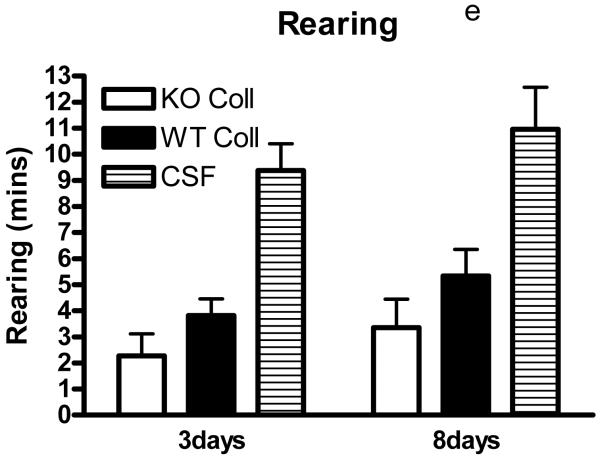
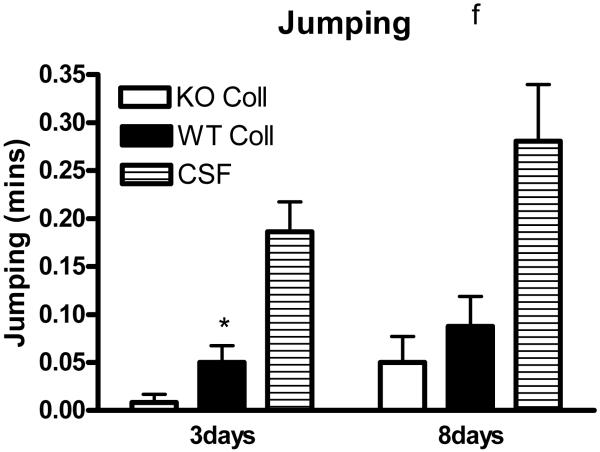
Preliminary observations using these ICH models suggested markedly reduced postoperative activity that persisted for several days after hemorrhage. In order to quantify this deficit, six hour video recordings were analyzed using a software program designed for mouse home cage behavioral assessment. Compared with surgical control mice injected with artificial CSF only, time spent walking, rearing, feeding, vertical hanging from cage top bars, and jumping were decreased in wild-type mice after collagenase injection (Fig. 5). The frequency of these behaviors was further reduced in hemopexin knockouts, with statistically significant differences compared with wild-type mice observed for the latter three. Total activity time 8 days after ICH was 30.8±4.3 minutes for wild-type mice, compared with 17.1±3.3 minutes for knockouts (P < 0.05).
Fig. 5.
Hemopexin knockout reduces home cage activity after collagenase-induced ICH. (A) Bar graph represents mean minutes of activity (sum of minutes spent walking, feeding, vertical hanging, rearing and jumping, ±S.E.M., 12–16/condition) during 6 hour evening video recordings conducted 3 days and 8 days after striatal injection of collagenase (WT Coll or KO Coll, n = 12–16) or artificial CSF (n = 31). WT and KO mice injected with artificial CSF had similar values, so results are combined for ease of display. (B-F) Bars represent minutes spent in each activity. *P< 0.05 compared corresponding KO condition, Bonferroni multiple comparisons test.
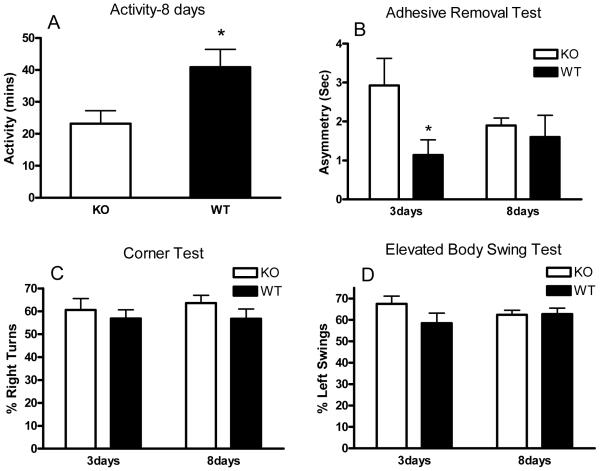
Wild-type mice injected with autologous blood were active for a total of 40.9±5.5 minutes of the 6-hour recording interval on day 8 (Fig. 6A). Consistent with the collagenase results, activity time was reduced to 23.2±4.1 minutes in knockouts (P < 0.05). Time spent in all individual behaviors as described above was reduced in knockouts, but differences reached statistical significance only for vertical hanging (4.7±1.6 minutes for wild-type vs. 0.9±0.2 minutes for knockouts). Neurologic deficits were also assessed with corner, adhesive removal, and elevated body swing tests (Fig. 6 B-D). A significant difference between knockouts and wild-type mice was observed only for the adhesive removal test at 3 days, which had resolved by 8 days.
Fig. 6.
Hemopexin knockout reduces home cage activity after striatal injection of autologous blood. Bars graphs represent mean minutes of activity (A, sum of minutes spent walking, feeding, vertical hanging, rearing and jumping, ±S.E.M., 18/condition) during 6 hour recordings conducted 8 days after striatal injection of 20 μl autologous blood, or indicated endpoints of adhesive removal, corner, or elevated body swing tests (B-D). *P < 0.05 compared with corresponding knockout condition.
Increased tissue heme in hemopexin knockouts
Striatal heme was quantified 3 and 9 days after injection of 20 μl autologous blood. At the former time point, the striatal hematoma is still present in this model; by 9 days, it has been resorbed, but staining by heme and its degradation products is still visible on fresh tissue preparations.4 Striatal heme associated with blood injection tended to be higher in hemopexin knockout mice at 3 days, but the difference did not reach statistical significance (Table 1). At 9 days, heme content was 2.7-fold higher in knockouts (P < 0.05).
Table 1.
Heme content (nanomoles/striatum)
Hemopexin knockout increases tissue heme after striatal blood injection. Bars represent mean tissue heme (±S.E.M.) 4 and 9 days after striatal injection of 20 μl autologous blood.
| KO | WT | |||
|---|---|---|---|---|
| 3 days | 35.05 ± 8.05 | n=5 | 24.73 ± 7.42 | n=5 |
| 9 days | 1.53 ± 0.39* | n=7 | 0.56 ± 0.15 | n=7 |
P< 0.05 compared with corresponding knockout condition.
Discussion
The results of these experiments indicate that physiologic concentrations of hemopexin attenuate tissue oxidation, cell death, and behavioral deficits after striatal ICH in mice. These observations are consistent with the effect of hemopexin against ischemic stroke,17 and suggest that binding of hemin to hemopexin may be protective in both disease processes.
Hemin in present in intracranial hematomas in high micromolar concentrations, exceeding the neurotoxic threshold in cultured cells by over 100-fold.16,27 In the absence of hemopexin, free hemin rapidly associates with albumin, the most abundant serum/CSF protein. However, this complex can participate in free radical reactions, and may actually enhance membrane oxidative damage by reducing cellular uptake and detoxification.28 In contrast, hemopexin has a much higher affinity for hemin, preventing its transfer to sites vulnerable to free radical attack, such as membrane lipids,12,42 and thereby preventing its neurotoxicity in vitro.17 In vivo, renal and endothelial injury produced by intravascular hemolysis or hemin injection is markedly exacerbated in hemopexin knockout mice,40,43 indicating that hemopexin has a primary role in intravascular heme scavenging and detoxification. The present results suggest a similar effect after experimental intracerebral hemorrhage. At least three mechanisms may account for the cytotoxicity of free heme. First, it directly and efficiently oxidizes membrane lipids by decomposing preformed lipid peroxides, thereby initiating free radical chain reactions.12,42 Second, it destabilizes membranes via an incompletely defined colloid osmotic mechanism that is not prevented by antioxidants.5 Third, in intact cells, heme breakdown may produce an iron-dependent oxidative injury to cell populations with limited iron-sequestering capacity, such as neurons.11 The latter mechanism may be protective if iron is not in excess, due to the antioxidant and anti-inflammatory effects of the other heme breakdown products, carbon monoxide and biliverdin. Although binding of heme to hemopexin inhibits lipid oxidation, its effects on colloid-osmotic cell lysis and heme breakdown by the heme oxygenases remain undefined.
Although the protective effects of hemopexin in acute injury models have been primarily attributed to heme scavenging and detoxification, the heme-hemopexin complex has pleiotropic effects on gene expression and cell signaling that may also promote cell survival in an oxidative environment (see Tolosano39 et al. for review). Perhaps most relevant to ICH, both heme oxygenase-1 and ferritin are potently induced by either heme-hemopexin or free hemin. The complex, however, has the advantage of upregulating these protective proteins without the collateral damage produced by heme-mediated oxidative reactions.9,17 Heme-hemopexin, but not free hemin, has been reported to activate c-Jun N-terminal kinase (JNK) in cultured hepatoma cells, leading to translocation of nuclear factor kappa-B (NFκB) to the nucleus, increased expression of the cell cycle regulatory proteins p21 and p53, and cell survival.9 However, this pathway is unlikely to account for the protective effect of hemopexin in the present study, since JNK activation after ICH appears to be deleterious.23 A recent study demonstrated that hemopexin decreased cytokine production in macrophages treated with lipopolysaccharide in vitro.18 While this observation suggests that hemopexin may downregulate innate immune system responses, its relevance to inflammation after ICH or other acute CNS injuries remains to be determined.
Striatal cell viability, assessed by conversion of the tetrazolium salt MTT to formazan, was reduced by approximately half in striata injected with collagenase, autologous blood, or hemin, and was further decreased in hemopexin knockouts. The MTT and related TTC assay methods for cell viability assessment are frequently used in cell culture and in vivo CNS injury models. In the present and prior studies, MTT reduction was assessed after tissue dissociation, which ensured that all striatal cells were exposed to the same concentration of MTT. This method provides information about cell viability that correlates well with that obtained by more labor-intensive cell counts of histologic sections,25,26 and is particularly suitable for use in mouse striatal injury models for three reasons. First, in fresh tissue preparations, the striatum is easily identified and removed by microdissection. Second, since it is a small structure (mean volume 23.6 ± 0.6 cubic mm32), the reducing capacity of the entire striatum can be quantified, eliminating any possibility of sampling bias. Third, the mouse striatum has a relatively uniform cellular composition, containing predominantly medium spiny neurons and few astrocytes.36 The significant reduction in striatal formazan production in hemopexin knockouts compared with wild-type mice after collagenase or autologous blood injection provides direct evidence that hemopexin is cytoprotective in these ICH models.
The CNS toxicity of whole blood lysate has recently been reported to be reduced in transgenic mice over-expressing haptoglobin.46 Consistent with prior observations,40 the mean serum haptoglobin level was significantly higher in hemopexin knockouts than in wild-type mice at baseline, and may have mitigated injury in knockouts. However, haptoglobin is an acute phase protein that is rapidly induced by surgical procedures.6 The increased levels in both wild-type and hemopexin knockout mice after striatal injection of either collagenase or artificial CSF demonstrates that this procedure per se was sufficient to induce an acute phase response, which obscured differences in wild-type and knockout mice 24 hours after collagenase injection. An acute phase response has also been observed immediately after clinical intracerebral hemorrhage,8 so its induction by striatal injections in the present study is unlikely to be a significant confounding factor.
Mice were noticeably less active after collagenase or blood injection. In order to determine if their inactivity was magnified by hemopexin knockout, six-hour video recordings were conducted in their home cages and were digitally analyzed. Assessment of locomotor deficits using this method is established in models of Huntington’s Disease and prion disease;35 a similar approach has recently been reported in a rat ICH model.24 All recordings were conducted from 4-10 PM, since mice are nocturnal and tend to sleep through morning and early afternoon hours when undisturbed. The significant reduction in a composite injury index consisting of minutes spent walking, rearing, eating, vertical hanging, and jumping at 8 days after collagenase or blood injection is consistent with cell viability and protein oxidation data, and suggests that physiologic levels of hemopexin improve behavioral outcome in this ICH model. Furthermore, the failure of corner, adhesive removal, and elevated body swing tests to detect any significant differences between knockout and wild-type mice at 8 days suggests that digital analysis of spontaneous motor activity may be a more sensitive behavioral outcome measure at this time point.
Compared with traditional methods of behavioral assessment in stroke models, digital analysis of home cage video recordings offers several advantages. First, it requires minimal investigator participation, reducing labor costs and the potential for bias. Second, it facilitates analysis of nighttime behaviors. The validity of daytime testing of nocturnal animals in stroke models has never been established, and is questionable given the behavioral inhibition and cognitive disturbances observed in mice during light phase testing.29 Third, it measures behaviors that are directly relevant to the animal’s functional status. Finally and perhaps most importantly, it eliminates the variability inherent in measuring brief episodes of behavior in animals stressed by handling and removal from their home cage. Although the replicability of behavioral test results in ICH models has not been intensively investigated, behavioral phenotyping studies suggest that laboratory-specific or investigator-specific factors often introduce significant variability that may obscure treatment effects.14
The mortality rate of 13.8% in wild-type mice was somewhat lower than previously reported using this model,3 which may reflect variability of activity in different lots of collagenase. Hemopexin knockouts tended to have a higher mortality rate (25.3%) but this difference did not quite reach statistical significance using an alpha level that was set a priori at 0.05 (P = 0.061). Although the data are suggestive of an increase in knockouts, an additional study with larger sample sizes is required to establish if physiologic concentrations of hemopexin attenuate mortality after experimental ICH.
The results of this study may be particularly relevant to ICH occurring in the setting of hemopexin deficiency. Uptake of the heme-hemopexin complex results in degradation of most of the hemopexin,13 resulting in very low serum levels in conditions producing chronic intravascular hemolysis. Muller-Eberhard et al.21 reported that three-quarters of sickle cell disease patients had serum hemopexin concentrations below 100 μg/ml (reference range 400-1500 μg/ml in humans7), due to increased catabolism without a compensatory increase in synthesis.10 Sickle cell disease is unfortunately associated with a markedly increased risk for stroke (prevalence 11% by age 20 and 24% by age 45); approximately one-third of strokes are primarily hemorrhagic, with the highest incidence in the third decade of life.22 The relationship between hemopexin levels and stroke outcome in sickle cell disease has not yet been the subject of a published study. The observations that hemopexin knockout mice sustained increased injury after both hemorrhagic and ischemic stroke17 suggest that it may be a worthy topic for future investigation.
Conclusion
Physiologic concentrations of hemopexin reduce peri-hematomal injury to an extent that is sufficient to ameliorate behavioral deficits in mice. These results suggest that hemopexin supplementation may be beneficial after hemorrhagic stroke when associated with hemopexin deficiency, which frequently accompanies sickle cell disease and other hemolytic disorders. It remains to be determined if increasing hemopexin above physiologic levels would provide any additional benefit. Given the limited availability and high cost of purified hemopexin, this question may be most efficiently answered by generating and testing transgenic mice that overexpress hemopexin, an approach recently used to demonstrate the efficacy of haptoglobin after ICH.46 The present data suggest that further investigation of the therapeutic potential of hemopexin is warranted.
Acknowledgements
This study was supported by a grant from the National Institutes of Health (NS42273), and by a Grant-in-Aid from the Great Rivers Affiliate of the American Heart Association to RFR.
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Alyash A. Redox and radical reactions of hemoglobin solutions: toxicities and protective strategies. In: Winslow R, editor. Blood Substitutes. Academic Press; London: 2006. pp. 197–205. [Google Scholar]
- 2.Bunn HF, Jandl JH. Exchange of heme along hemoglobins and between hemoglobin and albumin. J. Biol. Chem. 1968;243:465–475. [PubMed] [Google Scholar]
- 3.Chen M, Awe OO, Chen-Roetling J, Regan RF. Iron regulatory protein-2 knockout increases perihematomal ferritin expression and cell viability after intracerebral hemorrhage. Brain Res. 2010;1337:95–103. doi: 10.1016/j.brainres.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M, Regan RF. Time course of increased heme oxygenase activity and expression after experimental intracerebral hemorrhage: correlation with oxidative injury. J Neurochem. 2007;103:2015–2021. doi: 10.1111/j.1471-4159.2007.04885.x. [DOI] [PubMed] [Google Scholar]
- 5.Chou AC, Fitch CD. Mechanism of hemolysis induced by ferriprotoporphyrin IX. J. Clin. Invest. 1981;68:672–677. doi: 10.1172/JCI110302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conner JG, Eckersall PD, Ferguson J, Douglas TA. Acute phase response in the dog following surgical trauma. Res Vet Sci. 1988;45:107–110. [PubMed] [Google Scholar]
- 7.Delanghe JR, Langlois MR. Hemopexin: a review of biological aspects and the role in laboratory medicine. Clin Chim Acta. 2001;312:13–23. doi: 10.1016/s0009-8981(01)00586-1. [DOI] [PubMed] [Google Scholar]
- 8.Dziedzic T. Clinical significance of acute phase reaction in stroke patients. Front Biosci. 2008;13:2922–2927. doi: 10.2741/2897. [DOI] [PubMed] [Google Scholar]
- 9.Eskew JD, Vanacore RM, Sung L, Morales PJ, Smith A. Cellular protection mechanisms against extracellular heme. heme-hemopexin, but not free heme, activates the N-terminal c-jun kinase. J Biol Chem. 1999;274:638–648. doi: 10.1074/jbc.274.2.638. [DOI] [PubMed] [Google Scholar]
- 10.Foidart M, Liem HH, Adornato BT, Engel WK, Muller-Eberhard U. Hemopexin metabolism in patients with altered serum levels. J Lab Clin Med. 1983;102:838–846. [PubMed] [Google Scholar]
- 11.Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury on human neuron-like cells. J. Neurosci. Res. 2003;73:113–121. doi: 10.1002/jnr.10633. [DOI] [PubMed] [Google Scholar]
- 12.Gutteridge JMC, Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem. J. 1988;256:861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 14.Kafkafi N, Benjamini Y, Sakov A, Elmer GI, Golani I. Genotype-environment interactions in mouse behavior: a way out of the problem. Proc Natl Acad Sci U S A. 2005;102:4619–4624. doi: 10.1073/pnas.0409554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuross SA, Rank BH, Hebbel RP. Excess heme in sickle erythrocyte inside-out membranes: possible role in thiol oxidation. Blood. 1988;71:876–882. [PubMed] [Google Scholar]
- 16.Letarte PB, Lieberman K, Nagatani K, Haworth RA, Odell GB, Duff TA. Hemin: levels in experimental subarachnoid hematoma and effects on dissociated vascular smooth muscle cells. J. Neurosurg. 1993;79:252–255. doi: 10.3171/jns.1993.79.2.0252. [DOI] [PubMed] [Google Scholar]
- 17.Li RC, Saleem S, Zhen G, Cao W, Zhuang H, Lee J, et al. Heme-hemopexin complex attenuates neuronal cell death and stroke damage. J Cereb Blood Flow Metab. 2009;29:953–964. doi: 10.1038/jcbfm.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol. 2009;86:229–235. doi: 10.1189/jlb.1208742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu DZ, Cheng XY, Ander BP, Xu H, Davis RR, Gregg JP, et al. Src kinase inhibition decreases thrombin-induced injury and cell cycle re-entry in striatal neurons. Neurobiol Dis. 2008;30:201–211. doi: 10.1016/j.nbd.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLellan CL, Auriat AM, McGie SC, Yan RH, Huynh HD, De Butte MF, et al. Gauging recovery after hemorrhagic stroke in rats: implications for cytoprotection studies. J Cereb Blood Flow Metab. 2006;26:1031–1042. doi: 10.1038/sj.jcbfm.9600255. [DOI] [PubMed] [Google Scholar]
- 21.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32:811–815. [PubMed] [Google Scholar]
- 22.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 23.Ohnishi M, Katsuki H, Fujimoto S, Takagi M, Kume T, Akaike A. Involvement of thrombin and mitogen-activated protein kinase pathways in hemorrhagic brain injury. Exp Neurol. 2007;206:43–52. doi: 10.1016/j.expneurol.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Otero L, Zurita M, Aguayo C, Bonilla C, Rodriguez A, Vaquero J. Video-Tracking-Box linked to Smart software as a tool for evaluation of locomotor activity and orientation in brain-injured rats. J Neurosci Methods. 2010;188:53–57. doi: 10.1016/j.jneumeth.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J. Neurosurg. 2007;106:428–435. doi: 10.3171/jns.2007.106.3.428. [DOI] [PubMed] [Google Scholar]
- 26.Qu Y, Chen J, Benvenisti-Zarom L, Ma X, Regan RF. Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. J. Cereb. Blood Flow Metab. 2005;25:1466–1475. doi: 10.1038/sj.jcbfm.9600143. [DOI] [PubMed] [Google Scholar]
- 27.Regan RF, Chen J, Benvenisti-Zarom L. Heme oxygenase-2 gene deletion attenuates oxidative stress in neurons exposed to extracellular hemin. BMC Neurosci. 2004;5:34. doi: 10.1186/1471-2202-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson SR, Dang TN, Dringen R, Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Rep. 2009;14:228–235. doi: 10.1179/135100009X12525712409931. [DOI] [PubMed] [Google Scholar]
- 29.Roedel A, Storch C, Holsboer F, Ohl F. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Lab Anim. 2006;40:371–381. doi: 10.1258/002367706778476343. [DOI] [PubMed] [Google Scholar]
- 30.Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Rad. Biol. Med. 2003;35:872–881. doi: 10.1016/s0891-5849(03)00431-3. [DOI] [PubMed] [Google Scholar]
- 31.Rose K, Goldberg MP, Choi DW. Cytotoxicity in murine neocortical cell culture. In: Tyson CA, Frazier JM, editors. Methods in Toxicology; Part A: In Vitro Biological Systems. Vol. 1. Academic Press; San Diego: 1993. pp. 46–60. [Google Scholar]
- 32.Rosen GD, Williams RW. Complex trait analysis of the mouse striatum: independent QTLs modulate volume and neuron number. BMC Neurosci. 2001;2:5. doi: 10.1186/1471-2202-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadrzadeh SMH, Anderson DK, Panter SS, Hallaway PE, Eaton JW. Hemoglobin potentiates central nervous system damage. J. Clin. Invest. 1987;79:662–664. doi: 10.1172/JCI112865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 35.Steele AD, Jackson WS, King OD, Lindquist S. The power of automated high-resolution behavior analysis revealed by its application to mouse models of Huntington’s and prion diseases. Proc Natl Acad Sci U S A. 2007;104:1983–1988. doi: 10.1073/pnas.0610779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturrock RR. A comparative quantitative and morphological study of ageing in the mouse neostriatum, indusium griseum and anterior commissure. Neuropathol Appl Neurobiol. 1980;6:51–68. doi: 10.1111/j.1365-2990.1980.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 37.Tolosano E, Altruda F. Hemopexin: structure, function, and regulation. DNA Cell Biol. 2002;21:297–306. doi: 10.1089/104454902753759717. [DOI] [PubMed] [Google Scholar]
- 38.Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, et al. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood. 2002;100:4201–4208. doi: 10.1182/blood-2002-04-1270. [DOI] [PubMed] [Google Scholar]
- 39.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12:305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- 40.Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, et al. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood. 1999;94:3906–3914. [PubMed] [Google Scholar]
- 41.Van den Berg JJ, Kuypers FA, Qju JH, Chiu D, Lubin B, Roelofsen B, et al. The use of cis-parinaric acid to determine lipid peroxidation in human erythrocyte membranes:comparison of normal and sickle erythrocyte membranes. Biochem. Biophys. Acta. 1988;944:29–39. doi: 10.1016/0005-2736(88)90313-6. [DOI] [PubMed] [Google Scholar]
- 42.Vincent SH, Grady RW, Shaklai N, Snider JM, Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Arch Biochem Biophys. 1988;265:539–550. doi: 10.1016/0003-9861(88)90159-2. [DOI] [PubMed] [Google Scholar]
- 43.Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol. 2008;173:289–299. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 46.Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci. 2009;29:15819–15827. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]