Abstract
Bronchiolitis obliterans syndrome is a pulmonary complication of allogeneic hematopoietic cell transplantation. Recent National Institutes of Health consensus diagnostic criteria for bronchiolitis obliterans syndrome have not been assessed in a clinical setting. Modified National Institutes of Health diagnostic consensus criteria for bronchiolitis obliterans syndrome were applied to evaluate its prevalence, risk factors and outcomes in the modern era of allogeneic hematopoietic cell transplantation. Pulmonary function tests from 1145 patients were screened to identify patients with new-onset airflow obstruction. Clinical records were reviewed to exclude pulmonary infection and other causes. The overall prevalence of bronchiolitis obliterans syndrome among all transplanted patients was 5.5%, and 14% among patients with chronic graft-versus-host disease. The median time from transplant to meeting spirometric criteria for bronchiolitis obliterans syndrome was 439 days (range 274–1690). Although many previously identified risk factors were not significantly associated, lower baseline FEV1/SVC ratio (P = 0.006), non-Caucasian race (P = 0.014), and lower circulating IgG level (P = 0.010), and presence of chronic graft versus host disease (P < 0.001) were associated with an increase in risk, with the latter associated with a 10-fold increase in risk. Multivariate analysis indicated that bronchiolitis obliterans syndrome conferred a 1.6 fold increase in risk for mortality after diagnosis. These results suggest that National Institutes of Health diagnostic criteria can reliably identify bronchiolitis obliterans syndrome, and that it is more prevalent than previously suggested. Spirometric monitoring of high-risk patients with chronic graft-versus-host disease may permit earlier detection and intervention for this often-fatal disease.
Keywords: Bronchiolitis Obliterans Syndrome, Chronic Graft-Versus-Host Disease, Allogeneic Hematopoietic Cell Transplantation
INTRODUCTION
Bronchiolitis obliterans syndrome (BOS) is a lung complication of allogeneic hematopoietic cell transplantation (aHCT) recipients that is characterized clinically by the development of fixed new-onset airflow obstruction (AFO) and pathologically by progressive circumferential fibrosis targeting the terminal bronchioles. Because BOS is always observed in the presence of chronic graft-versus-host disease (cGVHD), and is also commonly observed after lung transplantation as host-versus-graft disease, it is likely that BOS is caused by an alloimmune response of donor hematopoietic cells against host lung antigens. Although BOS patients are typically treated with immunosuppressive agents, there is no strong evidence that any specific therapies are effective in improving long-term outcomes. Patients affected by BOS carry a poor prognosis, with an overall 2-year survival rate of 44–45% and a 5-year survival rate of 13% (1–3).
There is much variation in the estimated prevalence of BOS. Most studies estimate the prevalence of BOS to be 2–3% among aHCT recipients, or 6% among patients with cGVHD (2, 4–6). However, some suspect the prevalence of BOS may be as high as 10–20% (3, 7, 8). This variability in prevalence estimates is largely due to a lack of consensus regarding the clinical diagnostic criteria for BOS. Indeed, there are at least 10 distinct clinical definitions for BOS after HCT in the published literature (2, 7, 9–15).
In 2005, the National Institutes of Health (NIH) proposed new consensus diagnostic criteria for BOS, defining this syndrome by the presence of 4 characteristics: 1) forced expiratory volume in 1 second (FEV1) < 75% predicted, 2) FEV1/forced vital capacity (FVC) ratio < 0.7, 3) evidence of air trapping, small airway thickening, or bronchiectasis on high-resolution computed tomography (HRCT) or residual volume (RV) > 120% predicted or pathologic confirmation, and 4) absence of respiratory tract infection (11). Recommendations for modifying the NIH criteria were recently made to improve the diagnostic accuracy of the consensus criteria (16). The purpose of the current study is to use these recommendations to assess the prevalence, risk factors, and outcomes of BOS in a cohort of aHCT recipients.
MATERIALS AND METHODS
This retrospective study was approved by the institutional review board at the FHCRC. All patients who received their first aHCT at the Fred Hutchinson Cancer Research Center (FHCRC)/Seattle Cancer Care Alliance (SCCA) between January 1, 2002 and June 30, 2006 were eligible for this study. The medical records of all patients who met spirometric criteria for BOS were reviewed for additional clinical, radiologic, microbiologic and treatment data. All patients were evaluated for respiratory infection according to standard clinical protocol. When indicated, additional investigations for infection such as nasal wash, sputum culture, and bronchoscopies were performed. Assays for bacterial, viral, and fungal pathogens were routinely performed on all bronchoalveolar lavages. Details regarding the clinical data and infectious evaluation are available in the online supplement.
BOS patients were classified according to recognition status as concurrently recognized, late recognized, or never recognized. Concurrent clinical recognition was defined as clinical documentation of BOS in the medical records within one month of meeting NIH spirometric criteria. Late recognized was defined as documentation in the medical records of BOS greater than one month of meeting NIH spirometric criteria. Never recognized was defined as the absence of documentation of BOS in the available FHCRC and non-FHCRC medical records despite meeting NIH spirometric criteria.
Pulmonary Function Testing
In order to avoid misclassification due to reversible changes in lung function during the first year after transplant, only PFTs obtained at or after 1 year post-transplant (365 ± 100 days) were evaluated for BOS. As part of clinical protocol, all patients who return for a 1 year evaluation receive a PFT. However, after the first year, PFTs are obtained at the discretion of their primary physician. For all PFTs, predicted values were calculated using published equations for children and adults (17, 18). All pulmonary function values, except for the FEV1/SVC ratio, were expressed as a percentage of predicted values.
One year post-transplant PFTs were defined at 365 ± 100 days. Patients surviving to at least 1 year (± 100 days) were screened for BOS using a modified NIH spirometry criteria: 1) FEV1 < 75% predicted, 2) FEV1/SVC ratio < 0.7 and 3) decrease of the FEV1 by ≥ 10% in 3comparison to the pre-transplant value. Patients were required to meet all three criteria to pass this first screen. Post-bronchodilator values were used whenever available to minimize misclassification of reversible AFO. The date of the initial PFT fulfilling the above modified NIH spirometry criteria was used as the date of BOS diagnosis, with the exception of those that were reclassified as non-cases following chart review.
Pulmonary function at the time of meeting NIH spirometry criteria and after was quantified using the NIH recommended lung function score (LFS). The LFS was calculated using the FEV1 and carbon monoxide diffusion capacity (DLCO) (≥80% = 1, 70–79% = 2, 60–69% = 3, 50–59% = 4, 40–49% = 5, and <40% = 6) (11). Scores for FEV1 and DLCO were then summed, categorized from 0 to 3, and defined according to NIH recommendations [LFS score 2 = category 0 (normal); LFS score 3–5 = category 1 (mildly abnormal); LFS score 6–9 = category 2 (moderately abnormal); or LFS score 10–12 = category 3 (severely abnormal)].
Statistical Methods
All statistical analyses were performed using STATA 10.0 (StataCorp, College Station, TX) and R 2.6.2. Two-sided p-values < 0.05 were considered statistically significant. T-tests were performed to compare baseline PFT measurements in cases and non-cases. Univariate analyses were performed using Pearson’s chi-squared test. Covariates with p < 0.1 in univariate analyses were considered in multivariate Cox regression using backward stepwise regression to assess their impact on risk for BOS. Chronic and acute GVHD were treated as time-dependent covariates. Both unadjusted and adjusted (for age, disease risk, and time-dependent cGVHD and aGVHD) models were fitted to evaluate the effect of BOS on risk of non-relapse mortality. Cumulative incidence of BOS was calculated considering death, relapse, and second transplant as competing risks. The Kaplan-Meier method was used to estimate overall non-relapse survival in BOS cases. Stratified Kaplan-Meier curves were also calculated to investigate group survival trends in variables of interest. The log-rank test was used to evaluate survival differences across groups. Treatment for BOS was assessed using a Cox model among all BOS cases. Mean changes in LFS were compared among recognition and treatment groups using ANOVA.
RESULTS
Cohort Characteristics
Between January 1, 2002 and June 30, 2006, 1145 patients received a first-time aHCT. Thirty nine patients were excluded from the final analysis due to missing pre-transplant PFTs and 160 were excluded due to missing 1 year post-transplant PFTs. The remaining 946 subjects are described in Table 1. A total of 2999 PFTs (946 pre-transplant, 2053 post-transplant) were evaluated; 2350 local and 649 non-local PFTs were screened using the NIH spirometry criteria for BOS. Pre-transplant PFTs were obtained at a mean of 30 days prior to transplant.
Table 1.
Clinical Characteristics of the BOS and Non-BOS Cases from the Study Cohort
| Clinical Variables | BOS (%)N = 63 | Non-BOS (%)N = 883 | P value |
|---|---|---|---|
| Age, median: 48.25, range: 13.0 – 74.5 | 0.474 | ||
| Male: Female Ratio | 1 : 0.6 | 1 : 0.7 | 0.484 |
| Sex Match, Donor—Recipient | |||
| Female—Male | 22 (35) | 230 (26) | 0.395 |
| Female—Female | 13 (21) | 172 (20) | |
| Male—Male | 18 (29) | 291 (33) | |
| Male—Female | 10 (16) | 189 (21) | |
| Race | 71% White | 84% White | 0.014 |
| Disease risk at transplant | |||
| Low | 12 (19) | 130 (15) | 0.623 |
| Intermediate | 26 (41) | 400 (45) | |
| High | 25 (40) | 353 (40) | |
| Donor HLA Status | |||
| Matched/related | 34 (54) | 477 (64) | 0.778 |
| Mismatched/related | 20 (32) | 245 (33) | |
| Unrelated | 1 (2) | 23 (3) | |
| Stem Cell Source | |||
| Bone Marrow | 59(94) | 749 (85) | 0.155 |
| PBSC | 4 (6) | 130 (15) | |
| Others | 0 (0) | 4 (1) | |
| Cytomegalovirus serologic status, recipient – donor | |||
| Negative – Negative | 20 (32) | 262 (30) | 0.320 |
| Negative – Positive | 9 (14) | 109 (12) | |
| Positive – Negative | 12 (19) | 262 (30) | |
| Positive – Positive | 22 (35) | 248 (28) | |
| Conditioning regimens | |||
| Reduced Intensity | 26 (41) | 312 (35) | 0.319 |
| Myeloablative | 37 (59) | 571 (65) | |
| TBI | 9 (56) | 195 (34) | |
| Non-TBI | 28 (44) | 376 (66) | |
| Busulfan-based Conditioning Regimen | 27 (43) | 356 (43) | 0.692 |
| Acute GVHD Grade | |||
| 0/1/2 | 60 (95) | 745 (87) | 0.061 |
| 3 | 3 (5) | 109 (13) | |
| Chronic GVHD | |||
| Positive | 63 (100) | 444 (65) | <0.001 |
| Negative | 0 (0) | 240 (35) | |
| Baseline FEV1 | |||
| ≥ 80% | 47 (75) | 712 (81) | 0.071 |
| 70 – 79% | 10 (16) | 96 (11) | |
| 60 – 69% | 6 (10) | 36 (4) | |
| < 60% | 0 (0) | 23 (3) | |
| Smoking | |||
| Never | 7 (11) | 128 (15) | 0.623 |
| Former | 1 (2) | 37 (4) | |
| Current | 0 (0) | 10 (1) | |
| Prior Interstitial Pneumonitis | 2 (3) | 91 (10) | 0.066 |
BOS Prevalence
Initial screening identified 102 patients who met the NIH criteria for new-onset AFO. Fourteen cases were removed due to invalid PFT results caused by procedural error. One case was excluded because the patient developed AFO following two aHCTs. An additional 24 cases were excluded due to alternative diagnoses including pneumonia (n=3), restrictive lung disease (n = 9), sinusitis (n = 3), BOOP (n = 4), asthma (n = 3), and interstitial pneumonitis (n = 2), and no clinical data to support the presence of BOS.
Among the remaining 63 cases, 33 (52%) had both lung volume measurements and HRCT scans of the chest available at the time of meeting spirometric criteria. Of these, 30 (91%) met full NIH criteria for BOS: 7 had a RV > 120% alone, 6 had a RV ≤ 120% but HRCT findings consistent with BOS, and 17 had both a RV > 120% and HRCT findings consistent with BOS. Two (6%) patients had evidence of BOS on HRCT but no lung volume measurements were documented. Supportive HRCT findings included 14 (22%) with air trapping, 1 (2%) with bronchiectasis, 1 (2%) with bronchial wall thickening, 1 (2%) with centrilobular opacities, and 8 (13%) with a combination of these findings. There were an additional 23 patients who had lung volumes, but not HRCT, available either at the time of meeting spirometric criteria or subsequently. Of these, 22 (96%) had an RV > 120% and met full NIH criteria for BOS. Four of the patients who met full NIH criteria for BOS also received a histopathologic diagnosis by lung biopsy. Of the remaining 9 patients, none had received an HRCT and 3 were missing lung volume measurements. Nevertheless, 5 of these patients were clinically recognized as having BOS. In total, 59 (94%) of these 63 patients met full NIH criteria for BOS or were clinically recognized as having BOS, and the remaining 4 patients met the NIH spirometric criteria for BOS. For the remainder of this report, all 63 patients were considered to have confirmed BOS. The overall prevalence of BOS for all patients receiving an aHCT (n = 1145) was 5.5%.
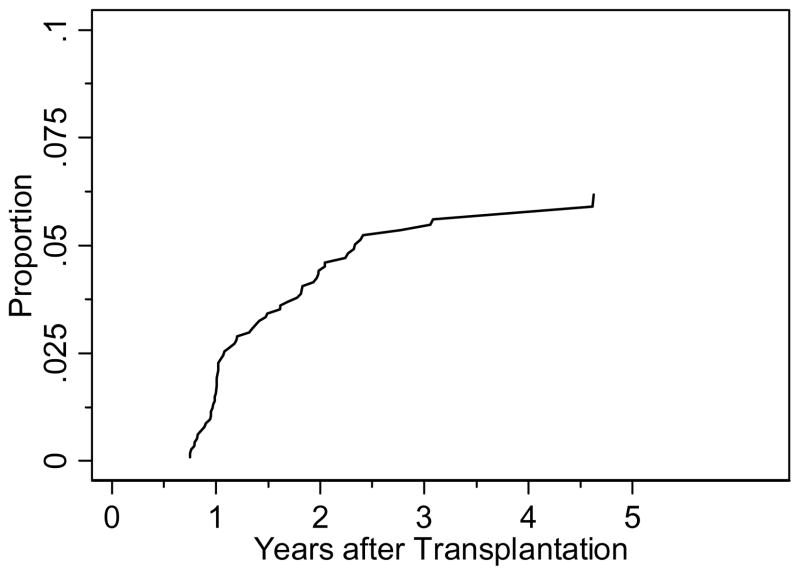
The prevalence of BOS among patients who developed cGVHD (n = 450) was 14%. The cumulative incidence curve for BOS with death as a competing risk is displayed in Figure 1; the majority of cases developed between 300–600 days post-transplant. The median time from transplant to meeting spirometric criteria for BOS was 439 days (range 274–1690). Among the 63 BOS patients, 52 cases were clinically recognized while 11 were clinically unrecognized. Of the 52 clinically recognized cases, 38 were recognized concurrently while 14 were recognized late, at a median time of 294 days (range 92–1219) after meeting spirometric criteria. The concurrently recognized cases included 4 patients who were clinically diagnosed with BOS prior to 1 year, before spirometric measurements were available. The median time to meeting spirometric criteria for the concurrently recognized, late recognized, and never recognized groups were 389 (range 274–1690), 492 (range 301–1217), and 719 (361–1685) days, respectively.
Figure 1.
Cumulative incidence of BOS among all transplanted patients considering death as a competing risk.
BOS Clinical Characteristics
The majority (n = 38, 60%) of BOS patients had no evidence of prior pulmonary disease. Twenty-five BOS patients had a documented prior medical history of pulmonary disease including 4 (6%) with COPD, 4 (6%) with asthma, 4 (6%) with chronic allergic rhinitis/sinusitis, 3 (5%) with radiation-induced lung injury, 2 (3%) with pre-transplant BOOP, 1 (1.6%) each with POEMS syndrome, pulmonary embolus, diffuse alveolar hemorrhage, or obstructive sleep apnea, and 4 (6%) with multiple diseases. Chart review also revealed that 1 BOS patient had a coexisting cryptogenic organizing pneumonia at the time of diagnosis. This case was pathologically confirmed.
Among BOS cases, the pre-transplant median percent predicted FEV1 was 87% (range 62–114), the median FEV1/SVC ratio was 0.76 (range 0.64–0.87), and the median percent predicted RV was 106% (range 57–203). In comparison, non-cases had higher FEV1 values (median: 93%, range 29–132, P = 0.025) and FEV1/SVC ratios (median: 0.79, range 0.53–1.00, P = 0.0013), with similar percent of predicted RV values (median: 104%, range 36–298, P = 0.5598). More BOS patients had a ratio < 0.7 prior to transplantation (22% versus 11%, P = 0.008). Table 2 summarizes PFT characteristics at time of meeting NIH criteria according to recognition status. Recognized cases tended to have lower FEV1 compared to never recognized cases (P = 0.049), and concurrently recognized cases had the highest magnitude of air trapping compared to the other groups (P = 0.008). Although the LFS of the concurrently recognized patients appeared to be worse than the late and never recognized patients, this was not statistically significant (P = 0.45).
Table 2.
Pulmonary function & clinical characteristics of BOS patients at time of meeting NIH criteria based upon clinical recognition status
| Measure | Concurrent n = 38 | Late n = 14 | Unrecognized n = 11 | p-value* | All Cases N = 63 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | Median | Range | ||
| FEV1 (%) | 58% | (16%, 74%) | 57% | (38%, 74%) | 64% | (44%, 73%) | 0.1750 | 59% | (16%, 74%) |
| SVC (%) | 73% | (41%, 99%) | 72% | (46%, 97%) | 75% | (57%, 91%) | 0.5871 | 73% | (41, 99%) |
| FEV1/SVC ratio | 0.61 | (0.29, 0.69) | 0.58 | (0.46, 0.68) | 0.64 | (0.54, 0.69) | 0.0646 | 0.63 | (0.29, 0.69) |
| TLC (%) | 99% | (75%, 153%) | 96% | (64%, 120%) | 90% | (66%, 119%) | 0.3360 | 96% | (64, 153%) |
| RV (%) | 155% | (65%, 334%) | 113% | (62%, 195%) | 116% | (57%, 163%) | 0.0275 | 135% | (57%, 334%) |
| DLCO (%) | 66% | (34%, 95%) | 59% | (9%, 98%) | 60% | (24%, 104%) | 0.2896 | 63% | (9%, 104%) |
| LFS | 9 | (4, 12) | 9 | (4, 12) | 8 | (4, 11) | 0.2282 | 9 | (4, 12) |
P-values were calculated using the Kruskal-Wallis test to compare the medians of the concurrent, late, and unrecognized groups.
All BOS cases were either concurrently (n = 62) diagnosed with cGVHD or diagnosed with cGVHD within 2 months after meeting NIH spirometric criteria (n = 1). Among the 52 clinically recognized cases, cGVHD status at the time of recognition included 31 with active cGVHD, 19 stable, 1 quiescent, and 1 without cGVHD. Sixty patients were on immunosuppression within the 3 months prior to meeting the NIH spirometric criteria; of these, 30 (48% of total BOS cases) were on an immunotherapy taper, while 8 were on a stable regimen, 3 were increased, and 19 were fluctuating. For those on immunotherapy tapers, these included systemic corticosteroids, tacrolimus, cyclosporine, and mycophenolate mofetil.
Risk Factors for BOS
Univariate analysis showed that BOS was not significantly associated with many previously identified risk factors for BOS, including a busulfan-based regimen (P = 0.692), peripheral blood stem cell source (P = 0.155), donor-recipient gender mismatch (P = 0.395), use of methotrexate for GVHD prophylaxis (P = 0.678), or respiratory viral infection within the first 100 days post-transplant (P = 0.376). However, BOS was significantly associated with cGVHD (P < 0.001), lower baseline FEV1/SVC ratio (P = 0.006), non-Caucasian race (P = 0.014), and lower circulating IgG level (P = 0.010), and borderline associated with lower baseline FEV1 (P = 0.071), lack of history of pneumonitis (P = 0.066), and absence of aGVHD (P = 0.061) (see Table 1). Additional analysis of IgG level revealed that this relationship was most significant when IgG levels were ≤ 350 ng/dL (HR: 2.28, 95% CI: (1.31, 3.96), P = 0.003). Univariate Cox regression analysis of the effect of cGVHD as a time-dependent covariate on risk of BOS revealed a hazard ratio (HR) for cGVHD of 10.65 (95% CI (3.33, 34.02), P < 0.001). Multivariate analysis that considered all the variables in Table 1 with a p-value < 0.1 level, with the exception of FEV1/SVC ratio which was colinear with baseline FEV1, revealed that only cGVHD and lower IgG levels remained significant risk factors for BOS (Table 3).
Table 3.
Multivariate Cox Regression for Risk of BOS.
| Variable | HR (95% CI) | P value |
|---|---|---|
| Pre-transplant FEV1 | ||
| > 80% | Referent | - |
| 70–79% | 1.15 (0.50, 2.65) | 0.745 |
| 60–69% | 1.59 (0.62, 4.10) | 0.338 |
| < 60% | - | - |
| Prior Interstitial | ||
| Pneumonitis | Referent | - |
| No | 3.97 (0.84, 18.68) | 0.081 |
| Yes | ||
| Chronic GVHD* | ||
| No | Referent | - |
| Yes | 8.51 (2.63, 27.48) | < 0.001 |
| Acute GVHD* | ||
| No | Referent | - |
| Yes | 0.43 (0.13, 1.47) | 0.178 |
| IgG level (ng/dL) | ||
| IgG ≥ 350 | Referent | - |
| IgG < 350 | 1.92 (1.09, 3.39) | 0.024 |
Chronic and Acute GVHD were included as time-dependent covariates.
Treatment and Outcomes for BOS
Of the 52 clinically recognized BOS cases, only 1 patient did not receive immunosuppressive therapy immediately following clinical diagnosis. Of the 51 patients who did receive therapy, 39 were intentionally treated for BOS, while 12 were monitored with a watchful waiting approach (no change in immunosuppressive regimen) but were kept on immunosuppression for coexisting cGVHD. Among those intentionally treated for BOS, 35 received oral prednisone at 1mg/kg/day, 23 received inhaled corticosteroids, 11 received azithromycin, and in rare cases montelukast (n = 3) or ECP (n = 1) were also administered. Most of these patients also received other immunosuppressants for coexisting cGVHD in other organs, including low-dose systemic corticosteroids, tacrolimus, cyclosporine, sirolimus, mycophenolate mofetil, ECP, azathioprine, and methotrexate. For the never recognized BOS cases, all 11 of them were on immunosuppressive therapies for coexisting cGVHD following meeting spirometric criteria for BOS.
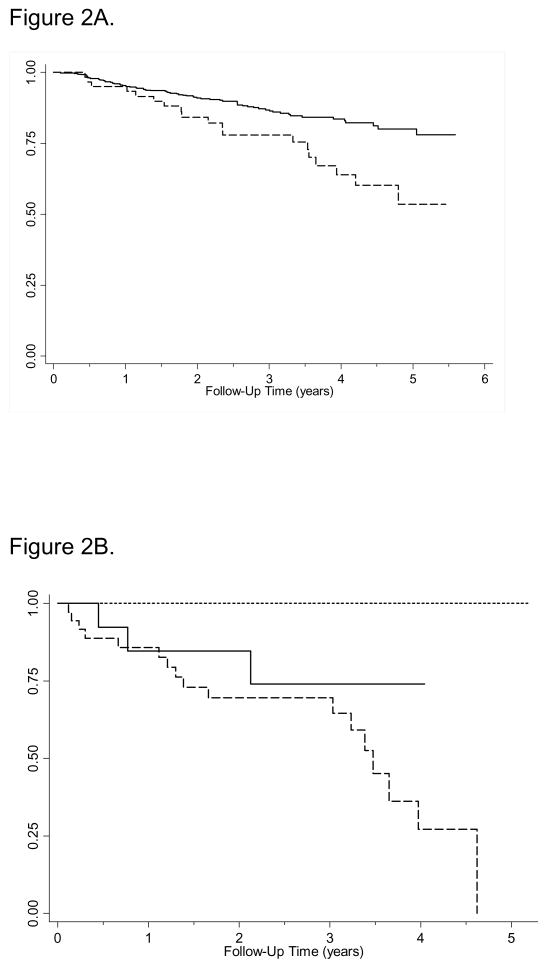
Survival analysis revealed a significant difference between subjects with and without BOS (P = 0.002; see Figure 2A). Sub-analysis of the BOS patients revealed that all of the deaths occurred among patients with clinically recognized BOS. While all patients without clinically recognized BOS survived the follow-up period, patients in the concurrently recognized group had the worst transplant related mortality, followed by the late-recognized group (P = 0.022, Figure 2B). Unadjusted analyses of transplant related mortality estimated a HR for BOS of 2.19 (95% CI (1.32, 3.65), P = 0.002), and were 3.29 (95% CI (1.91, 5.67), p<0.001) and 1.58 (95% CI (0.50, 5.01), p=0.77) when stratified for the concurrently recognized and late-recognized BOS groups. When adjusted for cGVHD and aGVHD, age, conditioning regimen, disease risk, and CMV serostatus, the estimated HR for BOS decreased to 1.62 (95% CI (1.03, 2.55), P = 0.035). Transplant related mortality did not differ significantly between cases that did or did not receive intentional therapy for BOS (P = 0.708). Transplant related mortality also did not differ significantly based on treatment regimen that specifically included high-dose systemic corticosteroids (P = 0.136), inhaled corticosteroids (P = 0.857), or azithromycin (P = 0.417) when compared to treatment regimens that did not. We also did not find evidence of significant differences in non-relapse survival between LFS categories at diagnosis (P = 0.222).
Figure 2.
Figure 2A. Kaplan-Meier survival estimates of transplant-related mortality comparing BOS to non-BOS cases (p=0.002). Non-cases are denoted by a solid line, cases are shown as a dashed line.
Figure 2B. Kaplan-Meier survival estimates of transplant-related mortality based on clinical recognition status among BOS cases (p=0.022). Concurrently recognized cases are denoted by a dashed line, late recognized cases with a solid line, and never recognized cases with a dotted line.
Among patients who had PFTs available at one year (n = 36) and two years (n = 23) after meeting BOS criteria, we evaluated the change in LFS among survivors. There was no difference in the change in LFS at one and two years after BOS diagnosis when comparing the clinically recognized versus clinically unrecognized patients. The mean change in LFS for clinically recognized and unrecognized patients was −0.313 ± 2.264 and 0.250 ± 2.500 at one year (P = 0.646) and −0.409 ± 2.720 and −1.000 ± 0.000 at two years (P = 0.834) respectively. There was also no significant difference in change of the LFS based upon treatment group. The mean change in LFS among patients who received and did not receive intentional therapy was −0.375 ± 2.722 and −0.292 ± 2.156 at one year (P = 0.930) and −2.000 ± 3.347 and 0.188 ± 2.287 at two years (P = 0.093) respectively.
We reviewed the charts of the patients who died after meeting the BOS criteria to determine the causes of death. Among 22 patients who died, 11 had a single cause of death, 8 were multifactorial, and 3 had no documentation of a cause of death. BOS was recognized as contributing to the cause of death in 11 (50%) of these patients—five recorded BOS as the sole cause of death, while 6 were multifactorial (disease relapse, GVHD, infection, pneumothorax). Other causes of death were disease relapse (n = 3), infection (n = 2), coronary artery disease (n = 1), relapse and GVHD (n = 1), and infection and GVHD (n = 1).
DISCUSSION
This study represents the first evaluation of BOS using the recent 2005 NIH consensus criteria to determine the prevalence, risk factors, and outcomes for BOS in the modern age of aHCT. Based upon our results, the 2005 NIH guidelines appear to be reliable for identifying BOS. However, meeting the spirometric criteria alone was nonspecific—obtaining HRCT and lung volumes as part of a clinical work-up is essential, as demonstrated by the fact that, among the BOS cases in our study who had both HRCT and lung volumes available, 100% ultimately met full NIH criteria for BOS.
The overall prevalence for BOS in our patient population was nearly 3-fold higher than the most comprehensive study published using the International Bone Marrow Transplant Registry, which found the prevalence of BOS to be 1.7% (6). Our higher prevalence may be attributable to our different study design and use of a different BOS definition. However, there is compelling evidence to suggest that even the never recognized patients were indeed true BOS cases. On average, the 11 clinically unrecognized patients experienced a 23% drop in their FEV1. A review of charts and clinical data revealed no alternative explanations for such a profound fixed decline in lung function. In addition, 5 of these 11 patients actually had evidence of air trapping on PFT and/or HRCT, allowing them to fully meet the NIH criteria for BOS.
The magnitude of the hazard for development of BOS associated with cGVHD was higher than anticipated. This finding provides strong support for the long-accepted hypothesis that BOS, which is also observed after lung transplantation, represents an alloimmune reaction in the lung. We were not surprised by the significantly increased risk for BOS associated with lower pre-transplant lung function, which others and we have documented in previous studies.(3, 19) The association of BOS with lower circulating IgG levels, which clearly demonstrated a threshold effect, is consistent with observations from prior studies.(4, 10) Although this association might be explained by increased susceptibility to infection, the mechanism by which lower circulating IgG levels might influence BOS risk is currently unknown and deserves additional attention.
Most surprising was the lack of significant association with many previously reported risk factors for BOS, including busulfan-based regimen, peripheral blood stem cell source, donor-recipient gender mismatch, aGVHD, prior history of pneumonitis, and early post-transplant respiratory viral infection. This observation might be related to two possibilities. First, our study may be simply underpowered to detect an association with the majority of these risk factors. We suspect this is unlikely given that most of the previous studies were performed using much smaller cohorts. A more likely explanation again is that all of the previous studies were conducted using several different BOS definitions.
Although our analysis found no significant difference in lung function change between patients who did and did not receive specific treatment targeted toward BOS, it is likely that these observations were subject to significant selection bias influenced by clinical symptoms. There was also a wide variation in the use of immunosuppressive agents for other manifestations of cGVHD, which made it difficult to accurately account for treatment-specific outcomes.
Our mortality observations based on recognition status may have significant clinical implications. The mortality analysis demonstrated that patients whose BOS was clinically recognized without delay had the worst survival. Because almost all of these patients received immunosuppressive therapy, one might expect these patients to have the best outcomes. However, it is more likely that the aggressive nature of these cases allowed the clinicians to easily recognize the syndrome. Conversely, our data also suggest that the clinically unrecognized patients remained such because they had a relatively milder and stable, albeit fixed, manifestation of BOS. Together, these results suggest that BOS may present along a clinical spectrum rather than manifesting as a single, unvarying disease.
There are a few limitations worthy of discussion. First, our retrospective study design limited availability of clinical data, making it impossible to gather all preferred clinical studies for assessment of the NIH criteria; for example, only 36 out of 63 BOS cases received an HRCT. For this reason, we took a primary approach of BOS screening through inclusion by NIH spirometric criteria and exclusion of alternative diagnoses, such as infection, by chart review. Second, exclusion of patients from the final study cohort due to missing PFT data may result in underestimation of the prevalence of BOS. Similarly, the current definition is also likely to miss patients who develop AFO in the setting of a restrictive process such as sclerotic skin GVHD, which can mask the obstructive pattern on PFTs. Thus, it remains possible that the prevalence in this study still represents an underestimation of the true prevalence of BOS.
Despite the availability of diagnostic criteria for BOS, our analysis suggests that BOS remains difficult to diagnose. We agree that pulmonary function testing and HRCT should play a significant role in diagnosing BOS and should guide treatment decisions, especially among patients with cGVHD during the first two years post-transplant. Future research in this area needs to focus on early detection of BOS and identifying the most effective and least morbid treatment regimens. If the risk-benefit ratio can be shifted toward decreased harm, physicians and patients may be more willing to treat BOS detected at an earlier stage, when lung function is more likely to be recovered and higher quality of life is more likely to be preserved.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health research grant HL088201
Footnotes
Financial Disclosure Statement: The authors declare no competing financial interests.
This article has an online data supplement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28:425–434. doi: 10.1038/sj.bmt.1703142. [DOI] [PubMed] [Google Scholar]
- 2.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 3.Clark JG, Schwartz DA, Flournoy N, Sullivan KM, Crawford SW, Thomas ED. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med. 1987;107:648–656. doi: 10.7326/0003-4819-107-5-648. [DOI] [PubMed] [Google Scholar]
- 4.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1988;72:621–627. [PubMed] [Google Scholar]
- 5.Marras TK, Chan CK, Lipton JH, Messner HA, Szalai JP, Laupacis A. Long-term pulmonary function abnormalities and survival after allogeneic marrow transplantation. Bone Marrow Transplant. 2004;33:509–517. doi: 10.1038/sj.bmt.1704377. [DOI] [PubMed] [Google Scholar]
- 6.Santo Tomas LH, Loberiza FR, Jr, Klein JP, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128:153–161. doi: 10.1378/chest.128.1.153. [DOI] [PubMed] [Google Scholar]
- 7.Curtis DJ, Smale A, Thien F, Schwarer AP, Szer J. Chronic airflow obstruction in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;16:169–173. [PubMed] [Google Scholar]
- 8.Yoshihara S, Tateishi U, Ando T, et al. Lower incidence of Bronchiolitis obliterans in allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning compared with myeloablative conditioning. Bone Marrow Transplant. 2005;35:1195–1200. doi: 10.1038/sj.bmt.1704985. [DOI] [PubMed] [Google Scholar]
- 9.Chan CK, Hyland RH, Hutcheon MA, et al. Small-airways disease in recipients of allogeneic bone marrow transplants. An analysis of 11 cases and a review of the literature. Medicine (Baltimore) 1987;66:327–340. doi: 10.1097/00005792-198709000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Ann Intern Med. 1989;111:368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 11.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Markopoulo KD, Cool CD, Elliot TL, et al. Obliterative bronchiolitis: varying presentations and clinicopathological correlation. Eur Respir J. 2002;19:20–30. doi: 10.1183/09031936.02.00282001. [DOI] [PubMed] [Google Scholar]
- 13.Philit F, Wiesendanger T, Archimbaud E, Mornex JF, Brune J, Cordier JF. Post-transplant obstructive lung disease (“bronchiolitis obliterans”): a clinical comparative study of bone marrow and lung transplant patients. Eur Respir J. 1995;8:551–558. [PubMed] [Google Scholar]
- 14.Yokoi T, Hirabayashi N, Ito M, et al. Broncho-bronchiolitis obliterans as a complication of bone marrow transplantation: a clinicopathological study of eight autopsy cases. Nagoya BMT Group. Virchows Arch. 1997;431:275–282. doi: 10.1007/s004280050099. [DOI] [PubMed] [Google Scholar]
- 15.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 18.Hsu KH, Jenkins DE, Hsi BP, et al. Ventilatory functions of normal children and young adults--Mexican-American, white, and black. I. Spirometry. J Pediatr. 1979;95:14–23. doi: 10.1016/s0022-3476(79)80075-x. [DOI] [PubMed] [Google Scholar]
- 19.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.