Abstract
The purpose of this paper is to summarize recent research on longevity, aging and adaptation in wild medfly populations and in a close relative of the medfly. The key findings include a new life table identity that relates age structure and the distribution of deaths in stationary populations, seasonal variation in the post-capture longevity of trapped medflies of unknown age, greater longevity of once-wild (wild-caught) adult medflies relative to never-wild (laboratory-emerged) individuals, differences in age specificity of different medfly field capture methods, large variation in the sex-specific longevity of six medfly global biotypes (e.g. Kenya; Brazil; Greece), and the extraordinary longevity of the natal fruit fly—a sister species of the medfly. The Discussion contains a listing of discoveries derived from this recent research that appear to be unique to the investigations on medfly aging in the wild. It is suggested that studies of aging in wild populations of Drosophila melanogaster have the potential to exploit this model organism in an entirely new aging research domain and thus complement the already deep literature on aging in this species.
Keywords: Ceratitis capitata, life table identity, captive cohort, age bias, medfly biotypes, natal fruit fly, Ceratitis rosa
INTRODUCTION
Tephritid fruit flies as models for aging research were originally introduced to the gerontology and biodemography literature nearly two decades ago with publication of the large-scale Mediterranean fruit fly (Ceratitis capitata) life table study showing that mortality slowed at advanced ages (e.g. Carey et al., 1992). This was the first of a number of papers that exploited one of the strengths of this model system—the availability of large numbers of individuals at low cost. Many of the biodemographic papers published after this early phase involving large-scale experiments focused on questions that required gathering individual-level data on age-specific egg production, disability and mating. Overviews of the results of these studies along with those of earlier large-scale life table experiments are contained in Carey (2003).
Although tephritid fruit flies continue to serve as laboratory models for aging research (Fanson et al., 2009; Papadopoulos et al., 2010; Zou et al., 2009), recent research efforts on medfly biodemography have expanded to include aging in the wild. This is an area of aging science that, with a few important exceptions (e.g. Begon, 1976; Bonduriansky and Brassil, 2002; Sherratt et al., 2010), has been largely neglected for Drosophila and other model organisms. Therefore the purpose of this paper is to provide an overview of recent findings concerned with medfly aging in the wild and discuss their implications and importance for aging research in general and Drosophila aging research in particular. The research results that I review in this paper are contained in seven different studies, six of which are grouped in two separate sections according to a general theme and one of which is summarized in a separate section. At the end I recap the original discoveries and developments that appear not to have precedents in the literature on the biology and demography of aging.
BACKGROUND
Importance of research on aging in the wild
All biologists are well aware that life in a captive laboratory environment for model organisms is vastly different from their lives if they were free-ranging animals living in the wild. Whereas laboratory animals are maintained under optimal physical conditions in cages, usually have ready access to food, water, and mates, and are protected from disease, parasites, and predators, these same animals living in the wild may be subject to disease, starvation, dessication, parasitisation, predation, hypothermia and hyperthermia. Because aging and longevity in the wild may differ so drastically from aging in the laboratory, it is impossible to characterize all of the actuarial properties of a species including its lifespan independent of its environment—a fly that is capable of living 6 months in the laboratory might live only a few days or weeks in the wild. Information on aging in both environments is thus complementary and mutually informing in at least two respects. First, knowledge of all aspects of aging and longevity in both environments including lifespan extremes, sex longevity differentials, actuarial aging rates, seasonality of frailty, and life table properties of biotypes will reveal which aspects are truly robust (i.e. aging traits that are present in both environments such as the sign of gender longevity differences) and those which are idiosyncratic to or an artefact of a particular environment. Second, inasmuch as the evolutionary theories of aging serve as the foundation for much of aging research, particularly for interpreting actuarial patterns at the advanced ages, the results of studies on aging and longevity in evolutionarily-relevant environments can be used to validate certain theories and reject others. Indeed, there is virtually no other way to test evolutionary theories of aging other than the use of data gathered on aging in the wild. This point is underscored by Williams and his co-workers (Williams et al., 2006) who noted that, because of the paucity of field data, the present weight of evidence has failed to establish George Williams’ classic hypothesis (Williams, 1957) that low adult death rates are associated with low rates of senescence as a general prediction of the way that environmental hazards shape aging schedules in the wild. It is remarkable that after over half a century that there is still no clear consensus on this classic theory because of lack of relevant field data.
Overview of medfly natural history
The medfly belongs to the dipteran family Tephritidae referred to as “true” fruit flies—a group of about 4,000 species distributed throughout most of the world (from Christenson and Foote, 1960). Tephritids lay eggs in intact fruit using their sharp ovipositor rather than on decaying fruit as do their distant relatives in the family Drosophilidae. Both genetic and phylogenetic evidence point toward tropical Africa as the medfly’s aboriginal home. However, the species is currently distributed throughout a wide range of climatic regions of the world including the Mediterranean, western regions of the Middle East, Central and South America, and the Pacific (Hawaii; western Australia). Generally speaking, the reproductive biology and life course of medflies is typical of other dipterans including Drosophilids—after emerging from a 3 week preadult phase (egg, larvae, pupae), adults of both sexes begin searching for mates and foraging for food. Following a 5 to 10 day maturation period females lay an average of 700 to 1,000 eggs (in laboratory) and survive for 4 to 6 weeks. Unlike Drosophila melanogaster most aspects of medfly ecology and behaviour in the field are well understood (e.g. Papadopoulos et al., 2001; Vargas et al., 1983). However, one aspect that is poorly understood is its demography in the wild, some insights of which are presented in the next three sections.
AGING, LONGEVITY AND ADAPTATION IN THE WILD
Medfly population aging: Analytical concepts and empirical studies
A life table equality: Death distribution reveals age distribution
A concept that appears not to have been considered in the development of techniques that can be used to study population aging and age structure in the wild concerns the information contained, not in dead individuals as in many of the historical approaches (e.g. egg load; cuticular hydrocarbons), but in live ones. The concept is this: If groups of individuals are collected from two populations separated in space or time, one for which the average age of individuals in it is greater than the other, the average of the remaining time-to-death of these captured individuals will be less in the older population. Further, the difference between the average ages to death will approximate the difference in the average ages between their populations.
For the idealized case of a stationary (replacement-only) population, the age distribution (and thus average age) of the population can be computed directly from the distribution of the remaining times to death of the individuals across all age classes in the current population. Thus if all individuals in a stationary population were marked at a given moment and their deaths monitored until the last individual died, the exact percentage that dies x-days after the moment of marking will equal the exact percentage of individuals age x in the stationary population. This is despite the mathematical fact that the percentage of all deaths that occur x-days after marking consists of individuals from all age classes.
This non-intuitive but mathematically true relationship is given in papers by Müller and his colleagues (Müller et al., 2004; Müller et al., 2007) and formally derived by Vaupel (2009). The key relations forming the life table identity are outlined in Table 1. The identity is revealed by the equality of the bold type columns cx and dx* in the leftmost and rightmost sub-tables. From these data on death distributions of randomly-marked individuals of unknown age in stationary populations it is possible to compute: (1) the age-specific survival schedule, lx; (2) population age structure; and (3) the probability that an individual chosen at random is one who has lived x years equals the probability the individuals is one who has that number of years left to live (Vaupel, 2009).
Table 1.
Illustration of the relationship between hypothetical ‘wild’ and ‘marked sample’ life tables in the stationary case (modified from Müller et al., 2004). The ‘wild’ population consist of Nx individuals at each age x with corresponding schedules of survival lx and age structure Cx=lx/∑ly with life table in the leftmost subtable. The ‘marked sample’ cohort consists initially of ‘marked’ individuals with the same age structure as the ‘wild’ cohort, all simultaneously entering the marked sample cohort at the age of capture and marking x*=0 (i.e. top row of the middle subtable). Remaining lifetimes are recorded for the marked sample and lx* is the survival schedule of the marked sample cohort, with death rates dx*=lx*+1 - lx*, as listed in the rightmost subtable. The survival schedules given separately for age cohorts x=0, x=l, x=2 and x=3 in dependency on marked sample cohort age x*, are listed in the corresponding columns of the subtable in the middle. In this hypothetical example, the initial marked sample cohort at marked sample cohort age x*=0 has an age structure identical to cx (row in bold type in the middle subtable) is identical to the bold type cx column of the leftmost subtable) and, in turn, identical to dx in the right-most column.
| Stationary Wild Population | Marked Sample of Wild Population | Fate of Captive Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Number | Survival | Age Structure |
Captive Age |
Age Distribution of Initial Marked Samplea | Sumb | Survivalb | Deaths | ||||
| x | Nx | lx | cx | x* | x=0 | x=1 | x=2 | x=3 | Σ | lx* | dx* | |
| 0 | 400 | 1.000 | 0.40 | 0 | 0.40 | 0.30 | 0.25 | 0.05 | 1.00 | → | 1.00 | 0.40 |
| 1 | 300 | 0.750 | 0.30 | 1 | 0.30 | 0.25 | 0.05 | 0.60 | → | 0.60 | 0.30 | |
| 2 | 250 | 0.625 | 0.25 | 2 | 0.25 | 0.05 | 0.30 | → | 0.30 | 0.25 | ||
| 3 | 50 | 0.125 | 0.05 | 3 | 0.05 | 0.05 | → | 0.05 | 0.05 | |||
| 4 | 0 | 0.000 | 0.00 | 4 | 0.00 | 0.00 | → | 0.00 | 0.00 | |||
Entries in each column at captive age x*=0 contain the proportion of the original wild population that are marked at each age (i.e. cx). That is, 0.40, 0.30, 0.25, and 0.05 of respective age classes 0, 1, 2 and 3 are marked since this is the fraction in the wild population of each age class. These individuals are then survived forward according to the life table rates contained in the left subtable.
Note that the row sum of the marked sample cohort at captive age x*=0 is the radix (=1.00) for the captive survival (lx*) and that subsequent row sums represent survival to the subsequent captive ages.
One of the most surprising aspects of this discovery is that, despite the extraordinary long history and extensive analytical studies on life tables (e.g. Preston et al., 2001), this identity was unknown prior to its publication in the paper by Müller and his colleagues. The life table equivalence is important because it sheds light on the relationship of age structure and death distributions in a population (albeit stationary and thus idealized) and, in turn, provides an initial analytical framework for constructing more realistic models such as the one used in the next section.
Population aging in wild medflies
Although ecologists have attempted to develop methods for estimating the age of individual insects (e.g. cuticular hydrocarbons; eye capsule pteridines; gene expression) and ultimately the age structure of their populations, the methods are often expensive and the results are always mixed. Indeed none of the technologies provide accurate estimates of individuals at more advanced ages.
An alternative approach to estimating population age structure was developed by Carey, Müller and their colleagues based on a method involving i) data on the remaining lifespans of live-captured individuals and from reference cohorts; and ii) a deconvolution model that is used to estimate population age structure (Carey et al., 2008; Müller et al., 2004; Müller et al., 2007).
This approach used to estimate the age structure of wild medfly populations in Greece, referred to as the captive cohort method, was based on the death distribution of over 4,000 medflies captured and monitored in the laboratory over three field seasons (Carey et al., 2008). The method assumed: (1) that relative changes in the patterns of death between cohorts of medflies captured during two or more sampling periods reflects relative changes in their respective population age distributions; (2) that changes in these post-capture death patterns can be used to estimate the actual age structure in the field if both laboratory-reared and wild-caught adult medflies experience the same mortality risk in the laboratory (i.e. memory-less assumption); and (3) the estimated age distribution of wild-caught flies can also be used to infer the actual age structure in the field (assuming flies are captured in proportion to their abundance).
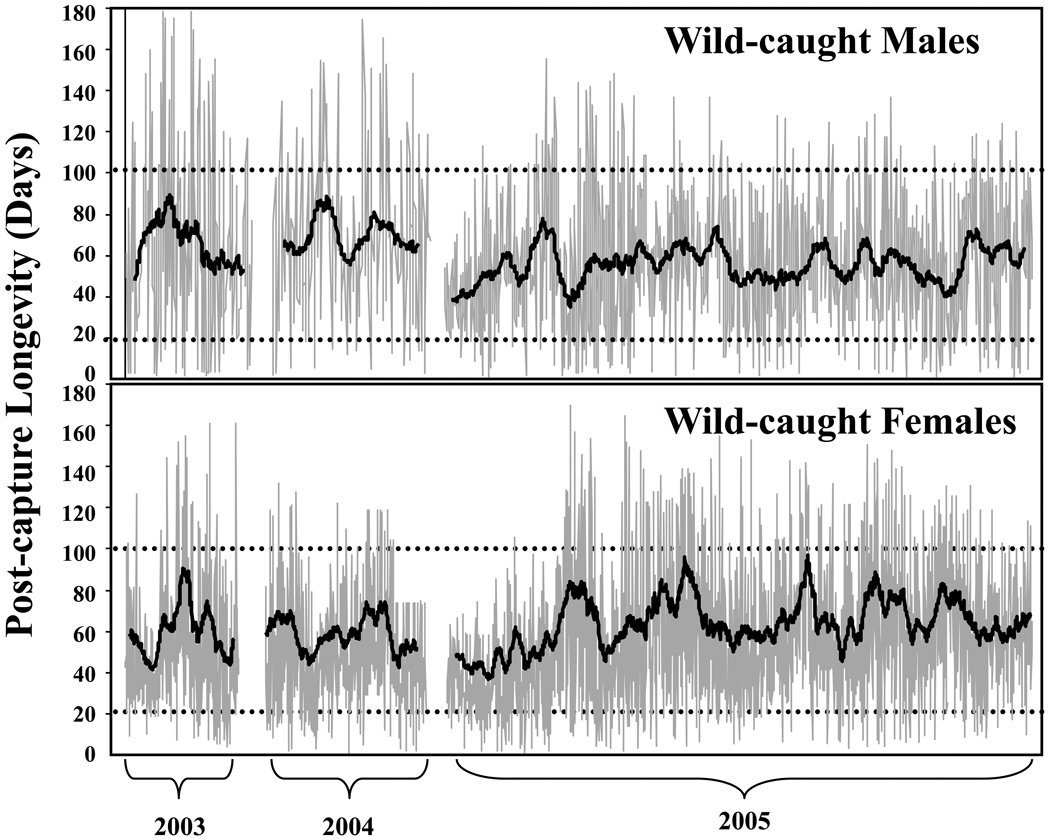
The results revealed that large seasonal differences (i.e. > 30 days) existed in the post-capture lifespans of medflies (Fig. 1). The empirical data and the modeling results suggested that: i) major shifts in population age structure (>30 days) occur in wild medfly populations; ii) middle-age (≈20–30 days old) and moderately old medflies (>50 days) are common throughout much of the field season; and iii) the life-span potential of once-wild (i.e. wild-caught) medflies was greater than that of never-wild medflies (i.e. medflies that were collected as larvae from the wild but maintained in the laboratory from eclosion through death). This last result is of stand-alone interest and is thus described next.
Figure 1.
Post-capture lifespans for 4,088 medflies (2653; 1435 males) trapped during the 2003–05 field seasons on Chios Island, Greece. The points (individual remaining lifespan) within each field season are connected by lines to depict both variation and seasonal trends. The running 30-day medians for each season are shown as the internal smoothed lines. The horizontal dashed lines show the 20-day (shorter-lived) and 100-day (longer-lived) post-capture age are included for reference (from Carey et al., 2008).
Once-wild medflies live longer than never-wild individuals
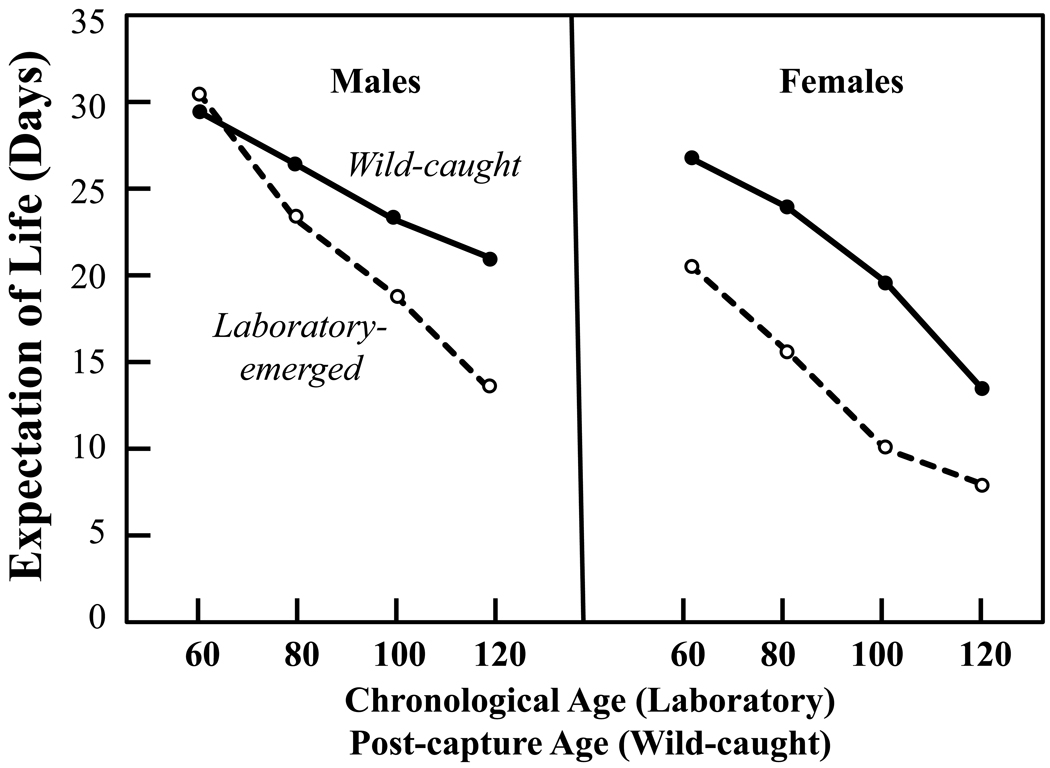
One of the more surprising results of medfly field studies (Carey et al., 2008) was that, despite being maintained under exactly the same conditions in the laboratory as adults, the captive lifespans of a substantial number of these captured flies of unknown age exceeded the lifespans of field-collected flies that were reared from field-infested fruit in the laboratory and thus were of known age at death (Fig. 2). With the exception of males at 60 days, the sex-specific expectation of life at ages 60, 80, 100 and 120 days for medflies that emerged in the laboratory from field-infested fruit of known age exceeded by 10 days or more the sex-specific expectations of remaining life of wild-caught flies (both sexes) of unknown age. There were also large differences in the number of wild-caught medflies living to extreme ages relative to the number of never-wild medflies living to extreme ages even though the never-wild (laboratory-reared) flies: i) were the exact biotype; ii) were not ‘domesticated’ since they were only the F1 generation out of the wild; and iii) they had to have been younger chronologically than any of the once-wild flies, all of which spent part of their lives in the wild prior to capture. For example, a total of 19 wild-caught females but no reference (never-wild) females survived in the laboratory for 140 days or more, and 6 wild-caught but no reference males survived in the laboratory for 170 days or more. Thus each of the 19 wild-caught females and the 6 wild-caught males had to have been more than 140 and 170 days old, respectively, when the last of their never-wild, known-age counterparts died.
Figure 2.
Expectation of remaining life at 60, 80, 100 and 120 days for medflies that emerged as adults in the laboratory from wild-collected hosts and for medflies of unknown ages that emerged in the field and were subsequently trapped as free-ranging adults.. ‘Chronological’ age applies known-aged flies that emerged in the laboratory and ‘post-capture’ age applies to the wild-caught flies and refers to the time that had elapsed since they were captured and brought to the laboratory (from Carey et al., 2008). The chronological age of known-age flies from the laboratory must have always been less than or equal to the post-capture age of wild-caught flies. Note that, with the exception of males at 60 days, remaining life expectancies in wild-caught flies for both sexes was always higher at all ages
The results raise the possibility that the higher survival of field-caught medflies was due to their experience (conditioning) in the wild—a concept that is important because it points to a mechanism that either suppresses longevity in the captive environment or enhances longevity in the wild. Genetic differences could not account for the exceptional longevity observed in captured medflies relative to reference medflies because the reference cohorts were derived from pupae collected from field-infested hosts over many different months during the 2003–05 seasons. Age bias of field captures cannot account for the increased frequency of survival to extreme ages since the reference cohorts were initiated entirely with newly-eclosed medflies; i.e. the youngest flies captured from the wild could not be younger than newly-eclosed (i.e. laboratory-emerged).
Although the presence of captured individual that live longer than any flies that emerged in the laboratory cannot be accounted for by selection arguments, the higher frequency of moderately long-lived individuals in the group of captured medflies can be accounted for by selection (Vaupel and Carey, 1993; Vaupel et al., 1979). Selection arguments might in principle account for these differences, but extended analysis indicated that selection is not likely to be strong enough to account on its own for the larger lifespans found among the once-wild flies (see Supplementary material in Carey et al., 2008). For such an explanation to hold valid, two claims would have to be true. First, death at young ages in the wild would have to be substantially correlated with reduced late-life survival potential. Early death could not be largely random, nor could it be tied primarily to traits associated with early-acting kinds of risks. Second, the raw amount of young mortality in the wild would have to be large enough to cull a substantial fraction of the weaker members of the population. Enough of the population would have to be removed to account for the special late survival of the remainder.
Carey and his co-authors (Carey et al., 2008) offered two hypotheses for the greater lifespan extremes observed in captured flies. The first was that the early-life experience of free-ranging adults may reduce their mortality costs of reproduction by inhibiting their ability to habituate in the captive environment and therefore reduce their reproductive costs. The second was that there is a window in the early adulthood of medflies when the presence in their diets of certain amounts or kinds of microorganisms is important and that wild flies have access to these micro-fauna but that reference flies in the laboratory do not.
The discovery that the potential longevity of wild-caught flies is substantially greater than flies that have emerged in the laboratory has been corroborated with recent findings from field studies (Alexander Diamantidis, Nikos Papadopoulos and James R. Carey, unpublished data). This more recent result affirms the earlier observation concerning the enhanced lifespan potential of once-wild medflies and thus underscores the importance of follow up research to identify the factor(s) that determine their extraordinary longevity.
Age bias in medfly capture methods
Despite the large amount of time, effort and resources that have been invested in sampling insect populations in both basic and applied contexts, it is remarkable that the literature contains little on age bias in insect capture techniques. Inasmuch as estimates of both absolute and relative changes in age structure using the captive cohort method will be influenced by age bias in adult sampling (live-capture) techniques, it is important to understand the extent to which the age of wild adults that constitute a captive sample is influenced by the capture method. Therefore Kouloussis and his co-workers (Kouloussis et al., 2009) developed a simple method for estimating relative differences in the age bias of different field sampling (collecting) techniques based on the concept that the survival rates of a mixture of medflies of different ages will reflect differences in their age composition. Three sampling methods were tested including: i) traps with a food-based synthetic attractant (both sexes); ii) traps with a male pheromone (males-only); and iii) aspiration of free-ranging medflies (both sexes).
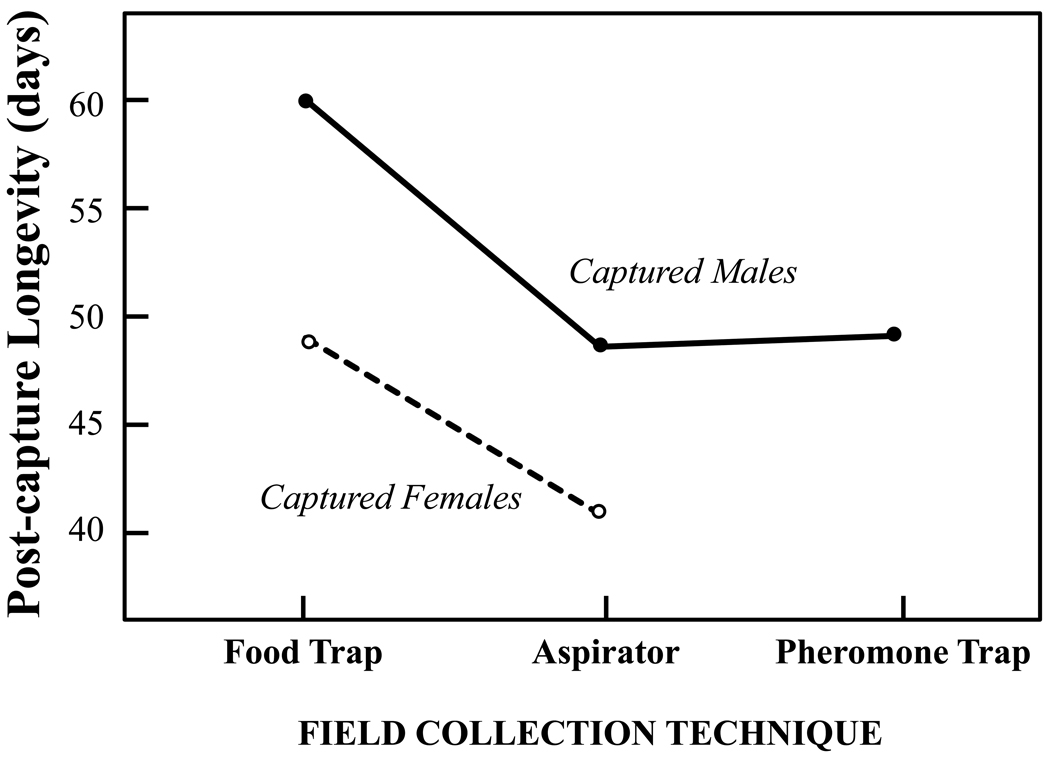
Although data gathered over multiple sampling dates were used in the study, the data gathered from a single date are given as an example here (Fig. 3). The age composition of the samples (both sexes), as inferred from differential post-capture survival rates, were significantly different for medflies captured using food traps relative to those captured using an aspirator (mean differences of 11 and 8 days for males and female, respectively). However, there were no significant differences in the post-capture survival rates for males captured by aspirators versus males captured using pheromone traps.
Figure 3.
Sex-specific post-capture longevity of wild medfly adults on Chios Island, Greece that were captured live on the same date (July 23, 2006) using either a food (protein-baited) trap (n= 211 and 268 for males and females, respectively, aspirator (n=233 and n=66 for males and females, respectively) or a pheromone (n=211 for males-only) (from Kouloussis et al., 2009). Means (±SD) for males were 59.9 (±35.4), 49.2 (±30.4), and 48.7 days (±27.6) for captures made by the food trap, the aspirator and the pheromone trap, respectively. Means for females were 49.1 (±30.7) and 41.1 days (±28.4) for captures made with the food trap and aspirator.
These findings have two practical implications. The first is that the results reveal an age bias in capture methods that needs to be taken into account when interpreting the results of studies using the captive cohort method of population age estimation. Indeed it is doubtful that the age structure of any sample of insects is an exact reflection of the age structure of the population as a whole. The second practical implication is that this life table assay provides a useful technique for estimating changes in the frailty of insect populations including populations of Drosophila. The importance of this simple method may increase as researchers attempt to connect the results of aging research in the laboratory with aging in the wild.
Medfly Longevity as Adaptation
Longevity differences in medfly biotypes associated with climate
Surprisingly little research has been conducted on how different environments shape life span in different insect species including in fruit flies. Because medflies have colonized a wide range of climatic regions ranging from desert and Mediterranean to subtropical and tropical, Diamantidis and his co-workers (Diamantidis et al., 2009) conducted a series of common garden studies to identify differences in the biodemographic characteristics of medflies from six global regions including three with tropical climates (i.e. Kenya; Hawaii; Guatemala) and three with Mediterranean climates (i.e. Portugal; Greece; north-eastern Brazil).
Two important results pertaining to the life table properties of each of these six medfly biotypes merit comment (Table 2). The first is that the six female biotypes could be classified into two categories according to their life expectancies: i) shorter-lived biotypes derived from tropical or subtropical regions (i.e. Guatemala, Hawaii, and Kenya); and ii) longer-lived biotypes derived from Mediterranean-type climatic regions (i.e. Portugal, Greece, and north-eastern Brazil). Diamantidis and his co-authors hypothesized that the greater longevity in female medflies from the global regions with Mediterranean-like climates (including semi-arid region of north-eastern Brazil) was due to selection for survival through exposure to extended dearth periods (e.g. extremes in seasonality; stochasticity). In contrast, the longevity of female medflies from tropical regions was lower due to reduced need to survive periods of resource scarcity that are less frequent in these types of environments.
Table 2.
Sex-specific expectation of life (ex) for x=0 and 100 days for medfly populations originating from six different geographic areas, (from Diamantidis et al., 2009).
| Population | Expectation of Life (days) |
|||
|---|---|---|---|---|
| e0 |
e100 |
|||
| Males | Females | Males | Females | |
| Guatemala | 68.0 | 48.1 | 0.0 | 0.0 |
| Hawaii | 106.5 | 52.1 | 45.5 | 11.5 |
| Kenya | 115.9 | 58.3 | 38.2 | 3.5 |
| Brazil | 122.3 | 75.7 | 36.0 | 8.5 |
| Portugal | 107.1 | 75.6 | 31.1 | 21.4 |
| Greece | 112.1 | 72.3 | 27.2 | 16.0 |
The second important result was that, with the exception of medflies from Guatemala, male medflies are extraordinarily long-lived, not only relative to female medflies, but relative to many other insect species. The life expectancy of newly-eclosed females ranged from around 50 to 75 days and of 100 day-old females from 0 to 21 days. In contrast, the life expectancies at eclosion for all male biotypes (excepting Guatemala) ranged from 107 to 122 days and at 100 days from 27 to 45 days. Clearly male medflies are not only the longer-lived sex in this species in particular, but also quite long lived relative to many other insect species more generally (Carey, 2001).
Medfly reproduction is adapted to its survival characteristics
Müller and his colleagues (Müller et al., 2009) used the data from the medfly biotypes (Diamantidis et al., 2009) to test what they referred to as the reproductive adaptation hypothesis—the temporal patterns of individual fertility schedules are influenced by the population survival schedule. It is therefore probable that at the population level, the ensemble of individual trajectories of fertility is affected by the survival schedule of the population. To investigate whether survival shapes reproduction, two subsamples were created from the original cohorts: a survival-adjusted and an unadjusted sample. The survival-adjusted sample was obtained by constrained randomization such that all cohorts have the same number of deaths in every 5 day interval.
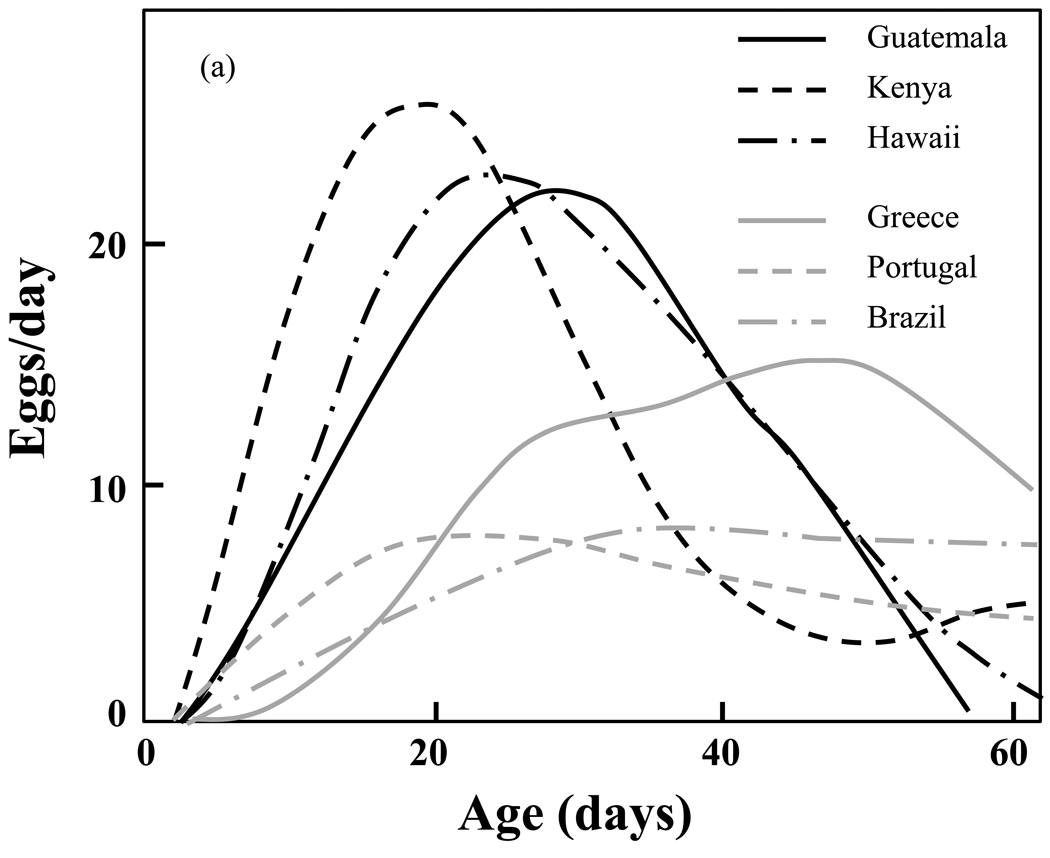
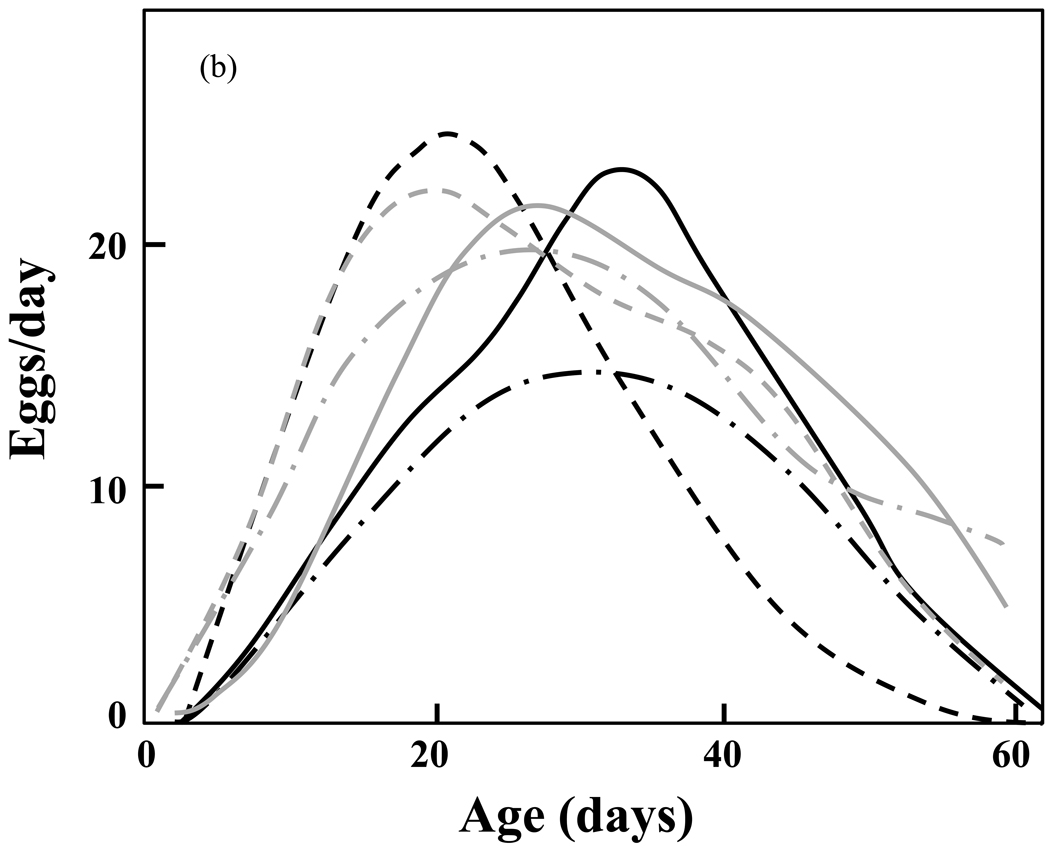
Large differences in reproductive schedules between the short-lived and the long-lived cohorts are evident when one compares the estimated mean functions of the unadjusted samples (Fig. 4a). These differences are substantially reduced in the survival-adjusted samples (Fig. 4b). The analysis demonstrates that the differences observed in reproductive trajectories can be largely explained by differences in survival. Müller and his colleagues therefore conclude that: (1) the observed differences in shapes and patterns of individual reproductive schedules are closely associated with differences in the survival schedules of the corresponding populations; and (2) the differences in reproductive patterns observed across populations disappear after adjusted for the population-specific differences in longevity. The consequences of these findings are two-fold. The first is that there exists a universal reproductive schedule that captures the essential features of reproduction and varies only little across the six populations. The second is that the close link of reproductive trajectories with survival provides support for the reproductive adaptation hypothesis.
Figure 4.
a,b. Estimated mean reproductive functions in the medfly biotypes for (1) the survival-unadjusted; and (b) the survival-adjusted samples (re-drawn from Müller, et al., 2009). Note the similarity of the adjusted rates (Fig. 4b) relative to the dissimilarity of the unadjusted rates (Fig. 4a).
Occasional negative environmental or season influences of the habitat have only limited impact if egg-laying periods are extended, but can have serious negative effects if egg-laying occurs in a very brief period. If all egg-laying takes place in a very short interval and external conditions during that time are not conducive to the maturing of eggs and young flies, the entire batch of eggs laid by individual flies would be lost. Inasmuch as lifespan evolution theory (Orzack, 2003) supports the notion that selection favors longer lifespans in both uncertain environments or in environments with dearth periods of resource availability, the lifespans of the long-lived group of medfly biotypes may have evolved in response to host-free summer periods owing to either chance or seasonal variation.
This finding maps into the classical literature concerned with the evolutionary biology of aging (e.g. Hamilton, 1966; Kirkwood, 2008; Rose and Charlesworth, 1980). As conditions arose that favored greater longevity for population replacement and growth, selection favored the individuals that were genetically predisposed to live longer and produce eggs at later ages. Thus the reproductive schedules of the longer-lived biotypes consisted of increasing fractions of individuals that survived to and reproduced at older ages resulting in the re-shaping of the cohort’s reproductive schedules—i.e. less pronounced peaks and longer tails in egg laying (as observed by Diamantidis et al., 2009). This process of ‘moulding’ both reproduction and mortality schedules was described over 4 decades ago in the classic paper by W. D. Hamilton (Hamilton, 1966)—i.e. the left-most part (younger) of the mortality schedule is ‘nibbled’ away faster than the right-most part (older) at the ages when reproduction ends. The greater the rate of genetic change, the greater the speed at which the mortality schedule is moulded.
Extraordinary longevity of a medfly sister species
Just as D. melanogaster is one of a large number of Drosophila species ranging from sibling species to more distant relatives such as the picture-winged Drosophila (e.g. D. mulli) from Hawaii, the medfly is also one of a large number of Ceratitis species. One such closely-related species is the natal fruit fly, C. rosa, a species that, unlike the medfly which is widespread, is restricted to its aboriginal home of Africa and the islands of Mauritius and Reunion (Duyck et al., 2007). Because C. rosa is virtually identical to the medfly in size, appearance, behavior, host range, and developmental rate from egg to adult, a straightforward demographic prediction is that its average longevity would be similar to that of the medfly.
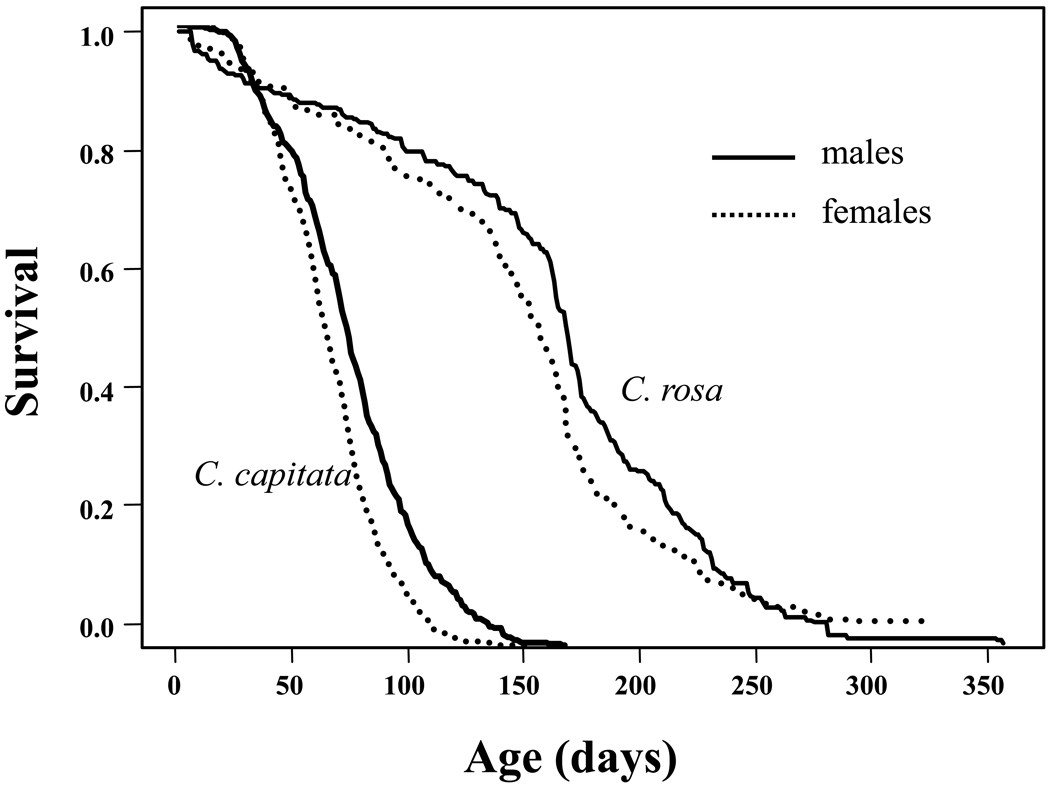
Remarkably survivorship (and thus lifespan) of C. rosa is much greater than that of the medfly as shown in Fig. 5. Whereas the life expectancy at eclosion for the medfly in most studies is typically around 50 to 70 days, (e.g. Carey et al., 2002; Carey et al., 2008), Duyck and his co-workers (Duyck et al., 2010) reported that life expectancies of male and female C. rosa were 160 and 143 days, respectively, with lifespans approaching one year (females=328 days; males= 357 days). Given the similarities in the life histories of C. rosa and C. capitata but the 2–3 fold difference in their adult lifespans, it is clear that the factors that select for extended longevity go beyond the basic dichotomy used by Diamanditis and his co-workers (Diamantidis et al., 2009) to classify shorter- and longer-lived medfly biotypes. An unknown factor or set of factors in the evolutionary ecology and life history of C. rosa selects for extraordinary longevity potential in this species that is either not present in the ecology or important to the life history of C. capitata. It is noteworthy that, whereas as the medfly as the shorter-lived species is a world-wide invasive pest (excepting Asia), the natal fruit fly as the long-lived species is not known for its ability to invade new regions (Duyck et al., 2007).
Figure 5.
Comparative sex-specific life expectancy of two Ceratitis species— the natal fruit fly, C. rosa (from Duyck et al., 2010) and the medfly, C. capitata (from Carey et al., 2008).
DISCUSSION
Unlike laboratory research on D. melanogaster in which both environmental and experimental conditions are carefully controlled, research on aging in the wild on insects such as the medfly is fraught with challenges including the lack of both controlled conditions and experimental controls, absence of information about basic genetic and demographic properties of the organisms being studied, and restricted periods for data collection (i.e. short seasons). Despite these obstacles, research that focuses on aging, longevity and adaptation in wild medflies provided Carey and his co-investigators the opportunity to develop new tools and generate original discoveries that are relevant to aging science. As examples, the following discoveries compiled from the research summarized in this paper appear not to have precedents in the aging literature. I first list the main finding and then note its importance.
Life table identity—equivalence of population age structure and the death distribution of randomly-marked individuals in a stationary population (Müller et al., 2004). This result is important because it establishes both the theoretical concept and analytical baseline relating population age structure to remaining time to death for individuals within an idealized, hypothetical population.
Captive cohort method—the age structure of wild populations can be estimated using the death distribution of wild-caught flies of unknown age, actuarial data from reference cohorts, and deconvolution models (Carey et al., 2008; Müller et al., 2004; Müller et al., 2007). These models are important because, unlike the model describing the life table identity which applies to an idealized (stationary) population, they can be applied to actual and not just hypothetical populations of medflies (as summarized next) but also populations of Drosophila melanogaster and other free-ranging insect species.
Large changes in age structure in wild medfly populations—shifts of up to 30 days in average age of medfly populations in the wild were documented using the captive cohort method of age structure estimation (Carey et al., 2008). This specific finding is important because i) a shift of this magnitude in the average age of a fruit fly population implies that many flies are living to middle ages as well as relatively advanced ages; and ii) shifts in age structure throughout the season documents the existence of population aging in wild populations of medflies as well as the seasonality, periodicity and scale of changes in age structure.
Extraordinary longevity of once-wild medflies—the post-capture lifespans of free-ranging medfly adults exceeded both the average and the extreme lifespans in medflies of the same biotype that emerged in the laboratory (Carey et al., 2008). This result is important because it opens up the possibility that there may be factors present in nature that extend longevity that have not been considered because all virtually all previous research on aging has focused exclusively on aging in model organisms in the laboratory.
Age bias in capture—the age composition of captured medflies is conditional on the field sampling method (Kouloussis et al., 2009). This result is important because it reveals i) the intuitive but poorly documented observation that individuals of different ages are neither randomly distributed nor have the same responses to stimuli in nature; ii) individuals of different ages are subject to differ risks due to their differences in location and behavior; and iii) the importance of understanding how different sampling methods have the potential to skew estimates of the age structure in wild populations regardless of the technique used to estimate population age (i.e. technology such as analysis of cuticular hydrocarbons or demographic approach using the captive cohort method).
Male medflies are unequivocally longer-lived than female medflies—evidence from both field and laboratory studies now leaves little doubt that male medflies live longer than female medflies (Carey, 2003; Carey et al., 2008; Diamantidis et al., 2009; Kouloussis et al., 2009). This observation is complemented by the finding by Duyck and his co-workers (Duyck et al., 2010) that the lifespan of males in a medfly sister species, the natal fruit fly (C. rosa), is also much greater than that of females. These findings are important because the general consensus in much of the gerontology literature is that females of most species live longer than males (Austad, 2006). However, this is not the case for medflies and therefore the generalization regarding the longevity advantage of females may need to be revisited.
Lifespan shapes and is shaped by reproduction—modeling using the survival and reproductive data on medfly biotypes supports the hypothesis that peak reproduction occurs before major mortality increases occur (Müller et al., 2009). This finding is important because it is consistent with and thus supports the theoretical underpinnings of the evolutionary theory of aging concerned with the evolution of extended longevity (Hamilton, 1966; Kirkwood, 2008; Rose and Charlesworth, 1980).
Large difference in longevity in closely-related species—the life expectancy of C. rosa, a species that is a close relative of the medfly, is over twice that of C. capitata even though the ecology and host ranges of these two species are nearly identical (Baliraine et al., 2004; Duyck et al., 2006). This result both highlights and underscores the extent to which Darwinian selection can alter the actuarial properties of species that are so closely related that they look nearly identical and have host ranges with nearly 100% overlap.
One of the obvious reasons these findings emerged from biodemographic research on the medfly in the wild rather than from research in the laboratory is that the focus in the medfly research is on ecological and demographic determinants, whereas the focus of laboratory research is typically on mechanisms (e.g. physiological; genetic; molecular). However, studies on aging, longevity and adaptation in the wild of model organisms used in aging research such as D. melanogaster have the potential, not only to answer questions and identify relationships similar to those on the medfly discussed in this paper, but also to validate laboratory findings and generate new sets of questions on aging in a complementary domain of aging research.
ACKNOWLEDGMENTS
I thank two reviewers for their extremely constructive comments, and Blanka Rogina for inviting me to submit this paper as part of this Special Issue that otherwise focuses exclusively on Drosophila research. Supported by NIA/NIH grants P01 AG022500-01 and P01 AG08761-10.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- Austad SN. Why women live longer than men: Sex differences in longevity. Gender Medicine. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- Baliraine FN, Bonizzoni M, Guglielmino CR, Osir EO, Lux SA, Mulaa FJ, Gomulski LM, Zheng L, Quilici S, Gasperi G, Malacrida AR. Population genetics of the potentially invasive African fruit fly species, Ceratitis rosa and Ceratitis fasciventris (Diptera: Tephritidae) Mol. Ecol. 2004;13:683–695. doi: 10.1046/j.1365-294x.2004.02105.x. [DOI] [PubMed] [Google Scholar]
- Begon M. Temporal variations in the reproductive condition of Drosophila obscura Fallen and D. subobscura Collin. Oecologia. 1976;23:31–47. doi: 10.1007/BF00351213. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R, Brassil CE. Rapid and costly ageing in wild male flies. Nature. 2002;420:377. doi: 10.1038/420377a. [DOI] [PubMed] [Google Scholar]
- Carey JR. Insect biodemography. Ann Review of Entomol. 2001;46:79–110. doi: 10.1146/annurev.ento.46.1.79. [DOI] [PubMed] [Google Scholar]
- Carey JR. The Biology and Demography of Life Span. Princeton: Princeton University Press; 2003. Longevity. [Google Scholar]
- Carey JR, Liedo P, Harshman L, Müller HG, Partridge L, Wang JL, Zhang Y. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Orozco D, Vaupel JW. Slowing of mortality rates at older ages in large medfly cohorts. Science. 1992;258:457–461. doi: 10.1126/science.1411540. [DOI] [PubMed] [Google Scholar]
- Carey JR, Papadopoulos N, Müller HG, Katsoyannos B, Kouloussis N, Wang JL, Wachter K, Yu W, Liedo P. Age structure changes and extraordinary life span in wild medfly populations. Aging Cell. 2008;7:426–437. doi: 10.1111/j.1474-9726.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson LD, Foote RH. Biology of fruit flies. Ann Review of Entomol. 1960;5:171–192. [Google Scholar]
- Diamantidis AD, Papadopoulos NT, Nakas CT, Wu S, Miiller H-G, Carey JR. Life history evolution in a globally-invading tephritid: Patterns of survival and reproduction in medflies from six world regions. Biol. Jour, of the Linnean Soc. 2009;97:106–117. [Google Scholar]
- Duyck PF, David P, Junod G, Brunei C, Dupont R, Quilici S. Importance of competition mechanisms in successive invastions by polyphagous tephritids in La Reunion. Ecol. 2006;87:1770–1780. doi: 10.1890/0012-9658(2006)87[1770:iocmis]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Duyck PF, Kouloussis N, Papadopoulos NT, Quilici S, Carey JR. Exceptional longevity in the tephritid, Ceratitis rosa, a close relative of the Mediterranean fruit fly. 2010 doi: 10.1603/ec11055. submitted. [DOI] [PubMed] [Google Scholar]
- Duyck PF, David P, Quilici S. Can more K-selected species be better invaders? A case study of fruit flies in La Réunion. Diversity and Distributions. 2007;13:535–543. [Google Scholar]
- Fanson BG, Weldon CW, Perez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8:514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The moulding of senescence by natural selection. Jour. of Theor. Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. A systematic look at an old problem. Nature. 2008;451:644–647. doi: 10.1038/451644a. [DOI] [PubMed] [Google Scholar]
- Kouloussis NA, Papadopoulos NT, Müller HG, Wang JL, Mao M, Katsoyannos BI, Duyck PF, Carey JR. Life table assay for assessing relative age bias in medfly capture methods. Entomol experimentalis et applicata. 2009;132:172–181. doi: 10.1111/j.1570-7458.2009.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HG, Wu S, Diamantidis AD, Papadopoulos NT, Carey JR. Reproduction is adapted to survival characteristics across geographically isolated medfly populations. Proc. of the Royal Soc of London. 2009;276:4409–4416. doi: 10.1098/rspb.2009.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HG, Wang JL, Carey JR, Caswell-Chen EP, Chen C, Papadopoulos N, Yao F. Demographic window to aging in the wild: Constructing life tables and estimating survival functions from marked individuals of unknown age. Aging Cell. 2004;3:125–131. doi: 10.1111/j.1474-9728.2004.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HG, Wang JL, Yu W, Delaigle A, Carey JR. Survival in the wild via residual demography. Theor. Popul Biol. 2007;72:513–522. doi: 10.1016/j.tpb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzack SH. How and why do aging and life span evolve? In: Carey JR, Tuljapurkar S, editors. Life Span: Evolutionary, Ecological, and Demographic Perspectives. Suppl. to Popul. and Devel Review. Vol. 29. New York: Population Council; 2003. pp. 19–38. [Google Scholar]
- Papadopoulos N, Katsoyannos BI, Carey JR, Kouloussis NA. Seasonal abundance of the Mediterranean fruit fly (Diptera: Tephritidae) in Northern Greece. Ann. Entomol. Soc of America. 2001;94:41–50. [Google Scholar]
- Papadopoulos NT, Liedo P, Miiller HG, Wang JL, Molleman F, Carey JR. Cost of reproduction in male medflies: The primacy of sexual courting in extreme longevity reduction. Jour. of Insect Physiol. 2010;56:283–287. doi: 10.1016/j.jinsphys.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SH, Heuveline P, Guillot M. Demography: Measuring and Modeling Popul. Processes. Maiden: Blackwell Publishers; 2001. [Google Scholar]
- Rose M, Charlesworth B. A test of evolutionary theories of senescence. Nature. 1980;287:141–142. doi: 10.1038/287141a0. [DOI] [PubMed] [Google Scholar]
- Sherratt TN, Laird RA, Hassall C, Lowe CD, Harvey IF, Watts PC, Cordero-Rivera A, Thompson DJ. Empirical evidence of senescece in adult damselflies (Odonata: Zygoptera) Jour of Animal Ecol. 2010;79:1034–1044. doi: 10.1111/j.1365-2656.2010.01719.x. [DOI] [PubMed] [Google Scholar]
- Vargas RI, Harris EJ, Nishida T. Distribution and seasonal occurence of Ceratitis capitata (Wiedemann) (Diptera:Tephritidae) on the island of Kauai in the Hawaiian Islands. Environ Entom. 1983;12:303–310. [Google Scholar]
- Vaupel JW. Life lived and left: Carey’s equality. Demographic Research. 2009;20:7–10. [Google Scholar]
- Vaupel JW, Carey JR. Compositional interpretations of medfly mortality. Science. 1993;260:1666–1667. doi: 10.1126/science.8503016. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evol. 1957;11:398–411. [Google Scholar]
- Williams PD, Day T, Fletcher Q, Rowe L. The shaping of senescence in the wild. Trends in Ecol and Evol. 2006;21:458–463. doi: 10.1016/j.tree.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Zou S, Carey JR, Liedo P, Ingram DK, Yu B, Ghaedian R. Prolongevity effects of an oregano and cranberry extract are diet-dependent in the Mexican Fruit Fly (Anastrepha ludens) Jour. of Geron.: Biol. Sci. 2009;65A:41–50. doi: 10.1093/gerona/glp176. [DOI] [PMC free article] [PubMed] [Google Scholar]