Abstract
Neoplastic cells are genetically unstable. Strategies that target pathways affecting genome instability can be exploited to disrupt tumor cell growth potentially with limited consequences to normal cells. Chromosomal instability (CIN) is one type of genome instability characterized by mitotic defects that increase the rate of chromosome mis-segregation. CIN is frequently caused by extra centrosomes that transiently disrupt normal bipolar spindle geometry needed for accurate chromosome segregation. Tumor cells survive with extra centrosomes because of biochemical pathways that cluster centrosomes and promote chromosome segregation on bipolar spindles. Recent work shows that targeted inhibition of these pathways prevents centrosome clustering and forces chromosomes to segregate to multiple daughter cells, an event triggering apoptosis that we refer to as anaphase catastrophe. Anaphase catastrophe specifically kills tumor cells with more than two centrosomes. This death program can occur after genetic or pharmacologic inhibition of cyclin dependent kinase 2 (Cdk2) and is augmented by combined treatment with a microtubule inhibitor. This proapoptotic effect occurs despite the presence of ras mutations in cancer cells. Anaphase catastrophe is a previously unrecognized mechanism that can be pharmacologically induced for apoptotic death of cancer cells. This is an appealing mechanism to engage for cancer therapy and prevention.
Keywords: anaphase catastrophe, cyclin E-Cdk2, multipolar anaphase, anti-neoplastic target
Background
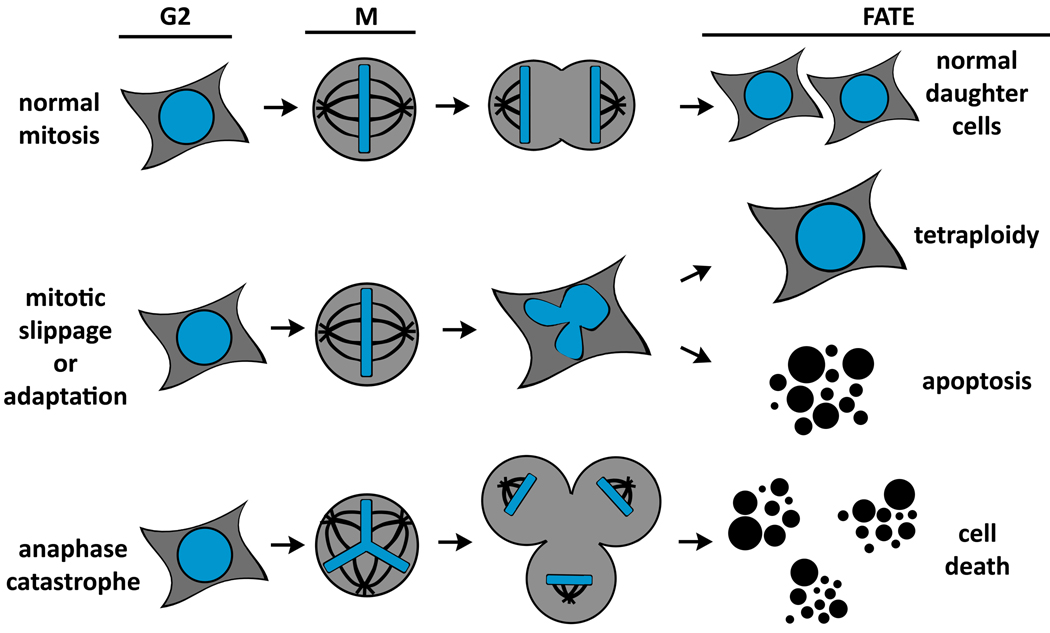
Anaphase catastrophe is a pro-apoptotic death mechanism observed in cancer cells with extra centrosomes that segregate chromosomes in the presence of multipolar spindles (1). The number of spindle poles in mitosis is determined by centrosomes, discrete organelles that nucleate spindle microtubules. Centrosome copy number is under strict cell cycle regulation. Centrosomes duplicate in S-phase so that normal cells enter mitosis with two centrosomes and equally segregate replicated chromosomes using bipolar spindles (Figure 1A and 2A) (2). Cells with extra centrosomes can undergo anaphase with multipolar spindles and segregate chromosomes improperly to more than two daughter cells (Figures 1C and 2B). This is a lethal event for each daughter cell (3, 4) that we call anaphase catastrophe. Anaphase catastrophe selectively targets cancer cells with extra centrosomes and spares normal cells that enter mitosis with only two centrosomes and, therefore, are incapable of segregating chromosomes to more than two spindle poles.
Figure 1. Fates of mitotic cells.
Cells can undergo diverse fates according to their status at anaphase. (A) Proper segregation of chromosomes in mitosis leads to the generation of two genetically identical daughter cells. (B) Gradual degradation of cyclin B in the presence of prolonged spindle checkpoint activation causes cells to exit mitosis without dividing chromosomes in anaphase, termed slippage. Cells that exit mitosis via slippage enter G1 as tetraploid cells. These cells may continue to cycle, senesce, or undergo apoptosis. (C) Anaphase catastrophe occurs when a cell with multiple centrosomes fails to coalesce centrosomes into two spindle poles and enters anaphase with a multipolar spindle. Segregation of chromosomes to more than two daughter cells causes cell death.
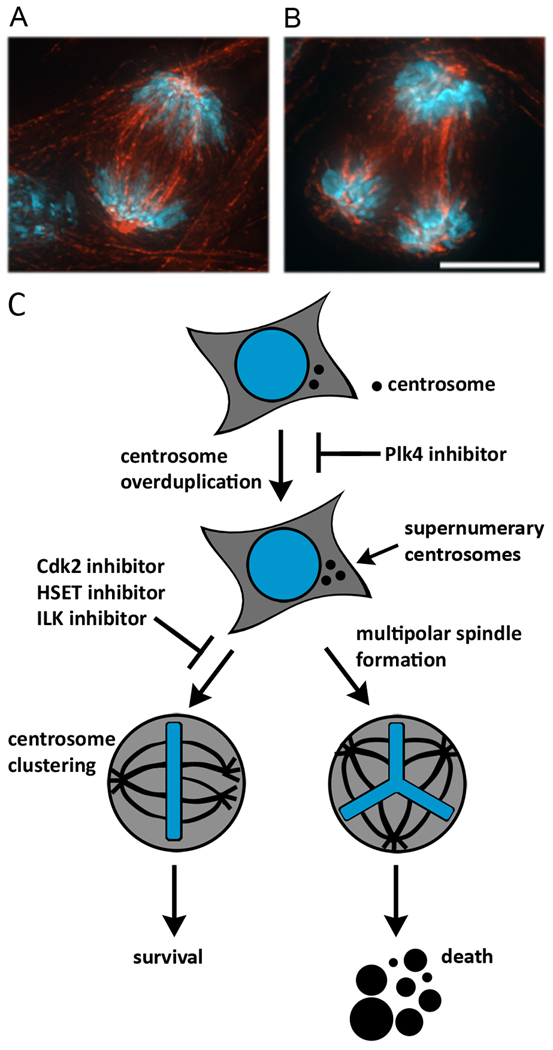
Figure 2. Inhibition of Cdk2 and other pathways triggers anaphase catastrophe in lung cancer cells.
Mouse lung cancer cells overexpressing cyclin E were treated with control siRNA (A) or Cdk2 targeting siRNA (B) for 24 hr. Cells were stained for microtubules in red and DNA in blue. A representative bipolar anaphase (A) and a representative multipolar anaphase leading to anaphase catastrophe (B) are shown. Scale bar, 10µm. (C) Pharmacologic or genetic inhibition of numerous pathways affects mitotic fidelity and can trigger anaphase catastrophe leading to cell death. Centrosome amplification leads to supernumerary centrosomes. This can be prevented with a Plk4 inhibitor. Centrosome clustering enables cancer cells to survive through mitosis in the presence of supernumerary centrosomes. Inhibition of Cdk2, HSET, or ILK inhibits centrosome clustering, resulting in multipolar spindle formation. This causes multipolar anaphases that trigger anaphase catastrophe.
Targeting Chromosomally Unstable Cancer Cells
Recent work revealed a mechanism that induces cell death preferentially in cancer cells with chromosomal instability (CIN) (1). This mechanism can be exploited therapeutically. CIN is common in aneuploid tumor cells and is usually caused by the persistence of inappropriate attachments of chromosomes to spindle microtubules (5–7). The prevalence of attachment errors increases sharply in cells with extra centrosomes, owing to the key role of centrosomes in determining the number of spindle poles during mitosis (3, 8). Cancer cells gain extra copies of centrosomes either from failure of cytokinesis or by deregulation of the strict cell cycle control of centrosome duplication. Because chromosome segregation is vital for cell survival, cancer cells with extra centrosomes assemble bipolar spindles in mitosis by clustering supernumerary centrosomes together at spindle poles (3, 4, 8).
There are many biochemical pathways that promote centrosome clustering during mitosis. Initial evidence shows that these pathways can be pharmacologically targeted to induce anaphase catastrophe (Figure 2C). A genome-wide screen using Drosophila cultured cells identified 133 distinct genes required for centrosome clustering (4). The products of these genes participate in diverse cellular processes including regulation of the actin cytoskeleton, spindle assembly, spindle assembly checkpoint (SAC) activity, and cell adhesion. Positive hits in that screen that represent potential targets for inhibition include the mitotic kinesin HSET, myosin 10A, and the enzyme tankyrase, which modifies proteins involved in spindle pole organization. HSET is not required for mitosis in normal somatic cells with two centrosomes (9). Loss of HSET function in cells with supernumerary centrosomes can induce anaphase catastrophe specifically within cells having extra centrosomes (4). This finding provides a proof of principle that targeted inhibition of these enzymes causes anaphase catastrophe and justifies the search for inhibitors of these enzymes or other targets that might cause anaphase catastrophe. The power of the genetic screens is offset by the fact that the strategy will only identify target genes whose products function at a specific phase of the cell cycle. Other biochemical pathways might participate in centrosome clustering. These represent additional opportunities for inducing anaphase catastrophe. As one example, lung cancers often overexpress cyclin E that can deregulate cyclin-dependent kinase (Cdk) activity (10). Targeting Cdk2, one of these deregulated kinases, triggers anaphase catastrophe (1).
Cell viability relies on the equal separation of replicated chromosomes during mitosis. Mitotic fidelity is enhanced by the SAC (11). The SAC prevents sister chromatid separation and anaphase onset until all chromosomes form appropriate bipolar attachments to spindle microtubules. A single unaligned or unattached chromosome is sufficient to maintain SAC activity and prevent entry into anaphase (12, 13). Thus, there is a defined sequence of events that is strictly followed to ensure accurate chromosome segregation in normal cells. Chromosomes form attachments to spindle microtubules and align at the equator of a bipolar spindle. That satisfies the SAC leading to activation of the ubiquitin ligase APC/C (anaphase promoting complex/cyclosome). APC/C subsequently triggers degradation of securin and the mitotic cyclin B to induce sister chromatid separation and exit from mitosis, respectively, so that daughter cells enter G1 phase of the next cell cycle with appropriate numbers of chromosomes (14).
Anaphase Catastrophe versus Mitotic Catastrophe
Chemotherapeutic drugs such as paclitaxel disrupt microtubule dynamics and chromosome attachment to spindle microtubules (15). This prolongs mitosis by preventing satisfaction of the SAC. The cellular response to prolonged mitotic arrest varies depending on the cell line and even among cells within the same cell line; the outcome of this response depends on processes that regulate cyclin B levels (16, 17). Cyclin B quantities gradually decrease and apoptotic signals gradually increase in mitotic cells. In some cells, mitosis can be sufficiently prolonged by anti-mitotic drugs. These promote apoptotic signals that can exceed a critical threshold and induce death in mitosis. In other cells, cyclin B levels drop below a critical threshold during mitotic arrest and cells exit mitosis and re-enter G1 as tetraploid cells without chromosome segregation. These tetraploid cells typically enter senescence or undergo apoptosis (Figure 1B), although some propagate and endocycle (16). Exit from mitosis without chromosome segregation following extended mitotic arrest has been termed adaptation because cells are said to ‘adapt’ to prolonged checkpoint activity (18). Perhaps a more appropriate term for this is mitotic slippage since cells slip out of mitosis without satisfying the SAC (17, 19). Notably, mitotic slippage or adaptation violates the temporal sequence of events needed for proper chromosome segregation because cells enter G1 of the next cell cycle without satisfying the SAC or adequately activating APC/C. Cancer cells with CIN are no more likely to continue cycling following mitotic slippage than are diploid cells (16), indicating that this alone does not selectively kill tumor cells. Nonetheless, it is proposed that substantial DNA damage conferred by chemotherapeutic agents or mutation of DNA damage response genes can promote cell death during mitosis or mitotic slippage (collectively known as mitotic catastrophe in (20)) of cancer cells. Cells that enter anaphase with multipolar spindles abide by the appropriate temporal sequence of biochemical events for mitotic exit and only initiate chromosome separation after all chromosomes are attached to the spindle. This is mechanistically distinct from mitotic slippage or adaptation, which is why we termed this anaphase catastrophe.
Clinical-Translational Advances
An established paradigm for cancer therapy involves targeting and killing dividing cells. Many chemotherapeutic agents target dividing cells during mitosis. This is a sensitive window in the cell cycle where chromosomes align and separate to form genetically identical daughter cells. Taxanes and vinca alkaloids successfully kill tumor cells during mitosis by targeting microtubules and disrupting normal chromosome movement. However, these drugs are not specific to cancer cells and disrupt microtubules in all cells leading to side effects such as neutropenia and neurotoxicity (15). Nevertheless, these drug effects establish that mitotic disruption is engaged by chemotherapy treatments. Based on this, compounds are being developed that inhibit proteins that only function during mitosis and would not target non-dividing cells. Clinical trials are underway to explore the efficacy of inhibiting molecular motors required for bipolar spindle organization (21) as well as of inhibitors of the essential Aurora and Polo-like kinases (22, 23). Yet, these drugs target actively dividing cells and do not necessarily exploit key differences between malignant and benign cells that might spare normal cells. For example, cells in most solid tumors are aneuploid with chromosome numbers that deviate from a multiple of the haploid genome. The role of aneuploidy in tumorigenesis is under study through experiments conducted in clinically-relevant animal models (24, 25). To date, those efforts have not identified specific treatment strategies that would selectively target aneuploid cells and spare diploid cells. However, the recent insights into the causes of CIN (3, 26) described above revealed that the pro-apoptotic mechanism of anaphase catastrophe can be pharmacologically targeted to selectively kill tumor cells.
We recently showed that targeted depletion of Cdk2, but not Cdk1 induced anaphase catastrophe in lung cancer cells; current work is elucidating Cdk2 targets that mediate this effect (Figure 2B; (1)). Cdk inhibitors exist and some such as flavopiridol and UCN-01 exert some clinical anti-tumor activity (27). Another inhibitor (seliciclib, CYC202, R-roscovitine) can preferentially inhibit Cdk2 at low concentrations, whereas at higher concentrations inhibition of Cdk1, Cdk7, or Cdk9 is observed (28). Anaphase catastrophe is induced when cells with extra centrosomes are exposed to seliciclib at dosages that should only target Cdk2 (1). Similar concentrations had minimal mitotic effects on immortalized pulmonary epithelial cells, implying differential activity against cancer cells (1). Even transient exposures (4 hours) to a Cdk2 inhibitor increased the number of cells undergoing anaphase catastrophe, suggesting that the drug acts by inhibiting cyclin-dependent kinase activity during mitosis and not during centrosome duplication in S phase (1). These and other findings provide a proof-of-principle for anaphase catastrophe induction after treatment with specific drugs. Future work should elucidate whether mechanisms seen in vitro can be observed in vivo via proof-of-principle clinical trials.
Consistent with this concept was the finding that integrin-linked kinase (ILK) activity is essential for centrosome clustering in cancer cells (29). ILK regulates actin and cell adhesion at focal adhesion sites as well as microtubule-associated components during mitosis likely through regulation of Aurora A/TACC3/TOG activity (30). Small molecule inhibitors of ILK can induce anaphase catastrophe within breast cancer cells with extra centrosomes, but not within normal cells or cancer cells without extra centrosomes (31). Therapeutic strategies that inhibit key target enzymes or pathways responsible for centrosome clustering are being uncovered that will selectively kill cancer cells with extra centrosomes while sparing normal cells. An important future direction for study is to identify downstream targets of Cdk2 and ILK that are essential for centrosome clustering. That search would likely reveal other candidate targets that induce anaphase catastrophe.
Intriguingly, drugs that induce anaphase catastrophe appear to cooperate with taxanes. Taxanes are routinely used in the treatment of breast, bladder, ovarian, head and neck, and lung cancer (15). These agents specifically target microtubules and disrupt the normal timing of mitosis by delaying the satisfaction of the SAC. Treatment of cells with combinations of either the Cdk2 inhibitor seliciclib and taxanes or the ILK inhibitor QLT-0267 and taxanes showed significant increases in cancer cell death (1, 31). In the case of combining seliciclib and taxanes, this increase was linked to augmented anaphase catastrophe (1). These combinations likely force cells to undergo catastrophic anaphase more efficiently than with either treatment alone. Combining an agent that induces anaphase catastrophe with a microtubule-targeting drug is an attractive regimen to consider in future clinical trials for appropriate cancers.
In this regard, pharmacogenomic analysis revealed that lung cancer cells with k-ras mutations are especially sensitive to Cdk2 inhibition (1). K-ras mutation typically predicts resistance to an epidermal growth factor-tyrosine kinase inhibitor (EGFR-TKI) (32). This mutation is found in most pancreatic cancers, in about 30% of lung cancers, and in many other cancers (33, 34). This finding suggests a regimen that augments anaphase catastrophe is appealing to consider for treating cancers that harbor ras mutations.
In conclusion, recent findings reveal anaphase in mitotic cells with multipolar spindles is a lethal event that is pharmacologically conferred. Anaphase catastrophe would selectively kill cancer cells with extra centrosomes and likely spare normal cells. This is a unique pathway identified in mitosis that could discriminate between cancerous and normal cells. Thus, anaphase catastrophe is a novel anitneoplastic mechanism to engage for cancer therapy and prevention.
Acknowledgements
This work was supported by National Institutes of Health (NIH) and National Cancer Institute (NCI) grants RO1-CA087546 (E.D.) and RO1-CA111422 (E.D.) as well as by RO1-GM51542 (D.A.C.), T32-GM008704 (S.L.T.) and a Samuel Waxman Cancer Research Foundation Award (E.D.). E. Dmitrovsky is an American Cancer Society Clinical Research Professor supported by a generous gift from the F. M. Kirby Foundation.
Footnotes
Disclosure of Potential Conflict of Interest
E. Dmitrovsky previously received a research grant from Cyclacel. The other authors disclosed no potential conflicts of interest.
References
- 1.Galimberti F, Thompson SL, Liu X, et al. Targeting the cyclin E-Cdk-2 complex represses lung cancer growth by triggering anaphase catastrophe. Clin. Cancer Res. 2010;16:109–120. doi: 10.1158/1078-0432.CCR-09-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doxsey S. Re-evaluating centrosome function. Nat Rev Mol Cell Biol. 2001;2:688–698. doi: 10.1038/35089575. [DOI] [PubMed] [Google Scholar]
- 3.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon M, Godinho SA, Chandhok NS, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22:2189–2203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhoum SF, Genovese G, Compton DA. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–129. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- 9.Mountain V, Simerly C, Howard L, Ando A, Schatten G, Compton DA. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 1999;147:351–366. doi: 10.1083/jcb.147.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonardo F, Rusch V, Langenfeld J, Dmitrovsky E, Klimstra DS. Overexpression of cyclins D1 and E is frequent in bronchial preneoplasia and precedes squamous cell carcinoma development. Cancer Res. 1999;59:2470–2476. [PubMed] [Google Scholar]
- 11.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 12.Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters J. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 15.Zelnak AB. Clinical pharmacology and use of microtubule-targeting agents in cancer therapy. Methods Mol. Med. 2007;137:209–234. doi: 10.1007/978-1-59745-442-1_15. [DOI] [PubMed] [Google Scholar]
- 16.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanni JS, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol. Cell. Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Castedo M, Perfettini J, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 21.Sakowicz R, Finer JT, Beraud C, et al. Antitumor activity of a kinesin inhibitor. Cancer Res. 2004;64:3276–3280. doi: 10.1158/0008-5472.can-03-3839. [DOI] [PubMed] [Google Scholar]
- 22.Warner SL, Stephens BJ, Von Hoff DD. Tubulin-associated proteins: Aurora and Polo-like kinases as therapeutic targets in cancer. Curr Oncol Rep. 2008;10:122–129. doi: 10.1007/s11912-008-0020-0. [DOI] [PubMed] [Google Scholar]
- 23.Schvartzman J, Sotillo R, Benezra R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat. Rev. Cancer. 2010;10:102–115. doi: 10.1038/nrc2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foijer F, Draviam VM, Sorger PK. Studying chromosome instability in the mouse. Biochim. Biophys. Acta. 2008;1786:73–82. doi: 10.1016/j.bbcan.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr. Biol. 2010;20:R285–R295. doi: 10.1016/j.cub.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 28.Collins I, Garrett MD. Targeting the cell division cycle in cancer: CDK and cell cycle checkpoint kinase inhibitors. Curr Opin Pharmacol. 2005;5:366–373. doi: 10.1016/j.coph.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Fielding AB, Lim S, Montgomery K, Dobreva I, Dedhar S. A critical role of integrin-linked kinase, ch-TOG and TACC3 in centrosome clustering in cancer cells. [cited 2010 Sep 21];Oncogene. 2010 doi: 10.1038/onc.2010.431. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20838383. [DOI] [PubMed] [Google Scholar]
- 30.Fielding AB, Dobreva I, McDonald PC, Foster LJ, Dedhar S. Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J. Cell Biol. 2008;180:681–689. doi: 10.1083/jcb.200710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalra J, Warburton C, Fang K, et al. QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model. Breast Cancer Res. 2009;11:R25. doi: 10.1186/bcr2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin. Cancer Res. 2007;13:2890–2896. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 33.Aviel-Ronen S, Blackhall FH, Shepherd FA, Tsao M. K-ras mutations in non-small-cell lung carcinoma: a review. Clin Lung Cancer. 2006;8:30–38. doi: 10.3816/CLC.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 34.Quinlan MP, Settleman J. Explaining the preponderance of Kras mutations in human cancer: An isoform-specific function in stem cell expansion. Cell Cycle. 2008;7:1332–1335. doi: 10.4161/cc.7.10.5927. [DOI] [PubMed] [Google Scholar]