Introduction
In the United States, the costs of diabetes mellitus are increasing. The cost of medical care for people with diagnosed diabetes increased from $1 billion per year in the 1970s to $116 billion per year in 2007. (1,2) Although people with diabetes comprise <6% of the U.S. population, approximately one in five health care dollars is spent caring for people with diabetes. (2) In 2007, the annual per capita health care expenditure for a person with diabetes was $11,700 and for a person without diabetes was $2,900. (2) The unadjusted per capita cost ratio for a person with diabetes compared to one without diabetes is 4.0. (2) People with diabetes are older than people without diabetes and health care costs increase with age, but even after adjusting for age, the per capita cost ratio is 2.3. (2)
Healthy lifestyle interventions for the general population and intensive lifestyle and medication interventions for high-risk individuals present opportunities for diabetes prevention. The entire population is at risk for diabetes. One in three Americans born today will develop diabetes over his or her lifetime. (3) To prevent diabetes, everyone in the population should be encouraged to eat a healthy diet, be physically active, and maintain an optimal weight. This approach, termed primordial prevention, seeks to address the underlying causative risk factors for disease by changing environmental conditions and social values to encourage positive health behaviors among children, adolescents, and young adults. Observational studies have suggested that both favorable and unfavorable changes in lifestyles are related to population level changes in disease burden. (4) Unfortunately, a recent large clinical trial of a school-based intervention program that involved changes in the schools’ food service environment and gym programs, and behavioral interventions to support healthy diet and physical activity at home had only a modest impact on risk factors for type 2 diabetes. (5)
More intensive, targeted interventions are appropriate for individuals who are at higher risk for diabetes including people who are older, obese, or who have impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or HbA1c levels indicative of increased risk for future diabetes. This approach, termed primary prevention, includes screening to identify high risk individuals and both intensive lifestyle interventions that may entail substantial costs and/or the use of medications that incur costs or may in fact cause harm. Randomized, controlled clinical trials from Asia, Europe, and North America have demonstrated that treating people who have glucose intolerance with intensive lifestyle interventions or medications can delay or prevent the development of type 2 diabetes. (6–11) In this article, we describe the costs of glucose intolerance and diabetes, the impact of glucose intolerance and diabetes on quality-of-life, and the cost-effectiveness of screening and primary prevention interventions for diabetes prevention.
The costs of glucose intolerance and diabetes
The costs of medical care increase along the continuum from normal glucose tolerance to glucose intolerance to diabetes mellitus to diabetes with complications. Compared to age- and sex-matched people without a future diagnosis of diabetes, people destined to develop diabetes experience increased medical costs for several years before the clinical diagnosis of diabetes mellitus. (12) Costs accelerate in the three years before diagnosis and in the final year before diagnosis, costs are nearly twice those of the previous year. (12) For several years before diagnosis, annual pharmacy costs are significantly greater for people who will develop diabetes, primarily related to the use of antihypertensive agents, antihyperlipidemic medications, and other cardiovascular drugs. (12) Outpatient costs for those destined to develop diabetes also increase gradually in the years before diagnosis and increased substantially in the year prior to diagnosis. (12) Although more variable, inpatient costs are also higher and account for the majority (53%) of the total additional costs. (12)
Studies that have examined costs of care in patients with IFG, IGT, or both compared to those with normal glucose tolerance have confirmed this finding. Mean annual direct medical costs are higher in persons with isolated IFG, isolated IGT, and both IFG and IGT compared to normoglycemic patients. (13) In general, differences are driven by inpatient costs. Microvascular complications add almost $1,900 and macrovascular complications add almost $3,900 to the annual age- and sex-adjusted per person medical costs of people with glucose intolerance compared to normoglycemic controls. (14)
The costs of medical care for people with diabetes are still higher than for people with normal glucose tolerance or glucose intolerance. In 2008, the American Diabetes Association published a cost-of-illness study to quantify the economic burden of diabetes. (2) The direct medical cost of diabetes (costs related to the treatment of diabetes, its complications and comorbidities, and general medical care) in the U.S. in 2007 was estimated to be $116 billion. The largest proportion of direct medical costs was incurred by people 65 years of age and older (56% of total direct medical costs). Thirty-five percent of total direct medical costs were incurred by people 45–64 years of age and 9% by people <45 years of age. The largest components of direct medical costs were for hospital inpatient care and nursing home care (56% of total direct medical costs). Lesser proportions went to pharmacy and supplies (24% of direct medical costs) and outpatient care (20% of direct medical costs). What can be inferred from the ADA's descriptive cost analysis is that much of the direct medical cost of diabetes is incurred by older patients with long standing diabetes and is attributable to long-term complications and comorbidities requiring hospital or nursing home care.
Additional studies have confirmed that a substantial proportion of the costs of diabetes arise from treating long-term complications, particularly cardiovascular and renal disease. (15) Per person costs increase over baseline by more than 50% after initiation of cardiovascular drug therapy (antihypertensives, antihyperlipidemics, digoxin, and antianginal medications) and/or co-management by a cardiologist. Costs increase by 360% after a major cardiovascular event such as stroke, myocardial infarction, revascularization procedure, or hospitalization for congestive heart failure. Abnormal renal function also increases the costs of diabetes care. (15) Microalbuminuria is associated with a 65% increase in costs, renal insufficiency (defined by estimated glomerular filtration rate <50 ml/min on at least 2 separate occasions) with a 195% increase, and end-stage renal disease (defined as long-term hemodialysis or renal transplantation) with a 771% increase in costs.
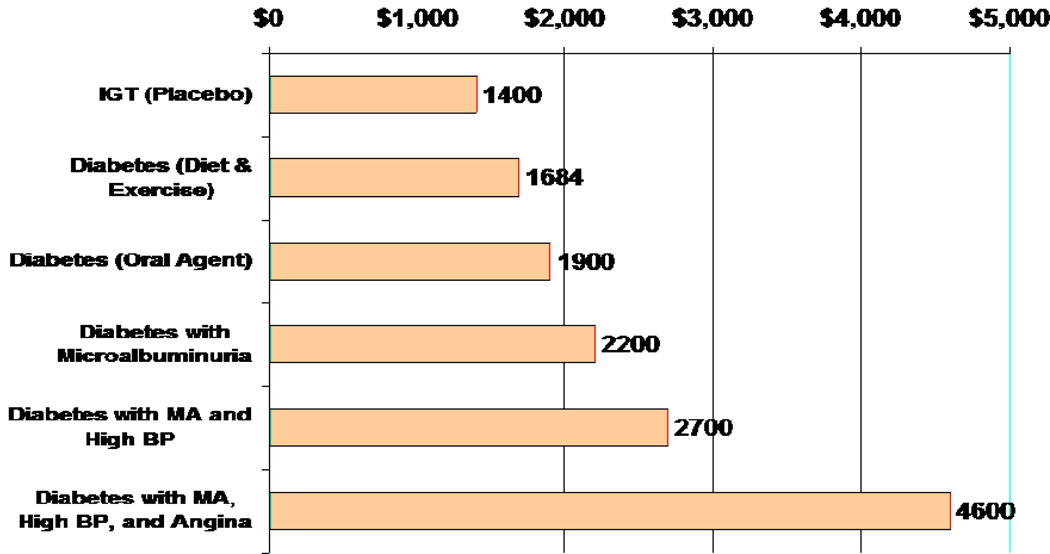
We have modeled cross-sectional data from a large population with type 2 diabetes to estimate the annual direct medical costs of care for people with glucose intolerance, diabetes, and diabetes with complications and comorbidities. (16) Figure 1 illustrates the annual direct medical costs for a man as he might progress from impaired glucose tolerance to diabetes to diabetes with complications and comorbidities. With progression from impaired glucose tolerance to diabetes treated with diet and exercise alone, to diabetes treated with an oral agent, to diabetes with complications and comorbidities, costs increase exponentially.
Figure 1. Annual Direct Medical Costs in a Man Progressing from IGT to Diabetes with Complications.
Adapted from Brandle M, Zhou H, Smith BRK, Marriott D, Burke R, Tabaei BP, Brown MB, Herman WH. The direct medical cost of type 2 diabetes mellitus. Diabetes Care 2003;26:2300–2304.
The costs of diabetic complications generally increase as a function of duration of diabetes. (17) In an analysis that used a simulation model to project the lifetime costs of complications resulting from type 2 diabetes in the U.S., macrovascular disease was the earliest and the largest cost component of the complications of diabetes, accounting for 85% of the cumulative cost of complications over the first 5 years and 52% of the costs over 30 years of diabetes. Microvascular and neuropathic complications contributed only about 15% to the cost of complications in the first 5 years of diabetes. They were, however, more important contributors to the late costs of type 2 diabetes. At 30 years duration of diabetes, nephropathy accounted for 21% of the costs of complications, neuropathy 17%, and retinopathy 10%. These results suggest that interventions to delay or prevent the development of diabetes have the potential to reduce the economic burden of cardiovascular comorbidities and delay or even prevent the costs of microvascular and neuropathic complications.
The quality-of-life impact of glucose intolerance and diabetes
In health-economic analyses, quality-of-life is assessed with the health-utility score where 1.0 represents perfect health and 0 represents health states equivalent to death. Scores are assigned to reflect the public’s preference for each health state. In general, quality-of-life decreases along the continuum from normal glucose tolerance to glucose intolerance to diabetes to diabetes with complications. A large portion of the decrement in quality-of-life associated with glucose intolerance compared to normal glucose tolerance appears to be related to obesity. A study of health-utility scores in an obese cohort (mean BMI 41.8 ± 6.7 kg/m2) compared to an age- and sex-matched non-obese cohort demonstrated lower mean utility scores in the obese group for each age category. (18) In another study of patients ≥45 years of age, health-utility scores were lower in obese patients (BMI ≥30 kg/m2) than in normal weight patients (BMI 18.5 - <25 kg/m2) even after controlling for patient characteristics (age, sex, and smoking status) and comorbidities (asthma, diabetes, stroke, heart disease, pain, arthritis, and cancer). (19)
In a study of health-utility scores in people without and with type 2 diabetes, BMI was lower for patients without diabetes (27.2 ± 5.1 kg/m2) compared to those with type 2 diabetes (28.9 ± 5.7 kg/m2). (20) In both the nondiabetic and type 2 diabetic patients, health-utility scores were correlated with BMI and decreased with increasing BMI category (normal, overweight, obese, and extremely obese). (20) The rate of change of utility scores attributable to BMI were not significantly different between groups even after adjusting for other known confounding factors. (20) This suggests that both obesity and diabetes have a significant negative effect on health utility scores.
Studies have also documented decrements in health utility scores for individuals with diabetes-related complications and comorbidities. In the United Kingdom Prospective Diabetes Study (UKPDS), diabetic subjects with microvascular complications had slightly, but not significantly lower health utility scores than diabetic patients without complications, and diabetic patients with macrovascular complications had significantly lower health utility scores than those without complications. (21) Among Dutch type 2 diabetic patients, older age, female sex, obesity, insulin therapy, and the presence of complications were associated with lower health utility scores. (22) Among Swedish patients with diabetic foot complications, those with foot ulcers and major amputations had lower health utility scores than those with primary healed ulcers. (23)
A recent study of health-related quality-of-life in patients with type 2 diabetes found that major diabetes-related comorbidities including stroke and/or transient ischemic attack, hospital admission for unstable angina, myocardial infarction, and peripheral revascularization and/or amputation had a major impact on utility scores. (24) More minor diabetes-related comorbidities and complications like currently treated hypertension and diabetic eye disease had a lesser impact. (24)
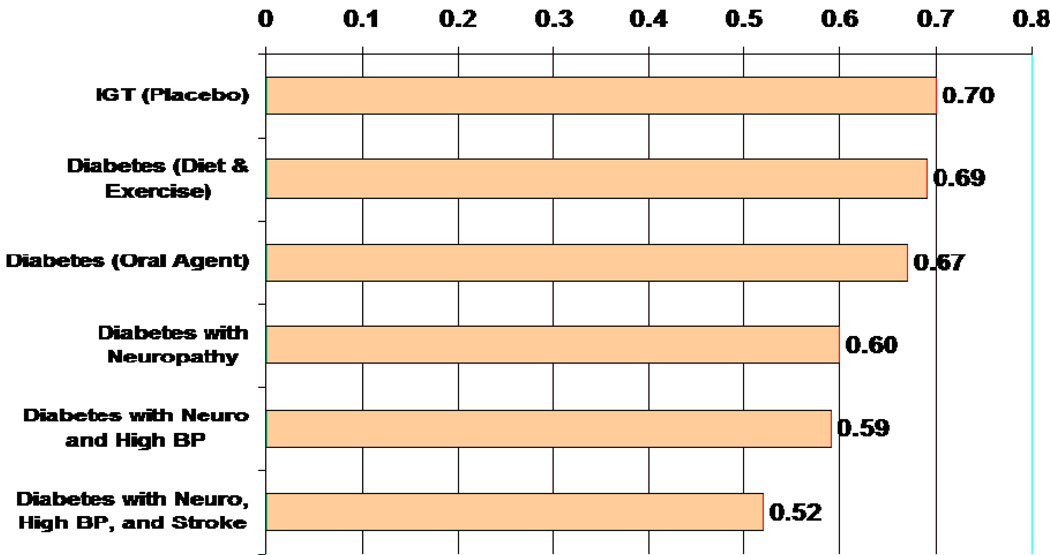
We have modeled cross-sectional data from a large population with type 2 diabetes to estimate health utility scores for people with glucose intolerance, diabetes, and diabetes with complications and comorbidities. (25) Figure 2 illustrates the health-utility scores for a man as he might progress from impaired glucose tolerance to diabetes to diabetes with complications and comorbidities. With progression from impaired glucose tolerance to diabetes treated with diet and exercise, to diabetes treated with an oral agent, to diabetes with complications and comorbidities, there is a progressive stepwise decrement in health-utility scores.
Figure 2. Health Utility Scores in a Man Progressing from IGT to Diabetes with Complications.
Adapted from Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, Engelgau MM, Kaplan R, Herman WH. Valuing health-related quality of life in diabetes. Diabetes Care 2002;25:2238–2243.
Cost-effectiveness analysis
Cost-effectiveness analysis is a tool that compares the costs and outcomes obtained with alternative treatments and shows the difference in cost per unit of health outcome obtained. (26) It shows the economic trade-offs of one treatment strategy compared to another and provides a measure of the value obtained with alternative treatment strategies for the money spent. (26) There are three types of economic analyses that explicitly compare costs and outcomes. (26) They differ in how outcomes are expressed. In cost-benefit analysis, outcomes are expressed in financial terms (dollars). A limitation of cost-benefit analysis is that it is often difficult to ascribe financial value to clinical outcomes such as disease, disability, and death. In cost-effectiveness analysis, outcomes are expressed in usual clinical terms such as cases of disease, complications, or comorbidities prevented, or years of life gained. A limitation of cost-effectiveness analysis is that analyses that report different clinical outcomes cannot be directly compared. A third type of analysis that addresses the limitations of both cost-benefit and cost-effectiveness analyses is cost-utility analysis. In cost-utility analysis, the outcome is expressed with a standardized metric, the quality-adjusted life-year (QALY), that can be compared across disease states. The QALY assesses both quality-of-life and length of life. QALYs are calculated as the sum of the product of the utility-score for each health state times the years of life that an individual lives in each health state.
In general, cost-utility analyses are most appropriate for comparing interventions in health and medicine. (26) The cost-utility of an intervention compared with usual care is defined as the difference in cost divided by the difference in health outcomes associated with the two approaches to treatment. The difference in cost is calculated as the cost associated with the intervention minus the cost associated with usual care and the difference in health outcomes is calculated as the QALYs accrued with the intervention minus the QALYs accrued with usual care. This measure, termed the incremental cost-effectiveness ratio (ICER), provides a measure of the value obtained for the money spent.
The cost-utility of diabetes prevention
The cost-utility of diabetes prevention is influenced by the costs and quality-of-life associated with the alternative interventions, the effectiveness of the interventions in delaying or preventing the development of diabetes and its microvascular and neuropathic complications, the impact of the interventions on cardiovascular risk factors and cardiovascular disease, the long-term safety of the interventions, and the costs and quality of life associated with the achieved health outcomes.
In the Diabetes Prevention Program (DPP), a variety of strategies were used to identify and recruit high risk patients. The lifestyle intervention involved a healthy, low-calorie, low-fat diet and moderate physical activity, such as brisk walking. The lifestyle intervention was implemented with a 16-lesson core curriculum covering diet, exercise, and behavior modification that was taught by case managers on a one-on-one basis, followed by individual sessions (usually monthly) and group sessions with case managers. The metformin and placebo interventions were initiated at a dosage of 850 mg once a day. At 1 month, the dosage of metformin or placebo was increased to 850 mg twice daily. Case managers reinforced adherence during individual quarterly sessions. All participants received standard lifestyle recommendations through written information and an annual 20- to 30-minutes individual session that emphasized the importance of a healthy lifestyle.
The potential value of interventions to prevent diabetes can be best understood by reviewing the DPP Research Group’s simulation of the impact of intensive lifestyle intervention and metformin on the lifetime incidence of diabetes among high risk persons with IGT (Figure 3). (27) If the entire DPP cohort were treated with the placebo intervention, approximately 50% would develop diabetes within 7 years. In contrast, it would take approximately 18 years for 50% of intensive lifestyle intervention-treated participants to develop diabetes and 10 years for 50% of metformin-treated participants to develop diabetes. Thus, compared with the placebo intervention, the lifestyle intervention delays the onset of diabetes by 11 years and the metformin intervention delays the onset of diabetes by 3 years. Over a lifetime, 83% of participants treated with the placebo intervention would develop diabetes as compared to 63% of those treated with the intensive lifestyle intervention and 75% of those treated with the metformin intervention. Thus, compared to the placebo intervention, the intensive lifestyle intervention reduces the absolute risk for developing diabetes by 20% and the metformin intervention reduces the absolute risk for developing diabetes by 8%. Since the risk of microvascular and neuropathic complications of diabetes, and to a lesser degree, the macrovascular complications of diabetes occur as a function of duration of diabetes (17), delaying the onset and preventing the development of diabetes substantially reduce the negative quality of life impact of diabetes treatment and the cost of both complications and comorbidities.
Figure 3. Simulated Cumulative Incidence of Diabetes in the DPP.
From Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, Hamman RF, Ackermann RT, Engelgau MM, Ratner RE. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332.
A number of investigators have assessed the cost-effectiveness of lifestyle and/or medication interventions compared to usual care for the primary prevention of type 2 diabetes. (27–33) A prospective economic analysis conducted by the DPP Research Group estimated that case finding cost approximately $140 per subject randomized. (34) The lifestyle intervention for diabetes prevention cost approximately $1,400 per person in its first year and approximately $700 per person per year thereafter. (34) Another analysis of a community-based lifestyle intervention program implemented through Young Men’s Christian Associations (YMCA) found that the cost of the intervention was substantially less, approximately $300 per person per year. (35) The average wholesale price of metformin at the dose used in the DPP was approximately $300 per year. (34) The cost of acarbose as used in the Stop-NIDDM Trial was approximately $1,400 per person per year (32) and the cost of rosiglitazone as used in the DREAM trial was approximately $2,000 per person per year. It is important to note, however, that metformin is now available as a generic medication and acarbose and thiazolidinediones will become generic. The usual cost of a generic medication is approximately 25% that of the brand medication cost. (27)
With respect to quality of life, the DPP Research Group demonstrated that quality of life is better with the intensive lifestyle intervention compared to metformin or usual care, and no different with metformin relative to usual care. (36) Clinical trials of acarbose and rosiglitazone for diabetes prevention have not included prospective utility assessments. The quality of life associated with these interventions may be no different than that associated with usual care, but it is possible that the gastrointestinal side effects associated with acarbose (10) and the weight gain associated with rosiglitazone (11) might be associated with some decrement in quality of life.
With respect to intervention effectiveness, it is clear that the lifestyle intervention and thiazolidinediones are most effective in preventing diabetes with relative risk reductions of between 29 and 58% for lifestyle interventions (6–9) and 55% and 60% (8,11) for thiazolidinediones. Metformin reduced the relative risk for development of diabetes by 26 to 31% (8,9) and acarbose by 25%. (10)
With respect to long-term health outcomes, the DPP demonstrated improved cardiovascular intermediate outcomes (blood pressure and lipids) associated with lifestyle and metformin interventions but no clear impact of these interventions on cardiovascular events (stroke, myocardial infarction) or survival. (8,37) The Stop-NIDDM study showed a statistically significant impact of acarbose on the incidence of cardiovascular disease but has not reported a survival benefit. (38)
With respect to intervention safety, the DPP and the Stop-NIDDM study suggested that lifestyle intervention, metformin and acarbose are safe. (8,10) Both increased risks of heart failure (11) and fractures (39) have been associated with thiazolidinedione treatment, potentially limiting its usefulness for diabetes prevention. The safety of thiazolidinediones with respect to cardiovascular disease and survival remain controversial. (40–41)
The literature on the cost-utility of diabetes prevention has recently been comprehensively reviewed. (42) Although there is no universally accepted rule to determine when an intervention is cost-effective, Laupacis and colleagues have proposed a system to rate economic evaluations on the likely magnitude of the net benefit associated with the intervention. (43) This system proposes rating interventions according to the cost per QALY-gained. Current consensus is that in the U.S., interventions that cost <$50,000 per QALY-gained are an appropriate way to use resources, those that cost $50,000 - $100,000 are probably appropriate, but those that cost >$100,000 may not represent a good value for money.
Table 1 summarizes the economic analyses that have adopted a single payer perspective, assessed QALYs, and discounted both costs and outcomes. As can be seen from the table, the costs of treatments for the primary prevention of type 2 diabetes range from <$1,000 to approximately $20,000 per QALY-gained. Of the six published analyses of lifestyle interventions, four found that lifestyle intervention was cost-saving or required only a modest expenditure per life-year or quality adjusted life-year gained making it extremely cost-effective. Similarly, three of the four published analyses of metformin therapy found it to be cost-saving or extremely cost-effective. The two shorter-term analyses of acarbose for diabetes prevention demonstrated it to be cost-saving or extremely cost-effective. No published studies have analyzed the cost-effectiveness of thiazolidinedione treatment for diabetes prevention.
Table 1.
Cost-Effectiveness of Interventions for the Primary Prevention of Type 2 Diabetes
| Intervention Type | Author, Year, (Reference) | Country | Time Horizon |
Cost per Life Year | Cost per QALY Gained |
|---|---|---|---|---|---|
| Lifestyle | Segal, 1998, (28) | Australia | 25 yrs | Cost-saving to A$2,600 (U.S. $1,659, 1998) |
—* |
| Palmer, 2004, (29) | Australia, France, Germany, Switzerland, UK | Lifetime | Cost-saving to €6,400 (U.S. $8,056, 2004) |
— | |
| Caro, 2004, (30) | Canada | 10 yrs | C$700 (U.S. $551, 2004) |
— | |
| DPP, 2005, (27) | US | Lifetime | — | $1,100 | |
| Eddy, 2005, (31) | US | 30 yrs | — | $143,000 | |
| Icks, 2007, (33) | Germany | 3 yrs | €4,664 (U.S. $6,269, 2007) |
||
| Metformin | Palmer, 2004, (29) | Australia, France, Germany, Switzerland, UK | Lifetime | Cost-saving to €5,400 (U.S. $6,836, 2004) |
— |
| Caro, 2004, (30) | Canada | 10 yrs | Cost-saving | — | |
| DPP, 2005, (27) | US | Lifetime | — | $1,800 | |
| Eddy, 2005, (31) | US | 30 yrs | — | $35,400 | |
| Acarbose | Caro, 2004, (30) | Canada | 10 yrs | Cost-saving | — |
| Josse, 2006, (32) | Spain, Germany, Sweden | 3 yrs | Cost-saving to €800 (U.S. $947, 2006) |
— |
Indicates that the results of the analysis were not reported.
A$ indicates Australian dollars.
€ indicates Euros.
C$ indicates Canadian dollars.
Summary and conclusions
The costs of diabetes in the United States are enormous and are growing. Although environmental and social interventions to address the underlying causative risk factors for diabetes including obesity and physical inactivity are promising, their effectiveness and cost-effectiveness are currently not established. Although more expensive than usual care, screening and intensive lifestyle interventions and interventions with metformin and acarbose that have targeted individuals at high risk for diabetes are effective and safe. Lifestyle interventions directly improve quality-of-life and delay or prevent the decrement in quality-of-life and the costs associated with diabetes and its complications. Metformin and acarbose interventions are effective in delaying or preventing the decrement of quality-of-life and the costs associated with diabetes and its complications. As a result, intensive lifestyle, metformin, and acarbose interventions are very cost-effective. Such interventions should be adopted by health systems and widely applied to at risk populations. Rigorous application of health economic principles to medical decision making may help to improve the value obtained for health care resources in the United States.
Acknowledgments
This work was supported by the Biostatistics and Economic Modeling Core of the Michigan Diabetes Research and Training Center funded by DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has nothing to disclose.
References
- 1.Herman WH. The economics of diabetes mellitus. In: Davidson JK, editor. Clinical diabetes mellitus: A problem-oriented approach. 3rd ed. New York: Thieme; 2000. pp. 815–828. [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 3.Narayan VKM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 4.Goldman L, Cook EF. The decline in ischemic heart disease mortality rates: An analysis of the comparative effects of medical interventions and changes in lifestyles. Ann Int Med. 1984;101:825–836. doi: 10.7326/0003-4819-101-6-825. [DOI] [PubMed] [Google Scholar]
- 5.The HEALTHY Study Group. A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363:443–453. doi: 10.1056/NEJMoa1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salmeinen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 10.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomized trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 12.Nichols GA, Glauber HS, Brown JB. Type 2 diabetes: Incremental medical care costs during the 8 years preceding diagnosis. Diabetes Care. 2000;23:1654–1659. doi: 10.2337/diacare.23.11.1654. [DOI] [PubMed] [Google Scholar]
- 13.Nichols GA, Arondekar B, Herman WH. Medical care costs one year after identification of hyperglycemia below the threshold for diabetes. Med Care. 2008;46:287–292. doi: 10.1097/MLR.0b013e31815b9772. [DOI] [PubMed] [Google Scholar]
- 14.Nichols GA, Arondekar B, Herman WH. Complications of dysglycemia and medical costs associated with nondiabetic hyperglycemia. Am J Manag Care. 2008;14:791–798. [PubMed] [Google Scholar]
- 15.Brown JB, Pedula KL, Bakst AW. The progressive cost of complications in type 2 diabetes mellitus. Arch Intern Med. 1999;159:1873–1880. doi: 10.1001/archinte.159.16.1873. [DOI] [PubMed] [Google Scholar]
- 16.Brandle M, Zhou H, Smith BRK, Marriott D, Burke R, Tabaei BP, Brown MB, Herman WH. The direct medical cost of type 2 diabetes mellitus. Diabetes Care. 2003;26:2300–2304. doi: 10.2337/diacare.26.8.2300. [DOI] [PubMed] [Google Scholar]
- 17.Caro JJ, Ward AJ, O’Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–481. doi: 10.2337/diacare.25.3.476. [DOI] [PubMed] [Google Scholar]
- 18.Anandacoomarasamy A, Caterson ID, Leibman S, Smith GS, Sambrook PN, Fransen M, March LM. Influence of BMI on health-related quality of life: Comparison between an obese adult cohort and age-matched population norms. Obesity. 2009;17:2114–2118. doi: 10.1038/oby.2009.121. [DOI] [PubMed] [Google Scholar]
- 19.Sach TH, Barton GR, Doherty M, Muir KR, Jenkinson C, Avery AJ. The relationship between body mass index and health-related quality of life: comparing the EQ-5D, EuroQol VAS and SF-6D. International Journal of Obesity. 2007;31:189–196. doi: 10.1038/sj.ijo.0803365. [DOI] [PubMed] [Google Scholar]
- 20.Lee AJ, Morgan CL, Morrissey M, Wittrup-Jensen KU, Kennedy-Martin T, Currie CJ. Evaluation of the association between the EQ-5Dindex (health-related utility) and body mass index (obesity) in hospital-treated people with Type 1 diabetes, Type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22:1482–1486. doi: 10.1111/j.1464-5491.2005.01657.x. [DOI] [PubMed] [Google Scholar]
- 21.U.K. Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37) Diabetes Care. 1999;22:1125–1136. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 22.Redekop WK, Koopmanschap MA, Stolk RP, Rutten GE, Wolffenbuttel BH, Niessen LW. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care. 2002;25:458–463. doi: 10.2337/diacare.25.3.458. [DOI] [PubMed] [Google Scholar]
- 23.Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications. 2000;14:235–241. doi: 10.1016/s1056-8727(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 24.Glasziou P, Alexander J, Beller E, Clarke P and the ADVANCE Collaborative Group. Which health-related quality of life score? A comparison of alternative utility measures in patients with type 2 diabetes in the ADVANCE trial. Heath and Quality of Life Outcomes. 2007;5:21–31. doi: 10.1186/1477-7525-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, Engelgau MM, Kaplan R, Herman WH. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25:2238–2243. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]
- 26.Herman WH. Economic analyses of diabetes interventions: rationale, principles, findings, and interpretation. The Endocrinologist. 1999;9:113–117. [Google Scholar]
- 27.Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, Hamman RF, Ackermann RT, Engelgau MM, Ratner RE. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323–332. doi: 10.7326/0003-4819-142-5-200503010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal L, Dalton AC, Richardson J. Cost-effectiveness of the primary prevention of non-insulin dependent diabetes mellitus. Health Promot Int. 1998;13:197–209. [Google Scholar]
- 29.Palmer AJ, Roze S, Valentine WJ, Spinas GA, Shaw JE, Zimmet PZ. Intensive lifestyle changes or metformin in patients with impaired glucose tolerance: modeling the long-term health economic implications of the diabetes prevention program in Australia, France, Germany, Switzerland, and the United Kingdom. Clin Ther. 2004;26:304–321. doi: 10.1016/s0149-2918(04)90029-x. [DOI] [PubMed] [Google Scholar]
- 30.Caro JJ, Getsios D, Caro I, Klittich WS, O’Brien JA. Economic evaluation of therapeutic interventions to prevent type 2 diabetes in Canada. Diabet Med. 2004;21:1229–1236. doi: 10.1111/j.1464-5491.2004.01330.x. [DOI] [PubMed] [Google Scholar]
- 31.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143:251–264. doi: 10.7326/0003-4819-143-4-200508160-00006. [DOI] [PubMed] [Google Scholar]
- 32.Josse RG, McGuire AJ, Saal GB. A review of the economic evidence for acarbose in the prevention of diabetes and cardiovascular events in individuals with impaired glucose tolerance. Int J Clin Pract. 2006;60:847–855. doi: 10.1111/j.1368-5031.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 33.Icks A, Rathmann W, Haastert B, Gandjour A, Holle R, John J, Giani G on behalf of the KORA Study Group. Clinical and cost-effectiveness of primary prevention of type 2 diabetes in a ‘real world’ routine healthcare setting: model based on the KORA Survey 2000. Diabetic Medicine. 2007;24:473–480. doi: 10.1111/j.1464-5491.2007.02108.x. [DOI] [PubMed] [Google Scholar]
- 34.Herman WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, Lachin JM, Engelgau MM. The Diabetes Prevention Program Research Group: Costs associated with the primary prevention of type 2 diabetes mellitus in the Diabetes Prevention Program. Diabetes Care. 2003;26:36–47. doi: 10.2337/diacare.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community. The YMCA Model. The Diabetes Educator. 2007;33:69–78. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 36.Herman WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, Lachin JM, Engelgau MM. The Diabetes Prevention Program Research Group: Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518–2523. doi: 10.2337/diacare.26.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 39.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G for the ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 40.Graham DJ, Oeullet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 41.Wertz DA, Chang CL, Sarawate CA, Willey VJ, Cziraky MJ, Bohn RL. Risk of cardiovascular events and all-cause mortality in patients treated with thiazolidinediones in a managed-care population. Circ Cardiovasc Qual Outcomes. 2010;3:538–545. doi: 10.1161/CIRCOUTCOMES.109.911461. [DOI] [PubMed] [Google Scholar]
- 42.Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: A systematic review. Diabetes Care. 2010;33:1872–1894. doi: 10.2337/dc10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Can Med Assoc J. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]