Abstract
The small heat shock proteins (sHSPs) are ubiquitous stress proteins proposed to act as molecular chaperones to prevent irreversible protein denaturation. We characterized the chaperone activity of Synechocystis HSP17 and found that it has not only protein-protective activity, but also a previously unrecognized ability to stabilize lipid membranes. Like other sHSPs, recombinant Synechocystis HSP17 formed stable complexes with denatured malate dehydrogenase and served as a reservoir for the unfolded substrate, transferring it to the DnaK/DnaJ/GrpE and GroEL/ES chaperone network for subsequent refolding. Large unilamellar vesicles made of synthetic and cyanobacterial lipids were found to modulate this refolding process. Investigation of HSP17-lipid interactions revealed a preference for the liquid crystalline phase and resulted in an elevated physical order in model lipid membranes. Direct evidence for the participation of HSP17 in the control of thylakoid membrane physical state in vivo was gained by examining an hsp17− deletion mutant compared with the isogenic wild-type hsp17+ revertant Synechocystis cells. We suggest that, together with GroEL, HSP17 behaves as an amphitropic protein and plays a dual role. Depending on its membrane or cytosolic location, it may function as a “membrane stabilizing factor” as well as a member of a multichaperone protein-folding network. Membrane association of sHSPs could antagonize the heat-induced hyperfluidization of specific membrane domains and thereby serve to preserve structural and functional integrity of biomembranes.
Small heat-shock proteins (sHSPs) with molecular masses of 15–42 kDa (1) are a ubiquitous class of molecular chaperones, which are sequence-related to the eye lens α-crystallins. Sequence homologies among sHSPs are restricted to short motifs at the carboxyl-terminal domain. In vitro, sHSPs can bind nonnative proteins, prevent their aggregation, and maintain them in a refolding-competent state. This reservoir for stably misfolded proteins can then interact with ATPase chaperones, such as DnaK/DnaJ/GrpE (the DnaK system) or GroEL/GroES, and restore the inactive proteins to functional states (2–6). In their native state, sHSPs form oligomers of 9–32 subunits or display a continuum of variable and dynamic quaternary structures (7, 8).
sHSPs are abundant in some nonstressed cells and tissues, like in mammalian lens. Typically, the synthesis of sHSPs is induced by various stresses and by a wide variety of additional signals (9, 10). There is growing evidence that both αB-crystallin and mammalian HSP27 provide protection against ischemic injury in cardiac myocytes (11). Several experiments support a role for sHSPs in the acquisition of thermotolerance in mammalian cells, yeast, plants, and prokaryotes (6, 12, 13).
A causal link between the level of sHSPs and thermotolerance in a cyanobacterium, Synechocystis sp. PCC6803, was published recently. Inactivation of the single Synechocystis sHSP, HSP17, resulted in a greatly reduced activity of photosynthetic oxygen evolution in heat-stressed cells. In addition, HSP17 was shown to be involved in the development of induced thermotolerance (14, 15). It was demonstrated both in nonstressed and heat-stressed cells that part of the HSP17 protein is associated with thylakoid membranes and that Hsp17 transcription is strongly regulated by subtle changes in membrane physical order (15, 16). Constitutive expression of the sHSP homologue from Synechococcus vulcanus in Synechococcus PCC7942 conferred cellular thermotolerance and thermal protection to the photosystem II complex and the light-harvesting phycobilisomes (17).
Although the mechanism of thermal death is not completely understood, membranes are known to be among the most sensitive targets of heat-stress damage in cells, as demonstrated by leakage of intracellular substances, loss of membrane components, sensitization to a variety of inhibitors, or penetration of hydrophobic dyes (18). The irreversible increase in membrane permeability clearly has profound repercussions on the viability of cyanobacteria (19). Supporting this view, the fluidization of the thylakoid membrane increased the heat sensitivity of Synechocystis (16). Long-term heat hardening of the photosynthetic apparatus has been correlated with a reduction in the level of lipid unsaturation and an elevation of the protein-to-lipid ratio in parallel with an overall increase in microviscosity (decrease in fluidity) of thylakoid membranes. An increased thylakoid molecular order is seen also upon a rapid heat acclimation of Synechocystis (20). Yet, membranes can be efficiently protected during and/or repaired after the sublethal heat stress. We report here that HSP17 acts like an amphitropic protein (21), i.e., binds weakly, reversibly, and specifically to membrane lipids. Interaction of HSP17 with membranes during sublethal heat stress can serve as a membrane protection mechanism. Using genetic and in vitro approaches, HSP17 was found to provide a short-term mechanism to protect membranes from thermal damage by increasing the membrane physical order, thus stability. Concomitantly, it can serve as a reservoir for stress-inactivated proteins to be recovered by the chaperone network after the stress. Thus, sHSPs assume the dual role of membrane and protein chaperones in the protection and recovery of thermally injured cells.
Materials and Methods
Materials.
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phosphoglycerol (DOPG), and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were purchased from Sigma; 1,6-diphenyl-1,3,5-hexatriene (DPH) and 1,1′-bis(4-anilino)naphthalene-5,5′-disulfonic acid (bis-ANS) were from Molecular Probes. Escherichia coli GroES7 and GroEL14 were purified as described (22). E. coli DnaK, DnaJ, and GrpE were overexpressed in E. coli and purified according to ref. 4. Pig heart mitochondrial malate dehydrogenase (MDH) was from Roche Molecular Biochemicals.
Expression and Purification of Synechocystis 6803 HSP17.
The hsp17 gene was amplified from the genomic DNA of Synechocystis by PCR using two primers: 5′-ATCATATGTCTCTCATTCTTTAC (forward), and 5′-TTAGGAAAGCTGAACTTTCAC (reverse), respectively. An NdeI site was introduced into the forward primer to simplify future cloning procedures. The amplified DNA fragment was cloned into pT7Blue vector (Novagen). The coding region of the hsp17 was then cloned into the NdeI and SmaI sites of the expression vector pAL781 (Invitrogen). The resultant plasmid, designated pAL-hsp17, contained the unmodified complete hsp17 coding region under the control of a tryptophan-inducible promoter. E. coli cells (strain GI724; Invitrogen) were transformed with pAL-hsp17, and hsp17 was expressed as recommended by the manufacturer. Cells were harvested and resuspended in 50 mM Tris (pH 7.5), 150 mM KCl, 20 mM Mg acetate, 2 mM DTT and disrupted with a Bead-Beater (Biospec Products, Bartlesville, OK), using 0.1-mm glass beads. Soluble proteins of the 30,000 × g (15 min) supernatant were precipitated by adding ammonium sulfate to a final concentration of 67%. After centrifugation (30,000 × g, 15 min), the pellet was resuspended and dialyzed against 50 mM Tris (pH 8.2), 100 mM KCl, 10 mM Mg acetate overnight. The protein solution was applied to a Superose 6 h 10/30 size exclusion HPLC column (Amersham Pharmacia) and eluted with 50 mM Tris (pH 8.2), 100 mM KCl, 10 mM Mg acetate at 0.5 ml/min. Fractions containing HSP17 were applied on a ResourceQ ion-exchange column (Amersham Pharmacia) and eluted with a linear gradient from 50 mM Tris (pH 8.2), 100 mM KCl, 10 mM Mg acetate to 50 mM Tris (pH 7.5), 700 mM KCl, 20 mM Mg acetate at a flow rate of 1.4 ml/min. Fractions containing HSP17 were combined and diluted five times with 50 mM Tris (pH 8.5), 10 mM Mg acetate and reloaded on the ResourceQ column and eluted with a linear gradient from 50 mM Tris (pH 8.5), 50 mM KCl, 20 mM Mg acetate to 50 mM Tris (pH 7.5), 700 mM KCl, 20 mM Mg acetate. HSP17-containing fractions were washed and concentrated by using a Centricon 100 concentrator (Amicon) in 100 mM Tris (pH 7.5), 150 mM KCl, 20 mM Mg acetate (buffer A) and stored at −80°C. The identity of recombinant HSP17 was confirmed by N-terminal amino acid sequencing.
Monitoring Temperature-Induced Binding of bis-ANS to HSP17.
Fluorescence of bis-ANS was measured by using a Quanta Master QM-1 fluorescence spectrometer (Photon Technology International, Princeton). To protein mixtures of HSP17 (1 μM) and/or MDH (0.8 μM) in buffer A, aqueous solution of bis-ANS was added to a final concentration of 10 μM. The excitation and emission wavelengths were 390 and 490 nm, respectively (3-nm slits). Temperature was controlled and measured as in ref. 22.
Generation of Isogenic Synechocystis hsp17 Deletion and Replacement Strains.
A Synechocystis sp. PCC6803 strain in which hsp17 had been deleted and replaced by a kanamycin resistance gene was obtained from H. Fukuzawa (Kyoto University, Kyoto, Japan) (strain HK-1). In this strain, the entire hsp17 coding region, as well as 160 bp of downstream sequence, comprising the entire intragenic region between hsp17 and a downstream ORF, were deleted. Plasmids to create isogenic deletion and replacement strains were made either with or without the hsp17 gene and the spectinomycin resistance gene (aadA). In the plasmid used to replace hsp17, the construct included the hsp17 promoter followed by the hsp17 gene with the 160-bp downstream intragenic fragment, then the spectinomycin resistance gene and the subsequent 3′ ORF. To create the isogenic spectinomycin-resistant deletion strain, an identical plasmid was constructed, but without the hsp17 gene. The spectinomycin hsp17 replacement and deletion plasmids were then used to transform the kanamycin-resistant deletion strain (HK-1), replacing the kanamycin resistance gene and creating new strains with or without hsp17, but with the same antibiotic resistance properties. Transformation and selection was carried out by standard techniques (23), and full segregation of the locus was confirmed by PCR analysis of genomic DNA from both strains.
Assay of Chaperone-Assisted Refolding of Heat-Denatured MDH.
Mitochondrial MDH (1 μM) was heat-denatured in buffer A containing 10 mM DTT for 30 min at 47°C as in ref. 24, in the presence of indicated amounts of HSP17 with/or without large unilamellar vesicles (LUVs) made of lipids as specified in Fig. 7. After heat treatment, DnaK, DnaJ, GrpE, and/or GroEL, GroES (4, 0.8, 0.4, 4, 8 μM, respectively), were added. Refolding was initiated at 25°C with 2 mM ATP in the presence of 2 mM phosphoenolpyruvate and 20 ng/ml pyruvate kinase as in ref. 4.
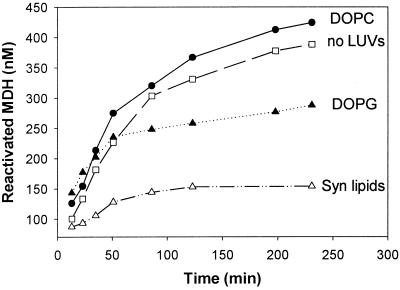
Figure 7.
Effect of lipids on the refolding of heat-denatured MDH by the multichaperone HSP17-KJELS system. MDH (1.2 μM) was denatured (30 min 47°C in buffer A containing 10 mM DTT) in the presence of HSP17 (7.2 μM). LUVs made of the indicated lipids were added to a final lipid concentration of 360 μM before the heat treatment. Recovery of MDH activity was followed at 25°C in the presence of ATP (1 mM), an active ATP-regeneration system supplemented with KJELS chaperones (derived from E. coli) (4, 0.8, 0.4, 4, and 6 μM, respectively). The final concentration of MDH, HSP17, and LUVs were 1 μM, 6 μM, and 300 μM, respectively.
Other Methods.
The measurement of steady-state fluorescence anisotropy and the isolation of thylakoid membranes were carried out as described (16). Monolayer experiments and DPH fluorescence anisotropy measurement on LUVs were done in buffer A essentially as in ref. 22. Protein concentrations were determined by the Micro BCA assay (Pierce). Fourier transform infrared spectroscopy (FTIR) experiments were carried out as in ref. 25. Lipid isolation, separation, and analysis from Synechocystis thylakoids were made as in ref. 16. Synechocystis HSP17 antibody was produced in rabbit by standard procedure. Western blot was made by the Amersham Pharmacia ECL method; HSP17 antibody was used in 1:3,000, anti-rabbit IgG in 1:8,000 dilution.
All experiments were repeated at least three times. In the figures, the results of one typical experiment are shown.
Results
HSP17 Stabilizes Heat-Denatured MDH for Subsequent Refolding by the Chaperone Network.
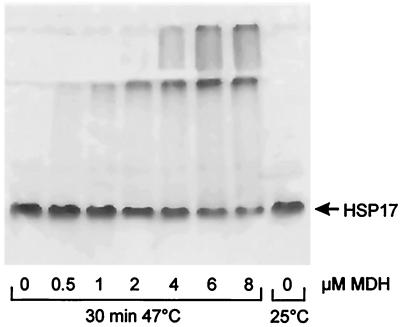
Small HSPs, such as IbpB from E. coli, have been suggested to form hollowed globular structures of variable sizes (10). In contrast, Synechocystis HSP17 formed distinct oligomeric patterns, although with apparent molecular masses that varied with the detection method. On “nondenaturing” gel, it migrated as a 70-kDa species, with very minor bands at 140 and 210 kDa, suggesting a gel-electrophoresis-resistant HSP17 tetramer that can form weaker octamers, dodecamers, etc. (Fig. 1). The oligomeric state of the 70-kDa species remained unaffected by a heat treatment alone, as revealed by nondenaturing PAGE (Fig. 1). In contrast, when HSP17 was incubated at 47°C in the presence of increasing amounts of denaturing MDH, the two proteins formed increasing and saturating amounts of gel-electrophoresis-resistant oligomers, which were larger than 800 kDa at the expense of the initial low molecular mass species (Fig. 1). The large, gel-electrophoresis-resistant HSP17-MDH complexes were mostly soluble, because they entered the stacking gel and some of the resolution gel. Note that neither native nor aggregated MDH alone entered the nondenaturing gels because it migrated toward the cathode.
Figure 1.
HSP17 forms stable complexes with heat-denatured MDH. Increasing amounts of MDH (0, 0.5, 1, 2, 4, 6, 8 μM) were heat-denatured (30 min at 47°C) or kept at 25°C in buffer A containing 10 mM DTT in the presence of HSP17 (16 μM) and analyzed by nondenaturing PAGE (6%).
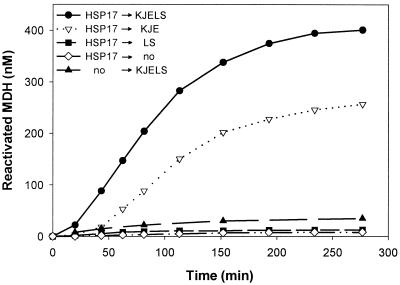
In vitro, heat-denatured MDH in association with Synechocystis HSP17 was efficiently transferred to the DnaK/DnaJ/GrpE (DnaK system) and GroEL/ES chaperone systems for subsequent ATP-dependent refolding (Fig. 2). Interestingly, when heat-denatured in the presence of cyanobacterial HSP17, MDH was more readily reactivated by the DnaK chaperone system alone, than when denatured in the presence of E. coli IbpB (4). After chaperone-assisted refolding of HSP17-bound MDH, native gels revealed that the HSP17 component of all high molecular weight HSP17-MDH oligomers reverted to the original low molecular weight tetrameric species (not shown).
Figure 2.
Chaperone-assisted refolding of heat-denatured MDH. MDH (1.2 μM) was 99.8% heat-denatured during 30 min at 47°C, either in the absence (no) or in the presence of 12 μM HSP17. After heat shock (→) recovery of MDH, activity was followed at 25°C in the presence of ATP (1 mM), an active ATP-regeneration system, without additional chaperones (no), or with E. coli DnaK, DnaJ, GrpE, GroEL, GroES (4, 0.8, 0.4, 4, 5 μM, respectively). The final concentration of MDH and HSP17 was 1 μM and 10 μM, respectively.
High Temperature Promotes the Exposure of Hidden Hydrophobic Domains.
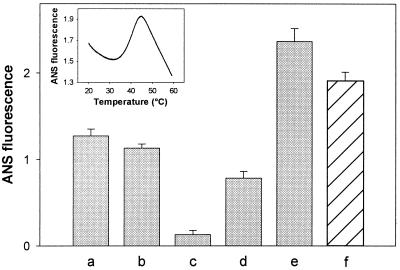
Bis-ANS is widely used as a probe to assess surface exposure of hydrophobic sites in proteins. The fluorescence emission spectra of bis-ANS (excited at 390 nm) in an aqueous buffer displayed a maximum at around 520 nm. Parallel with a strong increase in the fluorescence quantum yield, the presence of HSP17 shifted the maximum of the spectrum to 495 nm (data not shown). These data supported the existence of certain surface-exposed hydrophobic patches on native HSP17. To determine whether an increase in temperature would lead to a further exposure of hidden hydrophobic regions, the temperature dependence of the fluorescence intensity of bis-ANS in the presence of HSP17 was investigated (Fig. 3 Inset). Based on similar studies with α-crystallin (26), we concluded that, because of the thermal inactivation of the excited state of bis-ANS, the initially observable drop (20–35°C) and the decrease above 44°C in fluorescence intensity is unrelated to the conformational state of the protein. A progressive increase of bis-ANS emission intensity with an apparent maximum temperature of about 44°C, however, reflected an elevated binding of the probe indicating the temperature-driven exposure of buried hydrophobic regions in HSP17. Accessibility of hydrophic sites may result from heat-induced dissociation of the HSP17 oligomer, as previously described for yeast HSP26 (5).
Figure 3.
Temperature-induced exposure of hydrophobic surfaces of HSP17 measured by bis-ANS binding. HSP17 or MDH were kept at 25°C (a, HSP17; c, MDH) or incubated at 47°C for 30 min alone (b, HSP17; d, MDH) or together (e). The last column (f) shows the calculated sum of ANS fluorescence intensity measured separately for heat-treated HSP17 (b) and MDH (d), as well. (Inset) Fluorescence intensity (490 nm) of bis-ANS (10 μM) was measured in the presence of HSP17 (1 μM) as a function of temperature.
Bis-ANS binding to the HSP17 oligomers at 25°C remained virtually the same before and after a 30-min heat treatment at 47°C (Fig. 3). In contrast, the same heat treatment increased 6-fold the bis-ANS binding capacity of MDH, indicating that MDH has become irreversibly denatured. When MDH was treated for 30 min at 47°C in the presence of HSP17, the bis-ANS fluorescence subsequently measured at 25°C was 20% higher than the calculated sum of the fluorescence signals of separately heat-treated MDH and HSP17. Further hydrophobic regions, otherwise hidden in free HSP17 oligomers or MDH aggregates, likely became exposed in the HSP17-MDH complex, thus increasing the chances for optimal exposure to the DnaK chaperone system.
HSP17 Specifically Interacts with Various Lipids and Increases Molecular Order in the Fluid Lipid Bilayer.
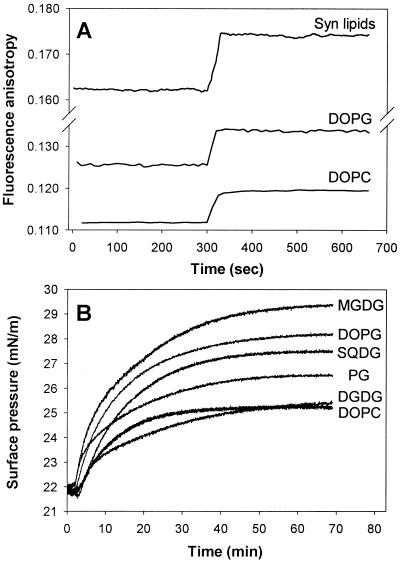
Because sHSPs, including HSP17, lack a leader sequence or putative transmembrane regions, the mechanism by which they become membrane-associated remains elusive. At 25°C, 1 g of HSP17 binds 50–60% more bis-ANS molecules than 1 g of native MDH (calculated based on Fig. 3), suggesting significant exposure of the hydrophobic patches on the surface of HSP17. To test whether the preexisting hydrophobicity results in an effective lipid interaction for HSP17, LUVs made of either negatively charged (DOPG) or zwitterionic (DOPC) phospholipids esterified with identical fatty acids (oleic acid) or of purified total polar lipids of Synechocystis thylakoids were used. The fluorescence anisotropy of DPH-labeled LUVs, a value inversely related to the fluidity of the bilayer, was used to monitor the dynamic properties of membranes (Fig. 4A). LUV-HSP17 interaction resulted in a strong increase in the molecular order of the membranes, notably the highest with Synechocystis lipids. In contrast to GroEL, however, shown to be located on the surface of lipid bilayers (22), the deeply membrane embedded DPH probe reported significant penetration of HSP17 into the membrane hydrophobic core. It should be noted that this effect is unique to HSP17, because saturating amounts of α-crystallin were reported to interact only with the external surfaces of membranes (27).
Figure 4.
Interaction of HSP17 with lipids. (A) Effect of HSP17 on the membrane physical state of LUVs. Steady-state fluorescence anisotropy of DPH (0.1 μM) embedded in LUVs (50 μM) made of DOPC, DOPG, or total polar lipid extract of Synechocystis (Syn lipids) was measured at 20°C in buffer A. At 300 s, HSP17 (1.5 μM) was added. (B) Effect of HSP17 on the surface pressure of different lipid monolayers. Monolayers of different lipids were spread at an air-buffer A interface from CHCl3 lipid solution to give an initial surface pressure of 22 mN/m. Hsp17 was added to the subphase at t = 2 min to a final concentration of 0.5 μM.
We further explored the specificity of the interaction of HSP17 with particular lipids, namely DOPG, DOPC, and each of the individual lipid classes of Synechocystis, i.e., MGDG, SQDG, DGDG, and PG, using the monolayer technique (Fig. 4B). The interaction of HSP17 with lipid monolayers spread at an air-buffer interface resulted in a surface pressure increase, which can be explained by the specific insertion of hydrophobic protein segments into the lipid monolayers. Studies on the effect of lipid head-group on the protein insertion revealed high specificity of HSP17 for negatively charged lipid such as DOPG, as compared with zwitterionic DOPC, both esterified with identical fatty acids. It should be noted, however, that injection of HSP17 beneath a film of neutral MGDG, the most abundant class of Synechocystis membrane lipids, caused by far the most dramatic increase in the interfacial pressure.
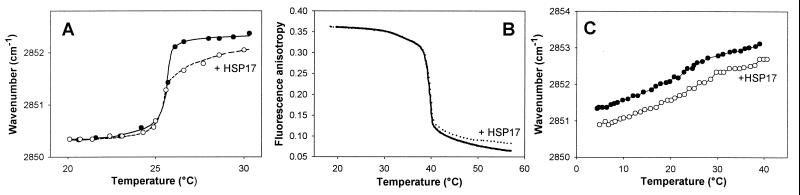
The gel-to-fluid thermal phase transition also can be monitored by FTIR following changes in the wavenumber of the CH2 stretches, which undergo an increase in frequency as the lipid acyl chains melt (25). In agreement with fluorescence anisotropy data gained with HSP17 containing DPPC vesicles (Fig. 5A), FTIR measurements did not reveal a significant change in the gel-to-liquid crystalline transition with DMPC membranes (Fig. 5B). However, similar to our previous findings with GroEL-lipid interaction (22), HSP17 association with lipids increased the membrane order predominantly in the liquid crystalline (“fluid”) state of membranes (above 24°C and 40°C for DMPC and DPPC, respectively), and either no association or no membrane ordering could be detected once the membrane lipids were adapted to a gel phase (Fig. 5 A and B). A similar ordering effect was observed upon addition of HSP17 to liposomes made from isolated Synechocystis membrane lipids, which do not form gel phase in the temperature range investigated (Fig. 5C). By assuming that even the high melting lipid components of membranes adapt a fluid or “hyperfluid” state during heat stress (19, 20, 28), the apparent lipid phase state specificity of the HSP17-lipid interaction highlights a potentially important physiological role in membrane thermostabilization.
Figure 5.
HSP17 induced increase of the molecular order of fluid lipid membranes. Effect of HSP17 on the CH2 stretching mode of DMPC (A) or of total polar lipid extract of Synechocystis (C) liposomes during heating (mol protein/mol lipid = 1:35) measured by FTIR. (B) DPH (0.1 μM) fluorescence anisotropy of DPPC LUVs (50 μM) in buffer A in the presence or absence of 1.5 μM HSP17.
Association of HSP17 with Thylakoids Reduces Membrane Fluidity.
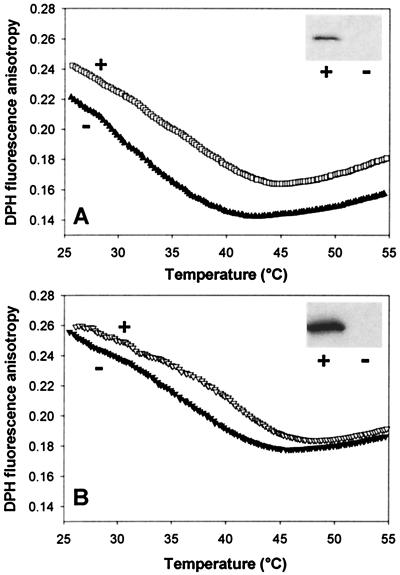
Earlier, we demonstrated that a portion of de novo synthesized HSP17 was associated in vivo with thylakoid membranes (16). The thylakoids of a Synechocystis hsp17 deletion mutant displayed abnormally small intramembrane spaces and was thermolabile (15). Recent studies of Nakamoto et al. (17) revealed that the constitutive expression of the sHSP (HspA) of S. vulcanus in Synechococcus PCC 7942 increased the thermal resistance of photosystem II. To estimate the contribution of HSP17 to the overall physical properties of the thylakoid membrane hsp17 deletion (hsp17−) and isogenic revertant (hsp17+), Synechocystis strains were examined. We first determined that the fatty acid and lipid composition of thylakoids of mutant and revertant cells were identical (data not shown). Thylakoid membrane fluidity was measured before and after a conditioning heat treatment. Western blot analysis of the thylakoid fraction of hsp17+ cells showed that a pool of HSP17 is associated with the thylakoid membrane, and its level is markedly elevated upon heat treatment (Fig. 6 A and B, Insets). As expected, HSP17 was completely absent in the mutant thylakoid preparations. DPH anisotropy was monitored over a wide temperature range to assess thylakoid fluidity. Data reveal that membrane fluidity of hsp17− cells grown at 30°C was remarkably higher than that of in the hsp17+ cells (Fig. 6A). After a heat treatment at 42°C for 3 h, the fluidity of the thylakoid of both cell types decreased considerably, suggesting that the cells were able to adapt to the higher temperature at the membrane level (Fig. 6B). The fluidity gap detected between thylakoids of 30°C grown mutant and isogenic revertant cells has been reduced significantly after heat treatment.
Figure 6.
Thylakoid membrane fluidization in vivo by HSP17 deletion. The fluorescence anisotropy of DPH (0.2 μM) embedded in thylakoid membranes (1 μg chlorophyll a/ml) isolated from hsp17− mutant (−) and hsp17+ isogenic revertant (+) Synechocystis cells grown at 30°C (A) or exposed to 42°C for 3 h (B) was measured in 10 mM TES-NaOH (pH 7.0) as a function of temperature as described (16). Western blots to estimate HSP17 levels in isolated thylakoid are shown (Inset). Equal amounts of protein were loaded on 17% polyacrylamide gel.
Liposomes Modulate Protein Refolding by the Multichaperone Network.
HSPs traditionally have been described as cytosolic chaperones that facilitate the correct folding of proteins or mediate the degradation of improperly folded ones. One may speculate, however, that the capability of HSPs to interact with lipids as presently documented for HSP17, or earlier for GroESL (22) and HSP70 (29) as well, also may modulate their specificity and affinity for heat-denatured substrates and/or other components of the KJELS folding system and thus, affect the activity of the entire protein-refolding process. First, we determined that the activity of native MDH was not affected by LUVs (data not shown). LUVs made of DOPG and DOPC lipids, which bind in distinct ways to HSP17 in monolayer studies (Fig. 4B), had different effects on the HSP17-KJELS-mediated reactivation of heat-denatured MDH. Whereas vesicles made of DOPC slightly activated, DOPG LUVs substantially reduced the refolding yield. Surprisingly, LUVs made of the mixture of Synechocystis membrane lipids, including the highly HSP17-interactive MGDG lipid molecular species, caused a strong inhibition of the refolding process (Fig. 7).
Discussion
Synechocystis sp. PCC 6803, the first photosynthetic organism for which the complete genome sequence has been determined, offers an excellent system to elucidate the function of sHSPs. We first identified HSP17 as a single sHSP encoded by a “fluidity gene” (16). Besides high temperature and light conditions, transcription of Synechocystis hsp17 was shown to be strongly regulated by changes in membrane physical order. In addition, most of the newly synthesized pool of HSP17 was found bound to the thylakoid membranes (16). Functional disruption of thylakoid-associated sHSPs, or addition of exogenous sHSPs to chloroplasts revealed that sHSPs are able to protect photosystem II (17), and thus, sHSPs could account for heat acclimation of electron transport in thermally adapted plants (30).
As we report here, HSP17 forms stable complexes with denatured MDH, and as was shown for IbpB (4) and plant Hsp18.1 (6), it appeared to serve as a reservoir for unfolded protein by binding and transferring MDH to other chaperones for subsequent active refolding. Depending on the lipid composition, however, LUVs altered the kinetics of HSP17-KJELS-mediated reactivation of denatured MDH. LUVs made of Synechocystis lipids inhibited most effectively the refolding process. Thus, our preliminary results suggest a fundamental role of membrane lipids in modulating the action of protein refolding by the multichaperone network. Additional studies should determine how membrane lipids affect the interaction of substrate with the folding machinery, the stability of the folding intermediates, and the ATPase activity of the chaperones.
Lipid interaction of Synechocystis HSP17 is specific with preference to the fluid lipid phase. By measuring either anisotropy changes using a fluorescent membrane probe or monitoring wavenumber alterations of the CH2 stretches of lipid acyl chains by FTIR, the ability of HSP17 to increase membrane physical order, using synthetic and cyanobacterial membrane lipids, was obvious. The assumption, that a membrane-associated pool of HSP17 may actively participate in the control of thylakoid physical state was further supported by using the hsp17 deletion mutant and isogenic revertant Synechocystis cells. In line with the model membrane studies, thylakoids derived from either the nontreated or heat-hardened HSP17− cells displayed a remarkable increase in thylakoid fluidity as compared with its isogenic revertant counterpart. Our finding that this preexisting difference observed in thylakoid fluidity levels is narrowed after heat pretreatment of the cells could be explained by the existence of additional adaptive heat-induced membrane alterations that are superimposed on the suggested membrane rigidifying effect of HSP17. Thus, translocation of GroEL or other chaperones to membranes may elevate lipid molecular order in both the hsp17− mutant and isogenic revertant cells during sublethal heat treatment (22, 31).
Further investigations should help us to understand the principles governing both the fatty acid and head-group specificity of the lipid-HSP17 interaction. Our recent FTIR studies on lipid-HSP17 interactions revealed considerable changes at different depths in the membranes, represented by lipid phosphate bands, interfacial region and at the hydrophobic core of membrane as well, reflecting the high complexity of lipid-sHSP association (N.M.T., I.H., Z.T., J.H.C., and L.V., unpublished work). The apparent lipid specificity of HSP17 also implies that the membrane binding of sHSPs through specific HSP/lipid interaction confines the location of sHSPs to one or more membrane domains or “rafts” (32).
Underlying our model concerning the biological significance of membrane-associated sHSPs, the presence of sHSPs in the membranes is widely documented from prokaryotes to plants, yeast, and mammalian cells (33–37).
Proteins functioning in transduction of signals generated in cell membranes (like protein kinase C, phospholipase C, DnaA, etc.) are commonly regulated by amphitropism (21). Regulatory switches that control their affinity for membranes include modulation of the membrane lipid composition and modification of the protein itself by ligand binding, phosphorylation, or acylation. Provided that a subclass of HSP17 acts like amphitropic proteins, their lipid-binding specificity and affinity can be the subject to regulation. Conversely, lipid interaction also may modulate the chaperone function of sHSPs. Such a double-sided regulatory mechanism might include changes in the composition and physical state of membrane lipids and the exposure of hydrophobic patches on the surface of HSP17, both as a function of temperature, pH, or ionic forces.
In conclusion, our present results imply that: (i) the thermally controlled membrane association of HSP17 previously described in vivo (16) is, at least partially, lipid mediated and therefore (ii) a portion of HSP17 may function primarily as a “membrane stabilizing factor” rather than a member of a multichaperone network. The membrane-associated pool of HSP17 is able to regulate rapidly and directly those key membrane attributes like membrane fluidity and consequently permeability, which are known elements of membrane (and cell) thermostability. We suggest that one very probable function of HSP17 is to influence the integrity of membranes, especially in the initial phases of stress conditions, preceding adaptation by lipid readjustment. Irrespective of their chaperone activity displayed together with the DnaK and GroEL systems, the ability of sHSPs to alter directly membrane physical parameters provides a rapid, reversible, and powerful tool for cellular thermoadaptation. It can antagonize the heat-induced disorganization of membranes, which is caused by the “hyperfluid” state of certain lipid domains, and might serve to preserve its structure and function (like activity of photosystem II) under heat stress. In addition, the thylakoid lipid phase may act as a cellular thermometer where thermal-stress-induced membrane perturbation(s) is sensed and transduced into a cellular signal leading to the activation of HS genes (16). One can speculate that membrane-rigidifying association of sHSPs documented here together with that shown for GroEL chaperonins before (22) may lead to a down-regulation of HSP gene expression. Such a cross talk between the primary sensor in the membranes and HSPs would suggest a feedback mechanism in the regulation of heat-shock genes (38) and provide a comprehensive model for the function of sHSPs in the cell.
Acknowledgments
We thank Kim Giese for preparation of the Synechocystis strains and Dr. H. Fukuzawa for providing the initial Synechocystis cultures and Hsp17 plasmids. We thank Anat Ben-Tsvi for the help in HSP17 purification and assays. This work was supported by an Israeli–Hungarian TéT Grant, a Bolyai Fellowship to Z.T., Hungarian National Scientific Research Foundation (OTKA) Grants T29883 and T025748 (to I.H. and L.V., respectively), National Institutes of Health Grant NIHGM42762 (to E.V.), and Grant 00-04-48421a from the Russian Foundation for Basic Research (to D.L.).
Abbreviations
- sHSP
small heat-shock protein
- DPH
1,6-diphenyl-1,3,5-hexatriene
- bis-ANS
1,1′-bis(4-anilino)naphthalene-5,5′-disulfonic acid
- LUV
large unilamellar vesicle
- MDH
malate dehydrogenase
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPG
1,2-dioleoyl-sn-glycero-3-phosphoglycerol
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- FTIR
Fourier transform infrared spectroscopy
References
- 1.Roy S K, Hiyama T, Nakamoto H. Eur J Biochem. 1999;262:406–416. doi: 10.1046/j.1432-1327.1999.00380.x. [DOI] [PubMed] [Google Scholar]
- 2.Ehrnsperger M, Graber S, Gaestel M, Buchner J. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee G J, Roseman A M, Saibil H R, Vierling E. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veinger L, Diamant S, Buchner J, Golubinoff P. J Biol Chem. 1998;273:11032–11037. doi: 10.1074/jbc.273.18.11032. [DOI] [PubMed] [Google Scholar]
- 5.Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White H E, Chen S, Saibil H E, Buchner J. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee G J, Vierling E. Plant Physiol. 2000;122:189–197. doi: 10.1104/pp.122.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K K, Kim R, Kim S H. Nature (London) 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- 8.Haley D A S, Bova M P, Huang Q-L, Mchaourab H S, Stewart P L. J Mol Biol. 2000;298:261–272. doi: 10.1006/jmbi.2000.3657. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz J. Semin Cell Dev Biol. 2000;11:53–60. doi: 10.1006/scdb.1999.0351. [DOI] [PubMed] [Google Scholar]
- 10.Shearstone J R, Baneyx F. J Biol Chem. 1999;274:9937–9945. doi: 10.1074/jbc.274.15.9937. [DOI] [PubMed] [Google Scholar]
- 11.Golenhofen N, Htun P, Ness W, Koob R, Schaper W, Drenckhahn D. Mol Cell Cardiol. 1999;31:569–580. doi: 10.1006/jmcc.1998.0892. [DOI] [PubMed] [Google Scholar]
- 12.van den Ijssel P R, Overcamp P, Knauf U, Gaestel M, de Jong W W. FEBS Lett. 1994;355:54–56. doi: 10.1016/0014-5793(94)01175-3. [DOI] [PubMed] [Google Scholar]
- 13.Soto A, Allona I, Collada C, Guevera M A, Casado R, Rodriguez Cerezo E, Aragoncillo C, Gomez L. Plant Physiol. 1999;120:521–528. doi: 10.1104/pp.120.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Prochaska D J, Fang F, Barnum S R. Curr Microbiol. 1998;37:403–407. doi: 10.1007/s002849900400. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Owen H A, Prochaska D J, Barnum S R. Curr Microbiol. 2000;40:283–287. doi: 10.1007/s002849910056. [DOI] [PubMed] [Google Scholar]
- 16.Horváth I, Glatz A, Varvasovszki V, Török Z, Páli T, Balogh G, Kovács E, Nádasdi L, Benko S, Joó F, Vígh L. Proc Natl Acad Sci USA. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamoto H, Suzuki N, Roy S K. FEBS Lett. 2000;483:169–174. doi: 10.1016/s0014-5793(00)02097-4. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchido T, Aoki I, Takano M. J Gen Microbiol. 1989;135:1941–1947. doi: 10.1099/00221287-135-7-1941. [DOI] [PubMed] [Google Scholar]
- 19.Inoue N, Emi T, Yamane Y, Kashino Y, Koike H, Satoh K. Plant Cell Physiol. 2000;41:515–522. doi: 10.1093/pcp/41.4.515. [DOI] [PubMed] [Google Scholar]
- 20.Glatz A, Vass I, Los D A, Vígh L. Plant Physiol Biochem. 1999;37:11–12. [Google Scholar]
- 21.Johnson J E, Cornell R B. Mol Membr Biol. 1999;16:217–235. doi: 10.1080/096876899294544. [DOI] [PubMed] [Google Scholar]
- 22.Török Z, Horváth I, Goloubinoff P, Kovács E, Glatz A, Balogh G, Vígh L. Proc Natl Acad Sci USA. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams J G K. Methods Enzymol. 1988;167:766–778. [Google Scholar]
- 24.Goloubinoff P, Diamant S, Weiss C, Azem A. FEBS Lett. 1997;407:215–219. doi: 10.1016/s0014-5793(97)00348-7. [DOI] [PubMed] [Google Scholar]
- 25.Tsvetkova N M, Phillips B L, Crowe L M, Crowe J H, Risbud S H. Biophys J. 1998;75:2947–2955. doi: 10.1016/S0006-3495(98)77736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das K, Surewicz WK. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Zeng J, Yin H, Borchman D, Peterson C A. Curr Eye Res. 1999;18:56–61. doi: 10.1076/ceyr.18.1.56.5387. [DOI] [PubMed] [Google Scholar]
- 28.Mejia R, Gomez-Eichelman M, Fernandez M S. Biochem Mol Biol Int. 1999;47:835–844. doi: 10.1080/15216549900201923. [DOI] [PubMed] [Google Scholar]
- 29.Mamelak D, Lingwood C. Glycoconjugate J. 1997;14:715–722. doi: 10.1023/a:1018569417218. [DOI] [PubMed] [Google Scholar]
- 30.Heckathorn S A, Downs C A, Sharkey T D, Coleman J S. Plant Physiol. 1998;116:439–444. doi: 10.1104/pp.116.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovács E, Török Z, Horváth I, Vígh L. Plant Physiol Biochem. 1994;32:285–293. [Google Scholar]
- 32.Fishov I, Woldringh C L. Mol Microbiol. 1999;32:1166–1172. doi: 10.1046/j.1365-2958.1999.01425.x. [DOI] [PubMed] [Google Scholar]
- 33.Kitagawa M, Matsumara Y, Tsuchido T. FEMS Microbiol Lett. 2000;184:165–171. doi: 10.1111/j.1574-6968.2000.tb09009.x. [DOI] [PubMed] [Google Scholar]
- 34.Lunsdorf H, Schairer H U, Heidelbach M. J Bacteriol. 1995;177:7092–7099. doi: 10.1128/jb.177.24.7092-7099.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaczinski H, Kloppstech K. Eur J Biochem. 1988;173:579–583. doi: 10.1111/j.1432-1033.1988.tb14038.x. [DOI] [PubMed] [Google Scholar]
- 36.Sales K, Brandt W, Rumbak E, Lindsey G. Biochim Biophys Acta. 2000;1463:267–278. doi: 10.1016/s0005-2736(99)00215-1. [DOI] [PubMed] [Google Scholar]
- 37.Chandrasheker G, Cenedella R J. Exp Eye Res. 1997;64:423–430. doi: 10.1006/exer.1996.0228. [DOI] [PubMed] [Google Scholar]
- 38.Vígh L, Maresca B, Harwood J. Trends Biochem Sci. 1998;23:369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]