Abstract
The Norrie disease gene (Ndp) codes for a secreted protein, Norrin, that activates canonical Wnt signaling by binding to its receptor, Frizzled-4. This signaling system is required for normal vascular development in the retina and for vascular survival in the cochlea. In mammals, the pattern of Ndp expression beyond the retina is poorly defined due to the low abundance of Norrin mRNA and protein. Here we characterize Ndp expression during mouse development by studying a knock-in mouse that carries the coding sequence of human placental alkaline phosphatase (AP) inserted at the Ndp locus (NdpAP). In the CNS, NdpAP expression is apparent by E10.5 and is dynamic and complex. The anatomically delimited regions of NdpAP expression observed prenatally in the CNS are replaced postnatally by widespread expression in astrocytes in the forebrain and midbrain, Bergman glia in the cerebellum, and Müller glia in the retina. In the developing and adult cochlea, NdpAP expression is closely associated with two densely vascularized regions, the stria vascularis and a capillary plexus between the organ of Corti and the spiral ganglion. These observations suggest the possibility that Norrin may have developmental and/or homeostatic functions beyond the retina and cochlea.
Keywords: Norrin, Norrie disease, Frizzled-4, mouse, brain development, vascular development
1. Results and discussion
In humans, mutations in the Norrie Disease Protein gene (Ndp) are responsible for Norrie Disease (ND), a severe X-linked retinal vascular disease (Berger and Ropers, 2001). The Ndp gene codes for a small secreted protein, Norrin, that is highly conserved among vertebrates. Frizzled-4 (Fz4), the Norrin receptor, is the only Frizzled family member that binds to Norrin with high-affinity (Smallwood et al., 2007), and in conjunction with a co-receptor, Lrp5 or Lrp6, and a an associated integral memebrane protein, Tspan12, this interaction potently activates canonical Wnt signaling (Xu et al., 2004; Junge et al., 2009; Ye et al., 2009). Norrin/Fz4/Lrp/Tspan12 signaling in endothelial cells plays a central role in retinal vascular development, and partial or complete loss of any of these signaling components in humans or mice results in retinal hypovascularization, which typically leads to retinal damage and vision loss (Richter et al., 1998; Xu et al., 2004; Luhman et al., 2005; Ye et al., 2009, 2010). In mice, loss of Norrin or Fz4 also leads to progressive loss of the stria vascularis in the inner ear, accompanied by progressive hearing loss (Wang et al., 2001; Rehm et al., 2002; Xu et al., 2004); in humans, over one third of ND patients develop progressive sensorineural hearing loss (Berger and Ropers, 2001). In addition, ~50% of ND patients are mentally retarded (Berger and Ropers, 2001), indicating a function for Norrin beyond the eye and ear.
Previous attempt to analyze Ndp expression in the mouse by in situ hybridization were hampered by poor cellular resolution and low sensitivity (Hartzer et al., 1999). We have recently generated a human placental alkaline phosphatase (AP) reporter knock-in allele, NdpAP, at the Ndp locus for the purpose of analyzing Ndp expression histochemically (see Fig. 1E of Ye et al., 2009). AP is a GPI-anchored plasma membrane protein that can be localized with a highly sensitive histochemical reaction, facilitating the visualization of cell morphologies in a variety of contexts (e.g., Badea et al., 2003). In the Ndp knock-in allele, the AP coding region and 3′ UTR were inserted 84 bp 5′ of the Ndp initiator methionine codon without deleting any chromosomal sequences in or around the Ndp gene. The Ndp coding region starts in the second exon, which is separated by a 16.5 kb intron from the first exon and the adjacent promotor sequences. The frt-flanked phosphoglycerate kinase promotor-Neo used for drug selection in embryonic stem cells was subsequently removed by in vivo Flp-mediated recombination. We assume that the insertion of ~2 kb of AP and 3′ UTR sequences 16.5 kb from the Ndp promotor has little or no effect on Ndp gene transcription. Here we use the NdpAP reporter mouse to systematically analyze the spatial and temporal pattern of Ndp expression in the developing and adult nervous system.
Figure 1.

NdpAP expression in the developing retina.
AP stained tissues from NdpAP/y mice. The AP histochemical reaction product is purple, but can appear blue-tinted, red-tinted, or black, depending on the tissue and the lighting. (A) The rostral region of an E10.5 embryo with the midline oriented horizontally. The bilateral optic stalks are indicated by arrows, but only the upper one is labeled. H, hypothalamus. (B) A dissected E10.5 eye with the optic stalk attached. (C) E15.5 retina with optic nerve attached. (D) The stained retina in (C) viewed from the back. (E) P1 retina. (F) P3 retina. Scale bars, 200 μm
1.1 Ndp expression in the developing retina
Previously, we reported that at all postnatal ages the NdpAP gene is expressed by Müller glia, the radial glia that span the full width of the retina (Ye et al., 2009). This identification was facilitated by the availability of NdpAP/+ females, in which tissue mosaicism generated by X-chromosome inactivation provides information on cell morphology in the form of spatial contrast between AP-expressing and AP-non-expressing cells. Müller glia-derived Norrin activates the Fz4 receptor on both endothelial cells and mural cells and promotes retinal vascular proliferation, maturation, and stabilization. It is interesting to note that in mice most Müller glia are born postnatally (Young, 1985a; Young, 1985b), and the timing of Müller cell differentiation closely matches that of retinal vascularization.
To further analyze Ndp expression in the developing retina, we followed NdpAP expression from embryonic day (E) 10.5 to postnatal day (P) 3 by AP histochemistry (Fig. 1). At E10.5, no expression is detected in the retina, but the mesenchyme surrounding the optic stalk is AP positive (Fig. 1A and B). By E15.5, NdpAP expression is seen in the retina at the optic disc, and this pattern is maintained until P0 (Fig. 1C and D). From P0 to P3, with the birth of Müller glia, NdpAP expression rapidly increases throughout the retina, without any detectable spatial gradient (Fig. 1E). By P3, the retinas of hemizygous NdpAP/Y males are homogeneously AP positive, indicating that the adult-like expression pattern has been established (Fig. 1F). The pan-retinal AP staining that is apparent at P1 (Fig. 1E) is reminiscent of the appearance of the Muller cell marker SLC1A3 (the glutamate transporter GLAST), suggesting that by this age many Muller glia are already expressing markers indicative of the fully differentiated state (Vazquez-Chona et al., 2009).
During retinal vascular development, the growing vascular plexus spreads centrifugally along the vitreal surface of the retina. This centrifugal expansion is driven, at least in part, by a VEGF gradient produced by a network of astrocytes that grows on the vitreal face of the retina ahead of the vascular plexus (Chan-Ling et al., 1995; Gerhardt et al., 2003). The lack of a Norrin gradient in the developing retina, indicates that Norrin-induced retinal vascular growth does not require a spatial concentration gradient. This is in contrast to some Wnt-mediated processes in the Drosophila embryo and wing, where different positions along a Wingless concentration gradient are associated with different developmental responses (van den Heuvel et al., 1993; Cadigan, 2002). The lack of an Ndp expression gradient across the retina is consistent with previous work showing that an Ndp transgene controlled by a lens specific promoter fully rescues the retinal vascular defect of Ndp mutant mice, presumably by uniformly bathing the retina in lens-derived Norrin (Ohlmann et al., 2005). These data are consistent with a model in which Norrin regulates the competence of retinal endothelial cells but does not function as a directional guidance cue.
1.2 Ndp expression in the neural tube and brain
At E10.5, NdpAP expression is observed in the hindbrain and throughout the neural tube (Fig. 2A and B). Cross sections of NdpAP/Y embryos show that NdpAP expression in the spinal cord and hindbrain is restricted to the dorsal and mid-dorsal regions of the neuroepithelium, respectively. This pattern is similar to the Ndp expression pattern observed during chick embryonic development, as determined by in situ hybridization (Paxton et al., 2010). The conservation of the embryonic Ndp expression pattern, as well as the Norrin amino acid sequence, between mammals and birds suggests a conserved and still unknown function for Norrin in early vertebrate development.
Figure 2.

NdpAP is expressed in discrete regions of the central nervous sytem at E10.5.
(A) Whole-mount AP histochemistry of an E10.5 NdpAP/Y embryo. (B) Dorsal view of the hindbrain region of the E10.5 embryo shown in (A). (C and D) 100μm transverse sections across the posterior (C) and hindbrain (D) regions of the neural tube. Scale bars, 200 μm
Interestingly, canonical Wnt signaling has recently been identified as an important regulator of CNS angiogenesis, with Wnt7a and Wnt7b redundantly controlling vascularization of the ventral neural tube (Daneman et al., 2009; Stenman et al., 2008). Consistent with that function, both Wnt7a and Wnt7b are expressed by the ventral neuroepithelium. Canonical Wnt signaling is also required in endothelial cells for vascularization of the dorsal neural tube, although the identities of the dorsal ligand(s) and the relevant receptor(s) are unknown (Daneman et al., 2009; Stenman et al., 2008). As no single receptor or single ligand knockout mouse mutant has been reported to exhibit an angiogenesis defect in the dorsal neural tube, there could be functional redundancy at both the ligand and the receptor levels. The expression of Ndp and Fz4 at this time and location, as well as the ability of Norrin/Fz4/Lrp/Tspan12 to activate canonical Wnt signaling and promote angiogenesis in the retina, favors them as candidates for regulating angiogenesis in the dorsal neural tube.
NdpAP expression is down-regulated in the spinal cord during late prenatal development, but during the same time NdpAP expression increases in the brain. At E10.5, a cluster of NdpAP-expressing cells is observed between the two lobes of the telencephalon, the site of the developing hypothalamus (Fig. 1A). At E15.5, NdpAP expression is observed in several brain regions: the olfactory bulb and along the lateral olfactory tract, the inferior region of the subventricular zone of the lateral ventricles, the territories flanking the medial ganglionic eminence (MGE), the hypothalamus, the amygdala (the target of the lateral olfactory tract), a narrow transverse territory within the posterior thalamus, and the cerebellar primordium (Fig. 3).
Figure 3.
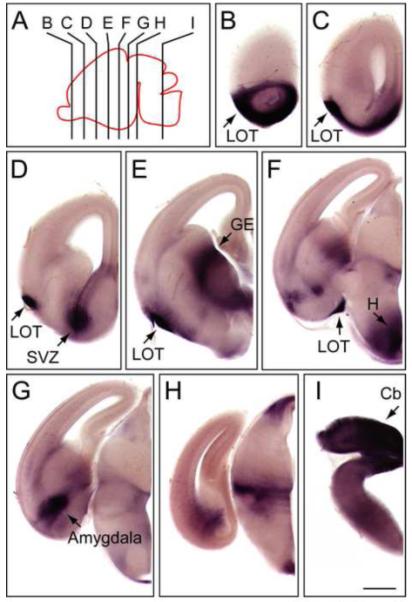
NdpAP expression in the E15.5 mouse brain.
(A) Diagram of an E15.5 mouse brain with the section planes shown for panels (B-I). Anterior is to the left. (B-I) AP stained 100 μm coronal brain sections from an E15.5 NdpAP/+ female embryo. The midline is at the right side of each panel. Cb, cerebellar primordium; GE, ganglionic eminence; H, hypothalamus; LOT, lateral olfactory tract; SVZ, subventricular zone. Scale bar, 500 μm.
At P0, a faint AP signal is detected in scattered cells with diffuse processes throughout the forebrain and diencephalon of NdpAP/Y mice (Fig. 4). In the mouse CNS, differentiation of astrocytes begins at this time (Mission et al., 1991; Kriegstein and Alvarez-Buylla, 2009). Since NdpAP is expressed in astrocytes in the adult brain (described below), it seems likely that the diffuse AP staining seen at P0 represents the beginning of astrocyte expression. At P0, the AP signal remains in the lateral olfactory tract and the subventricular zone, the germinal center for neurons and glia in the postnatal brain.
Figure 4.
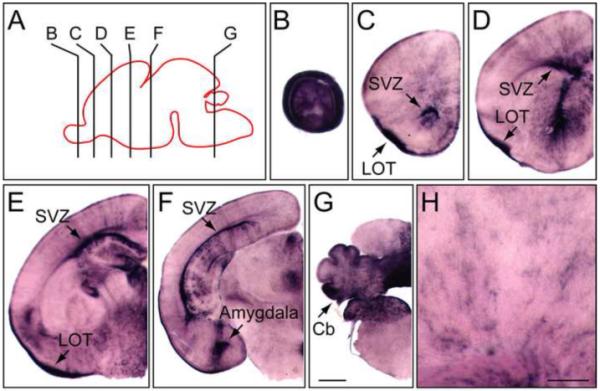
NdpAP expression in the P1 mouse brain.
(A) Diagram of a P1 mouse brain with the section planes shown for panels (B-G). Anterior is to the left. (B-G) AP stained 100 μm coronal sections from a P1 NdpAP/+ female mouse. The midline is at the right side of each panel. (H) Enlarged view of the cortex. Cb, cerebellum; LOT, lateral olfactory tract; SVZ, subventricular zone. Scale bars: B-G, 500 μm; H, 200 μm.
In the adult brain, NdpAP is widely expressed, with much of the brain - including the entire forebrain and diencephalon - of NdpAP/Y mice exhibiting intense and uniform staining. To more clearly visualize individual labeled cells, heterozygous female NdpAP/+ brains were AP stained (Fig. 5). This analysis revealed a relatively homogenous distribution of labeled cells with diffuse arbors, each occupying a ~50 um diameter spherical space - characteristics that match the morphology of astrocytes (Fig. 5F, H, and I). In the NdpAP/+ cortex, the AP-expressing cells show a subtle clustering into radial stripes, presumably reflecting radial migration from a subventricular zone that is mosaic for X-chromosome inactivation (Fig. 5F).
Figure 5.
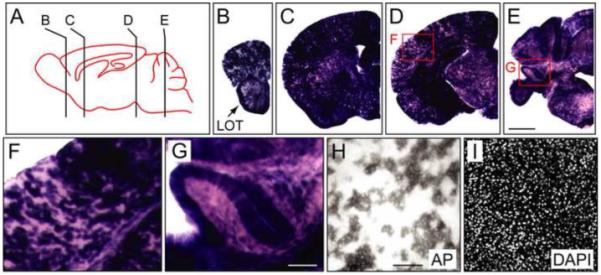
NdpAP expression in adult mouse brain.
(A) Diagram of an adult mouse brain with the section planes shown for panels (B-E). Anterior is to the left. (B-E) AP stained 200 μm coronal sections from an NdpAP/+ adult female. The midline is at the right side of each panel. (F and G) Enlarged views of the boxed regions in D and E. (H and I) 50 μm coronal sections from an NdpAP/+ adult female stained histochemically for AP; nuclei are labeled with DAPI. LOT, lateral olfactory tract. Scale bars: B-E, 1 mm; F, G, 200 μm; H, I, 100 μm
In the adult NdpAP/+ cerebellum, the AP signal is most intense in the molecular layer and is radially oriented; it is far less intense in the granule cell layer, the white matter, and the deep cerebellar nuclei (Fig. 5G). This AP localization is consistent with a previous report of Ndp expression in the Purkinje layer of the cerebellum by in situ hybridization with radioactive probes, presumably reflecting hybridization to cell bodies of Purkinje neurons and/or Bergman glia (Hartzer et al., 1999). Whereas both cell types extend a highly complex arbor radially into the molecular layer (Bellamy, 2006), only Purkinje cells extend processes beyond this layer, with each Purkinje cell also projecting an axon across the granule cell layer and white matter to the deep cerebellar nuclei. In previous studies, histochemical detection of the same AP reporter was observed to label all classes of neurites, including axons (Badea et al., 2003). Therefore, the absence of AP-positive axons traversing the molecular layer and white matter in the NdpAP/+ cerebellum implies that NdpAP expression is confined to Bergman glia.
1.3 Ndp expression in the inner ear
Norrin/Fz4 signaling is required for the maintenance of the stria vascularis in the inner ear, but the initial development of this vasculature is unaffected by loss of either Norrin or Fz4 (Rehm et al., 2002; Xu et al., 2004). The stria vascularis produces the endolymph within the scala media, and capillary loss in the stria vascularis is presumably the cause of progressive hearing loss in mice and humans with Ndp mutations. To identify the source of Norrin in the inner ear, we analyzed NdpAP expression in P2 and adult inner ears (Fig. 6). At P2, NdpAP is expressed in a highly vascularized zone between the organ of Corti and the spiral ganglion (Fig. 6B) and in the lateral wall adjacent to the stria vascularis (Fig. 6C). In Fig. 6B and C, the vasculature is decorated with fluorescent GS lectin, but the fluorescence is partially quenched by the purple AP histochemical reaction product. The P2 expression pattern is maintained essentially unchanged in the adult cochlea, consistent with the requirement for ongoing Norrin/Fz4 signaling for vascular maintenance (Fig. 6D and E).
Figure 6.
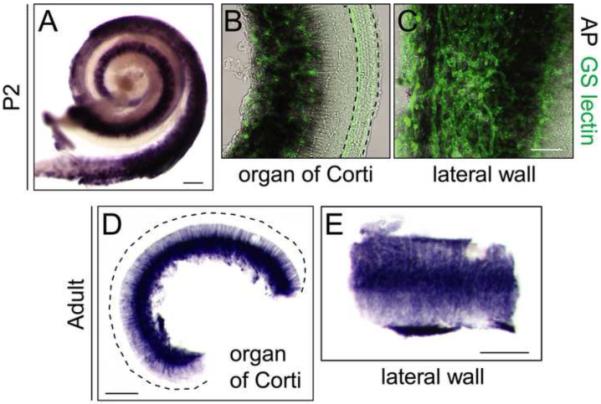
NdpAP expression in the postnatal mouse cochlea.
(A) AP stained whole mount of the cochlea from a NdpAP/+ P2 female. (B and C) AP stained whole mount of the organ of Corti (B) and the lateral wall of the cochlea (C), with the vasculature labeled by fluorescent GS lectin from an NdpAP/+ P2 female. Dashed lines in B outline the rows of auditory hair cells. (D and E) AP stained whole mount of the organ of Corti and the lateral wall from a two-month-old NdpAP/Y male. The dashed line in D outlines the outer edge of the organ of Corti. Scale bars: A, D, E, 200 μm; B, C, 50 μm.
1.4 Functional implications of the Ndp expressino pattern
The expression data presented here define the pre-natal, peri-natal, and adult patterns of Ndp expression in the mammalian CNS, and they complement and extend the Ndp expression patterns described recently in the early embryonic chicken CNS (Paxton et al., 2010). Taken together, the precisely defined territories in which Norrin is expressed in the developing CNS, the widespread expression of Norrin in glia in the adult CNS, and the expression of the Norrin receptor (Fz4) in essentially the entire vasculature from midgestation to adulthood, suggest the possibility that Norrin may have developmental and/or homeostasic functions beyond those identified thus far in the eye and ear.
2. Experimental procedures
2.1 Animal husbandry and genotyping
NdpAP/+ females were bred with wild-type males to produce NdpAP/y male and NdpAP/+ female progeny for AP histochemistry. PCR primers for genotyping are as follows. NdpAP/+ (sense strand: CTGCTGGAGACGGCCACTGCTCCCT; antisense strand: TGGCCAGCAGGGAGAGCATAGAAAT); Ndp WT (sense strand: CAGCTGTGCAGCACATACTGCTGTG; antisense strand: the same as above). DNA was extracted using the REDExtract-N-Amp™ Tissue PCR Kit (Sigma). PCR was performed with 35 cycles of 30 seconds denaturation at 94°C, 30 seconds annealing at 60°C, and 30 seconds elongation at 72°C. Mice were housed and handled in accordance with protocols approved by the Johns Hopkins University Animal Care and Use Committee and the IACUC guidelines.
2.2 Histology
Tissues were collected either fresh or after cardiac perfusion. Whole-mount embryos were fixed in 4% paraformaldehyde (PFA) in PBS overnight, and retinas and cochleas were fixed with 4% PFA in PBS for one hour at room temperature. For postnatal brain histology, mice were anesthetized with ketamine-xylazine and perfused with 4% PFA in PBS. Immersion fixed and dissected embryonic or P1 brains and perfusion fixed postnatal brains were embedded in 4% low melting point agarose in PBS, and sectioned on a vibratome at 50-200 μm thickness. Tissue sections were washed with PBS for 2 × 20 minutes at room temperature, and incubated at 70°C for 90 minutes to inactivate endogenous AP activity. AP staining was performed in 0.1 M Tris-HCl pH=9.5, 0.1 M NaCl, 50 mM MgCl2, 0.34 μg/ml nitroblue tetrazolium (NBT), and 0.175 μg/ml 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) (Boehringer Mannheim, Indianapolis, IN) at room temperature with gentle agitation overnight. After staining, tissues were washed 3 × 1 hour with PBST (PBS + 0.3% triton-X100), and postfixed in PBS with 4% PFA overnight. Adult brain sections and whole mount embryos were dehydrated in 50%, 75% and then 100% ethanol, and cleared with 2:1 benzyl benzoate:benzyl alcohol before imaging. GS-lectin (Invitrogen I21411, 20 μg/ml) staining was performed following AP histochemistry where indicated.
Acknowledgements
Supported by the National Eye Institute (NIH) and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J. Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy TC. Interactions between Purkinje neurones and Bergmann glia. Cerebellum. 2006;5:116–126. doi: 10.1080/14734220600724569. [DOI] [PubMed] [Google Scholar]
- Berger W, Ropers HH. Norrie disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Bases of Inherited Disease. Eighth Edition McGraw Hill; New York: 2001. pp. 5977–5985. [Google Scholar]
- Cadigan KM. Regulating morphogen gradients in the Drosophila wing. Semin. Cell Dev. Biol. 2002;13:83–90. doi: 10.1016/s1084-9521(02)00014-9. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest. Ophthalmol. Vis. Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzer MK, Cheng M, Liu X, Shastry BS. Localization of the Norrie disease gene mRNA by in situ hybridization. Brain Res. Bull. 1999;49:355–358. doi: 10.1016/s0361-9230(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann UF, Lin J, Acar N, Lammel S, Feil S, Grimm C, Seeliger MW, Hammes HP, Berger W. Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest. Ophthalmol. Vis. Sci. 2005;46:3372–3382. doi: 10.1167/iovs.05-0174. [DOI] [PubMed] [Google Scholar]
- Mission JP, Takahashi T, Caviness VS., Jr. Ontogeny of radial and other astroglial cells in murine cerebral cortex. Glia. 1991;4:138–148. doi: 10.1002/glia.440040205. [DOI] [PubMed] [Google Scholar]
- Ohlmann A, Scholz M, Goldwich A, Chauhan BK, Hudl K, Ohlmann AV, Zrenner E, Berger W, Cvekl A, Seeliger MW, Tamm ER. Ectopic norrin induces growth of ocular capillaries and restores normal retinal angiogenesis in Norrie disease mutant mice. J. Neurosci. 2005;25:1701–1710. doi: 10.1523/JNEUROSCI.4756-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton CN, Bleyl SB, Chapman SC, Schoenwolf GC. Identification of differentially expressed genes in early inner ear development. Gene Expr. Patterns. 2010;10:31–43. doi: 10.1016/j.gep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm HL, Zhang DS, Brown MC, Burgess B, Halpin C, Berger W, Morton CC, Corey DP, Chen ZY. Vascular defects and sensorineural deafness in a mouse model of Norrie disease. J. Neurosci. 2002;22:4286–4292. doi: 10.1523/JNEUROSCI.22-11-04286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Gottanka J, May CA, Welge-Lüssen U, Berger W, Lütjen-Drecoll E. Retinal vasculature changes in Norrie disease mice. Invest. Ophthalmol. Vis. Sci. 1998;39:2450–2457. [PubMed] [Google Scholar]
- Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. Mutational analysis of Norrin-Frizzled4 recognition. J. Biol. Chem. 2007;282:4057–68. doi: 10.1074/jbc.M609618200. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Klingensmith J, Perrimon N, Nusse R. Cell patterning in the Drosophila segment: engrailed and wingless antigen distributions in segment polarity mutant embryos. Dev. Suppl. 1993:105–14. [PubMed] [Google Scholar]
- Vazquez-Chona FR, Clark AM, Levine EM. Rlbp1 promoter drives robust Muller glial GFP expression in transgenic mice. Inv. Ophthalmol. Vis. Sci. 2009;50:3996–4003. doi: 10.1167/iovs.08-3189. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J. Neurosci. 2001;21:4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, Frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol. Med. 2010 doi: 10.1016/j.molmed.2010.07.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat. Rec. 1985a;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell proliferation during postnatal development of the retina in the mouse. Brain. Res. 1985b;353:229–239. doi: 10.1016/0165-3806(85)90211-1. [DOI] [PubMed] [Google Scholar]


