Abstract
Nicotine, varenicline, and cytisine are pharmacotherapies for tobacco dependence; the extent to which their in vivo effects vary as a function of differences in nicotinic acetylcholine receptor agonism is not clear. Male C57BL/6J mice responding under a fixed ratio 30 schedule of food delivery were used to establish the potency and time course of nicotine, varenicline, and cytisine; antagonism was examined with the non-competitive, non-selective antagonist mecamylamine and the competitive, α4β2 nicotinic receptor antagonist dihydro-β-erythroidine (DHβE). Intraperitoneal nicotine, varenicline, and cytisine dose-dependently decreased responding; nicotine was more potent (ED50 value = 0.83 mg/kg) than varenicline (ED50 value = 2.51 mg/kg) and cytisine (ED50 value = 2.97 mg/kg). The agonists had a similar time course including a rapid onset (5 min or less) and relatively short duration of action (30 min). Mecamylamine dose-dependently attenuated the rate-decreasing effects of a fixed dose of nicotine (1.78 mg/kg), varenicline (5.6 mg/kg), and cytisine (5.6 mg/kg). Mecamylamine (1 mg/kg) produced parallel rightward shifts in the dose- response curves for nicotine (3.3-fold), varenicline (3.1-fold), and cytisine (2.3-fold). In contrast, DHβE (3.2 mg/kg) produced 2-fold antagonism of nicotine and did not antagonize varenicline or cytisine. The data strongly suggest that nicotinic acetylcholine receptors mediate the effects of the agonists to decrease operant responding in mice. However, α4β2 receptor agonism appears to contribute partially to the rate-decreasing effects of nicotine but not to the rate-decreasing effects of varenicline and cytisine. Differential activation of α4β2 receptors in vivo might contribute to differences in the effectiveness of these smoking cessation aids.
Keywords: nicotine, varenicline, cytisine, mecamylamine, dihydro-β-erythroidine, behavior
1. Introduction
Cigarette smoking increases the risk of cardiovascular disease, respiratory disease, and cancer, and is a leading preventable cause of death. Nicotine, a chemical inhaled in tobacco smoke, is the prototypic agonist at acetylcholine receptor-gated ion channels and is primarily responsible for the abuse and dependence liability of cigarette smoking. Nicotine replacement therapy (e.g., nicotine patch and gum) is widely used to enhance smoking cessation. Alternative pharmacotherapies include the nicotinic acetylcholine receptor ligands cytisine (e.g., Tabex®; Etter, 2006) and varenicline (e.g., Chantix®; Rollema et al., 2007), both of which are administered orally. Although currently prescribed drugs are effective, there is margin for improvement, as significant numbers of cigarette smokers relapse despite receiving pharmacotherapy during quit attempts (George and O'Malley, 2004). Identifying receptor mechanisms responsible for the in vivo effects of nicotinic receptor ligands across a broad range of conditions could provide the basis for developing novel smoking cessation aids with enhanced therapeutic utility.
Nicotine, varenicline, and cytisine bind to nicotinic acetylcholine receptors, which are located on ion channels consisting of 5 protein subunits. Various types of subunits are differentially combined to produce multiple receptor subtypes; based on in vitro data, nicotinic receptor ligands vary in binding affinity and efficacy at the various receptor subtypes. Nicotine, varenicline, and cytisine were shown to have similar rank order binding affinity at receptor subtypes in the central nervous system as follows: heteromeric α4β2 > heteromeric α3β4 > homomeric α7subunits (Rollema et al., 2010). However, the drugs were reported to vary in agonist efficacy at α4β2 subunit-containing receptors in vitro, i.e., nicotine had higher efficacy than varenicline and cytisine (Coe et al., 2005). Whether in vivo effects vary as a function of nicotinic receptor binding affinity and efficacy remains a central issue underlying the development of nicotinic receptor-based therapeutics.
The goal of this study was to compare the effects of nicotine, varenicline, and cytisine in male C57BL/6J mice responding under a fixed ratio schedule of food presentation. First, experiments were conducted to establish the potency and time course of drugs to decrease fixed ratio responding. Second, the drugs were combined with nicotinic acetylcholine receptor antagonists to evaluate receptor mechanisms of action. Antagonists were the non-selective and non-competitive nicotinic acetylcholine receptor ligand mecamylamine and the competitive α4β2 subunit-containing receptor ligand dihydro-β-erythroidine (DHβE). Mecamylamine produced similar antagonism of nicotine, varenicline, and cytisine, whereas DHβE produced limited (nicotine) or no antagonism (varenicline and cytisine) up to the largest dose of DHβE that could be safely studied. Collectively, these results suggest that activation of α4β2 subunit-containing receptors plays a greater role in the behavioral effects of nicotine as compared to varenicline and cytisine.
2. Materials and Methods
2.1 Subjects
Sixteen male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were purchased at 9 weeks of age (approximately 15 g) and were housed individually on a 14/10-h light/dark cycle. Mice were maintained at 85% of free-feeding weight and received approximately 1 cc of 50% condensed milk during experimental sessions and 2 g of food (Dustless Precision Pellets 500 mg, Rodent Grain-Based Diet, Bio-Serv, Frenchtown, NJ) per day after sessions; water was available ad libitum in the home cage. Mice were habituated to the experimental room for 7 days before the first experimental session, and testing was conducted during the light period. Mice were maintained, and experiments were conducted in accordance with, the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio and with the “Principles of Laboratory Animal Care” and the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
2.2 Drugs
(-)-Nicotine hydrogen tartrate salt and cytisine were obtained from Sigma Chemical (St. Louis, MO, USA). Varenicline dihydrochloride, mecamylamine, and dihydro-β-erythroidine hydrobromide were obtained from The Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD, USA). Drugs were dissolved in 0.9% physiological saline and injected (i.p.) in a volume equivalent to 10 ml/kg. Nicotine dose was expressed in terms of the weight of the free base; dose of other drugs was expressed as the weight of the base and salt.
2.3 Apparatus
Commercially available mouse operant conditioning chambers (MedAssociates, St. Albans, VT) were placed in ventilated, sound-attenuating enclosures. The ceiling of each operant conditioning chamber contained a light (i.e., house light) and the side of one wall contained a recessed hole (2.2-cm diameter). The center of the hole was positioned 1.6 cm from the floor. The hole contained a photo beam, a light, and a dipper to which 0.01 cc of condensed milk could be delivered from a tray positioned outside the operant conditioning chamber. An interface (MedAssociates) connected the operant conditioning chambers to a computer, and experimental events were controlled and recorded with Med-PC software.
2.4 Operant conditioning procedure
Sessions were conducted once daily, seven days per week. During initial training, the mice were placed into the operant chamber for 30 min and the light inside the nose-poke hole was illuminated; a single response (i.e., disruption of the photobeam under a schedule of continuous reinforcement) resulted in 10-s access to 0.01 cc of milk. During the 10-s period of milk access, the light inside the nose-poke hole was extinguished, the house light was illuminated, and disruption of the photobeam had no programmed consequence. The schedule was increased every two sessions in the following increments: FR3, FR10, and FR30. The first two sessions at FR30 began with a 5-min timeout; during the timeout, the lights were off and there was no consequence for disruption of the photobeam. The timeout was followed by a 30-min period of milk availability. Starting with the third session at FR30, sessions were divided into six consecutive, multiple cycles; each cycle consisted of a 5-min timeout followed immediately by a 5-min period of milk availability. These session parameters (i.e. responding at an FR30 in multiple cycles) were used for the remainder of the study. The multiple-cycle procedure was used to establish the time course for the effects of nicotine, varenicline, and cytisine, as described below.
Before drug tests, mice received saline at the beginning of the first cycle. Sessions were conducted until responding for an individual mouse was ±20% of the mean rate for all cycles for 5 consecutive or 6 out of 7 days. After satisfying this criterion, drugs were administered during sessions; subsequent drug tests were conducted only when responding during the immediately preceding non-drug session was ±20% of the mean rate for all cycles during the 5 previous non-drug sessions. Dose-response functions were determined first for nicotine, varenicline, and cytisine by administering a dose at the beginning of the first cycle of a 6-cycle session; dose-response curves included an ineffective dose (i.e., a dose that did not modify response rate) up to a dose that resulted in delivery of no reinforcers in the first cycle. A dose-response curve for a particular agonist was completed in individual mice by administering all doses for that agonist in non-systematic order before determining the dose-response curve for the next agonist. The order of testing with agonists across mice was non-systematic. After examining the effects of nicotine, varenicline, and cytisine alone, antagonism by mecamylamine and DHβE was studied. To establish inhibition curves, nicotine (1.78 mg/kg), varenicline (5.6 mg/kg), and cytisine (5.6 mg/kg) were combined with mecamylamine (0.032-1 mg/kg) or DHβE (1.78 and 3.2 mg/kg). To examine shifts in agonist dose-response curves, various doses of nicotine, varenicline, and cytisine were studied in combination with 1 mg/kg of mecamylamine or 3.2 mg/kg of DHβE. Mecamylamine and DHβE were administered 5 min before the agonists or saline; dose-response data were obtained 10-15 min after administration of the antagonists and 5-10 min after administration of nicotine, varenicline, cytisine, or saline. The order of testing with mecamylamine and DHβE in combination with the nicotinic receptor agonists was non-systematic. After completion of the antagonism studies, the dose-response curves for nicotine, varenicline, and cytisine were determined a second time.
2.5 Data Analysis
Of the 16 mice used for these studies, a minimum of 7 mice were used for each treatment and the same subgroup of mice (i.e., a within-subjects design) was used to compare dose-response curves. That is, antagonism of nicotine, varenicline, and cytisine by mecamylamine was studied in one group of mice and antagonism by DHβE was studied in a second group of mice. Control rate of responding (responses per s when milk was available under the FR30 schedule excluding responses that were made during the 10-s period of milk availability) was calculated as the average rate for all cycles in the 5 non-drug (i.e., saline) sessions immediately preceding a drug session. Drug-induced changes in response rate were expressed and analyzed as a percentage of the control for individual mice. The potency of drugs to decrease responding was calculated by simultaneously fitting the linear portion of individual dose-response data with straight lines by means of GraphPad Prism version 5.0 for Windows (San Diego, CA) with linear regression. Linear portions were defined by doses producing 20-80% of the maximum effect, including not more than one dose producing less than 20% of the maximum effect and not more than one dose producing greater than 80% of the maximum effect. Slopes were compared using an F-ratio test; if the slopes were not significantly different (i.e., P>0.05), then a common, best-fitting slope was used for further analyses (Kenakin, 1997). Doses corresponding to the 50% level of the effect (ED50), potency ratios, and their 95% confidence limits were calculated by parallel line analyses of data from individual subjects (Tallarida, 2000). When the 95% confidence limits of the potency ratio did not include 1, potencies were considered significantly different. Inhibition curves for mecamylamine and DHβE were analyzed separately with ANOVA for repeated measures followed by Tukey post hoc test to examine significant differences (i.e., P<0.05) from the saline control or agonist alone.
3. Results
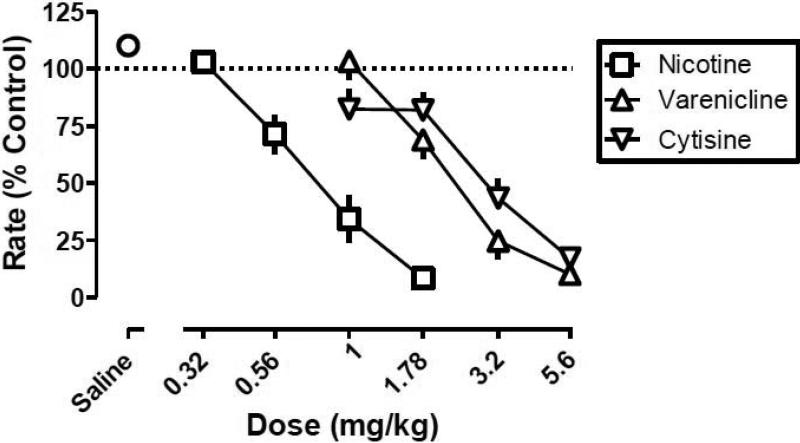
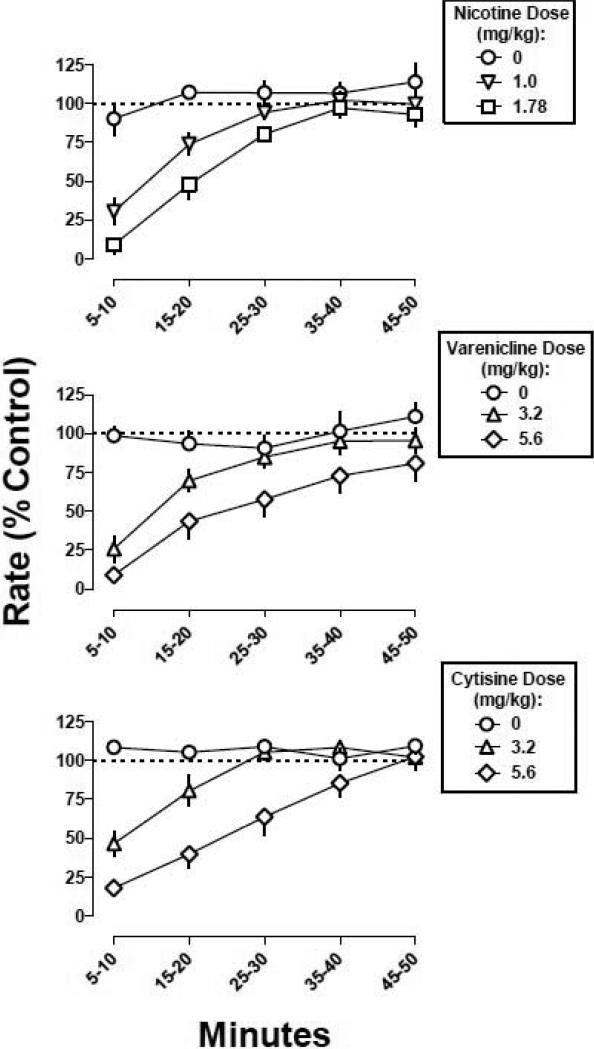
For control performance prior to drug testing, the median of absolute rate of responding among mice (n=16) was 3.1 responses per s; the range was 1.35-5.27 responses per s. Saline i.p. did not significantly modify response rate, whereas nicotine, varenicline, and cytisine dose-dependently decreased response rate (Fig. 1). For example, nicotine (1.78 mg/kg), varenicline (5.6 mg/kg), and cytisine (5.6 mg/kg) decreased responding to 8.4, 10, and 17% of control, respectively. The dose-response curves (i.e., ED50 value) for each agonist alone, one determined before and the other after the agonists were combined with the antagonists, were not significantly different from each other (P>0.05); therefore, the two controls were averaged for graphic presentation (Fig. 1) and further analysis. The slopes of the agonist dose-response curves did not significantly differ (P>0.05); nicotine (ED50 value = 0.83 mg/kg) was more potent than varenicline (ED50 value = 2.51 mg/kg) and cytisine (ED50 value = 2.97 mg/kg), which were equipotent (Table 1). Nicotine, varenicline, and cytisine had a similar time course of activity, which included a rapid onset (less than 5 min) and relatively short duration of action (30 min; Fig. 2).
Fig. 1.
Effects of nicotine, varenicline, and cytisine to decrease fixed ratio responding in C57BL6/J mice (n=13). Abscissa: dose in milligram per kilogram body weight or saline. Ordinate: mean (±S.E.M.) response rate expressed as a percentage of control (non-drug days) rate [Rate (% Control)].
Table 1.
ED50 values and 95% confidence limits for nicotine, varenicline, and cytisine alone (n=13) and in combination with mecamylamine (Mec; n=8) or DHβE (n=8). Potency ratios and 95% confidence limits are the ED50 values of varenicline and cytisine divided by the ED50 value of nicotine, or the ED50 values of the agonists in combination with an antagonist divided by the ED50 of the agonist alone.
| ED50 (mg/kg) | 95% Confidence Limits (mg/kg) | Potency Ratio | 95% Confidence Limits | |
|---|---|---|---|---|
| Agonists alone (n=13) | ||||
| Nicotine | 0.83 | 0.68-1.00 | ||
| Varenicline | 2.51 | 2.05-3.07 | 3.0 | 2.3-4.0 |
| Cytisine | 2.97 | 2.56-3.44 | 3.6 | 2.8-4.6 |
| Mecamylamine antagonism (n=8) | ||||
| Nicotine | 0.73 | 0.59-0.91 | ||
| + Mec (1 mg/kg) | 2.41 | 1.61-3.59 | 3.3a | 2.2-4.8 |
| Varenicline | 1.96 | 1.44-2.67 | ||
| + Mec (1 mg/kg) | 6.13 | 4.14-9.08 | 3.1a | 2.0-4.9 |
| Cytisine | 2.72 | 2.25-3.30 | ||
| + Mec (1 mg/kg) | 6.13 | 5.19-7.23 | 2.3a | 1.8-2.9 |
| DHβE antagonism (n=8) | ||||
| Nicotine | 0.93 | 0.75-1.15 | ||
| + DHβE (3.2 mg/kg) | 1.66 | 1.13-2.45 | 1.8a | 1.1-2.8 |
| Varenicline | 2.58 | 1.98-3.36 | ||
| + DHβE (3.2 mg/kg) | 3.22 | 2.46-4.21 | 1.1 | 0.7-1.8 |
| Cytisine | 3.22 | 2.54-4.10 | ||
| + DHβE (3.2 mg/kg) | 2.82 | 1.86-4.27 | 0.9 | 0.5-1.4 |
significant antagonism.
Fig. 2.
Time course for nicotine, varenicline, and cytisine to decrease fixed ratio responding in mice (n=13). Abscissa: time in minutes. Ordinate: mean (±S.E.M.) response rate expressed as a percentage of control (non-drug days) rate [Rate (% Control)].
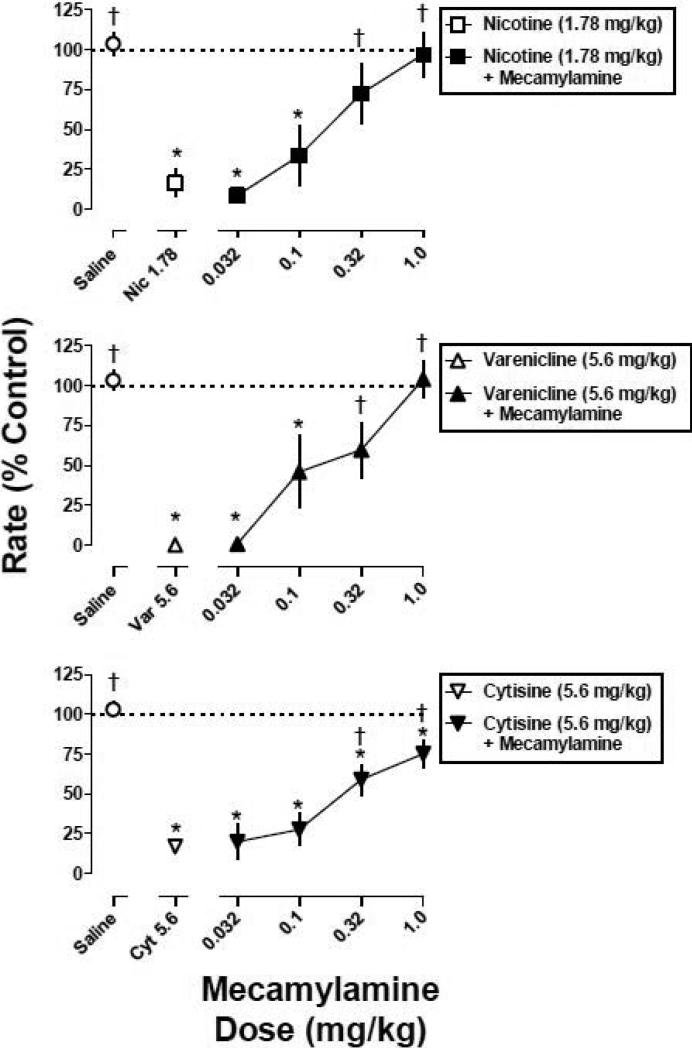
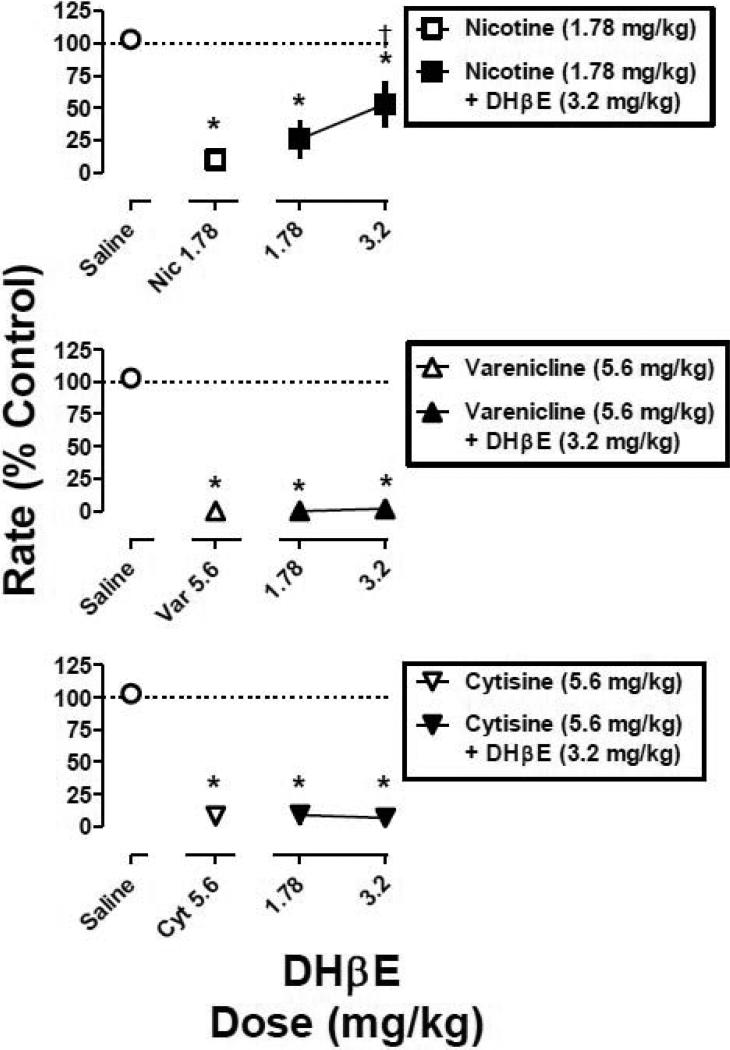
When combined with a dose that decreased mean response rate to less than 20% of control (i.e., 1.78 mg/kg of nicotine, 5.6 mg/kg of varenicline, and 5.6 mg/kg of cytisine), mecamylamine dose-dependently antagonized the rate-decreasing effects of all three agonists (Fig. 3).
Fig. 3.
The effects of various doses of mecamylamine in combination with a fixed dose of nicotine, varenicline, and cytisine. Abscissa: dose in milligram per kilogram body weight of mecamylamine or saline. Ordinate: mean (±S.E.M.) response rate expressed as a percentage of control (non-drug days) rate [Rate (% Control)]. *, significantly different from control (saline); †, significantly different from agonist alone (P<0.05).
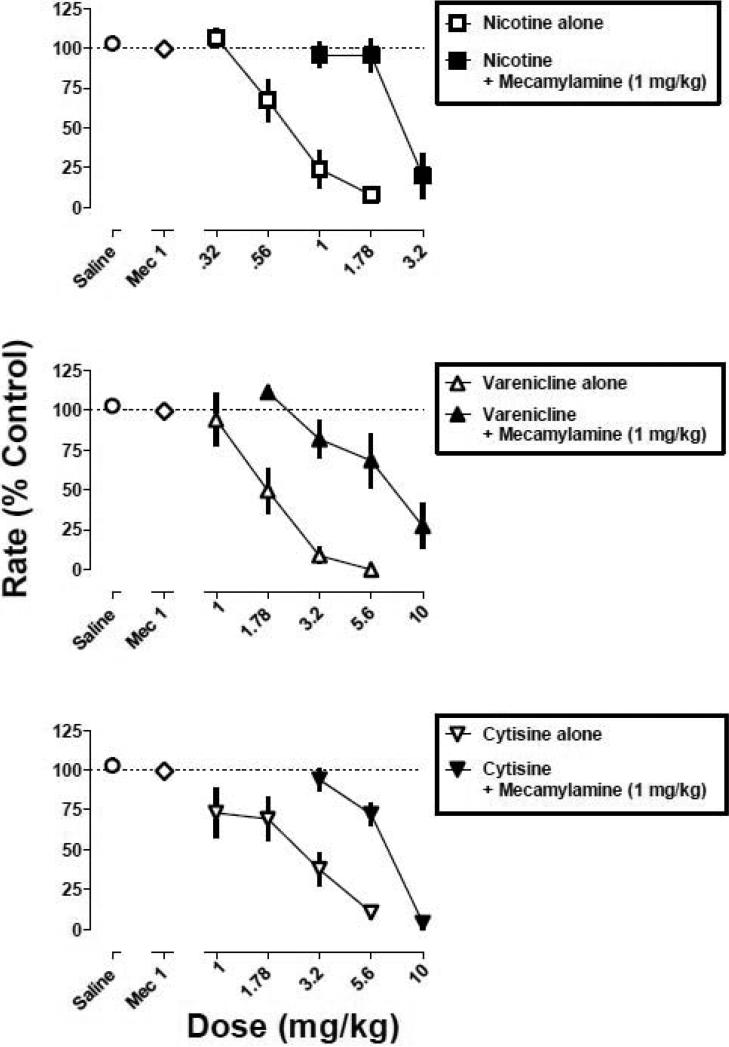
Responding in the presence of the largest dose (1 mg/kg) of mecamylamine in combination with nicotine (1.78 mg/kg), varenicline (5.6 mg/kg), and cytisine (5.6 mg/kg) was 97%, 104%, and 75% of control, respectively. Mecamylamine (0.32 and 1 mg/kg) fully antagonized nicotine and varenicline (i.e., responding was not significantly different from the saline control). In contrast, antagonism of cytisine by mecamylamine was incomplete (i.e., responding was significantly different from the saline control at all dose-combinations). A larger dose (3.2 mg/kg) of mecamylamine in combination with cytisine (5.6 mg/kg) was lethal. Mecamylamine alone (1 mg/kg) did not significantly modify response rate (Fig. 4, diamond above Mec 1). When the nicotine, varenicline, and cytisine dose-response curves were re-determined in the presence of mecamylamine (1 mg/kg), there were significant, parallel rightward shifts in the curves (Fig. 4), i.e., the slopes of the dose-response curves were not significantly different and the potency ratios of the 95% confidence limits did not include 1 (Fig. 4). The magnitude of rightward shift for cytisine (2.3-fold) was somewhat less than that of the rightward shifts for nicotine (3.3-fold) and varenicline (3.1-fold) (Table 1).
Fig. 4.
Dose-response curves for nicotine, varenicline, and cytisine, alone (open symbols) and in combination with mecamylamine (1 mg/kg; closed symbols). Abscissa: dose in milligram per kilogram body weight or saline. Ordinate: mean (±S.E.M.) response rate expressed as a percentage of control (non-drug days) rate [Rate (% Control)]. Control curves (open symbols) are plotted from a subset of mice contributing to the control curve in Fig. 1.
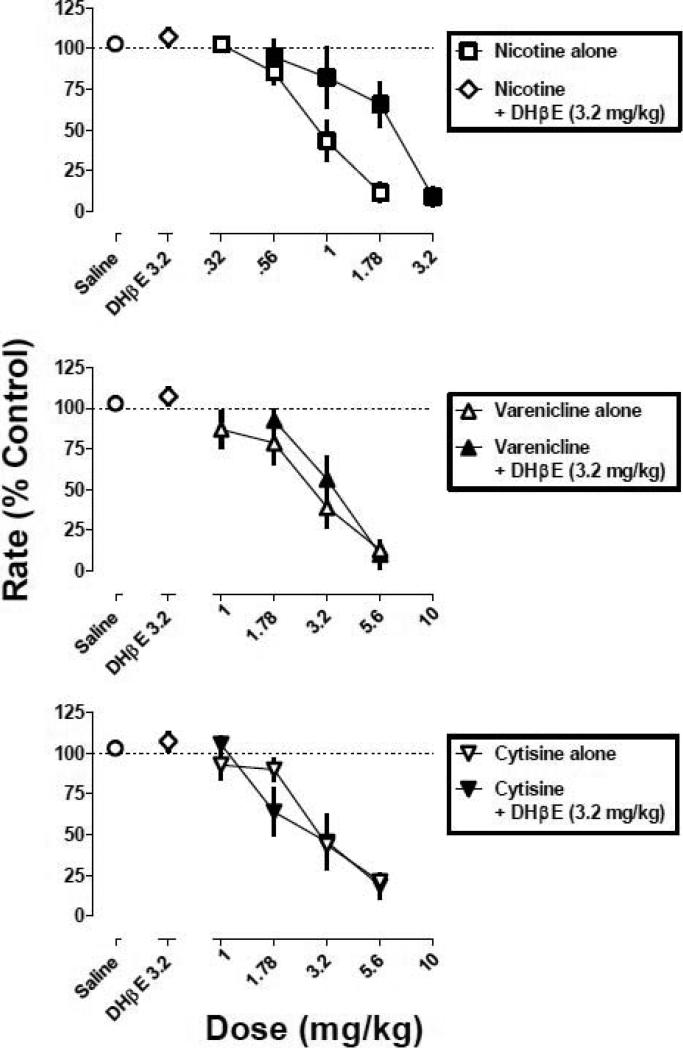
DHβE was less effective than mecamylamine as an antagonist of the rate-decreasing effects of the nicotinic receptor agonists. DHβE (3.2 mg/kg) produced partial antagonism of the rate-decreasing effects of nicotine (1.78 mg/kg). Response rate was 8% of control after nicotine alone (1.78) and was 53% of control when that dose of nicotine was combined with DHβE (Fig. 5). In contrast, DHβE (3.2 mg/kg) did not antagonize the rate-decreasing effects of varenicline (5.6 mg/kg) or cytisine (5.6 mg/kg). DHβE (3.2 mg/kg) alone did not significantly modify response rate (Fig. 6, diamond above DHβE) and significantly shifted the dose-response curve for nicotine 2-fold to the right (Fig. 6). In contrast, DHβE (3.2 mg/kg) did not significantly modify the dose-response curves for varenicline or cytisine (Table 1). A dose of 10 mg/kg of DHβE was lethal and therefore was not studied in combination with the agonists.
Fig. 5.
The effects of various doses of DHβE in combination with a fixed dose of nicotine, varenicline, and cytisine. Abscissa: dose in milligram per kilogram body weight or saline. Ordinate: mean (±S.E.M.) response rate expressed as a percentage of control (non-drug days) rate [Rate (% Control)]. *, significantly different from control (saline); †, significantly different from agonist alone (P<0.05).
Fig. 6.
Dose-response curves for nicotine, varenicline, and cytisine, alone (circles) and in combination with DHβE (3.2 mg/kg; squares). Abscissa: dose in milligram per kilogram body weight or saline. Ordinate: mean (±S.E.M.) response rate expressed as a percentage of control (non-drug days) rate [Rate (% Control)]. Control curves (open symbols) are plotted from a subset of mice contributing to the control curve in Fig. 1.
4. Discussion
This study examined pharmacologic mechanisms underlying the effects of nicotine, varenicline, and cytisine to decrease fixed ratio responding for food in C57BL/6J mice. Nicotine was 3-fold more potent than varenicline and cytisine in decreasing operant responding; varenicline and cytisine were equipotent. Nicotine, varenicline, and cytisine had a strikingly similar time course of activity, i.e., onset to peak effect was less than 5 min and the duration of action was 30-40 min. Mecamylamine produced similar antagonism of the rate-decreasing effects of nicotine, varenicline, and cytisine, demonstrating that nicotinic receptors mediated rate-decreasing effects. In contrast, DHβE produced 2-fold antagonism of nicotine and no antagonism of varenicline and cytisine, suggesting that activation of α4β2 receptors played a greater role in the effects of nicotine as compared to varenicline and cytisine in vivo.
Schedule-controlled responding has the advantage of providing a stable and highly quantifiable behavioral baseline for establishing receptor mechanisms underlying the in vivo effects of drugs, including nicotine (Spealman et al., 1981). Based on comparisons of relative potency, the pharmacologic mechanism(s) by which nicotine, varenicline, and cytisine decreased fixed ratio responding for milk appeared to be similar to the mechanism(s) by which the drugs produced other in vivo effects. For example, in previous studies with mice, nicotine was 3- and 4-fold more potent than varenicline in producing antinociception and hypolocomotion, respectively (Carroll et al., 2008) and at least 5-fold more potent than cytisine in producing antinociception (Damaj et al., 1998). Nicotine also was more potent than varenicline and cytisine when the drugs were assessed for discriminative stimulus effects in rats discriminating nicotine (LeSage et al., 2009; Smith et al., 2007). In some previous studies, varenicline and cytisine were not always able to produce the same maximum effect as that obtained with nicotine, consistent with differences in nicotinic acetylcholine receptor activation. In those previous studies, the effects of nicotine were sometimes antagonized by cytisine (Damaj et al., 1998) and varenicline (LeSage et al., 2009), suggesting cytisine and varenicline had lower efficacy then nicotine at a common receptor.
Nicotine, varenicline, and cytisine markedly decreased fixed ratio responding; underlying receptor mechanism(s) were examined by combining the drugs with prototype nicotinic receptor antagonists. Mecamylamine antagonized the effects of nicotine to decrease fixed ratio responding; similar results have been obtained previously in rats (Goldberg et al., 1989) and squirrel monkeys (Spealman et al., 1981). The current study demonstrated that mecamylamine produced similar antagonism of the rate-decreasing effects of nicotine, varenicline, and cytisine, i.e., the magnitude of shift produced by mecamylamine (1 mg/kg) was comparable for all three nicotinic receptor agonists. These results confirmed that nicotinic acetylcholine receptor activation was responsible for the rate-decreasing effects of all three nicotinic receptor ligands. However, given that mecamylamine lacks selectivity for various subtypes of nicotinic receptor (Papke et al., 2001), these data do not indicate whether the same or different receptor subtypes mediated the rate-decreasing effects of nicotine, varenicline, and cytisine.
Unlike mecamylamine, DHβE is thought to compete with nicotine at, and to have selectivity for, α4β2 nicotinic receptors (Williams and Robinson, 1984; Luetje and Patrick 1989). The α4β2 nicotinic receptor is the subtype most often implicated in the abuse and dependence liability of nicotine (McCallum et al., 2006; Nashmi and Lester, 2006). DHβE, up to the largest dose (3.2 mg/kg) that was not lethal, antagonized the rate-decreasing effects of nicotine. This result is consistent with previous studies demonstrating that DHβE antagonized the discriminative stimulus effects and, to a lesser extent, the rate-decreasing effects of nicotine in rodents (Stolerman et al., 1997). In contrast, DHβE did not antagonize the effects of varenicline and cytisine in the current study, suggesting that only the rate-decreasing effects of nicotine resulted from activation of α4β2 nicotinic receptors. This interpretation is consistent with the higher efficacy of nicotine, relative to both varenicline and cytisine, at α4β2 receptors subtypes as indexed by stimulation of electrophysiological responses in vitro (Papke and Heinemann, 1994; Coe et al., 2005; Mihalak et al., 2006). The efficacy of varenicline and cytisine at α4β2 receptors might have been insufficient for this particular subtype to contribute to rate-decreasing effects; therefore, activation of a receptor subtype aside from α4β2 was likely involved. Nicotine, varenicline, and cytisine were reported to have high efficacy at homomeric α7 receptors (Mihalak et al., 2006; Papke et al., 2007; Papke and Porter Papke, 2002); nonetheless, α7 receptors did not appear to mediate the effects of nicotine to decrease operant responding inasmuch as sensitivity to nicotine did not differ between transgenic mice lacking α7 receptors and wildtype mice (Naylor et al., 2004). It is not yet clear whether α7 receptors or other receptor subtypes (e.g., heteromeric α3β4 receptors) mediate the in vivo effects of varenicline and cytisine, as might be suggested by the in vitro binding profile of these ligands (Rollema et al., 2010).
Collectively, the results of the current study show that nicotine, varenicline, and cytisine have a strikingly similar time course of activity and decrease fixed ratio responding through activation of nicotinic acetylcholine receptors in mice. Based on the results obtained with DHβE, nicotine alone appeared to decrease fixed ratio responding for food through activation of α4β2 receptor subtypes. These results are consistent with varenicline and cytisine having lower agonist efficacy than nicotine at α4β2 receptors in vivo; differential agonist efficacy at α4β2 receptors has been hypothesized to be important for therapeutic effects. However, it is not clear whether the current results are predictive of the mechanism(s) responsible for the effects of nicotinic receptor agonists to promote smoking cessation. Nicotine tends to decrease operant responding at the same doses that disrupt locomotor behavior in rodents (Morrison, 1967; Stolerman et al., 1973; Tritto et al., 2004), suggesting that nicotine-induced decreases in schedule-controlled responding might be due to a disruption of motor activity. Nonetheless, the current results strengthen the position that smoking cessation treatments, even at relatively large doses and perhaps at smaller doses more relevant to therapeutics, vary in their capacity to stimulate α4β2 nicotinic receptors in vivo.
Acknowledgements
The authors are grateful to D. Schulze and C. Rock for excellent technical assistance. This work was supported by USPHS grant DA25267.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carroll FI, Yokota Y, Ma W, Lee JR, Brieaddy LE, Burgess JP, Navarro HA, Damaj MI, Martin BR. Synthesis, nicotinic acetylcholine receptor binding, and pharmacological properties of 3′-(substituted phenyl)deschloroepibatidine analogs. Bioorgan. Med. Chem. 2008;16:746–754. doi: 10.1016/j.bmc.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O'Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J. Med. Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J. Pharmacol. Exp. Ther. 1998;284:1058–1065. [PubMed] [Google Scholar]
- Etter JF. Cytisine for smoking cessation: a literature review and a meta-analysis. Arch. Intern. Med. 2006;166:1553–1559. doi: 10.1001/archinte.166.15.1553. [DOI] [PubMed] [Google Scholar]
- George TP, O'Malley SS. Current pharmacological treatments for nicotine dependence. Trends Pharmacol. Sci. 2004;25:42–48. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl.) 1989;97:295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Pharmacologic analysis of drug-receptor interaction. 3rd ed. Lippincott-Raven Publishers; Philadelphia: 1997. [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol. Biochem. Behav. 2009;91:461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J. Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, McIntosh JM, Quik M. Differential regulation of mesolimbic alpha 3/alpha 6 beta 2 and alpha 4 beta 2 nicotinic acetylcholine receptor sites and function after long-term oral nicotine to monkeys. J. Pharmacol. Exp. Ther. 2006;318:381–388. doi: 10.1124/jpet.106.104414. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol. Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Morrison CF. Effects of nicotine on operant behaviour of rats. Int. J. Neuropharmacol. 1967;6:229–240. doi: 10.1016/0028-3908(67)90010-x. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Lester HA. CNS localization of neuronal nicotinic receptors. J. Mol. Neurosci. 2006;30:181–184. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- Naylor C, Quarta D, Fernandes C, Stolerman IP. Tolerance to nicotine in mice lacking alpha7 nicotinic receptors. Psychopharmacology. 2005;180:558–563. doi: 10.1007/s00213-005-2187-5. [DOI] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J. Neurochem. 2007;101:160–167. doi: 10.1111/j.1471-4159.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- Papke RL, Heinemann SF. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta 2 subunit. Mol. Pharmacol. 2004;45:142–149. [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br. J. Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J. Pharmacol. Exp. Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, 3rd, Coe JW, O'Neill BT, Tseng E, Wang EQ, Mather RJ, Hurst RS, Williams KE, de Vries M, Cremers T, Bertrand S, Bertrand D. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br. J. Pharmacol. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl.) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR, Gardner ML. Behavioral effects of nicotine: schedule-controlled responding by squirrel monkeys. J. Pharmacol. Exp. Ther. 1981;216:484–491. [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharmacology (Berl.) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Fink R, Jarvik ME. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973;30:329–342. doi: 10.1007/BF00429192. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose-effect data analysis. Chapman & Hall/CRC; Boca Raton: 2000. [Google Scholar]
- Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, Marks MJ. Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob. Res. 2004;6:145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- Williams M, Robinson JL. Binding of nicotinic cholinergic antagonist, dihydro-β-erythroidine, to rat brain tissue. J. Neuroimmunol. 1984;4:2906–2911. doi: 10.1523/JNEUROSCI.04-12-02906.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]