Abstract
Hypoxic/ischemic disruption of ionic homeostasis is a critical trigger of neuronal injury/death in the brain. There is, however, no promising strategy against such pathophysiologic change to protect the brain from hypoxic/ischemic injury. Here, we present a novel finding that activation of δ-opioid receptors (DOR) reduced anoxic Na+ influx in the mouse cortex, which was completely blocked by DOR antagonism with naltrindole. Furthermore, we co-expressed DOR and Na+ channels in Xenopus oocytes and showed that DOR expression and activation indeed play an inhibitory role in Na+ channel regulation by decreasing the amplitude of sodium currents and increasing activation threshold of Na+ channels. Our results suggest that DOR protects from anoxic disruption of Na+ homeostasis via Na+ channel regulation. These data may potentially have significant impacts on understanding the intrinsic mechanism of neuronal responses to stress and provide clues for better solutions of hypoxic/ischemic encephalopathy, and for the exploration of acupuncture mechanism since acupuncture activates opioid system.
Keywords: δ-Opioid receptor, Na+ channels, Na+ influx, Hypoxia, Cortex
Introduction
It has been well recognized that hypoxic/ischemic disruption of ionic homeostasis, especially massive Na+ influx and K+ leakage, is a critical trigger of neuronal injury/death in the brain [1–6]. There is, however, no promising medical strategy against such pathophysiologic changes to protect the brain from hypoxic/ischemic injury in spite of intensive research in the past on neuroprotection [1, 3, 4, 7, 8]. Recently, we have shown that the activation of δ-opioid receptor (DOR) is neuroprotective from hypoxic/ischemic injury [9–12], which is also documented by many other investigators [13–20]. In the mechanistic exploration, our initial data suggest that the DOR-induced neuroprotection may be attributed to the stabilization of Na+–K+ homeostasis across the membrane under anoxic stress [21–24]. We showed that DOR protection against anoxic derangement of K+ homeostasis is largely abolished by low Na+ perfusion and by blockade of TTX-sensitive voltage-gated Na+ channels [23, 24]. Since Na+ channels are the major route of Na+ entry into neurons, our preliminary observations prompt us to raise the following question: does DOR regulate Na+ channel function and thus protect the brain from anoxic disruption of Na+ homeostasis. More specifically, the questions we might ask are: does DOR activation reduce anoxic Na+ influx in the brain, and does DOR expression/activation indeed affect Na+ channel function? There is, however, no direct information available in the literature.
Our previous studies imply a potential interaction between DOR and Na+ channels in the cortex. For example, after being exposed to prolonged hypoxia during postnatal development, cortical neurons are more sensitive to subsequent stress, which is largely attenuated by tetrodotoxin (TTX), a Na+ channel blocker [25]. Interestingly, an increased Na+ channel density [25] and decreased DOR density [26] occurred in the exposed brain. Furthermore, we observed that, in a mutant brain with epileptic seizures, cortical neurons are hyper-excitable with up-regulation of voltage-gated Na+ channels [27] and down-regulation of DOR expression [28]. All these observations, though circumstantial, suggest that DOR may mediate an inhibitory regulation of Na+ channels in the brain under pathological conditions and then influence Na+ and K+ homeostasis in the brain. Since Na+ channel up-regulation has been proven to participate in pathological changes in several neurological disorders, such as hypoxic/ischemic dysfunction/injury and epilepsy [25, 27, 29], the DOR-mediated inhibition on Na+ channels may provide clues for pursuit of better solutions for hypoxic/ischemic encephalopathy and other neurological disorders, such as epilepsy.
We therefore performed this study to address two fundamental questions regarding the role of DOR in ionic homeostasis under anoxic condition. Firstly, does DOR activation directly reduce anoxic Na+ influx? Secondly, does DOR truly play an inhibitory role in the regulation of Na+ channels? Correspondingly, we performed two sets of experiments to answer these questions, i.e., examining the effect of DOR activation on anoxic Na+ influx in the cortex measured with Na+-sensitive electrodes, and determining the effect of DOR expression and/or activation on Na+ channel currents in Xenopus oocytes with expression of Nav1.2 channels that are highly expressed in the cortex [27, 30].
Materials and methods
Animals
Male C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). All procedures on the mice were performed in accordance with the guidelines of the Animal Care and Use Committee of Yale University School of Medicine, which is accredited by the American Association for Accreditation for Laboratory Animal Care.
Adult female Xenopus laevis with a body weight of 200–250 g were provided by the Institute of Biochemistry and Cell Biology, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, and were housed in a tank with constantly filtered and re-circulated water maintained at 20°C. The Xenopus laevis were fed with beef and pig hearts. The experimental protocol was approved by the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Chemicals and reagents
Drugs and chemicals used in the study were as follows: Na+ ionophore VI (Fluka 71739), 2-nitrophenyl octyl ether (Fluka 73732), potassium tetraphenylborate (Fluka 72018), hexamethyldisilazane (Fluka 52619), 3-(N-morpholino) propane sulfonic acid (MOPS, Amresco, USA), tetramethylammonium chloride (TMA; Shymax Chemical, China), mineral oil (Bio-Rad, USA), liberase blendzyme 3 (Roche, USA), ciprofloxacin (ICN, USA), tetrodotoxin (Sigma, USA), naltrindole (Sigma, USA). UFP-512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid), a specific and potent DOR agonist [31] was synthesized by our research team. RNA transcription kit, Transcription kit Sp6 and RNA Transcription kit T7 were all products from Ambion (USA).
Slice preparation
Cortical slices were prepared from 24- to 32-day-old mice as described in our previous studies [21–24]. In brief, the brain was rapidly removed from the skull after decapitation under inhalational anesthesia with ether and placed in chilled carbogen-saturated ACSF for 1–2 min. Transverse cortical slices (400 μm) were cut on a vibrotome containing carbogen-saturated ice-cold ACSF. Slices were then transferred to an incubation holder placed in a beaker containing 150 ml ACSF vigorously aerated with carbogen at ~35°C. Standard ACSF consisted of (in mM) NaCl 125, KCl 3.1, NaHCO3 26, CaCl2 2.4, MgSO4 1.3, NaH2PO4 1.25, and d-glucose 10 at pH 7.4. After an equilibration period of at least 90 min in carbogen-saturated ACSF at ~35°C, slices were used for recording.
Induction of anoxia in cortical slices
A slice was transferred to the recording chamber (Model RC-22C; Warner Instrument, Hamden, CT, USA) after equilibration with carbogen-saturated ACSF. The chamber was perfused with carbogen-saturated ACSF (35.5 ± 0.5°C) with a flow rate of ~3 ml/min. Slices were completely submerged ~0.5 mm below the ACSF surface in the tissue chamber and kept under normoxic conditions for at least 15 min at 35.5°C before experimental measurements were taken. Anoxia was induced by switching from the control superfusate (95% O2, 5% CO2) to one continuously bubbled with 95% N2 and 5% CO2. Each slice was subjected to a single period of anoxia that continued for about 1.5 min after the onset of anoxic depolarization (as assessed by a sudden drop of extracellular [Na+] that usually occurs within 10 min after the onset of anoxia), or for a period of 20 min if anoxic depolarization did not occur.
Measurements of extracellular [Na+]
Extracellular Na+ concentrations were measured using Na+-sensitive microelectrodes. Na+-sensitive microelectrodes were prepared as described previously [32]. Glass capillary-pulled electrodes were silanized by exposure to hexamethyldisilazane for the Na+ electrode, and subsequently baked at about 180°C for at least 2 h. The microelectrode tips were then broken back to ~2 μm in diameter. The internal filling solution (150 mM NaCl + 10 mM HEPES) was injected from the back into the electrode. A column of optimized membrane phase (10% Na+ ionophore VI, 89.5% 2-nitrophenyl octyl ether, and 0.5% potassium tetraphenylborate), with height of about 1 mm was sucked into the microelectrode tips. The reference electrode was a Ag/AgCl bridge electrode embedded in 2% agar in 3 M KCl. Calibrations were carried out by detecting the responses generated in NaCl solutions (5, 10, 20, 50, 80, 100, 120, 150 mM NaCl) in triplicate. For each concentration, the average of voltage changes in three separate tests was used as the final voltage change. Over this range electrode response was near ideal, showing a logarithmic relationship to [Na+].
Electrical signals were monitored on an oscilloscope and recorded by a DC amplifier (Model IE-210, LPF 200; Warner Instruments, Hamden, CT, USA) and digitized by an Axon mini-digitizer acquisition system (Model miniDigi 1A; Axon Instruments, Union City, CA, USA) at a sampling rate of 100 Hz. For assessment of Na+ activities, the maximal drop in extracellular Na+ concentration induced by anoxia and the recovery duration of Na+ drop’s return to basal level during reoxygenation were analyzed.
After recording of a stable baseline for at least 5 min, the slices were subject to experimental treatments. The electrophysiological recordings were continuously performed at least 60 min.
Xenopus oocytes preparation
The oocytes were obtained from females of the clawed toad Xenopus laevis and treated with 5 ml of ORI containing liberase blendzyme 3 (0.3 U/ml) after clearing away the dead oocytes and debris. After 4 h of enzymatic digest on a shaker (40 rounds/min) at 20°C, the oocytes were moved into a centrifuge tube, and then gently washed three times with Ca2+-free ORI solution (90 mM NaCl, 2 mM KCl, 5 mM MOPS, pH 7.4 with Tris base), and five times with standard ORI solution. Large and healthy oocytes at stage V characterized by clear pigments in the animal poles were chosen for experiments.
Preparation of Nav1.2 cRNA and DOR cRNA
The increase, isolation, and purification of the plasmids were performed according to the standard protocols [33]. Nav1.2 cRNA was transcribed from Not I linearized rat Nav1.2 cDNA templates (generously provided by Dr. Goldin, Department of Microbiology and Molecular Genetics, University of California, USA) using RNA Transcription Kit T7 generously provided by Shanghai University Life-Science College. DOR cRNA was transcribed from linearized mouse DOR cDNA (kindly provided by Dr. Gang Pei, Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences) using RNA Transcription kit SP6. The cRNA yield was estimated by agar gel analysis and spectrophotometer and then used for microinjection (see below).
Microinjection and Nav1.2 and DOR expression in Xenopus oocytes
The microinjection into the oocytes was made based on the methods described by Guille [34]. Glass capillary-pulled electrodes were used as a microinjection needle, which was connected to a microsyringe pump controller (WPI, USA) and fixed on the micromanipulator. The manipulation was made on ice. Under the microscope, the electrode containing cRNA to be injected was moved onto the surface of one group of oocytes aligned in a dish, and inserted into the oocyte at its demarcation line between the animal and vegetal poles (about 300 μm in depth). The cRNA was injected with the desired amount in a rate of 5 nl/min controlled by the microsyringe controller. The amount of injected Nav1.2 cRNA in our experiments was 1, 5, and 10 ng per oocyte. For the co-injection group, Nav1.2 cRNA at 5 ng and various amounts of DOR cRNA (1, 5, 10, 20 ng) were injected. A batch of non-injected oocytes and another batch of deionized water injected oocytes were used as the controls.
After a batch of oocytes was injected, they were moved to six-well culture plates filled with ORI solution containing ciprofloxacin (70 mg/L), and cultured in an incubator at 20°C for 40–72 h. The culture media was changed every 20 h, and the dead oocytes were removed. After at least 40 h of culture, oocytes were used for electrophysiological recording.
Two-electrode voltage-clamp recording
For each experiment, two-microelectrode voltage clamp was performed to record the current in the oocytes at room temperature. Both the potential and current electrodes were pulled on a Narishige PC-10 puller from filament glass pipettes (1.2 mm OD, 0.69 mm ID), and filled with KCl (3 mol/L) with the resistance of 1–5 MΩ. Recordings were conducted using a TURBO TEC-03 amplifier (NPI Electronic, Germany). After two-electrode configuration was formed, oocytes were voltage-clamped at a membrane potential of −100 mV. Sodium currents were elicited by applying a series of voltage steps from the holding potential of −100 mV to the test potential of +70 mV with an increment of 10 mV/step and an interval of 6 s between steps. Current signals were sampled at 10 kHz with TURBO TEC-03 amplifier (NPI Electronic). Currents obtained from oocytes were digitized with an AD interface (Cellworks 5.5.1; NPI Electronic). The signals were displayed and stored on a PC computer using the data acquisition program Cellworks 5.5.1 (NPI Electronic) with a simultaneous recording on chart paper by on-line recorder (Servogor SE102-2; Kipp & Zonen, Delft, Netherlands). Oocytes were perfused with normal ORI or 0 Na+ ORI (90 mM TMA, 2 mM KCl, 2 mM CaCl2; 5 mM MOPS, pH 7.4 with Tris base) at a rate of 3–5 mL/min. All drugs were prepared with ORI or ORI without Na+ and administrated to the oocyte via perfusion.
Data analysis
All values were subjected to statistical analysis (ANOVA, Student’s t test, Newman Keuls test, etc) with n representing the number of the cells/slices examined. For ionic electrode experiments, one way ANOVA followed by Newman Keuls test was used for multiple pair-wise tests and two-tailed, unpaired Student’s t test was used for comparison of two experimental groups. For two-microelectrode voltage clamp experiments, all the data collection and analysis were carried out using Cellworks Reader 3.6 and Origin Pro 7.0 software (OriginLab, USA). Normalized currents were calculated by I/I 70, where I is the current amplitude measured during the test depolarization, I 70 is the current at the last step of the test potential (+70 mV). The voltage-dependent activation was determined using standard protocol. The conductance (G) was calculated according to G = I max /(V − V Na ), where I max is the peak current amplitude, V is the command potential, and V Na is the reversal potential which is determined by its I–V curve. G was then fitted by Boltzmann equation, G/G max = 1/(1 + exp[(V + V 50)/K m]), where G max is the maximum Na+ conductance, V 50 is the voltage where G is half of G max, and K m indicates the slope of the relationship between channel activation and membrane voltage. Statistical significance was defined by p < 0.05.
Results
DOR activation reduced anoxic Na+ influx in the cortex
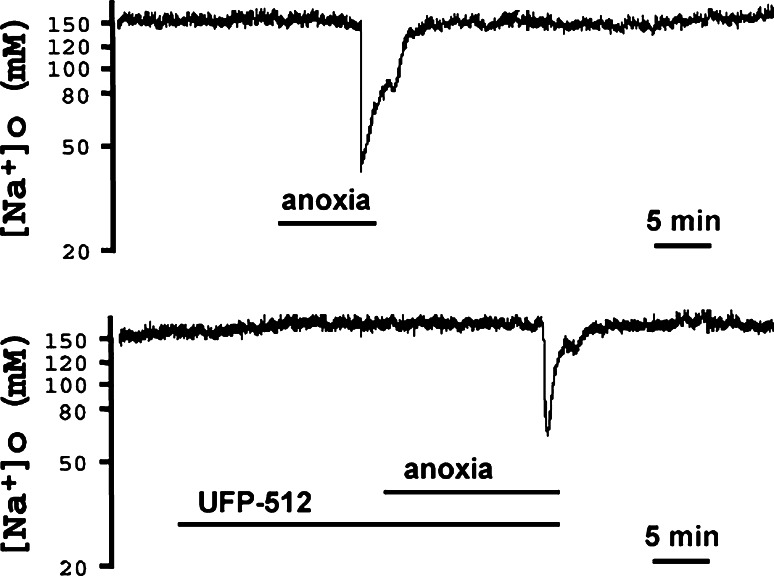
To obtain the first evidence as to whether DOR activation reduces Na+ influx in anoxia, we measured the changes in extracellular [Na+] induced by anoxia in mouse cortical slices. When the slices were equilibrated in artificial cerebrospinal fluid (ACSF), the baseline of extracellular [Na+] was found to be around 152 mM at rest and was stable for at least 60 min. In response to anoxic stress, extracellular [Na+] displayed a sudden drop from its baseline to 45.93 ± 3.67 mM (n = 5) (Fig. 1). After re-introducing the slices to oxygen, extracellular [Na+] gradually recovered to the baseline in 7.7 ± 0.9 min (n = 5). The response of extracellular [Na+] to anoxia decreased when UFP-512 (1–5 μM), a specific and potent DOR agonist [31], was applied to the slices starting 20 min before anoxia and lasting for the whole period of anoxic stress, For example, UFP-512 at 5 μM significantly attenuated the drop of extracellular [Na+] (62.47 ± 4.84 vs 45.93 ± 3.67 mM in control, p = 0.026). UFP-512 also accelerated the recovery of the anoxia-induced drop of extracellular [Na+] (3.7 ± 0.6 min vs 7.7 ± 0.9 min in control, n = 5, p = 0.0053).
Fig. 1.
Effect of DOR activation on anoxia-induced drop in extracellular [Na+] in cortical slices. Anoxia induced a sudden drop of extracellular [Na+] from its baseline of 152 to 45.93 ± 3.67 mM with 7.7 ± 0.9 min for recovery from peak drop to baseline after re-introducing oxygen (n = 5) (upper trace). Activation of DOR with UFP-512 (5 μM) significantly attenuated this drop to 62.47 ± 4.84 mM (p = 0.026) (lower trace) and accelerated the recovery of anoxia-induced drop of extracellular [Na+] from 7.7 ± 0.9 min in control to 3.7 ± 0.6 min (n = 5, p = 0.0053), suggesting that DOR activation reduced the anoxia-induced Na+ influx in the cortex
Furthermore, we tested whether the effect of UFP-512 can be influenced by DOR antagonist, naltrindole, to ascertain the role of DOR in anoxic Na+ influx. The results showed that the addition of naltrindole (1 μM) completely blocked the UFP-512-induced inhibition of anoxic Na+ influx (62.47 ± 4.84 mM in UFP-512 alone, n = 5, vs 35.34 ± 1.14 mM in the group of UFP-512 plus naltrindole, n = 6; p < 0.0001).
These results suggest that DOR activation inhibits anoxia-induced Na+ influx in the cortex.
DOR expression and activation down-regulated Na+ channel function
Since the major subtype of the voltage-gated Na+ channels in the cortex is Na1.2 channels [27, 35], we further asked whether DOR truly plays an inhibitory role in the regulation of Na1.2 channels. Because there is no specific activator/blocker for individual subtype of Na+ channels, we expressed Na1.2 channels in Xenopus oocytes and then tested the effects of DOR expression and activation on Na1.2 channel function.
Injection of Na+ channel cRNA-induced TTX-sensitive inward currents
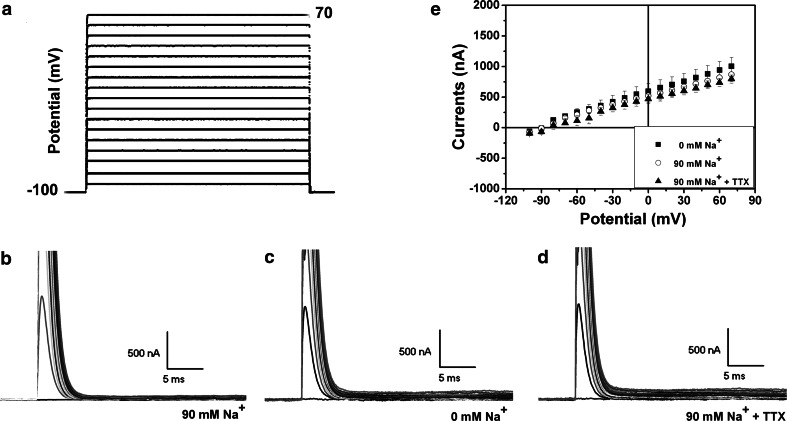
First, we examined whether there was any endogenous Na+ currents in the naïve oocytes in our experimental system. If there is a detectable amount of Na+ channels, inward currents should be elicited by a depolarized stimulating potential (e.g. steps from −30 to +30 mV), which actually stimulate Na+ influx. As shown in Fig. 2, a family of current traces was recorded in the oocytes in the solution with “normal” Na+ concentration. There were no recordable inward currents whatsoever (Fig. 2b). The addition of TTX (1 μM) did not affect the recordings (Fig. 2d). Similar recordings were also made in the buffer without the presence of Na+ ion (Fig. 2c). Figure 2e (n = 5) showed clearly that there was no appreciable inward current in the naïve oocytes without injection of exogenous cRNA in any conditions.
Fig. 2.
Representative recording traces from the naïve oocytes and the current–voltage relationship. The protocol of test potential is shown in a. b–d are families of representative currents traces that were recorded in the control oocytes in 90 mM Na+ solution (b), 0 Na+ solution (c), and 90 mM Na+ solution with 1 μM of TTX (d). e is the current–voltage (I–V) curve of the naïve oocytes (n = 5). Note that there was no appreciable inward current in naïve oocytes
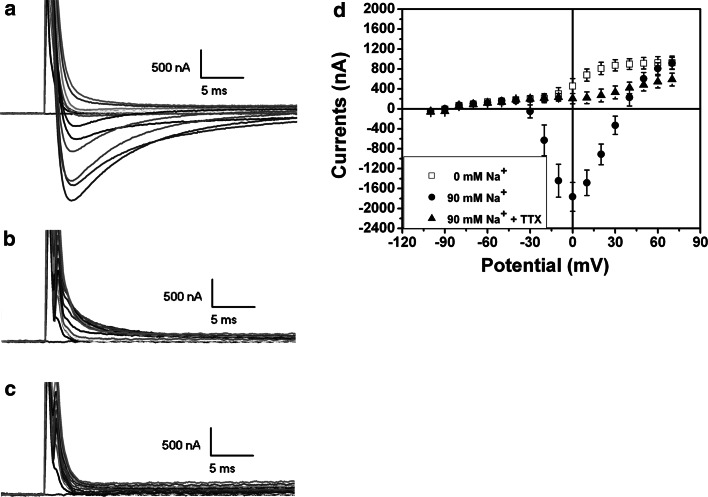
Large inward currents could be recorded after the injection of Nav1.2 cRNA (Fig. 3). Forty hours after the injection of 5 ng cRNA, the inward currents appeared from −30 to 30 mV in the depolarized mode. The mean value was about −1,765 ± 290 nA with the peak current at the potential 0 mV (n = 6) (Fig. 3a, d). Within the range of depolarization from −100 to 70 mV in 10 mV increments, the inward current opened to its max value at 50–60 ms during the simulated time frame. These inward currents could not been seen in the solution containing zero Na+ (n = 6; Fig. 3b, d). The application of TTX in the “normal” solution (90 mM Na+) completely abolished the inward currents (n = 6; Fig. 3c, d), suggesting that the inward currents were TTX-sensitive and Na+ channel-mediated currents. These Na+ currents were very likely based on the expression of exogenous Na+ channels since there was no significant trace of Na+ currents in the naïve oocytes under the same experimental condition.
Fig. 3.
Inward currents in Xenopus oocytes with Na1.2 channel expression. a A family of representative current traces were recorded in the oocytes with microinjection of 5 ng Nav1.2 cRNA in 90 mM Na+ solution (a, n = 6), 0 mM Na+ solution (b, n = 6), and 90 mM Na+ solution plus 1 μm of TTX. (c, n = 6). The I–V curves of each group were summarized in d. Note that Nav1.2 channel expression caused inward currents, which disappeared in 0 Na+ solution and could be completely blocked by TTX, suggesting that the inward currents are sodium currents
Na+ current size was dependent on the amount of injected cRNA and culture time
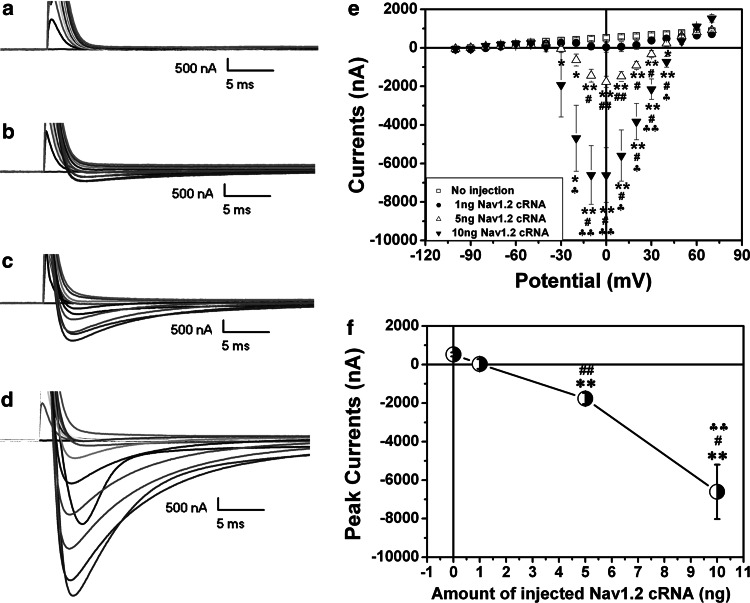
We measured the sodium currents in the oocytes with various amounts of injected Nav1.2 cRNA after 40 h of incubation. As shown in Fig. 4a, no inward currents were recorded in the group without injection of Na1.2 cRNA (n = 5). Only one out of three oocytes showed inward currents with the peak value being less than 500 nA when injected with 1 ng of Nav1.2 cRNA (Fig. 4b) (n = 3). At 5 ng of Nav1.2 cRNA, the sodium currents increased greatly (n = 6) (Fig. 4c). In the oocytes with injection of 10 ng Nav1.2 cRNA, the inward currents increased by several folds with the peak values being >6,000 nA (−6,604.8 ± 416.7 nA) (n = 6) (Fig. 4d). These results, as summarized in Fig. 4e and f, suggest that sodium current amplitude was proportionally related to the amount of the Nav1.2 cRNA injected into the oocytes. On the other hand, the amplitude of sodium currents increased with the time after the injection. For example, in contrast to the low peak current (−1,765.2 ± 290.5 nA) at the potential 0 mV in the oocytes with 5 ng of Nav1.2 cRNA after 40 h of incubation (Fig. 4c), the average value of the peak currents was more than 3,000 nA even with the expression of DOR (see below) after 72 h of incubation (Figs. 5a and 6).
Fig. 4.
The relationship between the amplitude of sodium currents and the amount of the injected Nav1.2 cRNA. A family of currents were elicited from control (a, n = 5), oocytes with injection of 1 ng (b, n = 3), 5 ng (c, n = 6), and 10 ng of Nav1.2 cRNA (d, n = 6), respectively. The I–V curves of each group were summarized in e. f shows the summary of the relationship between the peak currents and the amount of the injected Nav1.2 cRNA. *p < 0.05, **p < 0.01 vs control; # p < 0.05, ## p < 0.01 vs 1 ng group; ♣♣ p < 0.01 vs 5 ng group. Note that inward currents increased with the amount of injected Nav1.2 cRNA with no inward currents seen in naïve oocytes
Fig. 5.
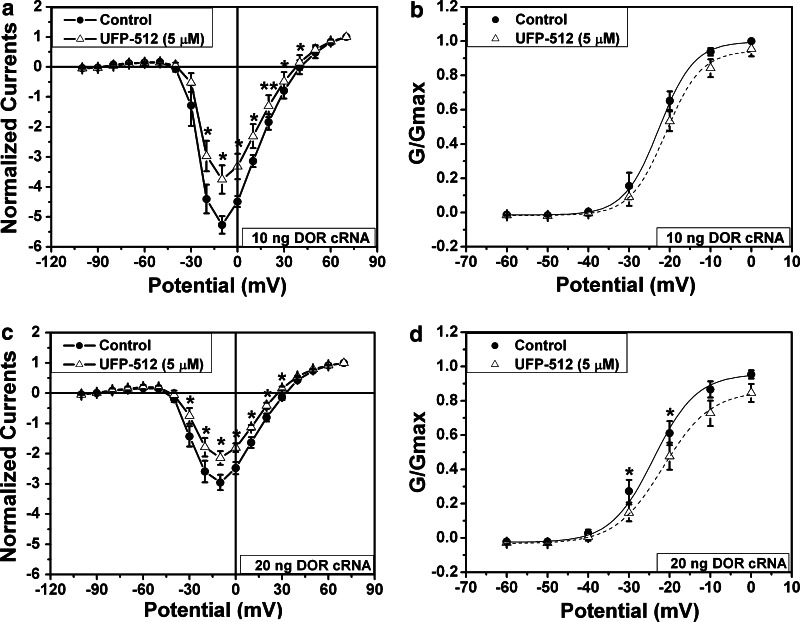
DOR activation induced inhibition of sodium currents in oocytes co-expressing DOR and Nav1.2. The recordings were performed in the oocytes expressing Nav1.2 expression (5 ng of injected Nav1.2 cRNA) with co-expression of DOR after 72 h of culture (a–b, 10 ng of injected DOR cRNA, n = 4; c–d, 20 ng of injected DOR cRNA, n = 9). DOR was activated by 5 μM of UFP-512 (open triangle). b and d are conductance/amplitude–voltage curves from a and c, respectively. *p < 0.05, **p < 0.01 vs control (no DOR activation). Note that the sodium currents were smaller in the oocytes with higher expression of DOR (20 ng of injected DOR cRNA) than in those with lower expression of DOR (10 ng of injected DOR cRNA). DOR activation reduced the amplitude of sodium currents and rightly shifted the conductance/amplitude–voltage curves
Fig. 6.
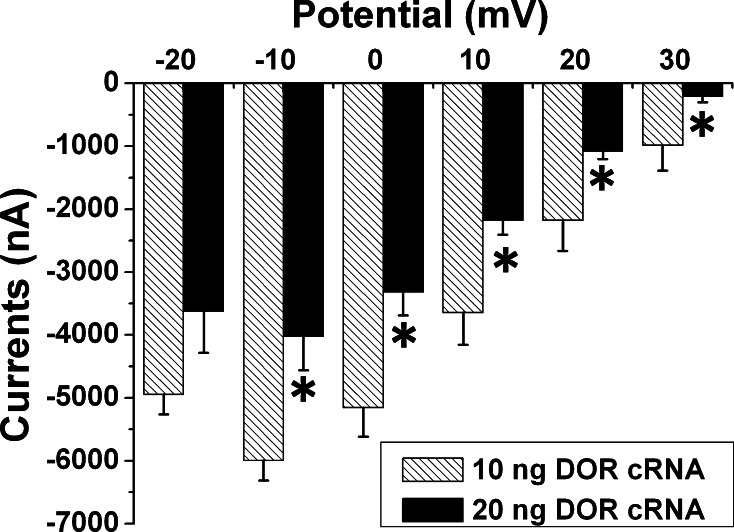
Effect of DOR expression on sodium currents. Five nanogram of Nav1.2 cRNA with 10 or 20 ng of DOR cRNA were injected into the oocytes and cultured for 72 h. *p < 0.05 vs 10 ng DOR cRNA. Note that the sodium current was significantly smaller in the oocytes with 20 ng DOR cRNA as compared to that of the oocytes with 10 ng DOR cRNA in the range of −10 to 30 mV of test potential, suggesting that an increase in DOR expression leads to a decrease in sodium current amplitude
Na+ currents were inhibited by DOR activation in oocytes with co-expression of DOR, but not in those without DOR expression
To determine the role of DOR in Na+ channel regulation, we co-expressed Na1.2 channels and DOR in the oocytes and then recorded sodium currents. Because DOR density critically affects neuronal responses to hypoxia [11], we expressed DOR at different levels by injecting various amounts of DOR cRNA into the oocytes and then tested their effects on sodium currents. To maximize effects of DOR expression, we extended the culture time to 72 h in this set of the experiments since the protein expression increases with time after the injection of cRNA as shown above.
In the group with the injection of 1 ng DOR cRNA, activation of DOR with 5 μM of UFP-512 had little effect on sodium currents (n = 5, data not shown). When the injected DOR cRNA was increased to 10 ng, DOR activation with the same amount of UFP-512 significantly reduced the sodium current by 27.4% (p = 0.012, n = 4) (Fig. 5a). When the injected DOR cRNA was increased to 20 ng, 5 μM of UFP-512 decreased sodium current amplitude by 35.2% (p = 0.030, n = 9; Fig. 5c). In the oocytes with a large amount of DOR cRNA (10 and 20 ng), UFP-512 significantly inhibited sodium currents within a broad range of depolarization potential (−30 to +40 mV) (Fig. 5a, c). On the other hand, UFP-512 (5 μM) had no significant effect on sodium currents in the oocytes expressing Nav1.2 alone (5 ng Nav1.2 cRNA) (p > 0.05, n = 10, data not shown). Interestingly, our data suggest that DOR expression alone (without DOR activation) could reduce the size of sodium currents. As shown in Fig. 6, the size of sodium currents was much smaller in oocytes with injection of 20 ng DOR cRNA than in those with 10 ng DOR cRNA.
To further characterize the DOR-induced inhibition of sodium currents, we analyzed the conductance/amplitude–voltage relationship by fitting with a Boltzmann equation in the oocytes with injection of 10 and 20 ng of DOR cRNA. Our results showed that DOR activation right shifted the curves (Fig. 5b, d), suggesting that DOR activation not only reduced the size of sodium currents but also increased the threshold of sodium channel activation.
In general, the peak current was around 0 mV of the potential in the oocytes with 5 ng of Nav1.2 cRNA alone (Fig. 3). With DOR co-expression, the evoked peak currents appeared at different depolarization potentials, typically four different types of recordings, i.e., 20, 0, −10, and −30 mV. Despite this difference, DOR activation could further inhibit sodium currents in all these oocytes with DOR expression (Fig. 7).
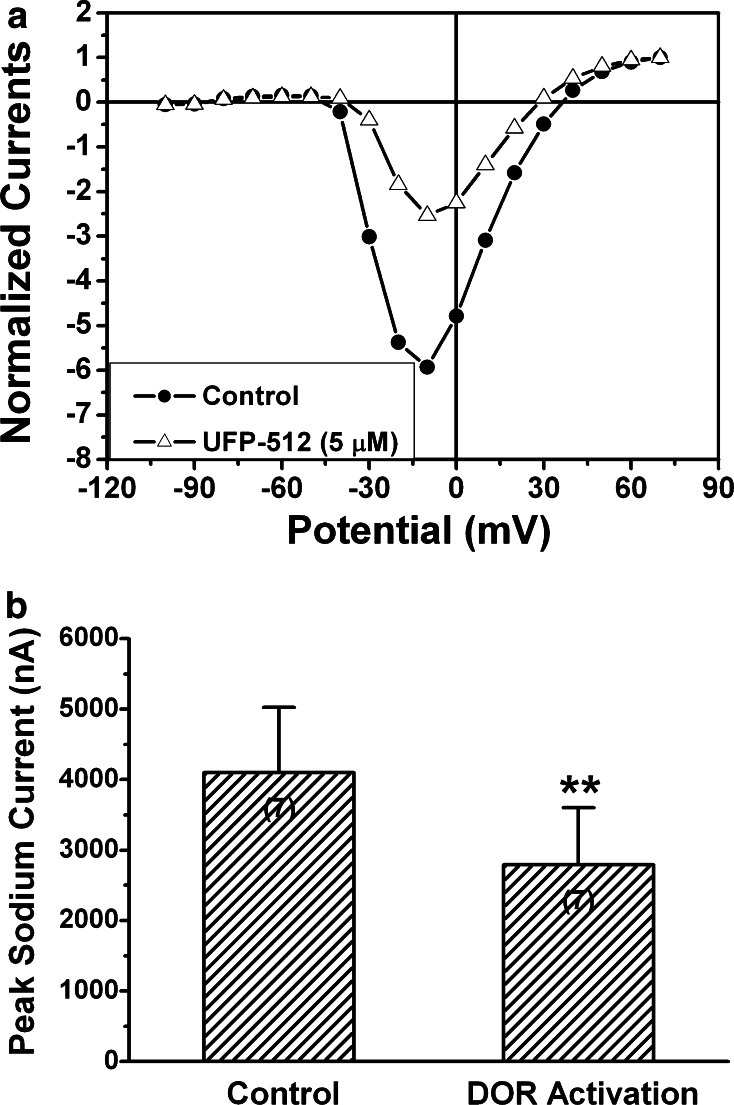
Fig. 7.
Effect of DOR activation on peak sodium currents in the oocytes co-expressing sodium channels and DOR. The recordings were performed in the oocytes co-expressing sodium channels (5 ng Nav1.2 cRNA) and DOR (10 ng DOR cRNA) after 72 h of culture. **P < 0.01 (n = 7) vs the control (No UFP-512). Note that although the peak currents were elicited at different depolarization potentials, DOR activation with 5 μM of UFP-512 could induce a significant reduction of peak sodium currents in all cases
DOR activation reduced Na+ channel currents in a dose–response manner
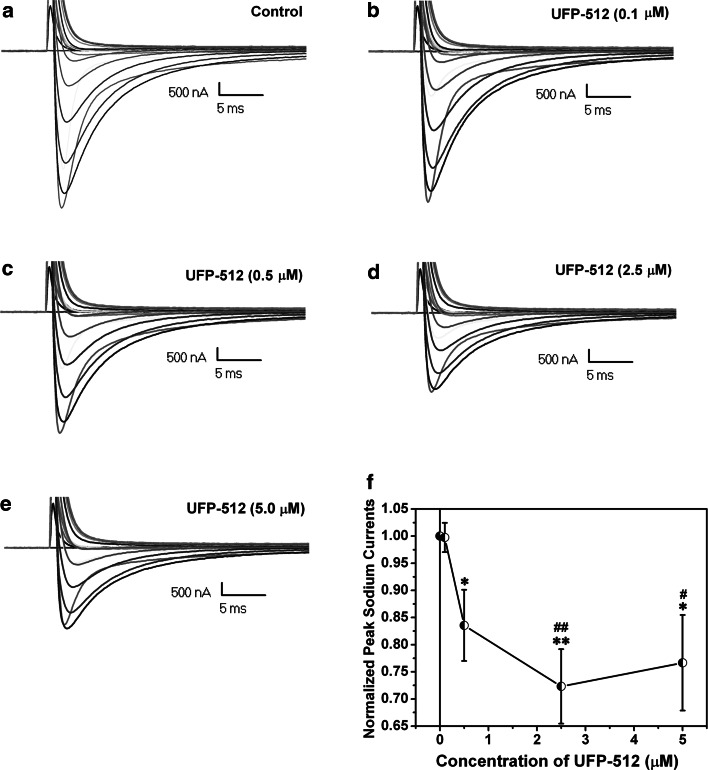
We performed a dose–response study to further clarify the DOR activation-induced inhibition of sodium currents. In the oocytes with co-expression of Na1.2 channels (5 ng cRNA) and DOR (10 ng cRNA) under the same experimental conditions, we applied UFP-512 at concentrations of 0, 0.1, 0.5, 2.5, and 5 μM and compared their effects on the sodium currents (Fig. 8). We found that 0.1 μM or less hardly inhibited sodium currents (Fig. 8b). However, at 0.5 μM, UFP-512 induced significant inhibition on the sodium currents (Fig. 8c). The maximal inhibition was seen at 2.5 μM (Fig. 8d). When the concentration was increased to 5 μM, the DOR-mediated inhibition of sodium currents could not be further strengthened (Fig. 8e, f), suggesting a saturable dose–response manner in terms of the DOR agonist-induced inhibition on the sodium currents.
Fig. 8.
Dose–responses of the DOR agonist-induced inhibition of sodium currents. The recordings were performed in the oocytes co-expressing sodium channels (5 ng of Nav1.2 cRNA) and DOR (10 ng of DOR cRNA). The families of representative currents traces show the inward currents recorded in control (a), 0.1 μM UFP-512 (b), 0.5 μM UFP-512 (c), 2.5 μM UFP-512 (d), and 5.0 μM UFP-512 (e), respectively. f shows the relationship curve of the current’s size vs concentration of UFP-512 (n = 4–5). *p < 0.05, **p < 0.01 vs control; # p < 0.05, ## p < 0.01 vs 0.1 μM UFP-512. Note that the DOR agonist-induced reduction of sodium current amplitude was in a dose–response manner
In control naïve oocytes, the application of UFP-512 (5 μM) had no effect at all on the electrophysiological recordings that could not detect any inward current (n = 9, data not shown).
Discussion
We have found in this work that DOR activation reduced anoxic Na+ influx in the cortex, which was completely blocked by the blockade of DOR. Furthermore, we presented the direct evidence that DOR indeed plays an inhibitory role in Na+ channel regulation. Since hypoxic/ischemic disruption of ionic homeostasis, especially Na+ influx and K+ leakage, is a key trigger of neuronal injury/death, the present data may improve our understanding of the intrinsic nature of neuronal response to environmental stress and provide clues for better solutions of hypoxic/ischemic encephalopathy.
Accumulating evidence has shown that Na+ influx and K+ leakage triggers neuronal injury or even death in the brain [1–6]. The DOR-induced reduction of Na+ influx presented in this study and K+ leakage as shown in our previous work [21–24] is certainly beneficial to neuronal adaptation to anoxic stress. The present data support our central hypothesis that DOR plays an inhibitory role in Na+ channel regulations and reveal an interesting mechanism underlying DOR’s important role in attenuation of anoxic disruption of Na+–K+ heomeostasis, i.e., DOR-mediated reduction of Na+ influx and K+ leakage in anoxic condition.
It has been shown that the pore-forming α subunits generate sodium currents with gating properties similar to native channels when expressed in Xenopus laevis oocytes [36]. In our work, the oocytes with injection of Nav1.2 cRNA showed large voltage-dependent inward currents that could be completely inhibited by TTX or in a zero Na+ solution, demonstrating that the inward currents we observed are indeed sodium currents. Therefore, it is reliable to use this model for investigating the response of Na+ channels to DOR activation.
Historically, there is only one published study suggesting that high doses of SNC80, a putative DOR agonist, caused an opioid-independent effect on sodium channels in the rat hippocampus [37]. However, we believe that the role of DOR in Na+ channel regulation needs to be re-clarified with more direct evidence because the hippocampus may differ from other brain region such as the cortex in terms of Na+ channel function and neuronal response to DOR activation. Our previous work has shown that the hippocampus has a very low density of DOR, while the cortex has a much higher density [38, 39]. This may lead to a major difference between the cortical and hippocampal neurons in neuronal properties including the responses of Na+ channels to endogenously released opioids and exogenous opioid agonists/antagonists. For instance, applying low concentrations of DOR agonists to hippocampal neurons may induce an insignificant effect because of low DOR density. On the other hand, high concentrations of DOR ligands, either SNC80, naloxone, or naltrindole, may induce a complicated, even adverse, effect on neurons because of the non-selective action on multiple membrane proteins. Therefore, the differential distribution and density of DOR among cells/tissues may greatly affect neuronal tolerance to hypoxic/ischemic stress and the response of Na+ channels to DOR activation. Indeed, there is evidence showing that the cortical neurons are more tolerant to hypoxic stress than the hippocampal neurons [40, 41]. In addition, some experimental procedures such as mechanical trituration and chemical digestion (e.g., Pronase) [37] may affect the function of DOR and Na+ channels as well as their interactions, causing complex cellular changes in the presence of DOR agonists/antagonists. To avoid these pitfalls and explore a “pure” interaction between DOR and Na1.2 channels, we used the advantages of Xenopus oocytes in this work.
Sodium channels are critical to the generation and conduction of the action potential. Of all the existent Na+ channel isoforms, neuronal Na1.2 channels are abundantly expressed in the central nervous system, especially in the cortex. We demonstrated that activation of DOR reduces the current amplitude and inhibits the excitability of Na+ channels, suggesting that DOR plays an inhibitory role in the regulation of Na+ channels. This is based on the following findings: (1) the amplitude of Nav1.2 currents was significantly reduced by the DOR agonist, UFP-512 [22, 31] in a dose-dependent manner, which is a typical agonist–receptor reaction, and (2) activation of DOR by UFP-512 induced a right shift of the activation curve of Nav1.2 currents, which was seen in the oocytes co-expressing Nav1.2 channels and DOR but not in those without DOR expression. If this inhibitory effect was not mediated by DOR, we would see this phenomenon in the oocytes without DOR expression. Interestingly, even DOR expression alone can down-regulate Na+ currents through an unknown mechanism. The potential mechanisms underlying the interaction between DOR and Na+ channels may be, at least partially, related to the regulation of G protein–protein kinase–MAPK pathway. Voltage-gated Na+ channels are important targets modulated by metabotropic receptors via G protein–protein kinases [42]. Therefore, DOR possibly regulates the activities of Na+ channels by modulating the activities of G protein–protein kinases. Indeed, we have observed that DOR attenuates anoxic K+ leakage which is through the inhibition of Na+ channels and relies on a PKC-dependent pathway [21, 23, 24]. MAPK pathways may play a role in the DOR-mediated regulation of Na+ channels because MAPK pathways are involved in both DOR signaling [11] and Na+ channel activity [43]. However, more in-depth investigations are needed to further clarify this issue.
Under acute pathophysiological conditions such as hypoxia/ischemia, excessive Na+ entry through Na+ channels [44, 45] may trigger a serial process of neuronal injury and death [1, 2, 6, 45]. The DOR-induced inhibition of such pathophysiological response is able to rescue neurons from hypoxic/ischemic injury. The inhibitory regulation of Na+ channels by DOR may play a role in the control of neuronal excitability. An impairment of such mechanism may lead to neuronal dysfunction/injury and eventually neurological diseases. For example, sodium channel mutation has been casually linked to human epilepsy [46, 47]. Na+ channel up-regulation has been demonstrated to critically lead to epileptic hyper-excitability and seizures [27, 29]. Interestingly, we observed that in the mutant brain exhibiting spontaneous epilepsy, Na+ channel was up-regulated [27] while DOR was down-regulated [28], suggesting a potential role of DOR impairment in the pathophysiology of epilepsy associated with genetic abnormality. Since Na+ channel up-regulation contributes greatly to epileptic hyper-excitability, and most of anti-epileptic drugs are actually inhibitors of Na+ channels [48], the DOR-mediated inhibition of Na+ channels may provide a novel clue for new solutions of epileptic seizures. In addition, the opposite changes in DOR and Na+ channel are also found in the brain and neurons exposed to prolonged hypoxia that have increased sensitivity to subsequent stress [25], while DOR activation attenuates Na+ channel dysregulation in hypoxia [49].
The expression and functional properties of voltage-gated Na+ channels in peripheral sensory neurons can be dynamically regulated following axonal injury or peripheral inflammation, suggesting that specific voltage-gated Na+ channels may play crucial roles in inflammatory, and possibly neuropathic pain [50]. Since DOR is inhibitory to Na+ channels, the anti-nociception effect of DOR may also be related to the inhibition of sodium channels.
In summary, we found that DOR activation reduced anoxic Na+ influx in the cortex. Moreover, we found that in oocytes with the co-expression of Nav1.2 channels and DOR, activation of DOR significantly decreased the amplitude of sodium currents in a dose-dependent fashion, and right-shifted the activation curve of Nav1.2 channels. Furthermore, DOR expression itself reduces the amplitude of sodium currents. Taken together with our previous work [24], our observations show that DOR expression/activation plays an inhibitory role in Na+ channel regulation, thus attenuating anoxic disruption of Na+ and K+ homeostasis in the brain, which may provide a potential clue for better solutions of certain neurological disorders and for exploring the mystery of acupuncture therapy since acupuncture activates DOR system in the brain.
Acknowledgments
This work was supported by the grants of STCSM-05DZ19745, 973 Program- 2006CB504509, NIH-HD34852, NIH-AT004422 and AHA-0755993T, and in part by University of Cagliari and the Intramural Research Program of the NIH and NIEHS.
Footnotes
The data of this manuscript were submitted on May 23, 2008 to The Beijing Joint Conference of Physiological Science 2008 and published in the format of abstract (Acta Physiologica Sinica 60: Supplement 1) and also presented at the Third Workshop on Scientific Approaches to Chinese Medicine in October 2008.
References
- 1.Breder J, Sabelhaus CF, Opitz T, Reymann KG, Schrőder UH. Inhibition of different pathways influencing Na+ homeostasis protects organotypic hippocampal slice cultures from hypoxic/hypoglycemic injury. Neuropharmacology. 2000;39:1779–1787. doi: 10.1016/S0028-3908(00)00027-7. [DOI] [PubMed] [Google Scholar]
- 2.Koike T, Tanaka S, Oda T, Ninomiya T. Sodium overload through voltage-dependent Na+ channels induces necrosis and apoptosis of rat superior cervical ganglion cells in vitro. Brain Res Bull. 2000;51:345–355. doi: 10.1016/S0361-9230(99)00246-4. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Gao TM, Gong LW, Zhuang ZY, Li X. Potassium channel blocker TEA prevents CA1 hippocampal injury following transient forebrain ischemia in adult rats. Neurosci Lett. 2001;305:83–86. doi: 10.1016/S0304-3940(01)01821-3. [DOI] [PubMed] [Google Scholar]
- 4.Wei L, Yu SP, Gottron F, Snider BJ, Zipfei GJ, Choi DW. Potassium channel blockers attenuate hypoxia- and ischemia-induced neuronal death in vitro and in vivo. Stroke. 2003;34:1281–1286. doi: 10.1161/01.STR.0000065828.18661.FE. [DOI] [PubMed] [Google Scholar]
- 5.Liu D, Slevin JR, Lu C, Chan SL, Hansson M, Elmer E, Mattson MP. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J Neurochem. 2003;86:966–979. doi: 10.1046/j.1471-4159.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- 6.Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- 7.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66:852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, Bresolin N, Comi GP, Corti S. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66:757–772. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JH, Gibney GT, Zhao P, Xia Y. Neuroprotective role of δ-opioid receptors in the cortical neurons. Am J Physiol Cell Physiol. 2002;282:C1225–C1234. doi: 10.1152/ajpcell.00226.2001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through δ-opioid receptor. Stroke. 2006;37:1094–1099. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- 11.Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen sensitive δ-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem. 2005;280:16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- 12.Yang YL, Xia XW, Zhang Y, Wang Q, Li L, Luo GH, Xia Y. δ-opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol. 2009;7:55. doi: 10.1186/1741-7007-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlongan CV, Hayashi T, Oeltgen PR, Su TP, Wang Y. Hibernation-like state induced by an opioid peptide protects against experimental stroke. BMC Biol. 2009;7:31. doi: 10.1186/1741-7007-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim YJ, Zheng S, Zuo Z. Morphine preconditions purkinje cells against cell death under in vitro simulated ischemia-reperfusion conditions. Anesthesiology. 2004;100:562–568. doi: 10.1097/00000542-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Su DS, Wang ZH, Zheng YJ, Zhao YH, Wang XR. Dose-dependent neuroprotection of delta opioid peptide [D-Ala2, D-Leu5]enkephalin in neuronal death and retarded behavior induced by forebrain ischemia in rats. Neurosci Lett. 2007;423:113–117. doi: 10.1016/j.neulet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Xiong LZ, Yang J, Wang Q, Lu ZH. Involvement of delta- and mu-opioid receptors in the delayed cerebral ischemic tolerance induced by repeated electroacupuncture preconditioning in rats. Chin Med J. 2007;120:394–399. [PubMed] [Google Scholar]
- 17.Iwata M, Inoue S, Kawaguchi M, Nakamura M, Konishi N, Furuya H. Effects of delta-opioid receptor stimulation and inhibition on hippocampal survival in a rat model of forebrain ischaemia. Br J Anaesth. 2007;99:538–546. doi: 10.1093/bja/aem220. [DOI] [PubMed] [Google Scholar]
- 18.Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta- opioid agonists in a rat model of global ischemia. Physiol Behav. 2008;93:502–511. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Pamenter ME, Buck LT. δ-opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex. J Exp Biol. 2008;211:3512–3517. doi: 10.1242/jeb.021949. [DOI] [PubMed] [Google Scholar]
- 20.Kao TK, Ou YC, Liao SL, Chen WY, Wang CC, Chen SY, Chiang AN, Chen CJ. Opioids modulate post-ischemic progression in a rat model of stroke. Neurochem Int. 2008;52:1256–1265. doi: 10.1016/j.neuint.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Chao D, Donnelly DF, Feng Y, Bazzy-Asaad A, Xia Y. Cortical δ-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2007;27:356–368. doi: 10.1038/sj.jcbfm.9600352. [DOI] [PubMed] [Google Scholar]
- 22.Chao D, Bazzy-Asaad A, Balboni G, Xia Y. δ-, but not μ-, opioid receptor stabilizes K+ homeostasis by reducing Ca2+ influx in the cortex during acute hypoxia. J Cell Physiol. 2007;212:60–67. doi: 10.1002/jcp.21000. [DOI] [PubMed] [Google Scholar]
- 23.Chao D, Bazzy-Asaad A, Balboni G, Salvadori S, Xia Y. Activation of DOR attenuates anoxic K+ derangement via inhibition of Na+ entry in mouse cortex. Cereb Cortex. 2008;18:2217–2227. doi: 10.1093/cercor/bhm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao D, Balboni G, Lazarus LH, Salvadori S, Xia Y. Na+ mechanism of δ-opioid receptor induced protection from anoxic K+ leakage in the cortex. Cell Mol Life Sci. 2009;66:1105–1115. doi: 10.1007/s00018-009-8759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia Y, Fung ML, O’Reilly JP, Haddad GG. Increased neuronal excitability after long-term O2 deprivation is mediated mainly by sodium channels. Brain Res Mol Brain Res. 2000;76:211–219. doi: 10.1016/S0169-328X(99)00338-1. [DOI] [PubMed] [Google Scholar]
- 26.Xia Y, Cao H, Zhang JH, Chen NY, Siegel K, Agulnik M, Haddad GG (2001) Effect of δ-opioid receptor activation on Na+ channel expression in cortical neurons subjected to prolonged hypoxia in culture. Soc Neurosci Abstract, Program No. 740.6
- 27.Xia Y, Zhao P, Xue J, Gu XQ, Sun X, Yao H, Haddad GG. Na+ channel expression and neuronal function in the Na+/H+ exchanger 1 null mutant mouse. J Neurophysiol. 2003;89:229–236. doi: 10.1152/jn.00488.2002. [DOI] [PubMed] [Google Scholar]
- 28.Zhao P, Ma MC, Qian H, Xia Y. Down-regulation of delta-opioid receptors in Na+/H+ exchanger 1 null mutant mouse brain with epilepsy. Neurosci Res. 2005;53:442–446. doi: 10.1016/j.neures.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal N, Alonso A, Ragsdale DS. Increased persistent sodium currents in rat entorhinal cortex layer V neurons in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia. 2003;44:1601–1604. doi: 10.1111/j.0013-9580.2003.23103.x. [DOI] [PubMed] [Google Scholar]
- 30.Jarnot M, Corbett AM. Immunolocalization of NaV1.2 channel subtypes in rat and cat brain and spinal cord with high affinity antibodies. Brain Res. 2006;1107:1–12. doi: 10.1016/j.brainres.2006.05.090. [DOI] [PubMed] [Google Scholar]
- 31.Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Jinsmaa Y, Bryant SD, Lazarus LH. Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J Med Chem. 2002;45:5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- 32.Műller M, Somjen GG. Na+ and K+ concentrations, extra- and intracellular voltage, and the effect of TTX in hypoxic rat hippocampal slices. J Neurophysiol. 2000;83:735–745. doi: 10.1152/jn.2000.83.2.735. [DOI] [PubMed] [Google Scholar]
- 33.Shah BS, Stevens EB, Gonzalez MI, Bramwell S, Pinnock RD, Lee K, Dixon AK. β3, a novel auxiliary subunit for the voltage-gated sodium channel, is expressed preferentially in sensory neurons and is upregulated in the chronic constriction injury model of neuropathic pain. Eur J Neurosci. 2000;12:3985–3990. doi: 10.1046/j.1460-9568.2000.00294.x. [DOI] [PubMed] [Google Scholar]
- 34.Guille M. Microinjection into Xenopus oocytes and embryos. Methods Mol Biol. 1999;127:111–123. doi: 10.1385/1-59259-678-9:111. [DOI] [PubMed] [Google Scholar]
- 35.Bartolomei F, Gastaldi M, Massacrier A, Planells R, Nicolas S, Cau P. Changes in the mRNAs encoding subtypes I, II and III sodium channel alpha subunits following kainate-induced seizures in rat brain. J Neurocytol. 1997;26:667–678. doi: 10.1023/A:1018549928277. [DOI] [PubMed] [Google Scholar]
- 36.Trimmer JS, Cooperman SS, Tomiko SA, Zhou JY, Crean SM, Boyle MB, Kallen RG, Sheng ZH, Barchi RL, Sigworth FJ. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989;3:33–49. doi: 10.1016/0896-6273(89)90113-X. [DOI] [PubMed] [Google Scholar]
- 37.Remy C, Remy S, Beck H, Swandulla D, Hans M. Modulation of voltage-dependent sodium channels by the δ-agonist SNC80 in acutely isolated rat hippocampal neurons. Neuropharmacology. 2004;47:1102–1112. doi: 10.1016/j.neuropharm.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Xia Y, Haddad GG. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res. 1991;549:181–193. doi: 10.1016/0006-8993(91)90457-7. [DOI] [PubMed] [Google Scholar]
- 39.Xia Y, Haddad GG. Major difference in the expression of μ- and δ-opioid receptors between turtle and rat brain. J Comp Neurol. 2001;436:202–210. doi: 10.1002/cne.1061. [DOI] [PubMed] [Google Scholar]
- 40.Dunn JF, Zaim WY, Meyerand E. Regional heterogeneity in the brain’s response to hypoxia measured using BOLD MR imaging. Magn Reson Med. 1999;41:850–854. doi: 10.1002/(SICI)1522-2594(199904)41:4<850::AID-MRM27>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Buresh Y, Koroleva VI, Korolev OS, Maresh V. Changes in the constant potential in brain structures in rats during focal ischemia and systemic hypoxia. Neurosci Behav Physiol. 1999;29:569–579. doi: 10.1007/BF02461150. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci. 2008;28:3190–3201. doi: 10.1523/JNEUROSCI.4403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fung ML, Croning MDR, Haddad GG. Sodium homeostasis in rat hippocampal slices during oxygen and glucose deprivation: role of voltage-sensitive sodium channels. Neurosci Lett. 1999;275:41–44. doi: 10.1016/S0304-3940(99)00728-4. [DOI] [PubMed] [Google Scholar]
- 45.Raley-Susman KM, Kass IS, Cottrell JE, Newman RB, Chambers G, Wang J. Sodium influx blockade and hypoxic damage to CA1 pyramidal neurons in rat hippocampal slices. J Neurophysiol. 2001;86:2715–2726. doi: 10.1152/jn.2001.86.6.2715. [DOI] [PubMed] [Google Scholar]
- 46.Mantegazza M, Gambardella A, Rusconi R, Schiavon E, Annesi F, Cassulini RR, Labate A, Carrideo S, Chifari R, Canevini MP, Canger R, Franceschetti S, Annesi G, Wanke E, Quattrone A. Identification of an Nav1.1 sodium channel (SCN1A) loss-of-function mutation associated with familial simple febrile seizures. Proc Natl Acad Sci USA. 2005;102:18177–18182. doi: 10.1073/pnas.0506818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu R, Thomas EA, Jenkins M, Gazina EV, Chiu C, Heron SE, Mulley JC, Scheffer IE, Berkovic SF, Petrou S. A childhood epilepsy mutation reveals a role for developmentally regulated splicing of a sodium channel. Mol Cell Neurosci. 2007;35:292–301. doi: 10.1016/j.mcn.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Armijo JA, Shushtarian M, Valdizan EM, Cuadrado A, de las Cuevas I, Adín J. Ion channels and epilepsy. Curr Pharm Des. 2005;11:1975–2003. doi: 10.2174/1381612054021006. [DOI] [PubMed] [Google Scholar]
- 49.Ma MC, Donnelly DF, Xia Y (2004) Neuronal preconditioning inhibits hypoxiainduced sodium channel up-regulation via d-opioid receptor. Soc Neurosci Abstract, 457.10, online
- 50.Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131:243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]