Abstract
Adenovirus particles can be engineered to display exogenous peptides on their surfaces by modification of viral capsid proteins, and particles that display pathogen-derived peptides can induce protective immunity. We constructed viable recombinant adenoviruses that display B-cell epitopes from the Plasmodium falciparum circumsporozoite protein (PfCSP) in the major adenovirus capsid protein, hexon. Recombinants induced high-titer antibodies against CSP when injected intraperitoneally into mice. Serum obtained from immunized mice recognized both recombinant PfCSP protein and P. falciparum sporozoites, and neutralized P. falciparum sporozoites in vitro. Replicating adenovirus vaccines have provided economical protection against adenovirus disease for over three decades. The recombinants described here may provide a path to an affordable malaria vaccine in the developing world.
Keywords: malaria vaccine, recombinant adenovirus, capsid display, immunogenicity
INTRODUCTION
Malaria imposes an immense burden of morbidity and mortality: more than 500 million clinical malaria infections and about 1 million deaths each year. Nearly all malaria mortality occurs in Africa, in children younger than 5 years of age. Malaria control methods based on drug treatment and vector control have succeeded in reducing disease in some places but are difficult to sustain and suffer from developing drug resistance in the parasite population and insecticide resistance in the vectors [1]. Development of an effective and economical malaria vaccine therefore is a high priority.
Immunization with radiation-attenuated sporozoites can provide immunity from malaria infection in animals and humans [2–5]. The immunodominant antigen in protection by sporozoites is circumsporozoite protein (CSP) [6], and subunit vaccines containing recombinant CSP protein have provided partial protection of humans from malaria infection [7–13]. Recombinant adenoviruses can effectively deliver CSP to mice: a single injection of an adenovirus recombinant that expressed P. yoelii CSP (PyCSP) induced high-titer PyCSP antibody and PyCSP-specific CD8+ and CD4+ T cells in mice and induced sterile protection in some individuals, with an overall 93% inhibition of liver stage development [14,15]. Recombinant adenoviruses that express CSP therefore may provide an effective tool for immunization against malaria in humans.
Like most recombinant adenovirus vaccine candidates, the PyCSP recombinant produces antigens intracellularly from a transgene that is expressed after the infection of cells by virus particles delivered in the inoculum. Alternatively, antigenic epitopes can be delivered by adenovirus recombinants whose capsids themselves incorporate and display exogenous peptides. Adenovirus particles that display foreign peptides can be potently immunogenic [16–18]. For example, an adenovirus recombinant that incorporates an immunodominant epitope from the Pseudomonas aeruginosa outer membrane protein F (OprF) in the major adenovirus capsid protein, hexon, induces strong humoral and cell-mediated immune responses and protects mice from lethal challenge by P. aeruginosa [18]. The central region of Plasmodium circumsporozoite proteins (CSP) include multiple repeats of short peptides. These CSP central repeats induce antibody responses when presented as recombinant proteins or synthetic peptides, and a protective antibody response to the CSP repeat has been demonstrated for P. yoelii in mice [19]. Because adenovirus recombinants that display exogenous peptides elicit strong antibody responses [18] and because antibody against CSP is an important component of immunity to sporozoite infection [19], adenovirus recombinants that display the CSP repeat may have potential in immunization against malaria. To assess this potential, we have constructed and characterized adenovirus recombinants that display peptides from the PfCSP central repeat in the context of the adenovirus hexon protein. Recombinants that display (NANP)5 or NANPNVDP(NANP)4 are highly immunogenic in mice and the (NANP)5 recombinant elicits sporozoite-neutralizing antibody. Adenovirus capsid display recombinants therefore are capable of inducing at least one of the elements of the immune response to sporozoites important in protection against malaria infection.
Material and Methods
Plasmids
pCP03 contains an intact wild-type adenovirus type 5 (Ad5) genome with a single NdeI restriction site in the hexon gene. pCP03 was created from pTG3602 [20] which contains an Ad5 genome including natural NdeI sites in the hexon gene (Ad5 nt 19548) and in the fiber gene (Ad5 nt 31082). To remove the NdeI site in the fiber gene, pTG3602 was digested with NdeI and PacI producing three viral DNA fragments and the pPolyII vector of pTG3602. The digested DNA was transfected into 293 cells [21] by CaPO4 transfection [22] along with a cloned viral DNA segment (nt 13255–27076) that spans the hexon NdeI site and an 86bp oligonucleotide that spans the fiber NdeI site and contains a silent mutation that destroys the NdeI recognition sequence. Recombination generated full length plaque-forming viral genomes lacking the fiber NdeI site. Fiber NdeI− mutant viral DNA was re-introduced into pTG3602 by recombination in E. coli SW102 [23] between purified virion DNA and a fragment of pTG3602 that bears the adenovirus ITRs and the pPolyII plasmid backbone, creating pCP03. To aid in screening of plasmids produced by recombination in bacteria and to reduce the background of wild type virus present following recombination in tissue culture, the lacZ expression cassette of pUC19 [24] was inserted into the unique Nde I site in the hexon gene in pCP03 by blunt-end ligation after NdeI digestion and repair of the cohesive end with T4 DNA polymerase. The resulting plasmid, pCP08, retains a single NdeI site located in the lacZ gene inserted into hexon. pJMG contains the Pme I (13255) to Bam HI (21562) fragment of Ad5 cloned into a derivative of pNEB193 (NEB) from which the Sac I site was removed by recombination with a mutant oligonucleotide.
Construction of recombinants
Insertions and substitutions in hypervariable region 1 of hexon were made by overlap extension PCR [25] using the primers listed in Table 1 and pJMG as the template. The primers encoding PfCSP peptides contained a novel BsiW1 restriction site to facilitate identification of recombinants. Final PCR products, about 1.5kb in length, were cloned into PCR-2.1-TOPO (Invitrogen) and modifications were verified by sequencing. PCR products were subcloned into pJMG using ApaI and SacI sites present in the viral portions of the overlap product.
Table 1.
Primers used in recombinant construction. Adenovirus sequences are in uppercase; CSP sequences are in lower case.
| Primer | Sequence |
|---|---|
| 5′ outside primer | 5′ CGGCGTGCTGGACAGGGGCCC3′ |
| 3′ outside primer | 5′ GCTGGCTCCGTCAACCC 3′ |
| G2 overlap (5′ side) | 5′ cattcgggttagcgttaggatttgcgttgggattggcattAGCTTCATCCCATTCGCAAGGATTTGGGG 3′ |
| G2 overlap (3′ side) | 5′ tcctaacgctaacccgaatgcaaaccccaacgccaatcctGTATTTGGGCAGGCGCCTTATTCTGG 3′ |
| II-g overlap (5′ side) | 5′ gcattcgggttagcgttaggatttgcgttaggatcgacgttgggattggcattAGCTTCATCCCATTCGCAAGG 3′ |
| II-g overlap (3′ side) | 5′ tcctaacgctaacccgaatgcaaaccccaacgccaatcctGCTACTGCTCTTGAAATAAACC 3′ |
Modified hexon fragments were incorporated into intact viral DNA by recombination either in bacteria or in transfected tissue culture cells. Bacterial recombination was performed as described [23]. The recipient plasmid was pCP08 cleaved once at the unique NdeI site within the hexon gene. For recombination in tissue culture, pCP03 was cut with NdeI and with PacI to release the viral DNA sequences from the plasmid. This DNA was introduced into 293 cells by CaPO4-mediated transfection along with plasmids carrying modified hexon fragments, also digested to release the viral sequences. DNAs were prepared from bacterial colonies (recombination in bacteria) or stocks prepared from picked plaques (recombination in tissue culture) and were screened for the presence of the novel restriction site incorporated into the PfCSP sequences. Virus was recovered from recombinant bacterial plasmids by transfection of 293 cells with PacI cleaved purified plasmid DNA. Stocks of recombinants were grown on 293 cells and purified by one discontinuous and two equilibrium CsCl density gradients [26]. Particle number in gradient-purified virus preparations was determined from the A260 of SDS-disrupted particles [27].
Immunoblotting
Immunoblotting was performed essentially as described [28]. Approximately 1 × 1010 purified virions or 1 × 104 sporozoites were disrupted by boiling in Laemmli sample buffer [29] and run on a 10% SDS- polyacrylamide gel. Blots were probed with anti-NANP monoclonal antibody 2A10 (MRA-183; [30]) at a dilution of 1:1000, with anti-serum against adenovirus late proteins (1:2000; [31]), or with immunized mouse sera (1:200).
Immunization of mice
4–6 week-old female C57BL/6 mice (Taconic Laboratories) were immunized with antigenically wild type adenovirus or capsid display recombinants by intraperitoneal injection of 150μl of phosphate-buffered saline containing 1010 CsCl gradient-purified virus particles. Mice were boosted identically at 3–4 weeks after the initial vaccination. Serum was collected from the tail vein prior to each immunization and periodically thereafter. All experiments were approved by the Animal Care and Use Committee of Johns Hopkins University.
ELISA
ELISA plates were coated with 1μg of either recombinant PfCSP or purified adenovirus particles. Recombinant PfCSP was a 6-His-tagged segment of the PfCSP protein that includes multiple copies of the central NANP repeat region encoded by plasmid pDS56-32/RBSII-CS27IVC-6XHis (Malaria Reference and Research Resource MRA-272). Recombinant PfCSP was purified from DH5α cell lysates by metal ion chromatography on a Ni-NTA agarose bead column (Qiagen) and was eluted and stored in 4M urea. Virus particles were purified as described above and disrupted with 4M urea before coating. Assays were performed in duplicate. The endpoint ELISA titer is reported as the highest dilution of serum at which the average absorbance was twice the value obtained using pre-immunization serum.
The isotypes of CSP- and Ad5-reactive antibodies were determined by ELISA, using a panel of isotype-specific secondary antibodies (Bio-Rad). Assays were performed on serum pools at dilutions of 1:500 (CSP) and 1:12,500 (Ad5) as recommended by the manufacturer. Data are presented as absorbance after 10 minutes development.
Indirect Immunofluorescence Assay (IFA)
Pooled sera from immunized mice were diluted in PBS containing 1% BSA and reacted with P. falciparum sporozoites air-dried onto multispot glass slides. After 1h incubation, slides were washed three times in PBS and incubated with an FITC-labeled goat anti-mouse IgG antibody for one hour. After three additional washes in PBS, slides were viewed with a fluorescence microscope.
Immunoelectron Microscopy
Immunoelectron microscopy was carried out as described [32]. NANP monoclonal antibody 2A10 (tissue culture supernatant) was used at a dilution of 1:500 and gold-conjugated secondary antibody (Jackson Immunoresearch) at 1:10.
Mosquito infection, parasite development and sporozoite production
Transgenic P. berghei that express CSP containing the central NANP region of the P. falciparum protein [33] were the generous gift of Dr. E. Nardin. To obtain sporozoites, Anopheles stephensi mosquitoes were fed on Swiss-Webster mice (Taconic Laboratories) that had been infected by intraperitoneal injection of blood stage transgenic parasites or by exposure to parasite-infected An. stephensi mosquitoes. 18–21 days after their last blood meal, mosquitoes were dissected and sporozoites were harvested from the salivary glands.
Quantitative transgenic sporozoite neutralization assay (TSNA)
TSNA was performed as described [34]. Briefly, 20,000 sporozoites freshly dissected from mosquitoes were incubated with a 1:6 dilution of mouse serum or monoclonal antibody 2A10 tissue culture supernatant for 45 minutes on ice and then added to human HepG2 cells grown to confluence in 24 well tissue culture plates. The medium was changed at 24 hours post-infection and total RNA was extracted after 48 hrs by the RNeasy (Qiagen) method as recommended by the supplier. Sporozoite replication was determined by qRT-PCR of P. berghei 18S RNA. Values were normalized against measurements of human actin mRNA in the same samples. Quantitative RT-PCR (qRT-PCR) was performed for transgenic P. berghei rRNA and cellular actin as previously described [35] modified to use a one step qRT-PCR kit (Invitrogen) according to manufacturer’s recommendations.
Image manipulation
Figures were prepared in Adobe Photoshop from digital and scanned images. In some cases, contrast and brightness were adjusted. Except as noted in the legends, any adjustments were made on complete images which were then cropped as needed.
RESULTS
Construction of capsid display recombinants
We prepared recombinants that express either of the P. falciparum CSP central repeat peptides (NANP)5 (recombinant G2) or NANPNVDP(NANP)4 (recombinant IIg) in the context of the adenovirus type 5 (Ad5) hexon protein. The (NANP)5 peptide was incorporated as a substitution for hexon amino acids 139–167; NANPNVDP(NANP)4 was inserted between hexon amino acids 138 and 139. Both of these modifications fall in hexon hypervariable region 1 (HVR1) [36].
To produce recombinants, an approximately 1.5 kb fragment of the hexon gene bearing the desired modification was first generated by overlap PCR. PCR fragments were cloned, their sequences were verified, and the modified PCR fragment was subcloned into a plasmid containing approximately 4kb of viral DNA on either side of the modification site to facilitate recombination with the viral genome. Modified hexon sequences were incorporated into intact viral genomes by recombination either with a viral genomic plasmid in bacteria (G2), or with a viral genome in transfected cells in tissue culture (IIg). Recombinants bearing modifications were identified by screening plasmid DNA (for recombination in bacteria) or viral DNA (for recombination in tissue culture) for a diagnostic restriction site incorporated into each modified hexon gene.
After plaque purification, stocks were prepared on 293 cells [21]. Recombinant virus particles were purified by banding in CsCl. Buoyant densities in CsCl were not noticeably different from wild-type virus, indicating that recombinant particles possess roughly normal ratios of DNA to protein. The yield of recombinant particles for both recombinants was within a factor of three of that of wild-type virus and the ratio of physical particles to plaque-forming virus determined after CsCl density gradient purification was similar to that of wild type Ad5 (15 – 25, data not shown), suggesting that recombinants grow normally and have normal stability during standard purification procedures. Insertion of CSP sequences in viral DNA was confirmed by analysis of restriction enzyme-digested virion DNA. Restriction patterns produced by both recombinants rule out unanticipated chromosomal rearrangements that result in retention of a wild type hexon gene in these viruses (data not shown).
Importantly, apart from the modified hexon gene, the recombinants are wild type Ad5. In particular, the E1 region is intact in recombinants and the recombinants therefore are viable on standard adenovirus host cell lines. Presumably, recombinants also would replicate in humans.
Immunological characterization of capsid display recombinants
To determine whether the PfCSP-derived peptides retain their immunologic reactivity when inserted into hexon, purified G2 [(NANP)5] and IIg [NANPNVDP(NANP)4] recombinant virus particles were probed in immunoblotting experiments with monoclonal antibody 2A10 [30], which recognizes a peptide [(NANP)3; [37]] that is present in both recombinants. Both G2 and IIg produce an antibody-reactive protein of approximately 120 kDa, consistent with the size of adenovirus hexon (Figure 1). Different recombinants are expected to have hexon proteins of slightly different sizes. The substitution in recombinant G2 of (NANP)5 for the 29 amino acids of HVR1 of native Ad5 hexon reduces the size of the protein by 9 residues, while the insertion of NVDP in HVR5 in recombinant IIg increases the length of hexon by 24 amino acids. Consistent with this prediction, the 2A10-reactive protein produced by G2 migrates slightly ahead of that produced by IIg in SDS-polyacrylamide gels, and the anticipated differences in the size of hexon among Ad5 and the recombinants are visible on Coomassie-stained SDS-polyacrylamide gels (Figure 1). We conclude that the hexon proteins made by the recombinants contain the intended CSP peptides and that the inserted NANP epitopes retain reactivity with an anti-NANP monoclonal antibody.
Figure 1.
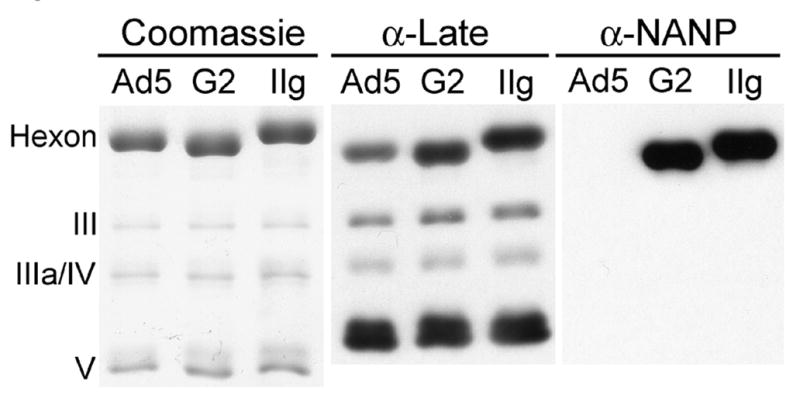
CSP epitopes are present in recombinant hexon proteins. 1010 purified Ad5, G2 or IIg virus particles were fractionated on SDS-polyacrylamide gels. The gels were stained with Coomassie Blue (left) or immunoblotted with Ad5 late protein antiserum (center) or anti-NANP monoclonal antibody 2A10 (right). G2 and IIg recombinant hexon proteins react with the monoclonal NANP antibody while wild type Ad5 hexon does not. G2 hexon contains a net 9 aa deletion and IIg hexon contains a 24 aa insertion, accounting for the differing mobilities of the three hexon proteins. The positions of major adenovirus capsid proteins, visible in the stained gel and the blot probed with anti-adenovirus serum, are marked on the left. The three panels are from different gels.
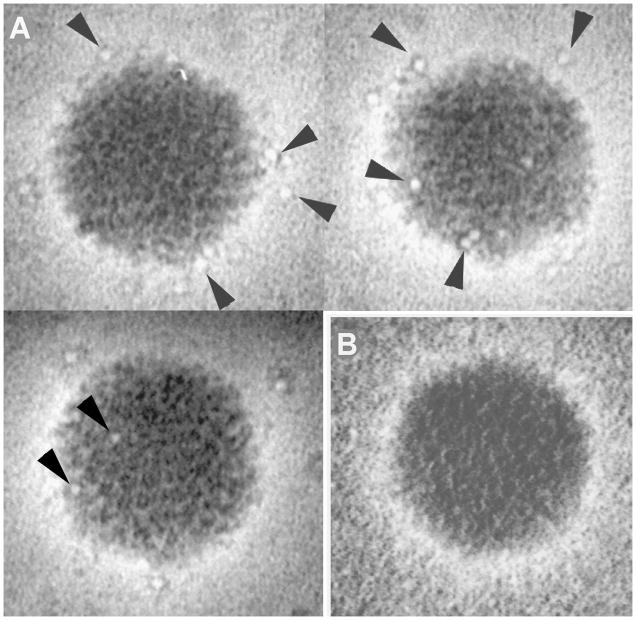
Peptides inserted into hexon hypervariable regions should be displayed on the surface of recombinant virus particles. To confirm that prediction, CsCl density gradient-purified recombinant G2 [(NANP)5] virus particles were examined by electron microscopy after immunoaffinity staining. Recombinant or wild type Ad5 particles were applied to carbon-coated grids, treated with (NANP)3 monoclonal antibody 2A10, and then treated with gold-conjugated secondary antibody. The grids were negatively stained with uranyl acetate and photographed with an electron microscope. The preparations of both recombinant and wild type virus contained adenovirus particles recognizable by size and shape (Fig. 2). Recombinant viruses were labeled with the gold conjugate (Fig. 2A) while none of the wild type viruses (Fig. 2B) were labeled. These results confirm that recombinant G2 displays the (NANP)3 epitope on the surface of the virus in a conformation that is recognized by neutralizing CSP antibody. The images also indicate that the modification made to hexon does not have gross effects on the overall structure of the virus.
Figure 2.
Immunogold labeling of capsid display recombinant G2. Purified G2 and wild type Ad5 virus particles were reacted first with anti-NANP monoclonal antibody 2A10 and then with rabbit anti-mouse IgG secondary antibody conjugated to 2nM gold beads. Electron micrographs of negatively-stained samples show that recombinant (A) but not wild-type Ad5 (B) virions are reactive with the NANP antibody as indicated by decoration by the gold-labeled secondary antibody (arrows). The four virions shown are taken from different negatives. Brightness and contrast were adjusted individually for clarity and consistency of appearance.
Immunization with capsid display recombinants induces antibodies against CSP that recognize P. falciparum sporozoites
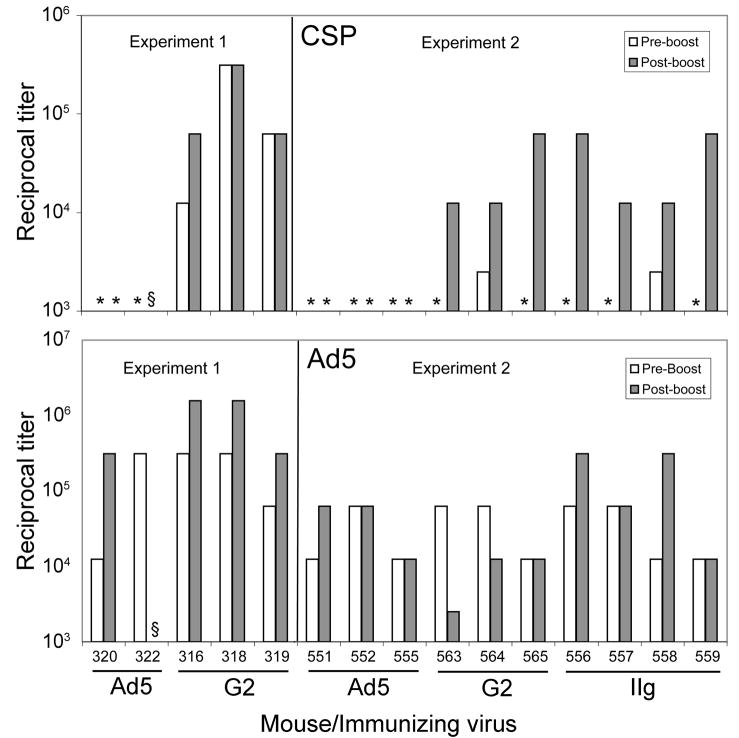
Ad5 neither replicates nor expresses late proteins (including hexon) in mice, and no recombinant protein will be produced in mice after immunization with capsid display recombinants. However, since the inserted CSP epitopes are displayed on the surface of recombinant virus particles, the particles themselves should be immunogenic. Therefore, we immunized groups of mice with recombinants G2 and IIg or with antigenically wild type Ad5. Immunization was by two or three intraperitoneal injections of 1010 CsCl gradient-purified virus particles at 3- to 4-week intervals. Sera were obtained from the tail vein prior to immunization and periodically after the initial injection. Serum samples from immunized and control mice were examined for Ad5 and CSP antibodies by ELISA using urea-disrupted purified virus particles or bacterial recombinant P. falciparum CSP, respectively, as the capture antigens. In two experiments, ELISA titers were determined for individual mice. All Ad5- and recombinant-immunized mice developed antibody to Ad5 after the first immunization (Figure 3, upper panel), with titers ranging from 1:12,500 to 1:312,500. Increases in Ad5 titer were observed in about half the mice after a second immunization. All mice immunized with CSP recombinants developed CSP antibody after either one or two immunizations (Figure 3, lower panel). Mice immunized with the Ad5 control developed no detectable CSP antibody.
Figure 3.
Antibody titers in immunized mice. Mice were immunized by two intraperitoneal injections of 1010 purified virus particles separated by 3 (Experiment 1) or 4 (Experiment 2) weeks. Sera collected immediately prior to the second immunization and 4 (Experiment 1) or 14 (Experiment 2) weeks post-boost were diluted in five-fold steps beginning at 1:2500 and antibodies against disrupted Ad5 virions (top panel) or recombinant CSP (bottom panel) were measured by ELISA. *: titer below 1:2500; §: sample lost.
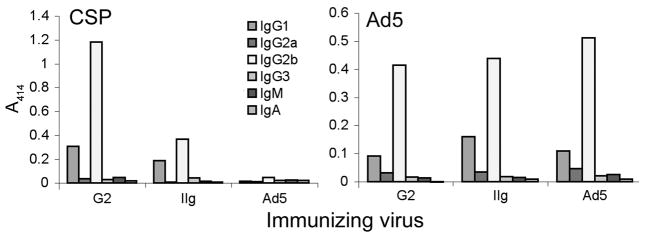
The isotype distribution of antibodies to CSP and Ad5 capsid proteins in serum pools also was determined (Figure 4). For both CSP and Ad5, the most prominent isotype was IgG2b, with substantial amounts of IgG1 also present. For CSP, little or no IgG2a was observed. Low levels of anti Ad5 IgG2a were detected in all pools, and an IgG2a CSP monoclonal antibody was sensitively detected (not shown). Little or no IgG3, IgA, or IgM was observed for CSP or for Ad5. Subcutaneous immunization of C57BL/6 mice with a capsid display recombinant that expressed an P. aeruginosa OmpF peptide, also induced primarily IgG2b antibodies, although in that study very little IgG1 was detected [18], which may reflect the differing routes of administration [38]. Monoclonal antibody Fab fragments are as effective as intact antibodies in protection of mice from P. berghei infection and in P. berghei sporozoite neutralization [39,40], suggesting that constant region-dependent processes are not crucial for protection and that isotype may not be a critical parameter in the humoral immune response.
Figure 4.
Isotype distribution of antibodies in immunized mice. Isotype-specific secondary antibodies were used in ELISAs to estimate levels of IgG1, IgG2a, IgG2b, IgA, and IgM in pooled sera from Experiment 2, week 14 (see Figure 3). For CSP, pooled sera were diluted 1:500; for Ad5, 1:12,500.
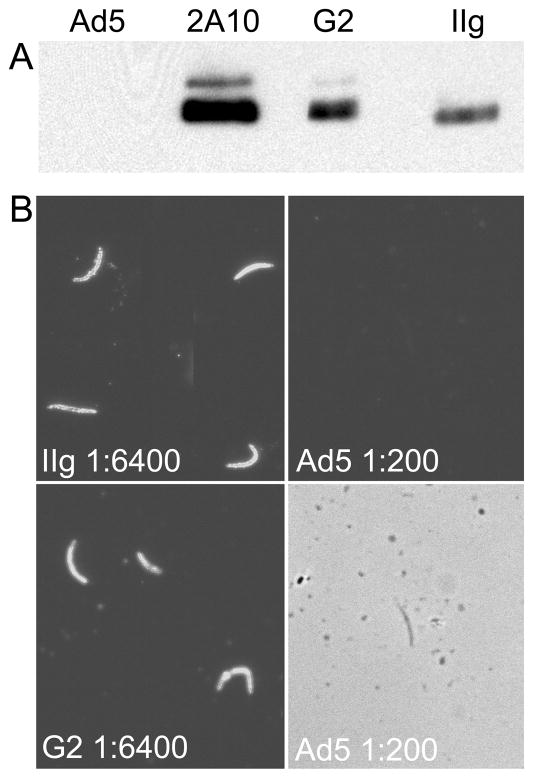
To confirm that sera from immunized mice recognize CSP produced by parasites, pooled serum from recombinant- and Ad5-immunized mice was used in immunoblotting experiments to probe whole sporozoite lysates of a transgenic P. berghei parasite that expresses a chimeric CSP gene that includes the P. falciparum NANP repeat region (CS(Pf) [33]). Recombinant immune serum, but not wild type Ad5 serum, detected a band in sporozoite lysates of slightly greater than 45kDa, the expected size of the hybrid CSP from CS(Pf) (Figure 5A).
Figure 5.
Capsid display recombinants induce antibodies that recognize authentic CSP. A. A transgenic CS(Pf) sporozoite lysate was fractionated by SDS-PAGE and immunoblotted with pooled sera from mice immunized with Ad5 or recombinants, or with NANP-specific monoclonal antibody 2A10. The monoclonal CSP antibody and sera from recombinant-immunized mice recognize a protein of the molecular weight predicted for CSP (about 50kDa,), while serum from Ad5-immunized mice do not. B. P. falciparum sporozoites air-dried onto glass slides were incubated with serially diluted pooled antisera from mice immunized with recombinants or Ad5, stained with FITC-conjugated secondary antibody, and photographed under UV illumination. For recombinant-immunized mice, the 1:6400 dilution is shown; for Ad5-immunized mice, the 1:200 dilution is shown. Exposures were identical. Recombinant panels show sporozoites from a single field, rearranged to save space. The lower Ad5 panel is a phase-contrast image of the area shown above, showing an unstained sporozoite.
To determine whether antibodies raised by recombinants recognize CSP in situ on the surface of sporozoites, air-dried P. falciparum sporozoites were treated with serially-diluted pooled mouse sera and with FITC-conjugated secondary antibody, and were examined with a fluorescence microscope. Pooled G2 and IIg sera each stained sporozoites at a dilution of 1:12,800 (Figure 5B; 1:6400 shown), while pooled serum from Ad5-immunized mice did not react with sporozoites at a dilution of 1:200. Thus, mice immunized with recombinants produce antibody that recognize authentic CSP in its normal context on the sporozoite surface.
Antibodies induced by capsid display recombinants neutralize sporozoites in vitro
Sporozoite-neutralizing antibodies can protect against malaria infection. Therefore, we investigated whether the antibodies induced by recombinant G2 were capable of neutralizing sporozoites in an in vitro assay (Transgenic Sporozoite Neutralizing Assay; TSNA; [34]). Pooled sera from recombinant G2- and Ad5-immunized mice, pre-immune sera, and the monoclonal antibody 2A10 were incubated with live transgenic P. berghei CS(Pf) sporozoites, which are sensitive to neutralization by antibodies to the P. falciparum CSP NANP repeat [33]. Treated sporozoites then were incubated with HepG2 liver cells in tissue culture. 48h post-infection, parasite replication was determined by quantitative RT-PCR (qRT-PCR) measurement of Plasmodium 18S ribosomal RNA (rRNA) in total RNA extracted from the infected cells. Results were normalized for RNA recovery by qRT-PCR measurement of cellular actin mRNA in the same samples. Experiments were conducted with sera collected after two doses of recombinant virus in two independent courses of immunization. In each experiment, serum from NANP-immunized mice reduced sporozoite infectivity ~6-fold compared to preimmune serum pools (Figure 6). Serum from IIg-immunized mice has not been tested. We conclude that capsid display recombinants can induce the humoral component of a potentially protective immune response to malaria.
Figure 6.
Capsid display recombinants induce neutralizing antibodies. Transgenic P. berghei sporozoites carrying the P. falciparum CSP NANP repeat were incubated with 1:6 dilutions of pre-immune serum, serum from Ad5- or G2-immunized mice, or CSP monoclonal antibody 2A10 (MAb). The treated sporozoites were added to liver cells in culture and parasite replication was measured 48h post-infection by qRT-PCR quantitation of P. berghei 18S rRNA in infected cells. Replication is expressed as the ratio between parasite rRNA and human actin mRNA for each RNA preparation. Ratios are the average of two biological replicates, each determined by three technical replicates. Error bars are the standard deviation of the mean of the two biological replicates. The monoclonal antibody and serum from G2-immunized mice reduced infectivity, while serum from Ad5-immunized mice did not. The right-most bar shows the 18S rRNA recovered from cells infected with killed (gamma-irradiated) sporozoites.
Discussion
The long-term goal of the work described here is a recombinant malaria vaccine patterned on vaccines used for nearly three decades to protect US military personnel from serious respiratory disease caused by adenovirus types 4 and 7 (Ad4 and Ad7). The military Ad4 and Ad7 vaccines are enteric-coated tablets containing low doses (~105 TCID50) of lyophilized live virus and are delivered as a single oral dose. Virus replication in the gut of a vaccinee induces both humoral and cell-mediated immune responses and protects against subsequent respiratory disease with an average efficacy in excess of 90% and with exemplary safety (reviewed in [41]). The properties of the oral adenovirus vaccines are well-suited for immunization in areas where public health resources are limiting. Immunization with the adenovirus vaccines is inexpensive, with an estimated cost to the military of $5 – $6 per vaccinee [42,43]. The single dose regimen eliminates the need for multiple visits to health care providers, minimizing demands on the public health infrastructure and mitigating compromised efficacy that can result from failure to comply with extended immunization schedules. Finally, the adenovirus vaccine’s oral route of delivery make it free of the hazards associated with the use of needles. Malaria exacts its greatest toll in the developing world, and live recombinant adenovirus vaccines may offer an approach that will be most valuable in the areas where malaria immunization presents its greatest challenges.
Circumsporozoite protein (CSP) is the immunodominant antigen in protection against malaria infection by immunization with irradiated sporozoites [6] and the recombinant CSP-based virus-like particle vaccine RTS, S is the only malaria vaccine candidate that has shown efficacy in large-scale human trials [11–13]. Several studies have shown that antibodies induced by CSP repeat peptides from P. berghei and P. yoelii are sufficient to provide protection from sporozoite infection [44]. Adenovirus particles that display foreign antigens can induce potent humoral immune responses [18], and adenovirus recombinants that display CSP epitopes therefore may induce a protective antibody response to CSP. To test this hypothesis, we prepared adenovirus recombinants that incorporate B-cell epitopes derived from the P. falciparum CSP central repeat [(NANP)5, or NANPNVDP(NANP)4] in the major adenovirus capsid protein, hexon. The recombinants are viable, have normal particle/plaque forming unit ratios, and can be purified by standard methods. Encouragingly, the recombinants produce hexon proteins and virus particles that are reactive with monoclonal antibodies against the NANP repeat.
Our recombinants are replication-competent and are designed to be used as live vaccines. Because human adenoviruses do not replicate in mice (or most other small laboratory animals), recombinants cannot be evaluated in those systems under the conditions of their intended use in humans. However, capsid display recombinants can elicit immune responses in mice despite failure to replicate [18]. Therefore, we sought to confirm the immunogenicity of CSP-bearing recombinants in mice. After intraperitoneal injection into mice, recombinants induce high titer anti-CSP antibodies detectable by ELISA. Both G2 [(NANP)5] and IIg (NANPNVDP(NANP)3 sera are reactive with intact sporozoites, and G2 immunized mouse serum neutralizes sporozoites bearing the P. falciparum NANP repeat in vitro (IIg was not tested). The immunogenicity of purified recombinant particles in mice strongly suggests that recombinants containing NANP-related epitopes will induce neutralizing antibody in immunized humans.
Cell mediated mechanisms also contribute to immunity to malaria in animals. Recombinant adenoviruses that express transgenes in animals induce strong T-cell responses to exogenous antigens [15,45–47]. However, our recombinants do not replicate in mice and no intracellular production of recombinant hexon proteins will have occurred in our immunized animals. Therefore, while we anticipate that in humans expression of malaria T-cell epitopes by replicating recombinants will induce T-cell responses that could contribute to protection, T-cell responses to these recombinants in mice are not likely to be a good indicator of the response in humans and were not characterized.
The data reported here show that P. falciparum CSP epitopes displayed on adenovirus particles can elicit neutralizing antibody, supporting the hypothesis that the antibody responses induced by the live oral military vaccines will extend to malaria epitopes present on capsid display recombinants. Extensive studies in primates of replicating adenovirus recombinants that express HIV antigens [45,47–50] further indicate that replicating recombinants can induce potent cell-mediated responses to exogenous antigens. These data and prior experience with live adenovirus vaccines argue strongly for the promise of live oral adenovirus-based recombinant vaccines against malaria as an efficient means to mitigate the burden of malaria in the developing world.
Acknowledgments
The authors wish to thank Dr. Godfree Mlambo for assistance in mosquito husbandry and Drs. David Sullivan, Ian Cockburn, Barry Falgout and Amy Baker for critical comments on the manuscript. Dr. Michael Berg and Amy Baker provided valuable advice and assistance on many aspects of this work. Eric Stevens ably assisted in mouse immunization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World malaria report 2005. World Health Organization; 2005. [Google Scholar]
- 2.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 3.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozoite-induced falciparum malaria. Am J Med Sci. 1973;266(3):169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24(3):397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 6.Kumar KA, Sano G, Boscardin S, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444(7121):937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JE, Giersing B, Mullen G, Moorthy V, Richie TL. Malaria vaccines: are we getting closer? Curr Opin Mol Ther. 2007;9(1):12–24. [PubMed] [Google Scholar]
- 8.Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 9.Sun P, Schwenk R, White K, et al. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4(+) and CD8(+) T cells producing IFN-gamma. J Immunol. 2003;171(12):6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 10.Heppner DG, Jr, Kester KE, Ockenhouse CF, et al. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine. 2005;23(17–18):2243–2250. doi: 10.1016/j.vaccine.2005.01.142. [DOI] [PubMed] [Google Scholar]
- 11.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364(9443):1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 12.Alonso PL, Sacarlal J, Aponte JJ, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366(9502):2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 13.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359(24):2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues EG, Zavala F, Eichinger D, Wilson JM, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol. 1997;158(3):1268–1274. [PubMed] [Google Scholar]
- 15.Rodrigues EG, Zavala F, Nussenzweig RS, Wilson JM, Tsuji M. Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine. 1998;16 (19):1812–1817. doi: 10.1016/s0264-410x(98)00181-9. [DOI] [PubMed] [Google Scholar]
- 16.Crompton J, Toogood CI, Wallis N, Hay RT. Expression of a foreign epitope on the surface of the adenovirus hexon. J Gen Virol. 1994;75 (Pt 1):133–139. doi: 10.1099/0022-1317-75-1-133. [DOI] [PubMed] [Google Scholar]
- 17.McConnell MJ, Danthinne X, Imperiale MJ. Characterization of a permissive epitope insertion site in adenovirus hexon. J Virol. 2006;80(11):5361–5370. doi: 10.1128/JVI.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worgall S, Krause A, et al. Hee KK, Vintayen EV, Hackett NR, Roelvink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J Clinical Investigation. 2005;115(5):1218–1219. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R, Charoenvit Y, Corradin G, et al. Induction of protective polyclonal antibodies by immunization with a Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine. J Immunol. 1995;154(6):2784–2793. [PubMed] [Google Scholar]
- 20.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70(7):4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 22.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 23.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33 (4):e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33 (1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 25.Pogulis RJ, Vallejo AN, Pease LR. In vitro recombination and mutagenesis by overlap extension PCR. Methods Mol Biol. 1996;57:167–176. doi: 10.1385/0-89603-332-5:167. [DOI] [PubMed] [Google Scholar]
- 26.Ketner G, Boyer J. Isolation, growth, and purification of defective adenovirus deletion mutants. In: Wold WSM, editor. Adenovirus Methods and Protocols. The Humana Press, Inc; Totowa, NJ: 1998. [DOI] [PubMed] [Google Scholar]
- 27.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70(11):7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 2001. [Google Scholar]
- 29.Laemmli UK, Quittner SF. Maturation of the head of bacteriophage T4. IV. The proteins of the core of the tubular polyheads and in vitro cleavage of the head proteins. Virology. 1974;62(2):483–499. doi: 10.1016/0042-6822(74)90409-7. [DOI] [PubMed] [Google Scholar]
- 30.Nardin EH, Nussenzweig V, Nussenzweig RS, et al. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982;156(1):20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ketner G, Bridge E, Virtanen A, Hemstrom C, Pettersson U. Complementation of adenovirus E4 mutants by transient expression of E4 cDNA and deletion plasmids. Nucleic Acids Res. 1989;17(8):3037–3048. doi: 10.1093/nar/17.8.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg M, DiFatta J, Hoiczyk E, Schlegel R, Ketner G. Viable adenovirus vaccine prototypes: high-level production of a papillomavirus capsid antigen from the major late transcriptional unit. Proc Natl Acad Sci U S A. 2005;102(12):4590–4595. doi: 10.1073/pnas.0500933102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol. 2002;169(12):6681–6685. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- 34.Kumar KA, Oliveira GA, Edelman R, Nardin E, Nussenzweig V. Quantitative Plasmodium sporozoite neutralization assay (TSNA) J Immunol Methods. 2004;292(1–2):157–164. doi: 10.1016/j.jim.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Bruna-Romero O, Gonzalez-Aseguinolaza G, Hafalla JC, Tsuji M, Nussenzweig RS. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc Natl Acad Sci U S A. 2001;98(20):11491–11496. doi: 10.1073/pnas.191380898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rux JJ, Kuser PR, Burnett RM. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods. J Virol. 2003;77(17):9553–9566. doi: 10.1128/JVI.77.17.9553-9566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zavala F, Tam JP, Hollingdale MR, et al. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- 38.Gahery-Segard H, Juillard V, Gaston J, et al. Humoral immune response to the capsid components of recombinant adenoviruses: routes of immunization modulate virus-induced Ig subclass shifts. Eur J Immunol. 1997;27(3):653–659. doi: 10.1002/eji.1830270312. [DOI] [PubMed] [Google Scholar]
- 39.Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollingdale MR, Zavala F, Nussenzweig RS, Nussenzweig V. Antibodies to the protective antigen of Plasmodium berghei sporozoites prevent entry into cultured cells. J Immunol. 1982;128(4):1929–1930. [PubMed] [Google Scholar]
- 41.Gaydos CA, Gray GC. Adenovirus Vaccine. In: Plotkin SA, Orenstein MD, editors. Vaccines. Saunders; Philadelphia PA: 2008. [Google Scholar]
- 42.Hyer RN, Howell MR, Ryan MA, Gaydos JC. Cost-effectiveness analysis of reacquiring and using adenovirus types 4 and 7 vaccines in naval recruits. Am J Trop Med Hyg. 2000;62(5):613–618. doi: 10.4269/ajtmh.2000.62.613. [DOI] [PubMed] [Google Scholar]
- 43.Howell MR, Nang RN, Gaydos CA, Gaydos JC. Prevention of adenoviral acute respiratory disease in Army recruits: cost-effectiveness of a military vaccination policy. Am J Prev Med. 1998;14(3):168–175. doi: 10.1016/s0749-3797(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji M, Zavala F. Peptide-based subunit vaccines against pre-erythrocytic stages of malaria parasites. Mol Immunol. 2001;38(6):433–442. doi: 10.1016/s0161-5890(01)00079-7. [DOI] [PubMed] [Google Scholar]
- 45.Buge SL, Richardson E, Alipanah S, et al. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71(11):8531–8541. doi: 10.1128/jvi.71.11.8531-8541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casimiro DR, Chen L, Fu TM, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77(11):6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng B, Wang LR, Gomez-Roman VR, et al. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J Virol. 2005;79(16):10200–10209. doi: 10.1128/JVI.79.16.10200-10209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patterson LJ, Malkevitch N, Zhao J, Peng B, Robert-Guroff M. Potent, persistent cellular immune responses elicited by sequential immunization of rhesus macaques with Ad5 host range mutant recombinants encoding SIV Rev and SIV Nef. DNA Cell Biol. 2002;21(9):627–635. doi: 10.1089/104454902760330165. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Roman VR, Robert-Guroff M. Adenoviruses as vectors for HIV vaccines. AIDS Rev. 2003;5(3):178–185. [PubMed] [Google Scholar]
- 50.Malkevitch N, Patterson LJ, Aldrich K, Richardson E, Alvord WG, Robert-Guroff M. A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques. J Immunol. 2003;170(8):4281–4289. doi: 10.4049/jimmunol.170.8.4281. [DOI] [PubMed] [Google Scholar]