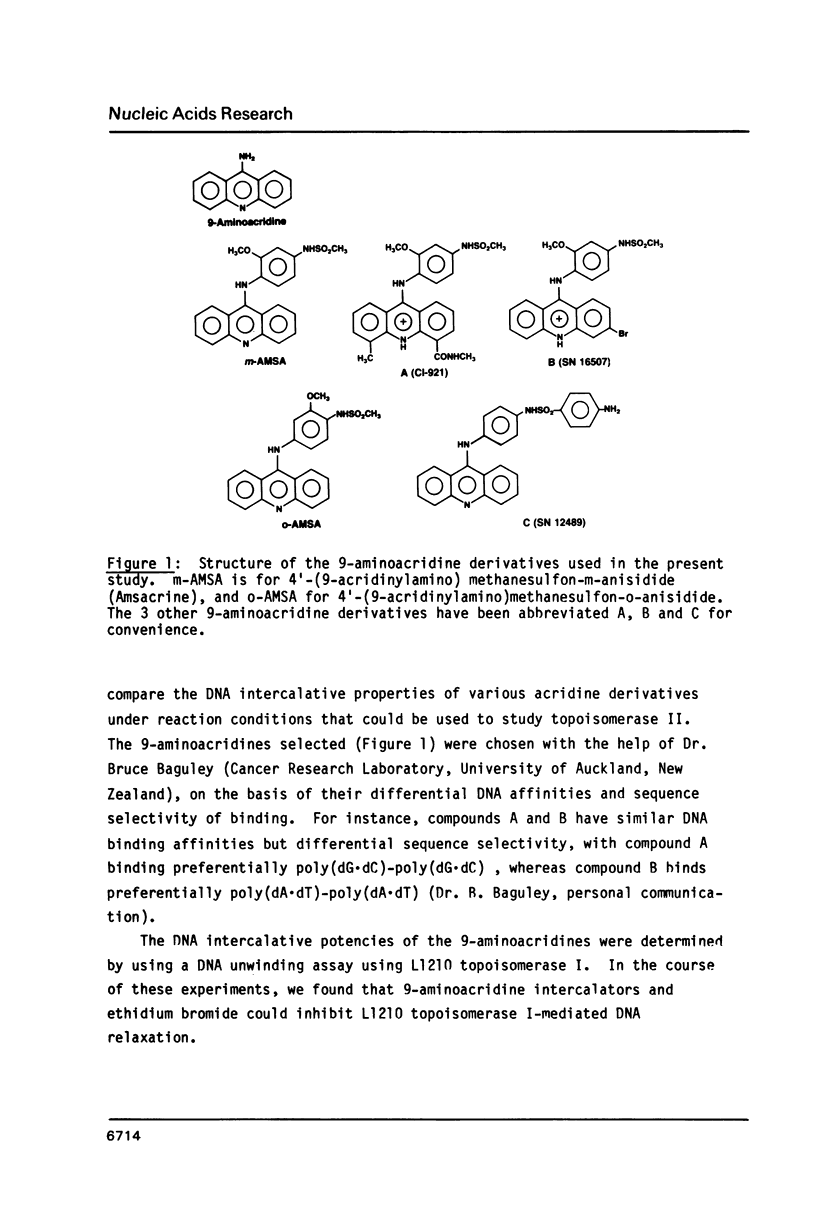
Abstract
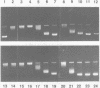
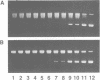
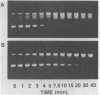
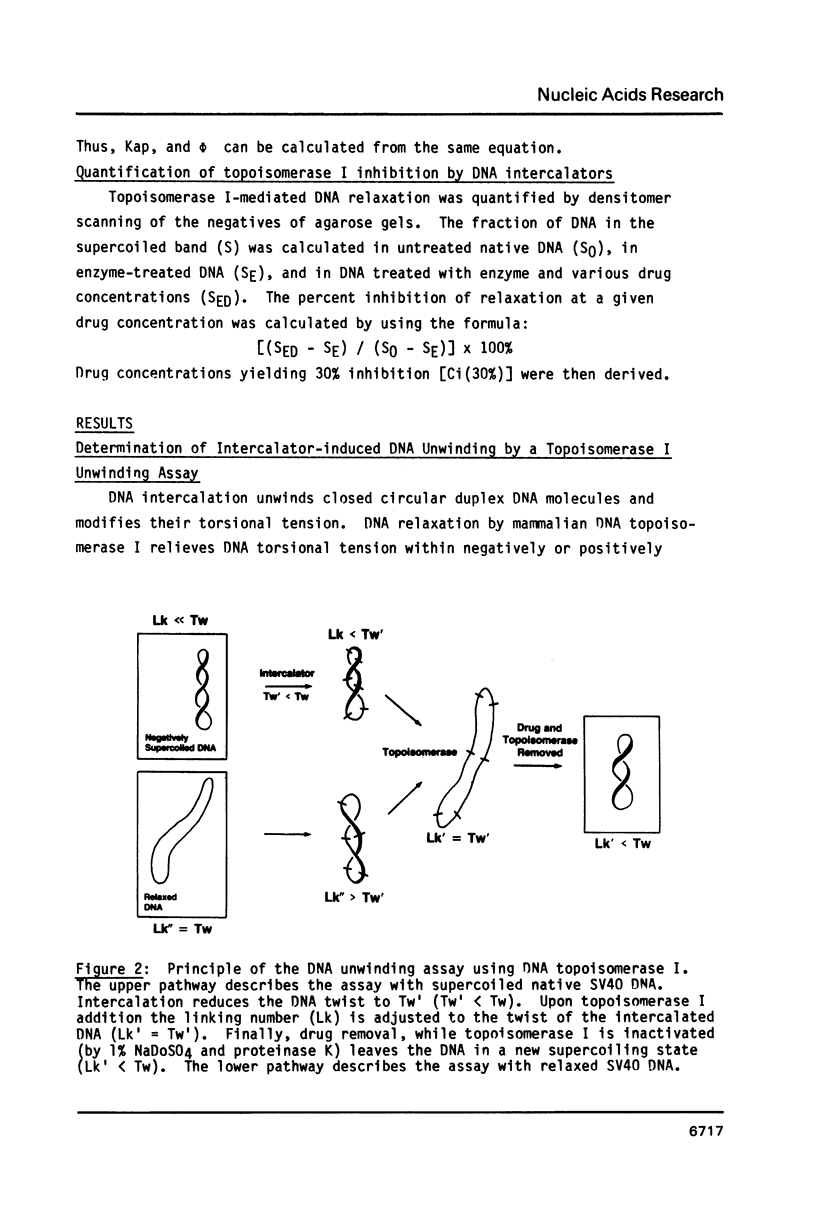
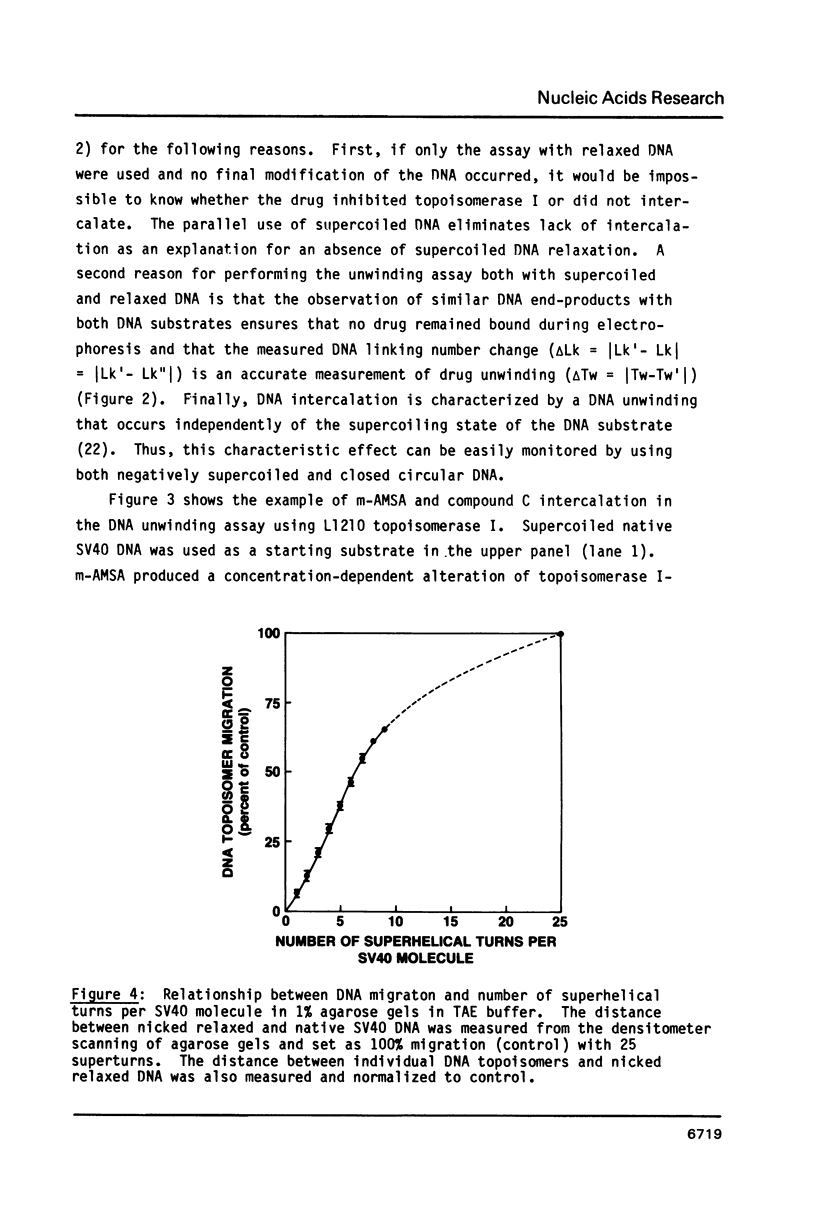
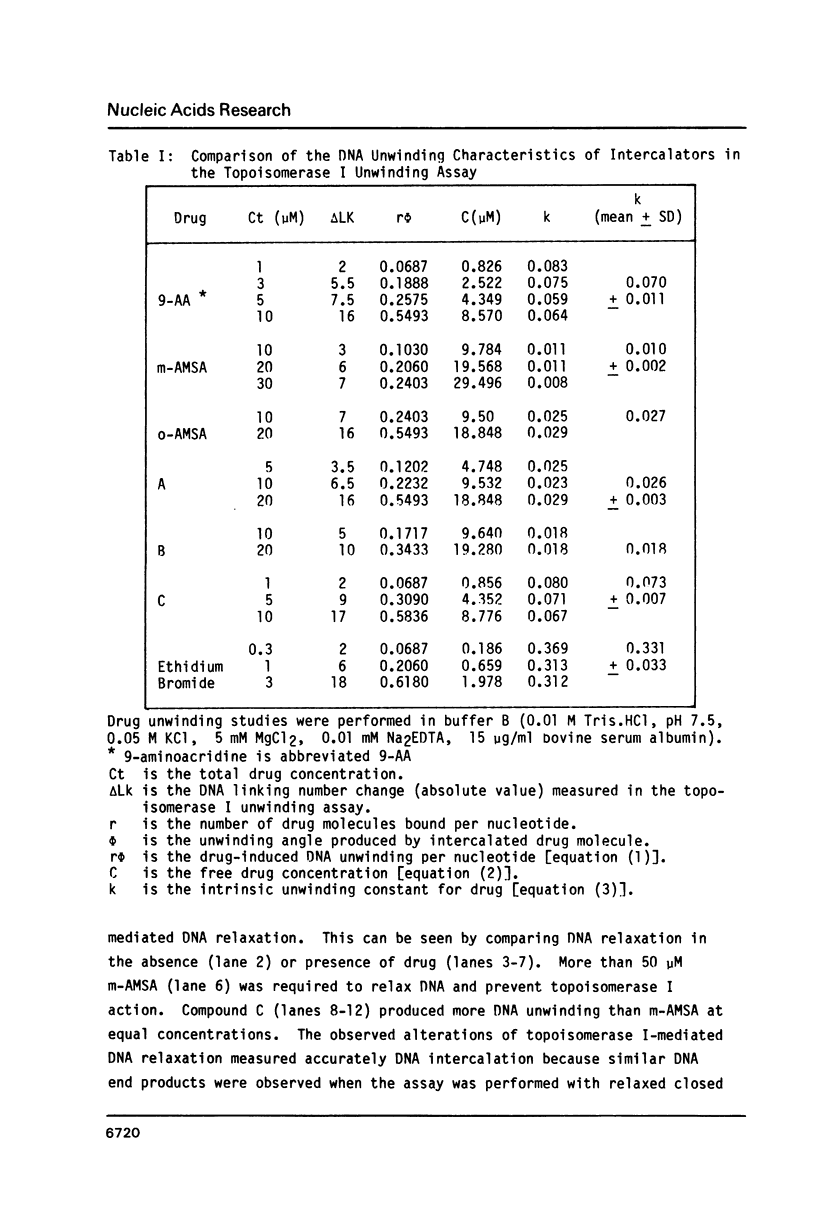
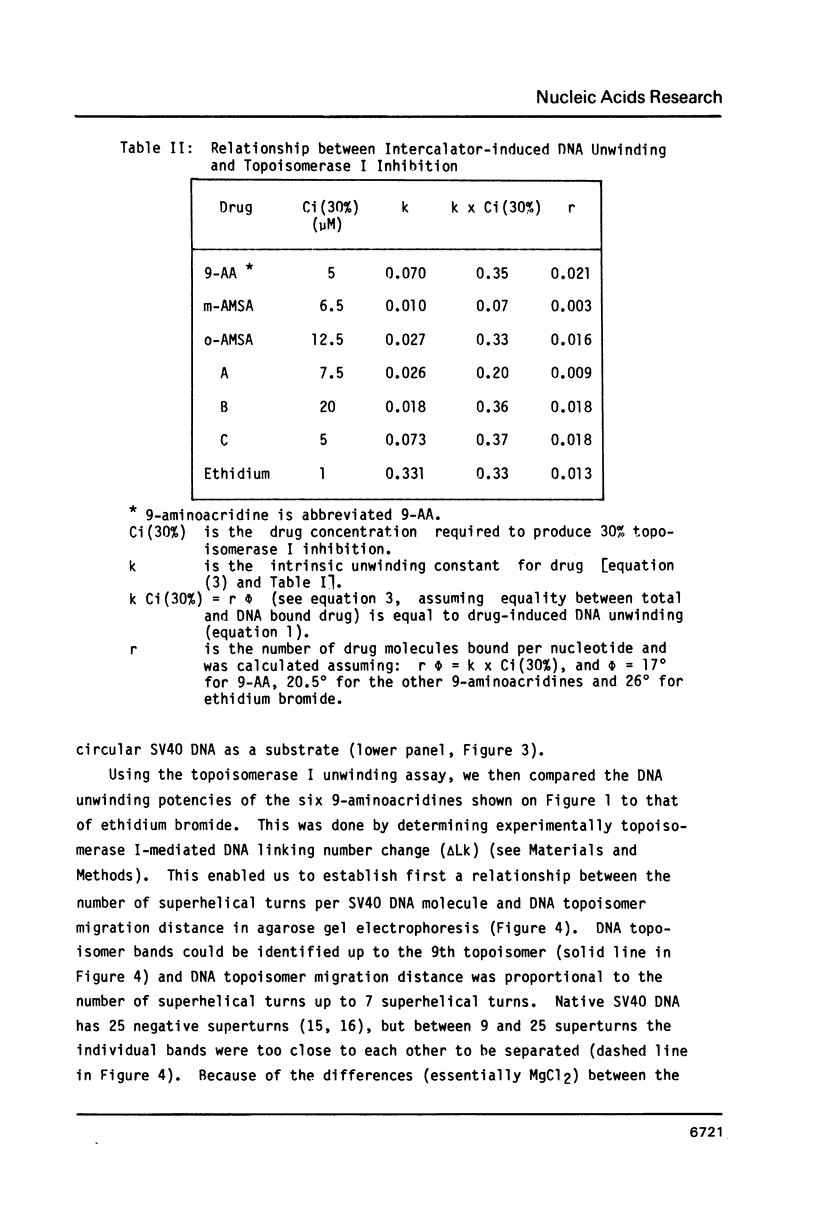
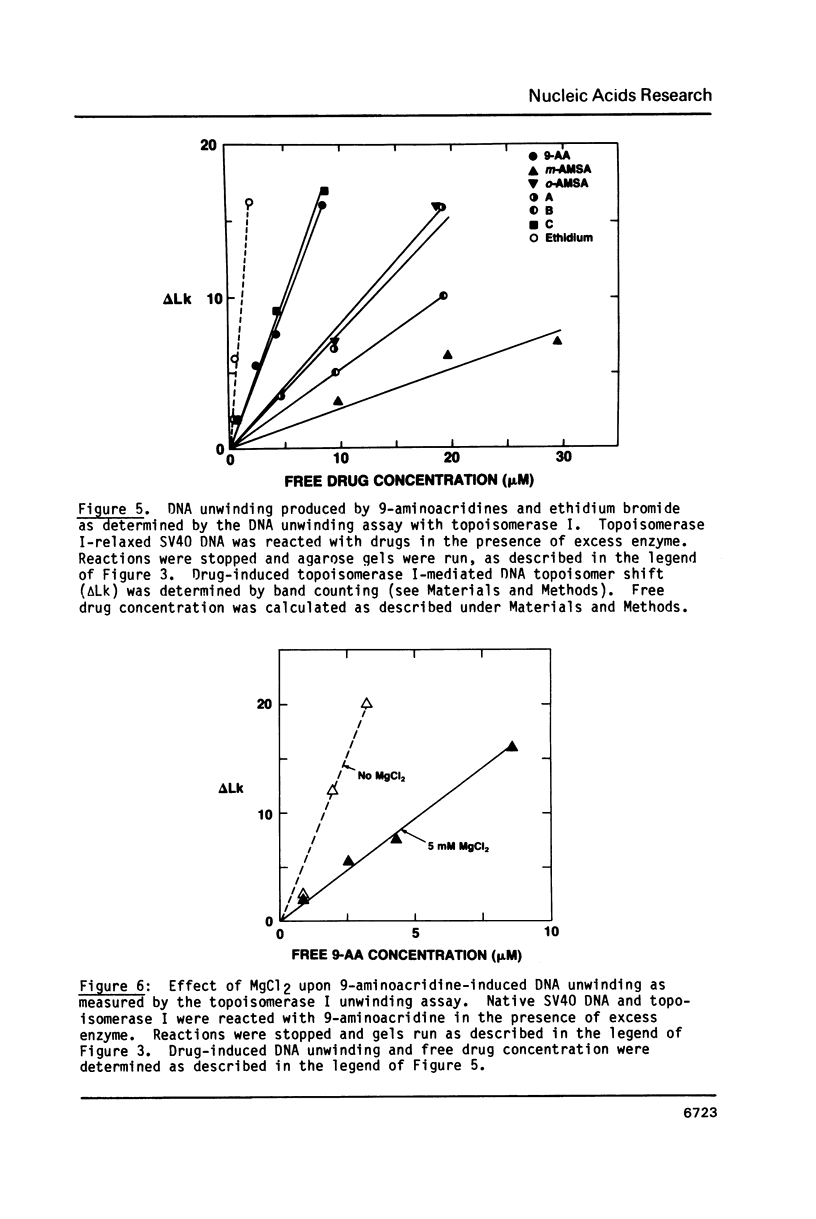
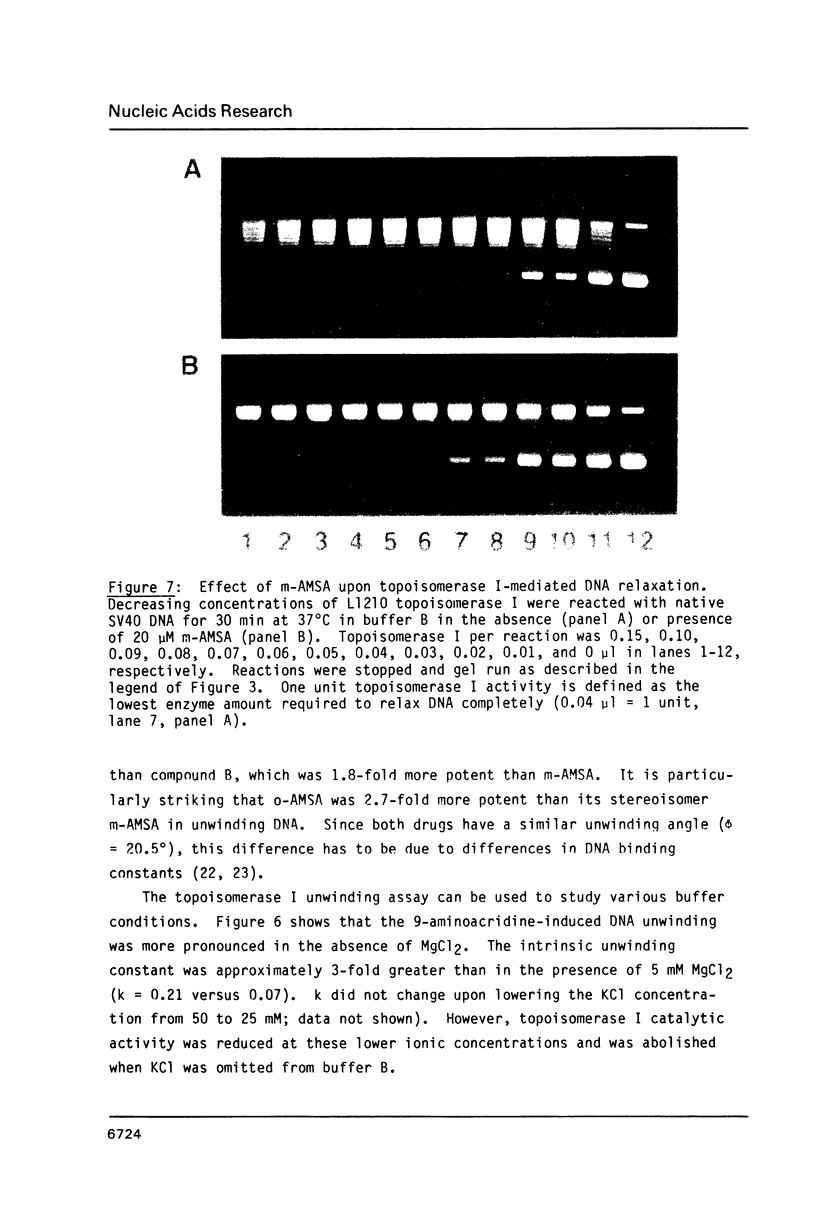
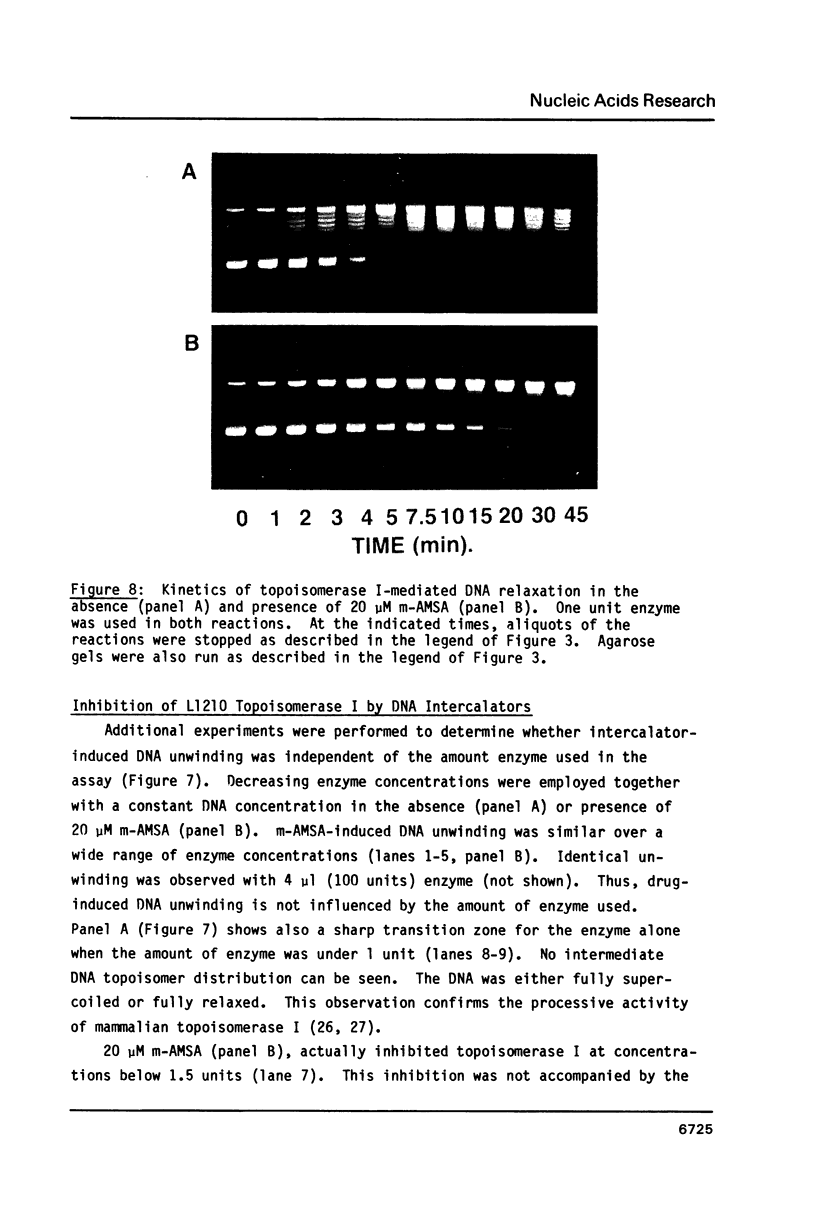
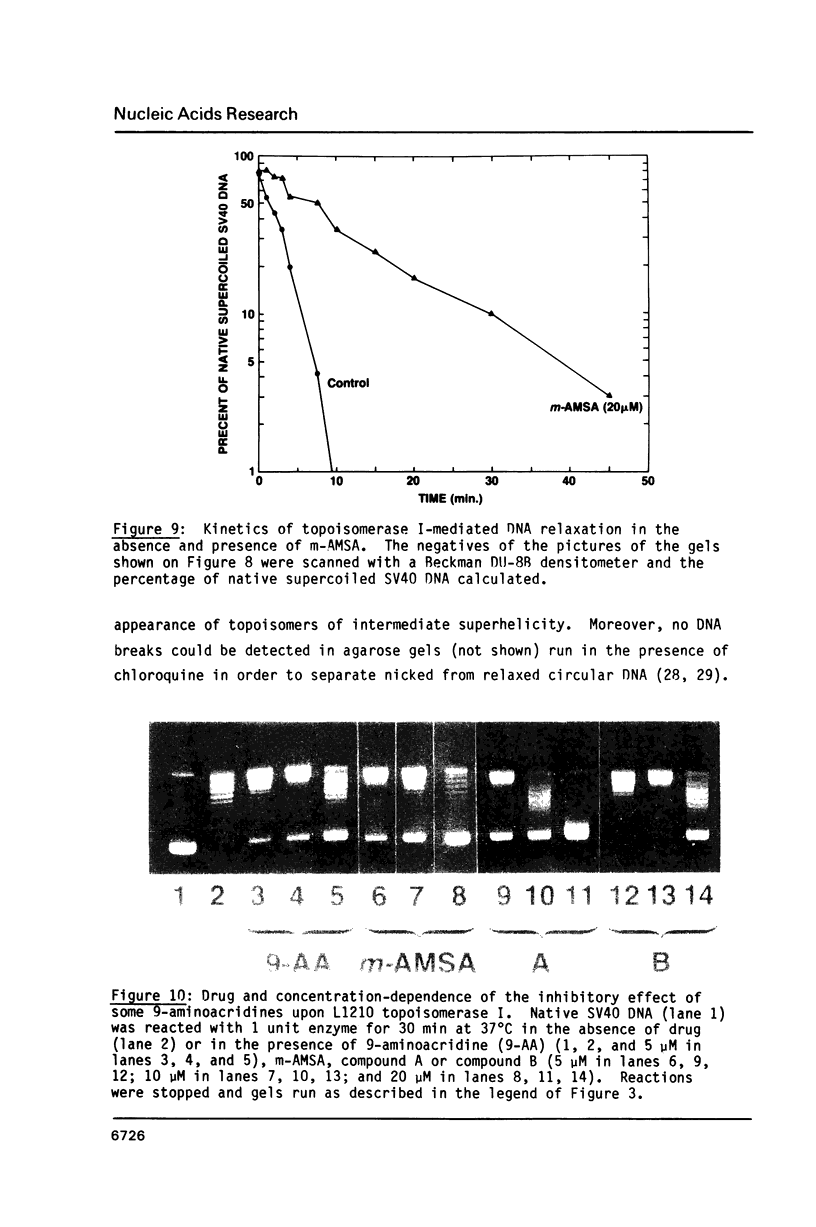
The DNA unwinding effects of some 9-aminoacridine derivatives were compared under reaction conditions that could be used to study drug-induced topoisomerase II inhibition. An assay was designed to determine drug-induced DNA unwinding by using L1210 topoisomerase I. 9-aminoacridines could be ranked by decreasing unwinding potency: compound C greater than or equal to 9-aminoacridine greater than o-AMSA greater than or equal to compound A greater than compound B greater than m-AMSA. Ethidium bromide was more potent than any of the 9-aminoacridines. This assay is a fast and simple method to compare DNA unwinding effects of intercalators. It led to the definition of a drug intrinsic unwinding constant (k). An additional finding was that all 9-aminoacridines and ethidium bromide inhibited L1210 topoisomerase I. Enzyme inhibition was detectable at low enzyme concentrations (less than or equal to 1 unit) and when the kinetics of topoisomerase I-mediated DNA relaxation was studied. Topoisomerase I inhibition was not associated with DNA swivelling or cleavage.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. R. Structure and reactions of closed duplex DNA. Annu Rev Biophys Bioeng. 1978;7:287–313. doi: 10.1146/annurev.bb.07.060178.001443. [DOI] [PubMed] [Google Scholar]
- Been M. D., Burgess R. R., Champoux J. J. Nucleotide sequence preference at rat liver and wheat germ type 1 DNA topoisomerase breakage sites in duplex SV40 DNA. Nucleic Acids Res. 1984 Apr 11;12(7):3097–3114. doi: 10.1093/nar/12.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain B. F., Atwell G. J., Denny W. A. Potential antitumor agents. 16.4'-(Acridin-9-ylamino)methanesulfonanilides. J Med Chem. 1975 Nov;18(11):1110–1117. doi: 10.1021/jm00245a013. [DOI] [PubMed] [Google Scholar]
- Cain B. F., Atwell G. J., Seelye R. N. Potential antitumour agents. 11. 9-anilinoacridines. J Med Chem. 1971 Apr;14(4):311–315. doi: 10.1021/jm00286a010. [DOI] [PubMed] [Google Scholar]
- Cain B. F., Atwell G. J. The experimental antitumour properties of three congeners of the acridylmethanesulphonanilide (AMSA) series. Eur J Cancer. 1974 Aug;10(8):539–549. doi: 10.1016/0014-2964(74)90079-6. [DOI] [PubMed] [Google Scholar]
- Cain B. F., Seelye R. N., Atwell G. J. Potential antitumor agents. 14. Acridylmethanesulfonanilides. J Med Chem. 1974 Sep;17(9):922–930. doi: 10.1021/jm00255a003. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Denny W. A., Cain B. F., Atwell G. J., Hansch C., Panthananickal A., Leo A. Potential antitumor agents. 36. Quantitative relationships between experimental antitumor activity, toxicity, and structure for the general class of 9-anilinoacridine antitumor agents. J Med Chem. 1982 Mar;25(3):276–315. doi: 10.1021/jm00345a015. [DOI] [PubMed] [Google Scholar]
- Douc-Rasy S., Kayser A., Riou G. F. Inhibition of the reactions catalysed by a type I topoisomerase and catenating enzyme of Trypanosoma cruzi by DNA-intercalating drugs. Preferential inhibition of the catenating reaction. EMBO J. 1984 Jan;3(1):11–16. doi: 10.1002/j.1460-2075.1984.tb01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G., Pilbrow J. R. Physico-chemical probes of intercalation. Int J Biochem. 1984;16(12):1179–1192. doi: 10.1016/0020-711x(84)90215-5. [DOI] [PubMed] [Google Scholar]
- Dougherty G. The unwinding of circular DNA by intercalating agents as determined by gel electrophoresis. Biosci Rep. 1983 May;3(5):453–460. doi: 10.1007/BF01121956. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Lebowitz J. A simple electrophoretic method for the determination of superhelix density of closed circular DNAs and for observation of their superhelix density heterogeneity. Anal Biochem. 1976 May 7;72:95–103. doi: 10.1016/0003-2697(76)90510-8. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. S., Bauer W. R. Temperature dependence of the gel electrophoretic mobility of superhelical DNA. Nucleic Acids Res. 1985 Mar 11;13(5):1665–1682. doi: 10.1093/nar/13.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Minford J., Pommier Y., Filipski J., Kohn K. W., Kerrigan D., Mattern M., Michaels S., Schwartz R., Zwelling L. A. Isolation of intercalator-dependent protein-linked DNA strand cleavage activity from cell nuclei and identification as topoisomerase II. Biochemistry. 1986 Jan 14;25(1):9–16. doi: 10.1021/bi00349a002. [DOI] [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini A. M., Ciarrocchi G. Inhibition of Micrococcus luteus DNA topoisomerase I by UV photoproducts. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1787–1791. doi: 10.1073/pnas.80.7.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Minford J. K., Schwartz R. E., Zwelling L. A., Kohn K. W. Effects of the DNA intercalators 4'-(9-acridinylamino)methanesulfon-m-anisidide and 2-methyl-9-hydroxyellipticinium on topoisomerase II mediated DNA strand cleavage and strand passage. Biochemistry. 1985 Nov 5;24(23):6410–6416. doi: 10.1021/bi00344a015. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Shure M., Tang D., Vinograd J., Vosberg H. P. Action of nicking-closing enzyme on supercoiled and nonsupercoiled closed circular DNA: formation of a Boltzmann distribution of topological isomers. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4280–4284. doi: 10.1073/pnas.72.11.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T. C., Chen G. L., Hsiang Y. H., Liu L. F. DNA damage by antitumor acridines mediated by mammalian DNA topoisomerase II. Cancer Res. 1986 Apr;46(4 Pt 2):2021–2026. [PubMed] [Google Scholar]
- Shure M., Pulleyblank D. E., Vinograd J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 1977;4(5):1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure M., Vinograd J. The number of superhelical turns in native virion SV40 DNA and minicol DNA determined by the band counting method. Cell. 1976 Jun;8(2):215–226. doi: 10.1016/0092-8674(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA-binding characteristics of acridinylmethanesulphonanilide drugs: comparison with antitumour properties. Eur J Cancer. 1976 Dec;12(12):995–1001. doi: 10.1016/0014-2964(76)90066-9. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Jones R. L. Intercalating drugs: DNA binding and molecular pharmacology. Adv Pharmacol Chemother. 1981;18:177–222. doi: 10.1016/s1054-3589(08)60255-0. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Erickson L. C., Ungerleider R. S., Nichols M., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4'-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981 Nov 10;20(23):6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]